Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Plasma and its derivatives are well-established clinical resources, but cost, risk of infectious disease transmission, although rare, and other adverse effects mandate their appropriate use. Even to this day, however, much still remains to be clarified regarding the appropriate clinical indications/protocols for plasma product use. Plasma can be separated from red blood cells (RBCs) through centrifugation of whole blood at the time of collection or can be collected by apheresis as a single product or as a by-product of platelet or RBC apheresis. Plasma can be processed into derivatives through cold ethanol fractionation (method of Cohn), additional factor purification steps, and pathogen inactivation. With the advent of genetic and cellular engineering technologies, the development of recombinant products with no human proteins is possible. In this chapter, the features and uses of plasma products, which include fresh frozen plasma (FFP), plasma frozen within 24 hours of phlebotomy (FP24), thawed plasma, liquid plasma, solvent detergent treated plasma (SD-plasma), pathogen-reduced plasma, cryoprecipitate, cryoprecipitate-reduced plasma (CRP), as well as plasma derivatives, including albumin, intravenous and intramuscular immunoglobulin, and purified and recombinant coagulation factor concentrates are discussed.
Plasma is the acellular, fluid compartment of blood and consists of 90% water, 7% protein and colloids, and 2% to 3% nutrients, crystalloids, hormones, and vitamins. The protein fraction contains the soluble clotting factors: fibrinogen, factor XIII, von Willebrand factor (vWF), factor VIII primarily bound to its carrier protein vWF, and the vitamin K-dependent coagulation factors II, VII, IX, and X. Primary indications for transfusion of plasma are either general clotting factor replacement or specific factor replacement when purified or recombinant products are not available. Clotting proteins are the constituents for which transfusion of plasma is required. Plasma products include FFP, FP24, thawed plasma, SD-plasma, and pathogen-reduced plasma, which are clinically equivalent.
Plasma frozen at −18°C or colder within 8 hours of donation (or within 6 hours with the use of some storage bags after apheresis collection) can be labelled as FFP. A second type of frozen plasma, the most commonly used in the United States, is FP24 plasma. FP24 is frozen at −18°C or colder within 24 hours of collection. FFP and FP24 may be stored up to 1 year before use, at which time it is thawed at 37°C for 20 to 30 minutes. After thawing, plasma is stored at 1°C to 6°C and transfused within 24 hours, after which it becomes thawed plasma (described below). A direct comparison between FFP and FP24 mean factor activity immediately post-thaw indicates that either product can be used interchangeably such that they are considered clinically equivalent ( Table 115.1 ).
| Factor | FFP | FP24 | Thawed Plasma at Day 5 | ∼Minimum Activity Required for Surgical Hemostasis |
|---|---|---|---|---|
| I (mg/dL) | 280 | 309 | 303 | 100 |
| II (IU/dL) | 97 | 97 | 96 | 20–40 |
| V (U/dL) | 85 | 86 | 59 | 25 |
| VII (IU/dL) | 105 | 89 | 77 | 10–20 |
| VIII (IU/dL) | 81 | 66 | 48 | 30 |
| IX (IU/dL) | 82 | 88 | 84 | 25–30 |
| X (IU/dL) | 94 | 94 | 91 | 10–20 |
Thawed FFP and FP24 stored at 1°C to 6°C for up to 5 days is termed thawed plasma and most coagulation factors remain relatively stable in it. Although there is evidence that activity levels for factors V, VII, and VIII decrease, this is not considered to be of clinical significance, as the mean factor activity levels for 5-day-old thawed plasma remain above the minimum activity required for surgical hemostasis (see Table 115.1 ). Compared to frozen plasma, stored thawed plasma improves patient care as there is no preparation time required resulting in a decreased turn-around time, and because of longer shelf life, it reduces wastage rate.
CRP, also known as cryosupernatant or cryoreduced plasma, is the remaining supernatant after the removal of cryoprecipitate from FFP, which is subsequently refrozen. This product has significantly decreased levels of factor VIII, factor XIII, vWF, fibrinogen, and fibronectin. The primary indication of CRP is for transfusion or plasma exchange in patients with thrombotic thrombocytopenic purpura (TTP). It can be thawed and stored for up to 5 days at 1°C to 6°C and is termed thawed plasma cryoprecipitate reduced.
Liquid plasma is plasma that is separated from whole blood at any time during storage and up to 5 days after the whole blood expiration date. Liquid plasma is never frozen and is maintained at 1°C to 6°C and stored for 26 days or 40 days depending on the anticoagulant preservative used to collect the whole blood. Clotting factor levels progressively decrease over time, particularly labile clotting factors (i.e., factors V and VIII). This product contains viable leukocytes, and thus would need product modifications (leukoreduction or irradiation) prior to transfusion in at-risk individuals (e.g., immunocompromised). It is used primarily for immediate treatment of patients undergoing massive transfusion due to massive hemorrhage.
Lyophilized plasma is produced and pooled from 10 or fewer apheresis plasma donors. The plasma undergoes a cryodesiccation process to form a 215 g powder in a sterile bottle. The plasma is then rehydrated for use via 200 mL of sterile water with soft agitation. While not in use yet in the United States (but used in Europe and in the US military), clinical trials in the US are underway. Studies suggest that the product is safe and efficacious for treatment of traumatic hemorrhage.
SD-plasma is US Food and Drug Administration (FDA)-approved (Octaplas, Octapharma USA Inc., Hoboken, NJ, USA; marketed as Octaplas LG in Europe and other markets) and is manufactured from ≤2500 pooled plasma products that have been treated with solvent (tributyl phosphate)/detergent (triton X-100) to inactivate lipid-enveloped viruses (HIV, hepatitis B, hepatitis C). Additionally, the product is tested for hepatitis A and parvovirus B19. The product is distributed in 200 mL containers, frozen at −18°C with a shelf life of 1 year. This pooled and treated plasma product has a lower rate of allergic reactions and has a substantially reduced risk of transfusion-related acute lung injury (TRALI), as the manufacturing process is considered to dilute neutrophil antibodies and soluble human leukocyte antigens (HLAs).
Other pathogen-reduction methods have been developed, including amotosalen photochemical treatment with ultraviolet (UVA) light (INTERCEPT plasma, Cerus Corp, Concord, CA), which is FDA approved. Riboflavin-treated plasma with UVA light (Mirasol Pathogen Reduction Technology, Terumo BCT, Denver, CO) and methylene blue-treated plasma (Theraflex System, MacoPharma, Mouvaux, France) are used outside of the United States, but these have not yet been FDA approved. Studies in Europe show that these methods are also quite effective at reducing the risk of pathogen contamination.
There are plasma products that are not used for transfusion but are used for further manufacturing into plasma derivatives. These products include recovered plasma that are derived from whole blood and are sent to a manufacturer from a collection facility through a “short supply agreement.” Source plasma, an FDA licensed product, is collected by apheresis which is intended for further manufacturing. The Plasma Protein Therapeutics Association promotes safe collection and manufacturing practices of plasma derivatives. Source plasma can be collected more frequently under special donor programs; the donors can be compensated for their time.
Convalescent plasma is plasma obtained from a patient who has recovered from or has been vaccinated against an infectious disease and has high titers of neutralizing antibodies (e.g., COVID-19). Convalescent plasma is transfused to infected patients to prevent or treat severe or life-threatening complications. COVID convalescent plasma potentially is beneficial if transfused early in the disease course or to patients who are immunocompromised. Convalescent plasma can also be pooled and treated, as described below, to create a hyperimmune globulin.
Most guidelines consistently support plasma transfusions for the treatment of bleeding in patients with multiple acquired coagulation factor deficiencies, as seen in liver failure, disseminated intravascular coagulopathy (DIC), massive transfusion, and in certain indications as a factor replacement during therapeutic plasma exchange (TPE; Table 115.2 ). However, the optimal transfusion strategy for these indications is not well established. Currently, there are no evidence-based laboratory value “triggers” for plasma administration, and any recommendation needs to be carefully weighed against the patient's presence, or risk, of bleeding. Plasma transfusion is not indicated for use in clotting disorders where purified, concentrated, or recombinant clotting factors are available (e.g., factor VIII and IX deficiencies).
| Indicated |
|
|
|
|
|
|
|
| Not Indicated |
|
|
|
|
|
Plasma is typically indicated when prothrombin time (PT) and/or partial thromboplastin time (PTT) are greater than 1.5 to 1.7 times normal paired with the presence of bleeding or anticipated bleeding. These current recommendations are justified as there is compelling evidence that plasma transfusions are ineffective in correcting mild to moderate abnormalities of coagulation screening tests. However, marked reductions in substantially elevated coagulation studies can occur with relatively modest plasma transfusion volumes. This response to plasma can be largely explained because of the nonlinear, exponential relationship between clotting factor activity levels and coagulation test results ( Fig. 115.1 ).
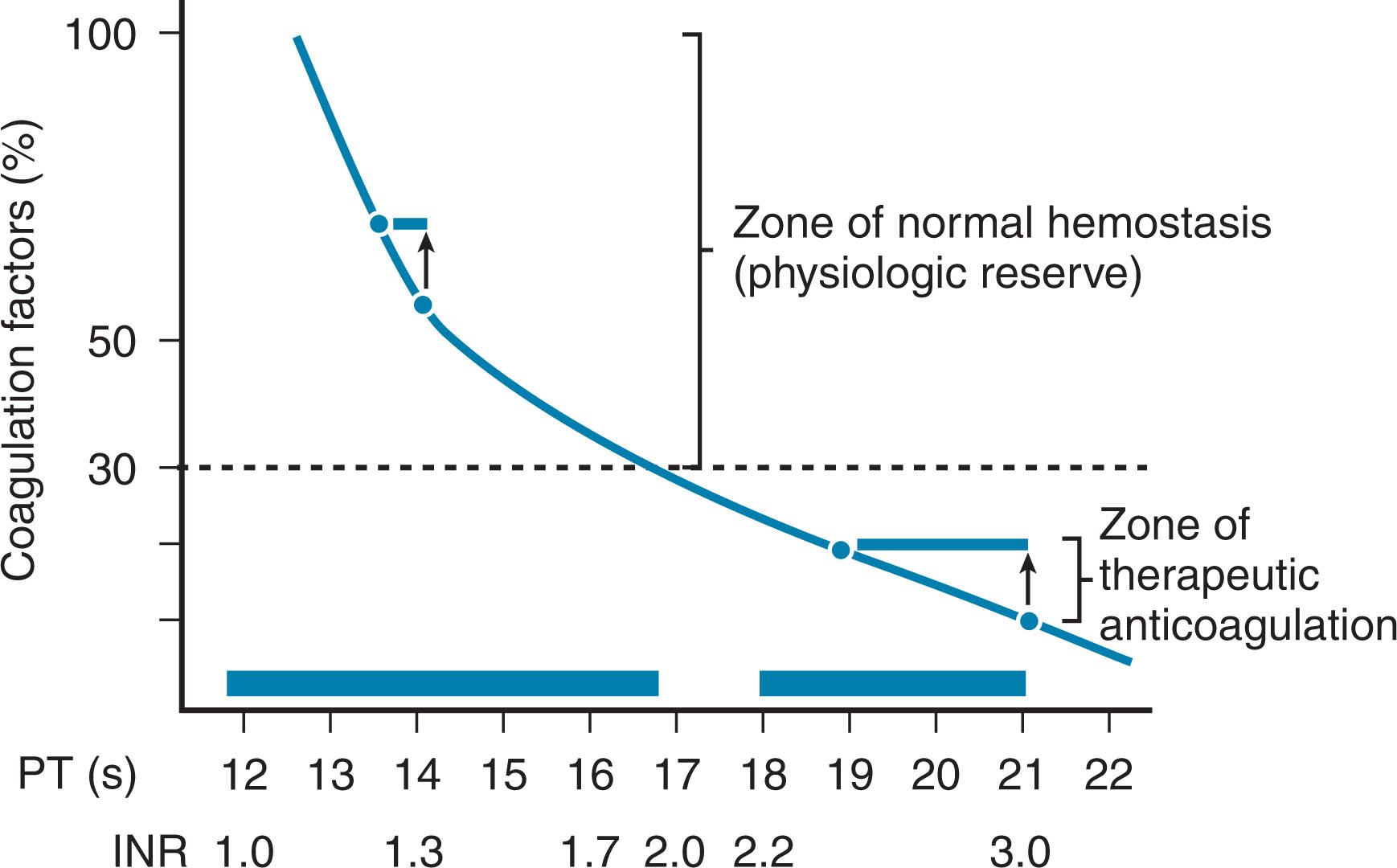
Audits of recent transfusion practices have consistently demonstrated that plasma product use is inappropriately high. Recent estimates suggest up to 83% (reported range: 10% to 83%) of plasma transfusions are not administered according to published guidelines. The most commonly cited reason for plasma administration is a preprocedural elevation in coagulation studies. This indication is not evidence-based, especially when the coagulation abnormality is mild-moderate. Moreover, plasma should not be used as a volume expander or as a source of nutrients. Clinical situations where plasma transfusions may be beneficial are described below.
Patients with liver failure may develop low levels of the vitamin K-dependent clotting factors (factors II, VII, IX, and X). These patients develop a prolonged PT/INR, PTT, and thrombin time. Fibrin split products may also be elevated in these patients, and in later stages, the fibrinogen level may be decreased. Hemorrhage, most often secondary to an anatomic lesion, may be worsened by the coagulopathy. Patients undergoing orthotopic liver transplantation complicated by preexisting severe liver disease and liver disease with DIC are two examples that may require large plasma transfusion volumes.
While elevations in coagulation tests are correlated with the incidence of bleeding in these patients, growing evidence now suggests that the PT and PTT are, in themselves, poor predictors of surgical bleeding. The reason for this lack of association may be twofold. First, PT and PTT values do not correlate well with plasma factor activity levels. One study identified that up to 50% of patients with abnormal coagulation tests had coagulation activity levels considered sufficient for adequate thrombus formation. Moreover, studies demonstrate that mild abnormalities in these coagulation tests do not correct—even with infusion of large quantities of plasma—because of the mathematical difficulty of infusing normal levels of factors into mildly deficient blood to get enough plasma to decrease the PT/PTT (see Fig. 115.1 ). Second, the lack of increased/excessive bleeding noted in some patients with liver disease and elevated coagulation tests may be caused by a parallel reduction in anticoagulant proteins, such as proteins C and S. Therefore, patients with liver disease may not bleed as much as expected because they retain a homeostatic balance between coagulant and anticoagulant proteins. Evidence suggests that the use of plasma in the context of severe liver disease and perioperatively during liver transplant does not significantly improve outcome. As a result, the use of plasma should be more limited in liver disease and hepatectomy patients. Plasma transfusion in these patients should be guided by a combination of clinical assessment, evidence and degree of bleeding, and coagulation test results. Plasma products are currently not recommended prophylactically before a surgical challenge or liver biopsy in these patients. However, as noted previously, plasma transfusions may be considered when the PT/PTT is greater than 1.5 to 1.7 times normal, or if the INR is 2.0 or greater when the risk of bleeding is considered high.
Massive transfusion is generally defined as receiving 10 or more units of RBCs within 24 hours (or one blood volume). Trauma patients may arrive at the hospital with a prolonged PT (termed acute trauma-induced coagulopathy) due to protein C activation, fibrinogen depletion, and platelet dysfunction. Acute trauma-induced coagulopathy is associated with increased mortality and increased use of blood products. Trauma patients can also develop a secondary coagulopathy, termed the lethal triad—dilutional coagulopathy, acidosis, and hypothermia. The dilutional coagulopathy is secondary to the administration of crystalloid and RBCs without coagulation factor support. Guidelines supported by randomized control trials recommend early use of tranexamic acid, an antifibrinolytic.
Studies have shown that the early use of plasma and platelets in trauma patients undergoing massive transfusion appears to decrease the incidence of secondary coagulopathy (lethal triad) and improve survival in these patients. The Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study demonstrated that early transfusion of plasma (within minutes of arrival to a trauma center) was associated with improved 6-hour survival after admission. The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial compared the effectiveness and safety of a 1:1:1 (plasma:platelet:RBC) transfusion ratio with a 1:1:2 transfusion ratio in trauma patients who were predicted to receive a massive transfusion. This randomized clinical trial found no overall difference in survival based on transfusion ratio, but did find that those who were randomized to receive more plasma (1:1:1 ratio) achieved hemostasis more quickly and frequently.
Questions regarding best practice remain. Currently, the use of cold stored whole blood is being evaluated and used in the prehospital and early hospital acute bleeding episodes. Studies support the use of whole blood early in trauma care prior to the need of specific blood components as a result of laboratory testing. Other studies are investigating the early use of cryoprecipitate or fibrinogen concentrates as part of massive transfusion protocols. Recently trials investigating the benefits of hospital plasma transfusion have been published. Prehospital plasma transfusion may benefit trauma victims who have longer transit time and suffered blunt trauma.
To improve the speed of identifying and treating coagulation abnormalities, some protocols incorporate thromboelastography (TEG) or other point-of-care tests. TEG technology provides a dynamic and global assessment of the coagulation process and can provide rapid assessments of the patient’s platelet function, coagulation cascade, and fibrinolysis, aiding in determining what medications or blood products the patient needs.
Massive transfusion in other conditions, such as liver, cardiac, or orthopedic surgery and obstetric hemorrhage, likely have a different pathophysiology and thus transfusion management of these patients may be different than trauma patients. Institutions should have policies in place for rapid availability of blood products and laboratory testing in these patient populations.
DIC may be secondary to sepsis, liver disease, hypotension, hypoperfusion, trauma, obstetric complications, leukemia (usually promyelocytic), or underlying malignancy. Successful treatment of the underlying cause is paramount. Recent guidelines suggest that plasma therapy should not be initiated based on abnormal laboratory results alone. Rather, patients with DIC and bleeding, those requiring an invasive procedure, and those at risk for bleeding complications should be given plasma in amounts sufficient to correct or ameliorate the coagulopathy or hemorrhagic diathesis. Large volumes of plasma are often necessary to correct the coagulation defect in these patients (up to 30 mL/kg). However, in patients with severe liver disease, bleeding, and DIC, plasma infusions often fail to normalize the PT and PTT.
Warfarin inhibits the hepatic synthesis of vitamin K-dependent clotting factors (factors II, VII, IX, and X) by blocking the recovery of the form of vitamin K that is active in the carboxylation of these proteins. Warfarin therapy induces functional deficiencies of these factors, which correct within 48 hours after the discontinuation of warfarin if diet and vitamin K absorption are normal.
The use of plasma in the context of warfarin anticoagulation is well established but is no longer the preferred treatment. If available, four factor prothrombin complex concentrate (PCC) should be chosen as the first-line therapy for rapid reversal of life- or limb-threatening warfarin anticoagulation. In patients anticoagulated with warfarin who have active bleeding, require emergency surgery, or have serious trauma, the deficient clotting factors can be immediately provided by PCC. PCC can reverse a warfarin-induced coagulopathy faster with lower mortality and less volume overload than plasma or vitamin K alone. Neither PCC nor plasma is indicated for warfarin reversal when the patient is not bleeding and has an INR less than 9, as vitamin K administration corrects the coagulopathy in 12 to 18 hours. INR levels need to be closely followed to ensure warfarin reversal is sustained. Plasma transfusion is only indicated for rapid reversal when no PCC is available.
In patients with TTP, TPE with plasma as the replacement fluid is life-saving. Plasma infusion or exchange is also critical in the treatment of individuals who have congenital TTP. TPE has decreased the mortality of TTP from over 90% to less than 10% (see Chapter 132 ). Six randomized control trials have demonstrated that TPE is most effective in patients who have an autoantibody to ADAMTS13 (ADAM metallopeptidase with thrombospondin type 1 motif 13). This is caused by both the removal of a patient’s plasma containing the inhibitor coupled with the addition of donor plasma containing the functional vWF-cleaving protease. The FDA has also approved the use of CRP for TTP. Some authorities advocate CPR use as a first-line therapy for TTP, as these products have a lower level of vWF than FFP, comparable ADAMTS13 activity, and lower amounts of ADAMTS13–larger vWF multimer complexes. However, the most recent multicenter prospective randomized trial comparing TPE with plasma and CRP for the initial treatment of TTP demonstrated the two products were equivalent.
The treatment of TTP has changed with the approval of caplacizumab, which inhibits the interaction between vWF multimers and platelets. Caplacizumab treatment is associated with faster platelet count recovery, lower incidence of TTP-related death or thromboembolism, and TTP recurrence.
TPE is not indicated in the treatment of individuals with diarrhea-associated hemolytic uremic syndrome (HUS) (also termed thrombotic microangiopathy, Shiga toxin mediated) unless there are severe neurological symptoms. Seven randomized control trials evaluated the efficacy of TPE for typical cases of HUS. These trials found that the plasma use was not superior to supportive therapies alone. TPE with plasma replacement fluid, however, is currently indicated in diarrhea-negative (atypical) HUS (aHUS), which is caused by several inherited and sporadic conditions that lead to the uncontrolled activation of the alternative complement system (i.e., deficiency or autoantibody to complement factor H). Typically, all patients diagnosed with aHUS are empirically treated with TPE or plasma transfusions until underlying disease or mutation is further defined, which then determines treatment. TPE has been theoretically proposed to effectively remove the potentially causative autoantibody or mutated circulating complement regulator, while replacing absent or defective complement regulators. However, the clinical response varies depending on the underlying cause (see Chapter 132 ).
TPE with plasma replacement may also be indicated in other thrombotic microangiopathies including those due to medications, most notably ticlopidine and clopidogrel, and potentially cyclosporine or tacrolimus. Another example is in the treatment of thrombotic microangiopathy associated with stem cell transplantation.
Plasma as replacement fluid, either partially or completely, for TPE is used in other diseases with risk of hemorrhage caused by the resulting coagulopathy, such as diffuse alveolar hemorrhage, liver failure, and perioperatively (see Chapter 116 ).
Studies have shown that prophylactic administration of plasma to nonbleeding recipients with abnormal coagulation studies (i.e., PT, PTT, INR) is unlikely to produce a clinical benefit and unnecessarily exposes the patient to the risks of plasma transfusion. Moreover, systematic reviews of the predictive value of a prolonged PT or PTT found no significant difference in the risk of bleeding between patients with a prolonged PT or PTT and those with normal clotting parameters in the settings of bronchoscopy, central vein cannulation, angiography, liver biopsy, or lumbar puncture. This is supported by randomized control trials and meta-analyses which have evaluated the efficacy of the prophylactic use of plasma products to reduce the risk of bleeding. One trial, the Northern Neonatal Nursing Initiative Group Trial, randomized 776 neonates and evaluated whether plasma transfusion prophylaxis could prevent intraventricular hemorrhage in comparison with volume expanders (gelofusine or dextrose-saline). In a second large randomized clinical trial, 275 patients were randomized to see whether plasma transfusions could prophylactically prevent bleeding in acute pancreatitis patients. Neither large study showed clinical benefit of prophylactic plasma use. In one systematic review, 55 other randomized clinical trials were reviewed and evaluated. Only 17 of these 55 involved a control group that did not receive plasma. Overall, like the two largest studies, the results of these randomized control trials failed to show evidence for the efficacy of prophylactic plasma use across multiple clinical and laboratory outcomes. Similarly, a second meta-analysis evaluated 25 independent studies of minor surgical procedures and found that there was no significant difference in bleeding risk between those who did and did not have a coagulopathy. Despite this evidence, current recommendations still indicate that a pretransfusion INR of ≥1.5 to 1.7 be used as a transfusion trigger, as the prophylactic use of plasma is theoretically justified when the clinical risk of bleeding is greater than potential harms of using plasma.
One unit of plasma derived from a unit of whole blood is 200 to 280 mL. When plasma is collected by apheresis, as much as 800 mL can be obtained from one individual (“jumbo” plasma units), but these are then divided into volume of 200 to 250 mL. On average, there is 0.7 to 1 unit/mL of activity of each coagulation factor per milliliter of plasma and 1 to 2 mg/mL of fibrinogen. The appropriate dose of plasma may be estimated from the plasma volume, the desired increment of factor activity, and the expected half-life of the factor being replaced (i.e., factor VII has a half-life of only 4 to 6 hours, and thus plasma doses should be repeated every few hours if replacing factor VII in a patient with factor VII deficiency). Alternatively, the plasma dosage may be estimated as 10 to 15 mL/kg, and with that dose all clotting factor activities will increase by ∼30% in the absence of rapid ongoing consumption Ideally, plasma should be ordered as the number of milliliters to be infused. The frequency of administration depends on the clinical response to the infusion and correction of laboratory parameters. Moreover, plasma infusions should be given as close to the time as it is needed to allow for its maximum hemostatic effect if given pre-procedure. Complete normalization of coagulation laboratory values will likely not be achieved with plasma infusion, and minimal elevations of PT/INR (e.g., INR <1.5) are usually not corrected with plasma administration.
Plasma is screened for unexpected RBC antibodies during product testing and should be ABO-type compatible with the patient (see below). However, following traumatic injury, the patient's blood type is often unknown. Emergency release plasma, traditionally group AB, is used until blood typing has been completed, and the plasma used can be switched to the patient's identified ABO type. The advantage in using group AB plasma is its lack of anti-A and anti-B antibodies, thus avoiding the risk of acute hemolytic transfusion reactions. However, only 4% of the population is group AB. In order to increase the availability of plasma for emergency use during massive transfusion, group A plasma, with or without assuring low titers of anti-B, is now being used. Studies demonstrate no increased risk to the recipient and no adverse effect on clinical outcomes. Since patients typically receive group O RBCs and about 80% of the population is group O or group A, the risk of hemolysis is low.
| Plasma Product ABO Type | ||||
|---|---|---|---|---|
| Patient ABO type | O | A | B | AB |
| O | Yes | Yes | Yes | Yes |
| A | No | Yes | No | Yes |
| B | No | No | Yes | Yes |
| AB | No | No | No | Yes |
Plasma transfusions, like all blood products, have the potential for infectious and noninfectious adverse events. Transfusion transmitted diseases, although rare, traditionally include HIV, hepatitis B, and hepatitis C (see Chapter 118 ). Noninfectious risks include allergic reactions, TRALI, transfusion-associated circulatory overload (TACO), and hemolytic reactions (see Chapter 117 ).
Cryoprecipitate is prepared from 1 unit of FFP thawed at 1°C to 6°C. The precipitate is then refrozen and stored at −18°C or colder for 1 year. Cryoprecipitate, volume of 10 to 15 mL, contains 80 to 100 units of factor VIII, 150 to 250 mg of fibrinogen, and 50 to 60 mg of fibronectin as well as vWF and factor XIII. Pathogen-reduced cryoprecipitated fibrinogen complex is now approved for use in acquired fibrinogen deficiency or for replacement of factor XIII or von Willebrand factor, but not for FVIII replacement, if no specific factor concentrates are available.
Cryoprecipitate takes 10 to 15 minutes to thaw at ∼37°C, and then requires pooling before infusion. Prepooled (pooled before storage) cryoprecipitate products are available, easing the burden of preparation on the transfusion services. Once pooled and thawed, cryoprecipitate is maintained at 20°C to 24°C and outdates in 4 hours (6 hours if unpooled or pooled in a closed system).
Cryoprecipitate is used predominantly to treat bleeding associated with fibrinogen deficiency and in the manufacturing of fibrin sealants and glue ( Table 115.3 ). Cryoprecipitate should not be used to treat factor XIII, vWF, and factor VIII deficiencies, as virally inactivated factor concentrates are available. Human fibrinogen concentrate is also available, and FDA approved, and is primarily used for congenital fibrinogen factor deficiency in the United States and broader indications in Europe.
| Indicated |
|
|
|
|
|
|
| Possibly Indicated |
|
|
|
Like plasma, recent studies also indicate that cryoprecipitate is not used appropriately in clinical practice. One large audit, for instance, demonstrated that across 25 Canadian hospitals and 4370 units of cryoprecipitate transfusion, only 24% of transfusions were considered clinically appropriate, and 34% of cryoprecipitate transfusions were deemed inappropriate according to published national guidelines (i.e., Transfusion Medicine Advisory Group of British Columbia, Canada Guidelines for cryoprecipitate transfusion).
Fibrinogen deficiency is the primary indication for cryoprecipitate transfusion. The deficiency may be caused by congenital afibrinogenemia or dysfibrinogenemia, severe liver disease, DIC, or massive transfusion. Patients with the latter indications often have concomitant decreases in clotting factor levels and require the coadministration of plasma products. It is important to obtain fibrinogen measurements because levels less than 100 mg/dL cause prolongation of the PT and PTT, despite adequate clotting factor replacement. Very low levels of fibrinogen occur during liver transplantation (<100 mg/dL), where transfusion support with cryoprecipitate is vital.
A specific purified human fibrinogen concentrate is an alternative for direct fibrinogen replacement in isolated fibrinogen deficiencies, such as inherited hypofibrinogenemia. Fibrinogen concentrates undergo viral inactivation and have a standardized fibrinogen content; these are used preferentially over cryoprecipitate in some countries, but studies have not demonstrated a clinical benefit over cryoprecipitate.
Fibrin glue/sealant results from the mixture of a fibrinogen source (from plasma, platelet-rich plasma, or allogeneic/autologous cryoprecipitate) with a thrombin source (bovine, human, or recombinant). The enhanced local hemostasis achieved by the sealant product is through the action of thrombin on fibrinogen. “Fibrin glue” is a non–FDA-approved thrombin/preparation, and it has been widely used in Europe for many years. Fibrin sealants are FDA-approved alternatives to fibrin glue and are advantageous over locally made fibrin glues, because of standard dosing. Fibrin glues/sealants can be used for multiple surgical purposes, including as a topical hemostat (creating a blood clot to halt bleeding), as a sealant (agents to prevent leakage of potentially nonclotting fluids [i.e., cerebrospinal fluid]), or as an adhesive (bonds different tissues together).
The safety profile of each product differs depending on the product components and source. Bovine thrombin has been reported to cause anaphylaxis (because of bovine allergies), coagulopathy through formation of antibodies to factor V or II, and rarely death caused by severe systemic hypotensive reactions. Consequently, bovine products have an FDA mandated black-box warning on their package inserts. Pooled human plasma sources have the potential risk of viral or prion disease transmission. Reports indicate that hepatitis A and parvovirus B19 are particularly difficult to remove from these products despite current cleansing and filtration methods, and it is recommended that patients be counseled about this risk, although, given the current safety of the blood supply, the infectious risks are extremely low. Recombinant products, while eliminating the risk of infectious transmission or antibody formation, may also cause allergic reactions because of the hamster or snake proteins used to manufacture the product. Lastly, autologous fibrin glue preparations have been used, although the infectious risks (e.g., HIV and hepatitis) associated with the use of heterologous fibrin glue are eliminated by replacement with the autologous source, but are resource intensive.
Alternatively, albumin mixed with glutaraldehyde has been used to form both an effective sealant and adhesive. The FDA has currently approved an albumin-based product to seal large blood vessel anastomoses and to reattach layers of the aorta in the context of an aortic dissection. Other successful reported uses include as a sealant in breast cancer surgery, and to reduce air leaks in lung volume reduction procedures. Side effects of this compound can be significant, however, and include nerve and muscle necrosis, sinoatrial node damage, calcium metabolism abnormalities, mucosal and skin irritation, adhesive emboli, limitation of aortic growth, and pseudoaneurysms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here