Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients who are injured or undergo extensive and complicated surgery manifest a profound acute phase reaction in response to tissue injury, reperfusion, and hemodynamic disturbances. A metabolic environment of increased catecholamines and cortisol orchestrates an increase in energy expenditure and protein turnover, leading to a state of heightened catabolism.
The resultant insulin resistance from this increased catabolic response is responsible for the decreased peripheral use of glucose and an increase in the rates of lipolysis and proteolysis for the provision of amino acids and fatty acids as fuel substrates. The conversion of peripherally mobilized amino acids (primarily alanine) to glucose by gluconeogenesis is not suppressed by hyperglycemia or the infusion of glucose solutions in this environment. The amino acid pool rapidly becomes depleted of essential amino acids as the branched chain amino acids are used preferentially as fuel in skeletal muscle, while large amounts of the conditionally essential amino acid glutamine are required for metabolic processes, especially in the intestinal mucosa. Decreased protein synthesis in skeletal muscle and in the gastrointestinal tract is accompanied by increased protein breakdown, with the shuttling of amino acids to lung, cardiac, liver, and splenic tissue, where protein synthesis is better maintained. As this process is perpetuated by cytokine activation, the critically ill and injured patient remains catabolic and consumes skeletal and visceral muscle and fat reserves rapidly. This catabolic state may be exacerbated by the development of anabolic resistance, as is seen in the newly recognized persistent inflammatory catabolic syndrome (PICS). All these disturbances can deplete important trace elements and vitamins, and their deficiencies may be associated with end-organ dysfunction.
Malnutrition may be generally defined as a state of relative nutrient deprivation and metabolic disturbance that causes increased protein turnover, compromises host defenses, and increases the risks of complications and death. For years, protein-calorie malnutrition has been characterized by weight loss, hypoalbuminemia, decreased skeletal muscle mass, reduced fat stores, and decreased total lymphocyte counts. In 1936, Hiram O. Studley first identified preoperative malnutrition as a specific operative risk factor in patients with peptic ulcer disease. He noted a 10-fold increase in mortality rate in patients who had lost over 20% of their body weight. He proposed that this might be reversible with a preoperative method for overcoming this deficit. Multiple studies performed since his time have confirmed that malnutrition results in poor wound healing, increased infection rates, prolonged postoperative ileus, lengthened hospital stay, and increased mortality.
In the stressed state, malnutrition may manifest as a functional deterioration in organ system function along with poor wound healing or wound breakdown. Respiratory muscle weakness can predispose to atelectasis, pneumonia, and prolonged ventilator dependence. In addition, all aspects of the immune response may be impaired by malnutrition. Host barrier function may be compromised together with cell-mediated and humoral immunity as cell growth and turnover are diminished.
As nutritional status is vital to patient outcomes, the Joint Commission has directed nutrition screening on all patients within 24 hours of hospital admission. In the surgical population, it is important to focus on patients that are at high risk for malnutrition based on their primary diseases, other comorbidities, and planned surgical procedures.
A thorough patient history, a detailed physical examination, and ongoing clinical monitoring are the key tools for assessing the adequacy of ongoing nutritional support. Laboratory markers, indirect calorimetry, and body weight and composition can also be used as adjuncts in the assessment of nutritional status.
The baseline assessment of a patient for nutritional support begins with identification of patients who are already malnourished. Many studies have attempted to determine the best way to identify malnutrition in such patients. Questions regarding unintentional weight loss and decreased caloric intake should be asked in an objective way so that the patient is not in a position to give a biased qualitative answer that denies the severity of their illness. In addition to a focused physical examination, accurate measurement of the patient’s weight, mid-arm circumference (MAC) or mid-arm muscle circumference, triceps skinfold thickness (TSF), and grip strength contribute to the initial assessment. A comparison of the patient’s current weight to their usual body weight and ideal body weight along with a calculation of the body mass index in kilograms/meter 2 (BMI) will contribute significantly to the diagnosis. When necessary, laboratory markers such as serum prealbumin, albumin, transferrin, and retinol-binding protein can all be used. Expert consensus uses the following parameters, with the presence of two or more of the following being diagnostic of malnutrition: insufficient energy intake, weight loss, loss of muscle mass or subcutaneous fat, fluid accumulation that may mask weight loss, and diminished handgrip strength.
The diagnosis of severe malnutrition can also be established using several clinical nutritional evaluation tools. One tool is the Subjective Global Assessment of Nutritional Status (SGA). It is based on a brief focused nutritional history and physical examination. Another tool developed in Europe is the Nutrition Risk Screening Tool. This uses historic information and clinical parameters to determine risk of malnutrition. A score of >3 signifies risk and a score of >5 signifies high risk (see Table 1 ). The Nutrition Risk Index uses the patient’s weight and laboratory markers to calculate the risk of malnutrition. The clinician uses historical information about recent food intake or unintentional weight loss and examines the patient for signs of nutritional depletion. Patients with multiple or severe stigmata of malnutrition or more than 15% weight loss within 6 months would be considered as seriously depleted. However, in patients with biopsy-proven carcinoma, a weight loss of only 10% in 6 months constitutes a high-risk group who would likely benefit from a course of preoperative nutritional support.
| Impaired Nutritional Status | Severity of Disease | ||
|---|---|---|---|
| Mild—score 1 | Weight loss > 5% in 3 months or food intake < 50%–75% in preceding week | Mild—score 1 | Hip fracture, chronic patients with acute complications: cirrhosis, COPD, ESRD, DM, malignancy (oncology) |
| Moderate—score 2 | Weight loss > 5% in 2 months or BMI 18.5–20.5 and impaired general condition or food intake 25%–50% in preceding week | Moderate—score 2 | Major abdominal surgery, stroke, severe pneumonia, malignancy (hematology) |
| Severe—score 3 | Weight loss > 5% in 1 month or > 15% in 3 months or BMI < 18.5 and impaired general condition or food intake 0%–25% in preceding week | Severe—score 3 | Head injury, bone marrow transplant, ICU patients (APACHE II>10) |
| Score: | Score: Age adjustment score * : 0–1 Total Score: |
||
* Find the highest score for impaired nutritional status and add it to the highest score for severity of disease; then if age > 70 years, add 1 to get the total score.
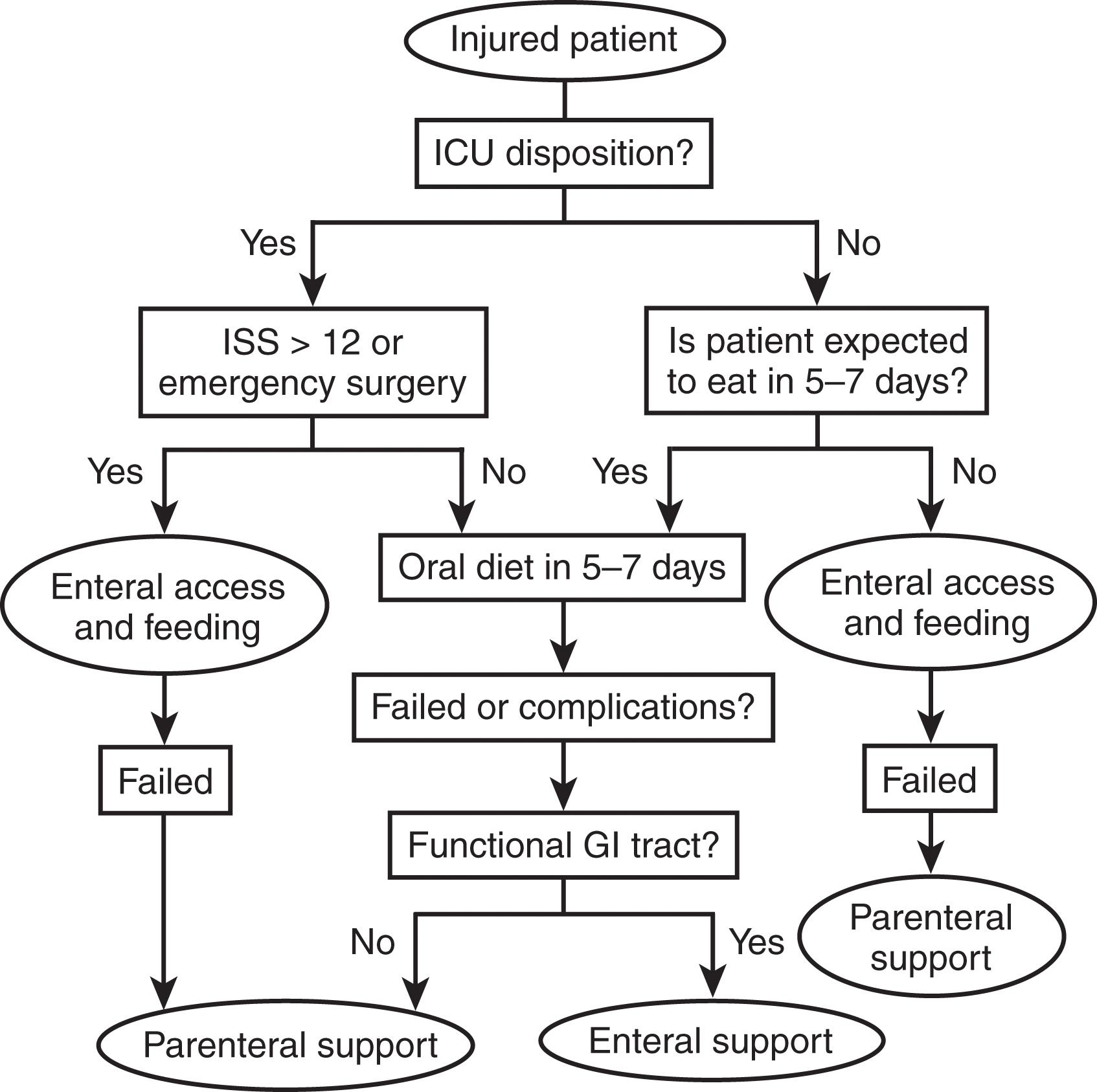
The determination of nutritional risk in acutely ill or injured patients can be more challenging due to a lack of historic information, fluid sequestration, and the inability to predict the clinical course. Heyland developed and validated a scoring system to determine the nutritional risk of such patients in 2011. The Nutritional Risk in Critically Ill Score (NUTRIC) was initially developed including the inflammatory marker IL-6. Due to the difficulty in obtaining this value clinically, the score was modified and subsequently validated without it. The remaining variables in the NUTRIC score are age, APACHE II score, SOFA score, number of comorbidities, and days from hospital to ICU admission. A Modified NUTRIC score of >5 defines a high-risk patient (see Table 2 ). In the case of injured patients, the clinical course may be determined more by their burden of injury than by prior comorbidities. An algorithm developed specifically for trauma patients was developed using ICU disposition and Injury Severity Scoring that was 80% to 88% predictive of the need for nutritional intervention ( Fig. 1 ).
| Variables in NUTRIC Score | Range | Points |
|---|---|---|
| Age | <50 | 0 |
| 50–74 | 1 | |
| ≥75 | 2 | |
| APACHE II | <15 | 0 |
| 15 to <20 | 1 | |
| 20–28 | 2 | |
| ≥28 | 3 | |
| SOFA | <6 | 0 |
| 6 to <10 | 1 | |
| ≥10 | 2 | |
| No. of comorbidities | 0–1 | 0 |
| 2+ | 1 | |
| Days from hospital to ICU admission | 0 to <1 | 0 |
| 1+ | 1 | |
| Total = |
Once the diagnosis of malnutrition has been made and a nutritional support plan has been implemented, continued measurement of the patient’s energy expenditure is necessary to ensure that the plan is appropriately addressing the patient’s needs. Indirect calorimetry remains the gold standard for measuring the patient’s resting energy expenditure (REE). Previously, isolated measurements of REE had been performed and rarely repeated during the patient’s hospital stay. The use of a static measurement of a patient’s REE to calculate nutritional needs during a hospital stay may result in overfeeding or underfeeding. More recent data obtained by Yeh et al in trauma patients suggest that continuous monitoring of REE via indirect calorimetry over a 14-day period shows great variability, with REE peaking on day 7 of injury and declining after day 14. Thus, frequent utilization of indirect calorimetry during the early phases of injury and then measurement of REE during steady state (after the 14-day period) is now the recommended protocol for accurately assessing a patient’s nutritional needs. These data emphasize the need for ongoing reassessment of patients’ nutritional needs during their hospital stay.
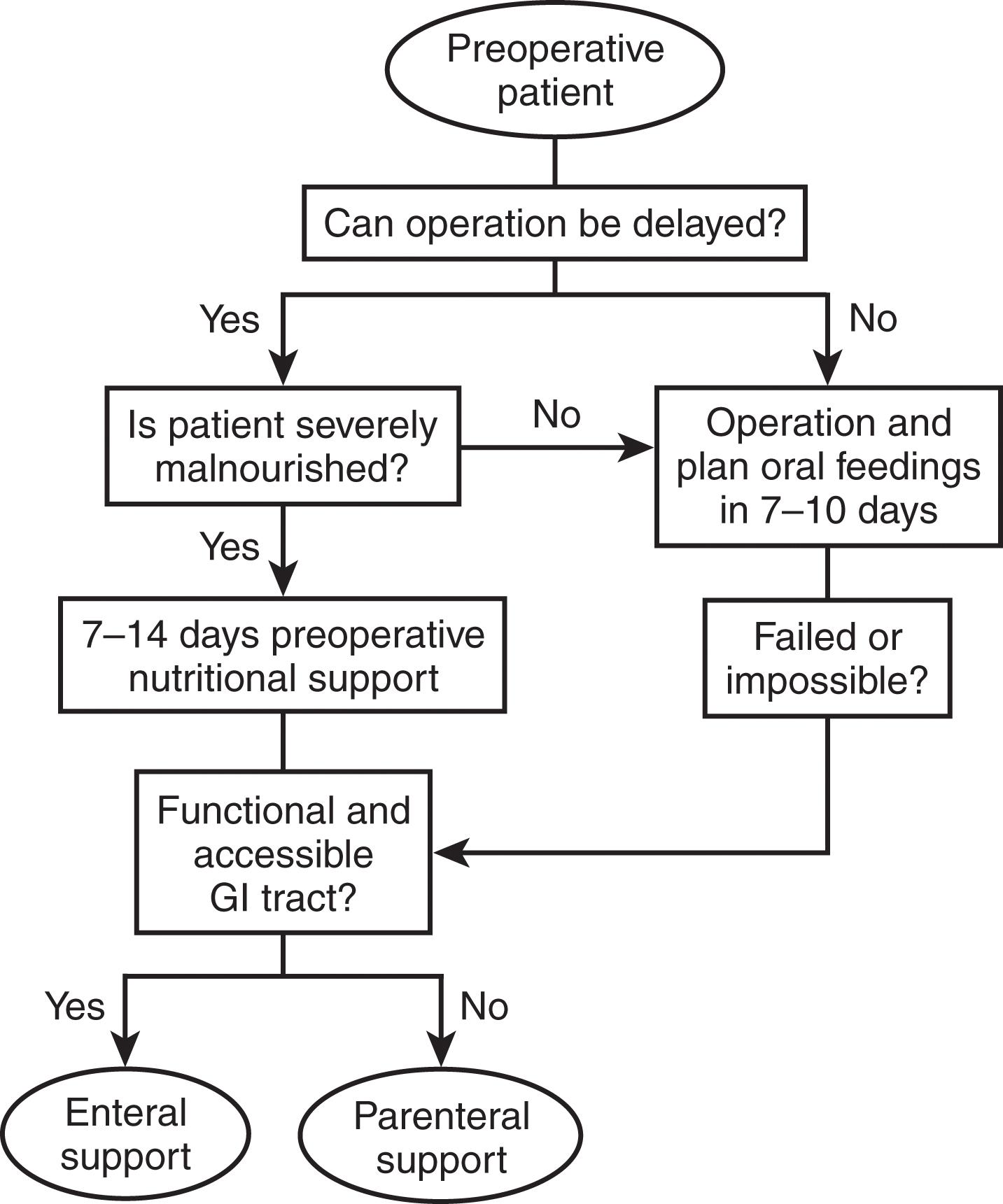
There will be occasions where patients will present with acute surgical problems, precluding the ability to optimize nutritional status. The focus in the acute period should be rapid identification of patients with nutritional risk as well as judicious correction during the perioperative phase. In more elective situations, there are two notable circumstances in which preoperative nutritional support should be considered a high priority. The first involves patients who will require major operative intervention but cannot undergo immediate surgery and will likely have a prolonged fast for more than 5 days. In the second circumstance, operative intervention must be delayed in order to rehabilitate patients with significant nutritional deficits that could increase postoperative morbidity if not ameliorated ( Fig. 2 ).
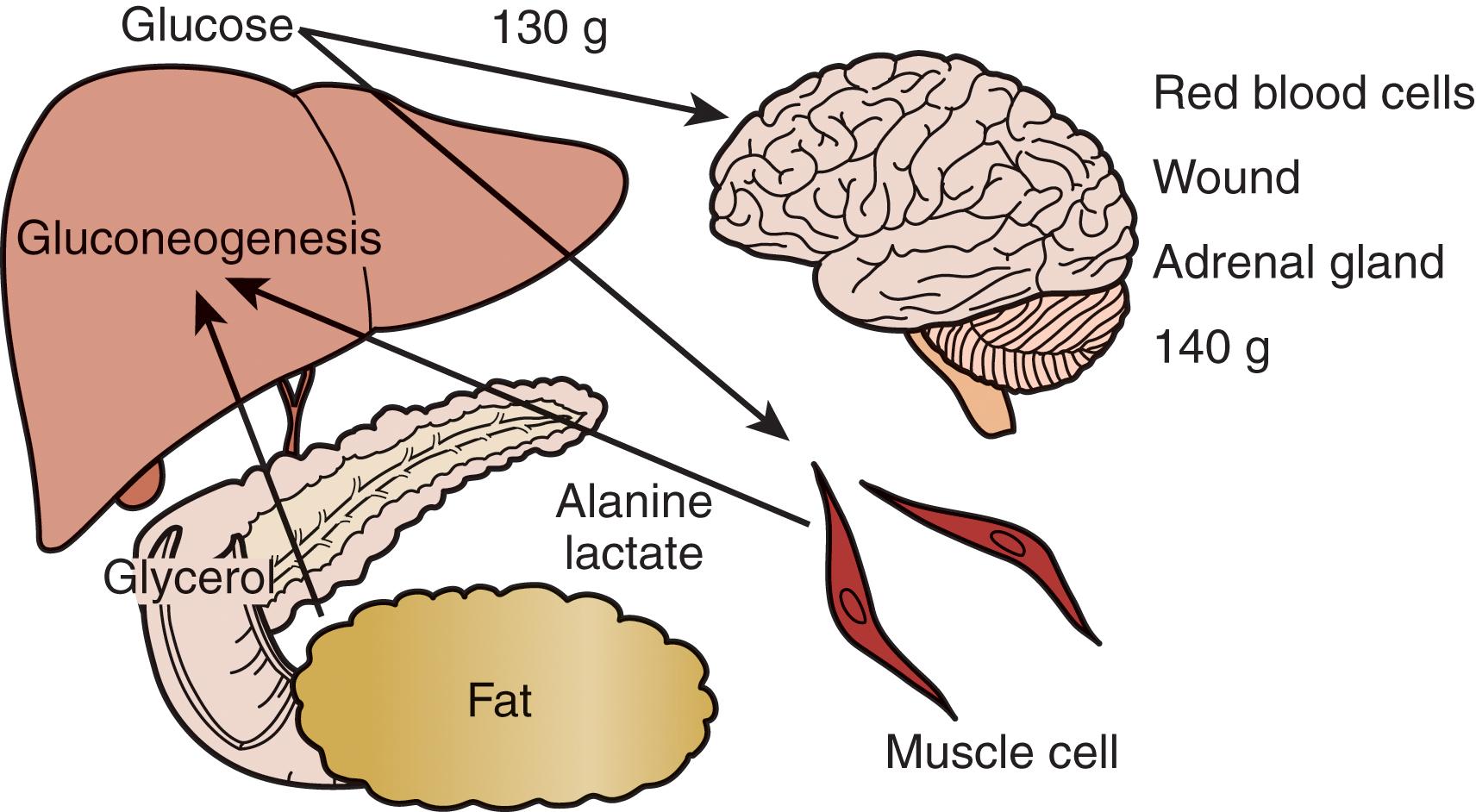
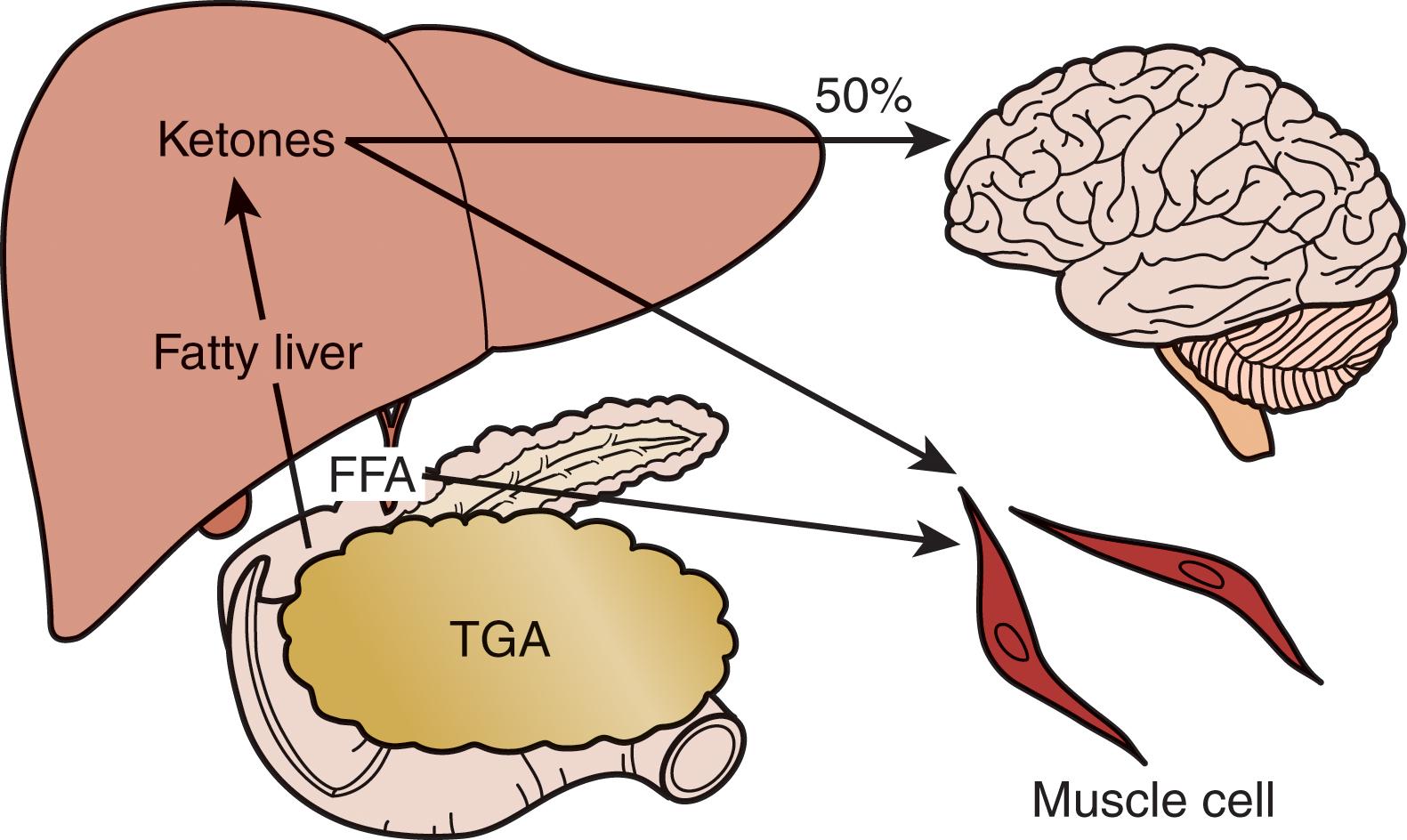
In the preoperative period, the response to starvation is associated with a redistribution of substrate flow from peripheral tissues to meet metabolic demands. The falling level of insulin promotes the release of fatty acids and amino acids from adipose tissue and skeletal muscle. Although most peripheral tissues can use fatty acids as fuel, proteolysis continues to fuel gluconeogenesis in order to support the fuel requirements of the glucose-dependent tissues ( Fig. 3 ). Over time, adaptation to starvation occurs as the brain metabolism adapts to use ketones for 50% of its fuel needs. As fat-derived fuel sources are used more, the dependence on protein catabolism decreases from 85% to 35% ( Fig. 4 ).
Patients with upper gastrointestinal tract malignancies have the highest incidence of protein-calorie malnutrition, with over 30% of patients demonstrating significant nutritional deficits. Preoperative chemotherapy and radiation, combined with cancer cachexia, obstruction, increased nutrient losses, and abnormal substrate metabolism, increase nutritional risk. Prospective studies have shown a decrease in major complications such as anastomotic leak and wound disruption when surgery is delayed and preoperative parenteral nutrition is administered to severely malnourished patients. However, an increase in infectious complications can occur without apparent clinical benefit when preoperative parenteral nutrition is administered to patients who are well nourished or only mildly malnourished.
After selection of a patient for preoperative nutrition, it is necessary to select an appropriate formulation and treatment course. Although the optimal duration of preoperative therapy has yet to be determined, nutritional therapy for 7 to 15 days is standard with protein administration at a dose of 1.5 to 1.8 g/kg of protein per day to support protein synthesis. Total nonprotein calories should be calculated at 150% of basal energy expenditure as measured using indirect calorimetry or derived from the Harris-Benedict equations ( Table 3 ). It is prudent to initiate a nutritional program in patients with severe malnutrition and starvation at less than basal energy dosages to prevent refeeding syndrome and uncontrolled hyperglycemia. Support may be increased daily to goal as tolerated under close metabolic surveillance. Frequent reassessment of the patient’s energy requirements is recommended to prevent underfeeding or overfeeding.
| BEE Women = 655 + (9.6 × weight in kg) + (1.7 × height in cm) − (4.7 × age in years) |
| BEE Men = 66 + (13.7 × weight in kg) + (5 × height in cm) − (6.8 × age in years) |
Additionally, electrolyte levels as well as serum osmolarity should be monitored to avoid hyperosmolar states and to replete and balance serum levels of potassium, phosphorus, and magnesium during refeeding. Otherwise, patients may develop fluid retention and electrolyte imbalances that can result in life-threatening cardiac dysrhythmias. Blood sugar levels must be carefully monitored, and hyperglycemia should be treated efficaciously to avoid the increased risk of infections and the development of hyperosmolar states.
Enteral nutritional support is the delivery of nutrients into the gastrointestinal tract, which may require a temporary or permanent feeding tube, and is the preferred method of administering nutrition when at all feasible. Surgical patients can benefit from enteral nutrition due to enhancement of mucosal blood flow and maintenance of the gut-associated lymphoid tissue (GALT) and the mucosal barrier. The initial gastrointestinal barrier function is provided by mucus-containing lactoferrin and lysozyme, both of which are effective, nonspecific inhibitors of microbial growth. Normal, undisturbed bacterial flora exerts a similar effect. Epithelial tight junctions form the next line of nonspecific defense, with junctional integrity being energy dependent, and at least partially reliant on the presence of intraluminal energy substrates. Specific intestinal immunity is governed by the GALT. The inductive sites in the Peyer patches provide an interface between antigen-presenting cells and circulating lymphocytes. Some animal studies have demonstrated improved immunity in enterally fed groups compared with the parenterally fed groups, but this finding has not been confirmed uniformly or unequivocally to date in controlled, prospective, randomized human studies.
Preoperative enteral nutritional support has been demonstrated to decrease postoperative complication rates by 10% to 15% compared with control subjects, but debate is ongoing over the length of therapy that is needed to achieve this advantage. The literature supports a course of enteral feedings for 5 to 20 days prior to surgery. Recently, it has been shown that there may be an advantage to using immune-enhancing formulas, either alone as preoperative supplements, or in combination with postoperative support, but this has not been demonstrated uniformly.
Patients may have inadequate appetite or gastrointestinal function to maintain optimal nutrition on oral intake alone. Enteral feeding has been used successfully to meet the nutritional needs of such patients with a wide range of surgical problems including cancer, inflammatory bowel disease, and pancreatic disorders. However, its use is contraindicated in cases of bowel obstruction, persistent intolerance of feedings, major gastrointestinal bleeding, and inability to access the gastrointestinal tract effectively and safely.
Once it has been decided to administer enteral nutrition, the optimal type of enteral access must be selected. Factors that determine the choice of enteral access include which components of the gastrointestinal tract are available, how long a course of enteral therapy is planned, whether the patient is at risk for aspiration, and finally, the nutritional status of the patient. When available, the gastric route is usually preferred. Postpyloric feeding into the duodenum or jejunum may be indicated in the presence of early satiety, gastric disease, or aspiration risk. Nasogastric and nasoenteric tubes are recommended for short-term feeding because of their ease of placement, low cost, and low complication rate. For longer-term feeding, gastrostomy tubes are most commonly placed by the percutaneous endoscopic technique. Interventional radiologists can also place feeding tubes percutaneously into the stomach as well as into the jejunum. If these less invasive techniques are not successful, feeding tubes may be placed by open or laparoscopic techniques. Note that invasive and percutaneous feeding access techniques require healing of the tract and may be hazardous in patients with severe malnutrition. A course of noninvasive feeding may be required to optimize the patient prior to a percutaneous gastrointestinal tract feeding access.
Natural foods can be blended to provide complete nutrition either orally or by tube directly into the stomach. Polymeric formulations are available composed of macronutrients in the form of isolates of intact protein, triglycerides, and carbohydrate polymers designed to provide complete nutrition. Monomeric (elemental) formulations are mixtures of protein moieties in the form of peptides and amino acids, fat as long-chain triglycerides (LCT) and medium-chain triglycerides (MCT), and carbohydrates as partially hydrolyzed starch maltodextrins and glucose oligosaccharides. These formulas are designed primarily for tube feeding below the duodenum into the jejunum or for patients with abnormalities of digestion or absorption.
The appropriate selection of enteral formulation requires knowledge of the physiologic mechanisms of the digestion and absorption of each macronutrient and the caloric values of enteral substrates ( Table 4 ). Sources of carbohydrate found in enteral formulas range from simple sugars to complex starches. The larger-molecular-weight starches exert less osmotic pressure in the intestinal lumen than simple sugars but require more time for digestion prior to absorption. Different enteral formulas contain variable amounts of carbohydrates, which can range from 28% to 70% of total calories. Patients with diabetes or carbon dioxide retention due to chronic obstructive pulmonary disease should be given formulas with a lower percentage of carbohydrate calories.
| Nutrient | Parenteral (kcal/g) | Enteral (kcal/g) |
|---|---|---|
| Carbohydrate | 3.4 | 4.0 |
| Fat | 9.0 | 9.0 |
| Protein | 4.0 | 4.0 |
Many enteral formulas now contain fiber, which may be soluble or insoluble. Insoluble fiber improves colonic function and bowel transit time but has no other nutritional benefit. In contrast, soluble fiber binds to cholesterol and bile salts and thus can lower serum cholesterol levels. Additionally, they function as a prebiotic as colonic bacteria digest soluble fiber and thereby produce short-chain fatty acids that are utilized as fuel by the colonocytes.
Enteral formulas contain fats usually derived from corn, soy, and safflower oil. Lipids serve as a concentrated energy source and enhance the flavor of enteral formulas without increasing osmolality. The absorption of fat-soluble vitamins requires the intake of a minimum of 15 to 25 g of lipid per day. Linoleic or linolenic acid must be provided to prevent essential fatty acid deficiency. Because omega-6 fatty acids have been shown to have immunosuppressive effects due to the production of inflammatory end products, omega-3 fatty acids, which counter these effects, have been added to some enteral products. Medium-chain triglycerides may also be a useful caloric source for patients with fat malabsorption, as they do not require pancreatic lipase for hydrolysis, are absorbed in the duodenum and upper jejunum into the portal circulation without micelle formation, and do not require carnitine for transport into the mitochondria.
Enteral protein may be provided in the form of intact protein such as casein, partially hydrolyzed oligopeptides, or crystalline l-amino acids. Intact protein and protein hydrolysates require further digestion by pancreatic and brush border proteases into short-chain peptides and amino acids. These nutrients are then freely absorbed by the enterocytes, primarily in the proximal small intestine. Patients with malabsorption may benefit from enteral protein in the form of short peptides and free amino acids.
Currently, more than 100 enteral products are available commercially, and most formulas have a caloric density between 1 and 2 kcal/mL, are lactose free, and can provide the recommended daily dietary allowances of vitamins and minerals in less than 2 L of formula per day. The majority of patients tolerate standard enteral formulas; however, monomeric (elemental) formulas may be necessary in patients with malabsorption. Excellent results with improved immune function and surgical outcomes have been obtained with the perioperative administration of immune-enhancing formulas.
Other disease-specific formulations have been compounded for patients with renal failure, pulmonary insufficiency, and diabetes. Renal failure formulas have higher percentages of the essential amino acids and very low levels of potassium and phosphorus. Patients with advanced pulmonary disease need to receive most of their calories as fat in order to decrease carbon dioxide production. Diabetic formulas also contain additional calories as fat, but also contain soluble fiber to decrease blood sugar levels. It is important to note that all of these specialty formulas are expensive and should only be used when a standard formulation with an appropriate nutrient profile has failed in providing optimal nutritional support to the patient. Because most formulas contain only 65% water, it may be necessary to administer supplemental hypotonic enteral fluid boluses to achieve satisfactory fluid and electrolyte balance.
Total parenteral nutrition (TPN) should be administered to preoperative patients who are severely malnourished and have compromised or nonfunctioning gastrointestinal tracts. Dextrose and fat emulsion formulas are used to provide nonprotein calories, usually in a 70:30 ratio. Adequate nonprotein calories must be administered to support protein synthesis in a 150:1 calorie-to-nitrogen ratio in anabolic patients, along with multivitamins and trace elements as part of the comprehensive nutritional regimen.
The caloric values of TPN substrates can be found in Table 4 . The amount of dextrose administered should range between 4 and 6 mg/kg/minute. However, in patients with chronic obstructive pulmonary disease carbohydrate administration should be maintained at 4 mg/kg/minute or less. In diabetic patients, it is recommended that dextrose administration be monitored and blood sugars kept tightly controlled between 120 and 180 mg/dL.
Fat emulsions can be used to supplement nonprotein calories; however, data suggest that preoperative intravenous lipid therapy should be limited to a maximum of 30% of the total calories. Lipids are usually administered as a 20% emulsion either separately or as part of a three-in-one formula composed of dextrose, amino acids, and lipid emulsion. Depending upon caloric needs and delivery method, quantities of 100 to 250 mL may be prescribed daily. Protein is administered as a free amino acid solution, including all of the essential amino acids.
While providing parenteral nutrition for patients with gastrointestinal dysfunction, it is important to consider fluid requirements. A more dilute, high-volume solution may be needed in patients with large fluid deficits or losses, although more concentrated, lower-volume solutions will be necessary in patients who have volume restrictions secondary to heart failure, renal failure, or hepatic insufficiency.
With protein-calorie malnutrition, intracellular losses of potassium, magnesium, and phosphorus occur simultaneously with gains in sodium and water. During injudicious refeeding, sodium balance may become markedly positive and result in water retention. Potassium, phosphorus, and magnesium plasma levels may drop precipitously upon initiation of nutritional support, as these ions are transported intracellularly together with the infused macronutrients and micronutrients. It is important to monitor electrolytes and fluid balance meticulously to avoid the risk of refeeding syndrome. In addition, potassium, phosphorus, and magnesium deficiencies must be corrected if optimal anabolism is to occur ( Table 5 ).
| Electrolyte | Daily Requirement | Fluid Compartment | Effects of Excessive Levels | Effects of Diminished Levels |
|---|---|---|---|---|
| Sodium (Na + ) | 60–120 mEq | Extracellular | Dry mucous membranes, maniacal behavior | Seizures, altered mental status |
| Potassium (K + ) | 40–120 mEq | Intracellular | Cardiac arrest, peaked T waves, wide QRS complex on electrocardiogram | Cardiac dysrhythmias, muscle weakness |
| Magnesium (Mg 2+ ) | 10–20 mmol | Intracellular | Cardiac dysrhythmias, hypotonia | Hypokalemia, hypocalcemia, seizures |
| Phosphorus (PO 4 2− ) | 14–20 mmol | Intracellular | Calcium phosphate salt deposits | Altered mental status, muscle weakness, hemolysis, paresthesias |
| Calcium (Ca 2+ ) | 5 mg | Intracellular | Lethargy, constipation | Tetany, hyperreflexia, seizures, cardiac dysrhythmias |
Trace minerals are inorganic compounds and vitamins are complex organic compounds that regulate metabolic processes ( Table 6 ). The majority of these micronutrients act as coenzymes or as essential elemental constituents of enzyme complexes regulating the biotransformation of carbohydrates, proteins, and fats. Iron, zinc, copper, chromium, selenium, iodine, and cobalt are known to be necessary for normal health and function in human beings. Furthermore, in malnourished and seriously ill patients, requirements for zinc and selenium should be assessed and supplemented as is necessary. If the fluid balance and electrolyte and blood sugar levels of the patient stabilize on parenteral support, the patient may be discharged home on cyclic overnight infusions for ongoing nutritional rehabilitation while awaiting surgery. The infusion cycle is gradually decreased from 24 hours to 18 hours and then to 14 to 16 hours daily as tolerated to allow the patient some freedom from the parenteral apparatus. A permanent central venous access is indicated for maximally safe and effective home parenteral nutrition.
| Vitamin or Mineral | Daily Requirement | Extra Losses Open Abdomen | Extra Losses EC/EA Fistula |
|---|---|---|---|
| Biotin | 60 μg | ||
| Chromium | 10–20 μg | ||
| Copper | 0.1–0.5 μg | * | * |
| Folic acid | 600 μg | * | |
| Iron | 0–2 mg | * | |
| Niacin | 50 mg | ||
| Pantothenate | 15 mg | ||
| Pyridoxine | 5 mg | ||
| Riboflavin | 5 mg | ||
| Selenium | 20–200 μg | ||
| Thiamine (B 1 ) | 5 mg | ||
| Vitamin A | 2500 IU | * | |
| Vitamin B 12 | 12 μg | * | |
| Vitamin C | 1000 mg | * | * |
| Vitamin D | 25–100 μg | ||
| Vitamin E | 50 IU | * | |
| Vitamin K | 1–2 mg | ||
| Zinc | 1–15 μg | * | * |
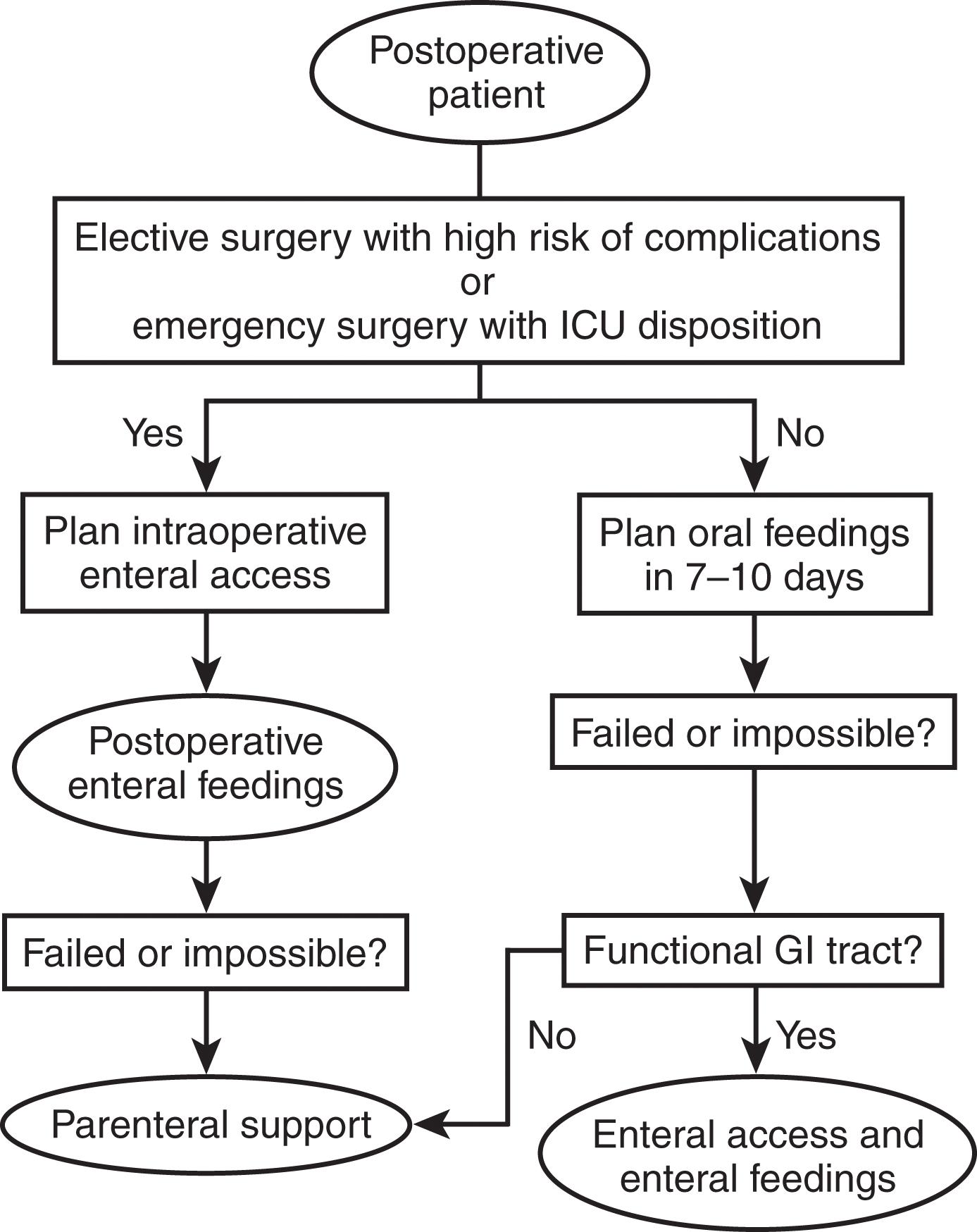
When developing a plan for postoperative or postinjury nutrition, it is important to anticipate the length of time that the patient may require ventilator support or experience ileus. The premorbid nutritional status of the patient and the state of catabolism must be assessed to optimize the opportunity for intraoperative enteral access ( Fig. 5 ). However, this type of detailed assessment may not be possible in patients scheduled for emergency surgery or in those with acute traumatic injury. In the acute setting, patients with significant total body surface area burns and significant trauma are at the greatest risk for protein-calorie malnutrition, and early nutritional support should be administered utilizing the previously described NUTRIC score ( Table 2 ) or traumatic injury algorithm for risk assessment. (See Fig. 1 .) Note that if the patient’s clinical condition deteriorates, the NUTRIC score should be recalculated. Patients who score as high risk should have nutritional support initiated within 4 days of injury or surgery.
As was previously mentioned, indirect calorimetry is the most accurate method of determining an individual patient’s energy determination, but it requires interpretation regarding therapeutic goals because of variations seen in the acute phase of injury. Ventilator support, renal replacement therapy, sedatives, and pain can interfere with results. Based on existing literature, a conservative interpretation would be best in the early phase of critical illness. The Harris-Benedict equations can also be utilized at basal to 1.3 times the basal rate when indirect calorimetry is not available ( Table 3 ). It important to note that these equations are most often unreliable in overweight and underweight patients. Simple weight-based equations can be utilized with 25 to 30 kcal/kg/day; however, accurate dry weights in the ICU setting may be difficult to obtain. In addition, this calculation does not factor in gender or age and may result in under- and overfeeding in younger and older populations, respectively.
Although protein requirements in stable adults are only 0.6 g/kg/day, the increased catabolism that occurs during critical illness increases the amount of protein required for balance. Improved nitrogen equilibrium occurs with increased protein synthesis in the majority of postoperative patients when adequate total calories are provided along with 1.5 to 2.0 g/kg of protein daily and up to 2.5 g/kg/day in patients on continuous renal replacement therapy (CRRT). There has been recent evidence that the amount of protein support plays an important role in patient outcomes and should be counted as part of the total energy supplied.
Carbohydrates should be administered at a maximum rate of 4 to 6 mg of dextrose/kg/minute, and blood sugar levels should be maintained between 120 and 180 mg/dL. Fat calories should not exceed 20% of total calories in most cases. Calories added from intravenous fluids and lipid-based medications must be taken into consideration as part of the nutritional support to avoid overfeeding. Supplements of enteral glutamine should be given in doses of 0.5 g/kg/day as well as multivitamins and trace minerals, including zinc and selenium.
Patients receiving postoperative nutrition should be monitored for blood sugar levels, electrolytes, fluid balance, and efficacy of therapy. Serum protein markers with short half-lives are most effective in measuring improvement in the visceral protein compartment ( Table 7 ). Failure to achieve improvement in these marker levels should prompt assessment of the nutrition administered over the past several days along with a search for untreated infection or inflammation. A simultaneous elevation in the C-reactive protein (CRP) level would suggest an ongoing inflammatory response as the etiology of suboptimal levels of protein markers. Nitrogen balance studies may be performed in which the amount of protein administered is evaluated relative to the amount of nitrogen lost in the urine, stool, and wound drainage ( Table 8 ). Critically ill patients should be maintained in neutral nitrogen balance, whereas anabolic patients should be maintained in a slightly positive nitrogen balance. Goals of therapy should include normalization of visceral protein markers, wound healing, and improvement in the somatic muscle mass through daily physical therapy.
| Protein Marker | Normal Values | Half-Life (Days) |
|---|---|---|
| Albumin | >3.5 g/dL | 20 |
| Transferrin | >200 mg/dL | 8.5 |
| Prealbumin | 20–30 mg/dL | 1.3 |
| Retinol-binding protein | 4–5 mg/dL | 0.4 |
| N balance = N (in) − N (out) |
| N (in) = protein/6.25 (g/day) |
| N (out) = total urinary N * (g/day) + gastrointestinal losses (2–4 g/day) + cutaneous losses (0–4 g/day) |
* Total urinary N can be either measured directly or estimated by measuring urine urea N and dividing by 0.8.
A substantial amount of data supports the enteral route of postoperative nutrition in patients following elective and emergency surgery as well as in patients who have sustained trauma and thermal injury. Delivery of nutrients by the enteral route attenuates the metabolic response to stress, provides more stable control of blood sugar levels, and is associated with fewer clinical infections. Enteral nutritional support has also been associated with increased intestinal anastomotic strength and higher rates of closure following damage control surgery.
In patients who undergo laparotomy, access for enteral feeding can be best achieved intraoperatively (see Figs. 1 and 5 ). Postoperatively, enteral nutrition support should begin between 12 and 72 hours following injury or surgery. Even if the total amount of required calories cannot be provided via enteral feeding, the immune protective effects of enteral nutrition can be seen with trickle feeding as low as 10 mL/hr. While previous data have suggested hemodynamic instability as a contraindication to enteral nutritional support, recent data support the administration of enteral nutrition in patients receiving deescalating levels of vasopressor support.
Administration of continuous tube feedings usually is initiated at 10 to 20 mL/hr and may be increased by the same volume every 8 to 24 hours depending on the clinical scenario and patient tolerance. Abdominal distention should prompt the immediate decrease in the tube feeding rate by half and should mandate cessation of feedings if distention persists. Bolus feedings into the stomach of 200 to 300 mL every 2 to 6 hours may also be given and may help to maintain adequate amounts of feeding despite the interruption in feedings related to other requirements of daily care and diagnostic tests. Postpyloric enteral nutrition should be considered in patients who are at risk for aspiration. Patients who have multiple returns to the operating room anticipated may also benefit from postpyloric enteral access, as holding tube feeds should not be required to undergo general anesthesia. Careful communication with the anesthesiology team is mandated to prevent long interruptions in nutritional support, which can inadvertently lead to malnutrition in a patient who is prescribed adequate caloric and protein intake but may not consistently receive it. The utilization of an enteral nutritional support bundle that targets and monitors goal feeding volumes using a multidisciplinary team approach has been demonstrated to provide more effective enteral nutritional support (see Table 9 ). This approach provides the same diligence in accomplishing enteral feedings as is routinely utilized in other treatment modalities employed in critical care. Note that it is important to continue enteral feedings until it has been documented that the patient is taking an adequate volitional oral diet of both calories and protein.
|
As previously discussed, special metabolic enteral formulas are available and designed for patients having unique metabolic requirements related to renal failure, hyperglycemia, pulmonary insufficiency, and trauma. In acutely injured trauma patients, immune-modulating formulas consisting of omega-3 fatty acids, RNA, and arginine have been associated with reduction of infections, wound complications, and hospital stays. However, these formulas are not recommended in septic patients. Other nutrients identified as potentially immune-modulating include carnitine, taurine, glutamine, N -acetylcysteine, antioxidant vitamins, and trace elements. Wound-healing formulas have been shown to be especially helpful in burn patients and contain higher levels of zinc, vitamin C, and vitamin A.
Because most well-nourished patients can tolerate inadequate nutrition for 7 days postoperatively following uncomplicated operative procedures, the use of routine postoperative parenteral nutrition in such patients is not justified (see Fig. 5 ). However, well-nourished patients with severe stress and preoperatively depleted patients should receive postoperative nutrition as early as possible whenever they are at high risk for inadequate nutrient intake. Management becomes more challenging when enteral feedings are not possible or inadequate. Occasionally, when full enteral feeding volumes cannot be met, protein powders can be given to meet protein requirements, and the additional energy provided via intravenous fluids and lipid-based medications may meet the threshold of 60% to 80% of caloric needs during this early period. Customized TPN can also be used as an adjunct to enteral nutrition when total caloric requirements cannot be provided through the enteral route, with careful assessment of all other nutritional sources to avoid overfeeding. Additional candidates for postoperative parenteral feedings are those who have been treated with preoperative parenteral nutrition and are unable to receive or absorb adequate postoperative enteral nutrition and those patients who develop complications that preclude use of the gastrointestinal tract. Parenteral nutrition may be lifesaving in patients with high-output proximal enterocutaneous fistulas, massive intestinal resection, or end jejunostomy syndrome.
Central venous access must be established to administer these high-osmolarity formulas (up to 2000 mOsm). When calculating a TPN formula, it is preferable to establish the nutrient components and then the desired fluid volume. Most current ordering systems will automatically calculate the osmolarity of the solution and indicate whether further dilution is necessary. For patients without central access who do not require fluid restriction, peripheral formulas may be prescribed but must have a maximum osmolarity of 900 mOsm. A balanced electrolyte solution should be added so as not to cause electrolyte derangements. Note that TPN solutions cannot be utilized to correct severe electrolyte disturbances.
It has been recommended to hold standard lipid therapy during the first week of recovery due to the immunosuppressive effects of the omega-6 fatty acid composition of standard lipid formulations. Newly Food and Drug Administration–approved lipid formulations containing a more balanced composition of omega-6, omega-3, medium chain triglycerides, and olive oil have recently become available for clinical use.
Certain new modalities may increase the efficacy of postoperative parenteral nutrition. Intravenous glutamine and antioxidants have also shown promise, and in the future, both may be indicated as a part of a nutritional regimen in critically ill surgical patients. Although scientific studies demonstrating potential benefits are available, additional clinical trials proving efficacy and safety are required to justify their routine use owing to high costs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here