Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Treatment for the muscular dystrophy group of disorders continues to gain momentum based on improvements in therapeutic strategies, facilitated by a greater availability of funding for translational research. This review emphasizes the progress made for Duchenne muscular dystrophy (DMD) and related dystrophinopathies as well as the limb girdle muscular dystrophies (LGMDs). Clinical gene therapy trials for DMD, Becker muscular dystrophy (BMD), and LGMDs are underway. Initial clinical trials used direct intramuscular gene delivery and are now moving forward in hopes of producing clinical benefit through regional vascular delivery. Proof of principle studies have also shown that packaging genes exceeding the usual limits of adeno-associated virus (AAV) look promising and expand the disease repertoire previously thought to limit clinical translation. Exon skipping using a phosphorodiamidate morpholino oligomer (PMO) by all measures enhances dystrophin expression and prevents disease progression. It is an exciting time in muscle disease therapeutics with continued expansion of skills to overcome treatment obstacles.
It has become apparent, perhaps now more than ever, that our best chance of helping patients with muscular dystrophy is to treat as early as possible. We have direct evidence that DMD is present at or shortly after birth. Creatine kinase (CK) is elevated at the time of delivery (>2000 U/L) and functional motor assessment demonstrates low scores measured by the Bayley-III motor functional scale for gross motor and fine motor function. In the neonatal period, most DMD boys perform more than a standard deviation below the mean and show loss of function and less maturational improvement with age. The time to treat DMD is at this early age, before muscle degeneration presents a challenge that is clearly difficult to overcome.
The stage for early intervention was set through an Ohio newborn screening study with the development of a 2-tier system for identifying DMD boys. In a cohort of nearly 40,000 newborns, using the dried blood spot card otherwise taken for mandated testing (regulated state by state), additional blood spots were available for both CK and DNA testing. If the CK was found to be 3 standard deviations above normal, DNA was extracted for whole genome amplification with assessment of single-/multi-exon deletions/duplications in the DMD gene using multiplex ligation-dependent probe amplification (MLPA) and for point mutations. When applied throughout the state of Ohio, 6 single-exon or multi-exon deletions were found ( Table 51.1 ). The Ohio system is more applicable than the screening paradigm previously used in virtually all other countries in the world, where a single-tier system had been used. In these countries, the single-tier approach first determines CK levels at birth; if elevated, infants are retested at 6 to 8 weeks of age or even later for validation. If the CK remains high, DMD gene mutation analysis is performed at the follow-up appointment. This system is most feasible in countries with a nationalized health care system with the capacity to track newborns through follow-up care. However, it is poorly adapted to a system without nationalized health care and where hospital discharges are planned within 24 to 48 hours postdelivery. In addition, privatized medicine brings less certainty for time and place of follow-up care. It is noteworthy that an International Workshop on Newborn Screening for DMD also advocated the 2-tier system of screening. Also of interest, the latest estimates of incidence of DMD in newborn males based on worldwide studies is approximately 1:5000, replacing the older estimates of 1:3500 ( Table 51.2 ).
| Gender | CK U/L | Gene | Mutation | Frame |
|---|---|---|---|---|
| Male | 2462 | DMD | Del Ex 50 | Out |
| Male | 2675 | DMD | Del Ex 5–41 | In |
| Male | 2003 | DMD | Del Ex 8–9 | Out |
| Male | 2466 | DMD | Del Ex 45 | Out |
| Male | 2791 | DMD | Del Ex 45–48 | Out |
| Male | 2688 | DMD | Del Ex 4–7 | Out |
| Year | Location | Number Screened | Incidence |
|---|---|---|---|
| 1979 | New Zealand | 10,000 | 1:5000 |
| 2 DMD | |||
| 1986 | W. Germany | 358,000 | 1:4589 |
| 78 DMD | |||
| 1988 | Manitoba, CA | 54,000 | 1:5400 |
| 10 DMD | |||
| 1989 | Lyon, Fr | 37,312 | 1:5330 |
| 7 DMD | |||
| 1991 | W. Pennsylvania, USA | 49,000 | 1:4900 |
| 10 DMD | |||
| 1998 | Cyprus | 30,219 | 1:6002 |
| 5 DMD | |||
| 2006 | Antwerp, Be | 281,214 | 1:5500 |
| 51 DMD | |||
| 2011 | Wales, UK | 335,045 | 1:5266 |
| 63 DMD | |||
| 2013 | Ohio, USA | 37,749 | 1:6291 |
| 6 DMD |
DMD has been the disease target for exon skipping. It is the most common, severe childhood form of muscular dystrophy. The very large size of the DMD gene results in spontaneous mutations and an unending trail of new cases and new carriers, emphasizing the compelling need to find a treatment. The importance of the early natural history data cannot be underestimated, establishing outcomes for clinical trials when intervention may be most advantageous. On average, however, most DMD boys are not diagnosed until 4 to 5 years of age, and we know that muscle biopsies at this time point show florid hallmarks of the dystrophic process with fiber loss, necrosis, and fibrosis.
The response to glucocorticoids is important to emphasize. DMD is the only dystrophy showing unequivocal evidence of functional benefit to steroids, convincingly established in a randomized, double-blind, controlled prednisone trial. Improved extremity muscle strength and function were observed, including time to rise from supine to standing, walking 9 m, and climbing stairs. Similar results were later reported with deflazacort, an alternative, sodium-sparing glucocorticoid. A high-dose, weekend regimen of prednisone (10 mg/kg/wk: half on Saturday and half on Sunday) showed equal efficacy to daily steroids. Glucocorticoids are accepted as the standard of care and are acceptable and encouraged for most clinical trials testing novel therapies such as exon skipping agents. It is generally accepted that glucocorticoids will extend ambulation by 2 years in the DMD population, usually wheelchair dependent by 10 to 12 years of age.
Exon skipping is targeted at the pre-mRNA level, allowing one or more exons to be omitted to restore the dystrophin reading frame. This is accomplished with splice-switching oligomers, typically 20 to 30 nucleotides in length and complementary in sequence to regions of the pre-mRNA transcript. Preclinical efficacy has been demonstrated in the mdx, dystrophin/utrophin knockout mouse, and the dystrophin-deficient dog using both 2′O-methyl-ribo-oligonucleoside-phosphorothioate (2′OMePS) and phosphorodiamidate morpholino oligomers (PMOs). The objective of exon skipping is to restore the disrupted reading frame of the DMD gene with the production of functional dystrophin, recognizing that it will not be full length and clinically may result in a Becker-like phenotype. Clinically, it is estimated that 70% to 80% of DMD mutations can be corrected by exon skipping. The early emphasis for clinical trials has been to target the hotspot region for deletions between exons 42 and 55, where mutations are generally amenable to exon skipping. Exon 51 skipping is applicable to the largest group of all DMD patients (13%) inclusive of deletions: 45–50, 47–50, 48–50, 49–50, 50; 52, or 52–63.
Proof-of-principle clinical trials in DMD used a 2′OMePS oligomer (PRO051/GSK2402968, ProsensaTherapeutics) or a PMO (AVI-4658/eteplirsen®; Sarepta Therapeutics) delivered directly to muscle, targeting exon 51. Using either oligomer, exon skipping was validated by RT-PCR and by demonstrating newly synthesized dystrophin protein correctly localized to the sarcolemma. Phase I/II extension studies were performed using systemically delivered oligomers. In the PRO051 trial, dose-related efficacy was achieved with evidence of new dystrophin expression in approximately 60% to 100% of muscle fibers in 10 of 12 patients with modest improvement in the 6-minute walk test (6MWT). In the phase II open-label study with AVI-4658 (eteplirsen®), 7 of 19 patients saw a modest response with a mean increase of sarcolemmal dystrophin from 8.9% to 16.4%. The dosing results were variable with one patient responding to eteplirsen treatment with 2 mg/kg, while all other responders received the higher dosing levels of 10 and 20 mg/kg.
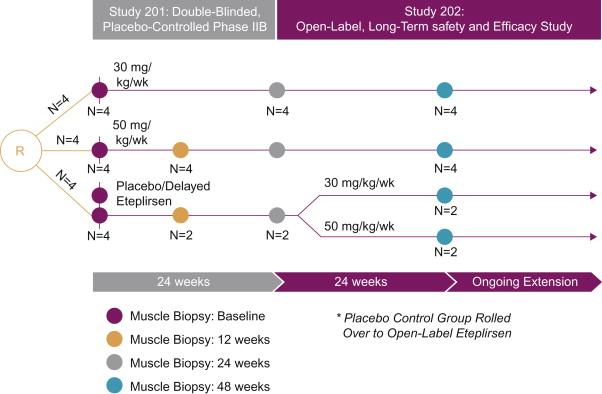
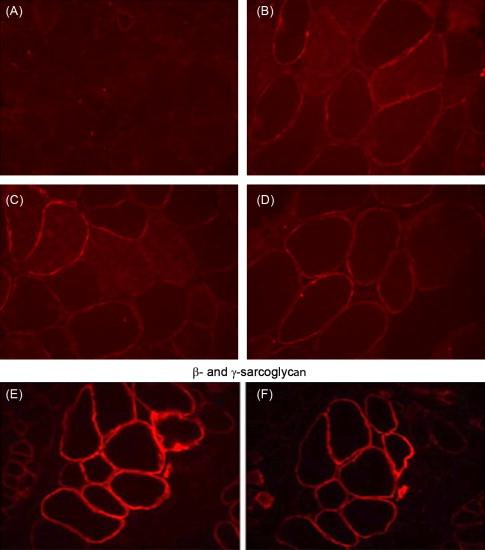
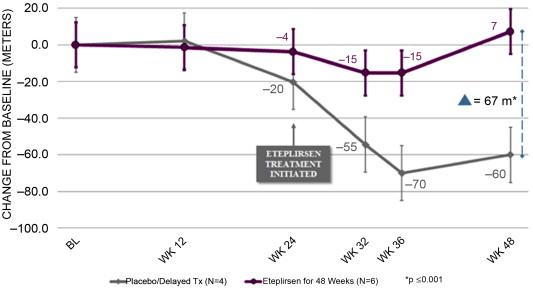
In the only double-blind randomized controlled trial of exon skipping using eteplirsen, high- and low-dose treated cohorts were compared to placebo for the first 24 weeks, after which all boys were on treatment. Dystrophin expression and distance walked on the 6MWT were outcome measures. Enrollment included DMD boys, ages 7 to 13, with confirmed DMD gene deletions correctable by skipping exon 51. All boys were on a stable dose of glucocorticoids (prednisone or deflazacort) for at least 6 months. The design of the trial is depicted in Figure 51.1 . The results of dystrophin production at all time points are shown in Figure 51.2 . At 12 weeks’ posttreatment, no significant dystrophin was found. Dystrophin production could be seen at week 24, and this further quantitatively increased after 48 weeks of eteplirsen. These findings are a clear indication that dystrophin production started sometime after week 12 and was more influenced by duration rather than dose of treatment. We also found restoration of sarcoglycans and nNOS binding accompanying dystrophin production ( Figure 51.3 ). The distance walked in the 6MWT was remarkably stable for boys taking eteplirsen from the start of the study without decline. In contrast the placebo cohort declined until week 36, consistent with natural history studies. Their walking distance stabilized 12 weeks after starting eteplirsen, consistent with dystrophin production; however, there had been a total decline in distance walked of 67.3 m compared to the treated group (p≤0.001) ( Figure 51.4 ). An important lesson from the trial was the need to modify enrollment criteria for future exon-skipping trials; two treated patients had to be excluded from the final account of walking distance because they lost ambulation prior to the initiation of dystrophin production by exon skipping. Their pretreatment profile (the tallest with the lowest baseline 6MWT values) predicted a rapid decline different from that of all others enrolled in the study.
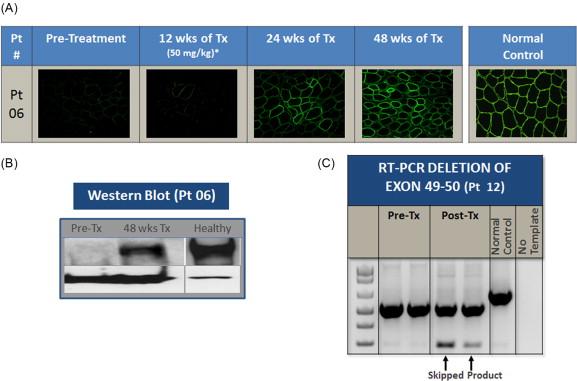
The safety profile of eteplirsen was impeccable. Not a single significant adverse event occurred following treatment. The study has now extended for more than 1.5 years, and stability and safety continue without deviation. Two patients had transient proteinuria, but with rechecking this was no longer present. This very safe and promising method of treatment is now extending to include other exons. None is as inclusive as targeting exon 51, but collectively a large part of the DMD population can be reached using this strategy.
Stop codons account for approximately 13% to 15% of DMD mutations. Effective treatment of this group has potential impact equivalent to exon skipping. Encouraging results were originally obtained in mdx mice where the aminoglycoside, gentamicin, successfully increased dystrophin expression to about 20%. Gentamicin-treated mice showed improved histology and protection against contraction-induced injury. Plasma CK levels were also reduced by 70%. Molecular studies of mutation suppression in the mdx mouse served as a stimulus to attempt a similar approach using gentamicin in DMD patients. The initial clinical trial reported in four DMD patients carrying premature termination codons in the DMD gene reported no improvement in clinical measures or dystrophin expression. Of interest, serum CK dropped significantly. A subsequent gentamicin trial reported more favorable results in a small cohort of four subjects. When gentamicin was given over two cycles, each for 6 days with a 7-week hiatus between dosing, muscle biopsy revealed increased dystrophin expression in 3 out of 4 patients. Serum CK decreased from day 2 to day 6 of the treatment but subsequently came back to the initial levels. No clinically meaningful outcomes were obtained in any of these patients.
A third clinical trial conducted by our group at the Nationwide Children’s Hospital consisted of a two-phase protocol. An initial short-term gentamicin, 14-day dosing study was carried out in 10 DMD boys with proven stop codon mutations. A biological effect was established with a 50% reduction of CK by day 14. The results were stop codon-dependent since serum CK did not change in a control group with frameshift mutations. Having demonstrated a biological effect in the short-term trial, a 6-month study was designed with weekly or twice-weekly gentamicin administration. Enrollment was dependent on normal hearing and kidney function, and negative testing for the known A1555G mitochondrial DNA mutation in the 12S rRNA gene predisposing to aminoglycoside-induced hearing loss. Renal function was monitored using cystatin C to avoid false negative findings related to decreased serum creatinine and creatinine clearance levels in the DMD population. Sixteen subjects with documented stop codon mutations received weekly (N=12) or twice weekly (N=4) gentamicin. No patient demonstrated a decline in renal or hearing function except one patient given a miscalculated dose (125% of recommended) for the first 4 administrations, resulting in a transient high-frequency hearing loss that returned to normal within 3 months. Owing to the small sample size, weekly and biweekly treated patients were analyzed as one group. Twelve subjects agreed to pretreatment and posttreatment muscle biopsies. In 3 of 9 subjects, dystrophin protein increased to 13.0–15% of wild-type levels ( Figure 51.5 ). In addition to dystrophin expression in muscle tissue, serum CK was mildly reduced after 6 months of gentamicin treatment (9851±2109 U/L to 5316±850 U/L, p=0.04). Additional clinical outcome measures were variable. The average muscle score (AMS), which was expected to decline by 0.2 units over 6 months showed little or no change (pretreatment AMS 5.37±0.39 compared with posttreatment AMS 5.35±0.38). However, no functional outcomes were improved, such as the time to walk 30 feet, climb four standard steps, or stand from a supine position on the floor.
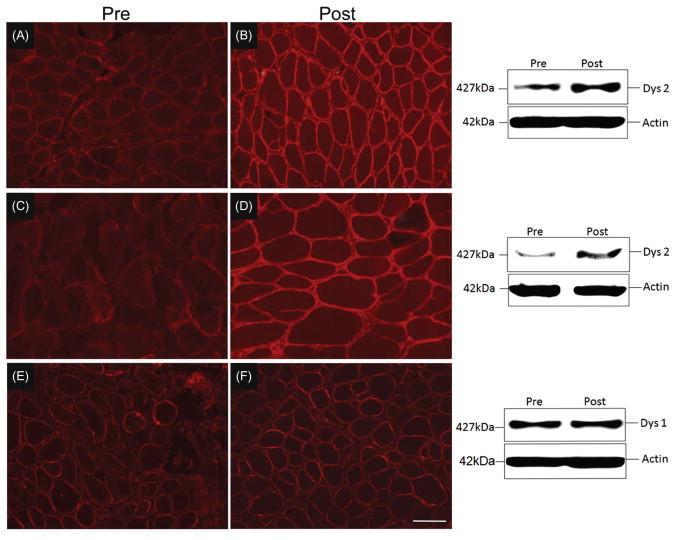
Overall, the clinical trial validates that mutation suppression or readthrough can potentially be translated to patients but would require more frequent or higher dosing to produce clinically meaningful results.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here