Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most ventricular arrhythmias (VAs) in patients without structural heart disease originate from the outflow tracts of the right ventricle (RV) and left ventricle (LV). However, up to 20% of idiopathic VAs may originate from non–outflow tract sites, including the tricuspid and mitral valve (MV) annuli, para-Hisian region, cardiac crux, moderator band (MB), and papillary muscles in the RV and LV.
Mapping and ablation of idiopathic VAs should follow an organized anatomic approach that starts with careful analysis of the 12-lead electrocardiogram (ECG). Examination of the QRS axis, precordial transition, QRS duration, and other specific ECG features allows formulation of a hypothesis about the likely site of origin. The next step involves a detailed characterization of the patient’s heart anatomy with the use of intracardiac echocardiography (ICE) followed by confirmation of the suspected site of origin by electroanatomic mapping. If ablation fails to suppress the arrhythmia after the first lesions, attention should focus again on the ECG and anatomy to look for the reasons of failure: Did the exit change? Is the source epicardial or intramural? Can the site of origin be better approached from a neighboring structure? Is the site of origin in an intracavitary structure instead of the ventricular wall? Is contact with the tissue (and hence ablation energy delivery) limited by mobility or by the presence of obstacles such as valve leaflets?
In this chapter we review the ablation strategy for VAs originating from the papillary muscles, MB, mitral annulus, and tricuspid annulus, in addition to some relevant anatomic considerations for approaching these arrhythmias.
The papillary muscles of the LV are a source of VAs both in structurally normal and abnormal hearts. Presentations include isolated premature ventricular contractions (PVCs), nonsustained ventricular tachycardia (VT), and sustained recurrent VT. PVCs arising from the papillary muscles may also play a role as triggers of ventricular fibrillation (VF), which has been postulated as the mechanism of sudden death in some patients with MV prolapse. Most papillary muscle VAs have a focal mechanism (abnormal automaticity or triggered activity) and are sensitive to catecholamines. , However, patients with prior myocardial infarction or arrhythmic MV prolapse may have evidence of papillary muscle scar, and in these cases the mechanism is likely reentrant. , , ,
The papillary muscles are an integral part of the MV and tricuspid valve (TV) apparatus. Typically, in the LV there are two papillary muscles: anterolateral and posteromedial ( Fig. 82.1.A ). They originate from the mid or apical third of the LV and give rise to multiple chordae tendineae, which attach to the MV leaflets. The anterolateral papillary muscle originates from the anterolateral LV wall and provides chordae to the anterolateral half of the anterior and posterior mitral leaflets. The posteromedial papillary muscle originates from the inferoseptal LV wall and provides chordae to the posteromedial half of both leaflets.
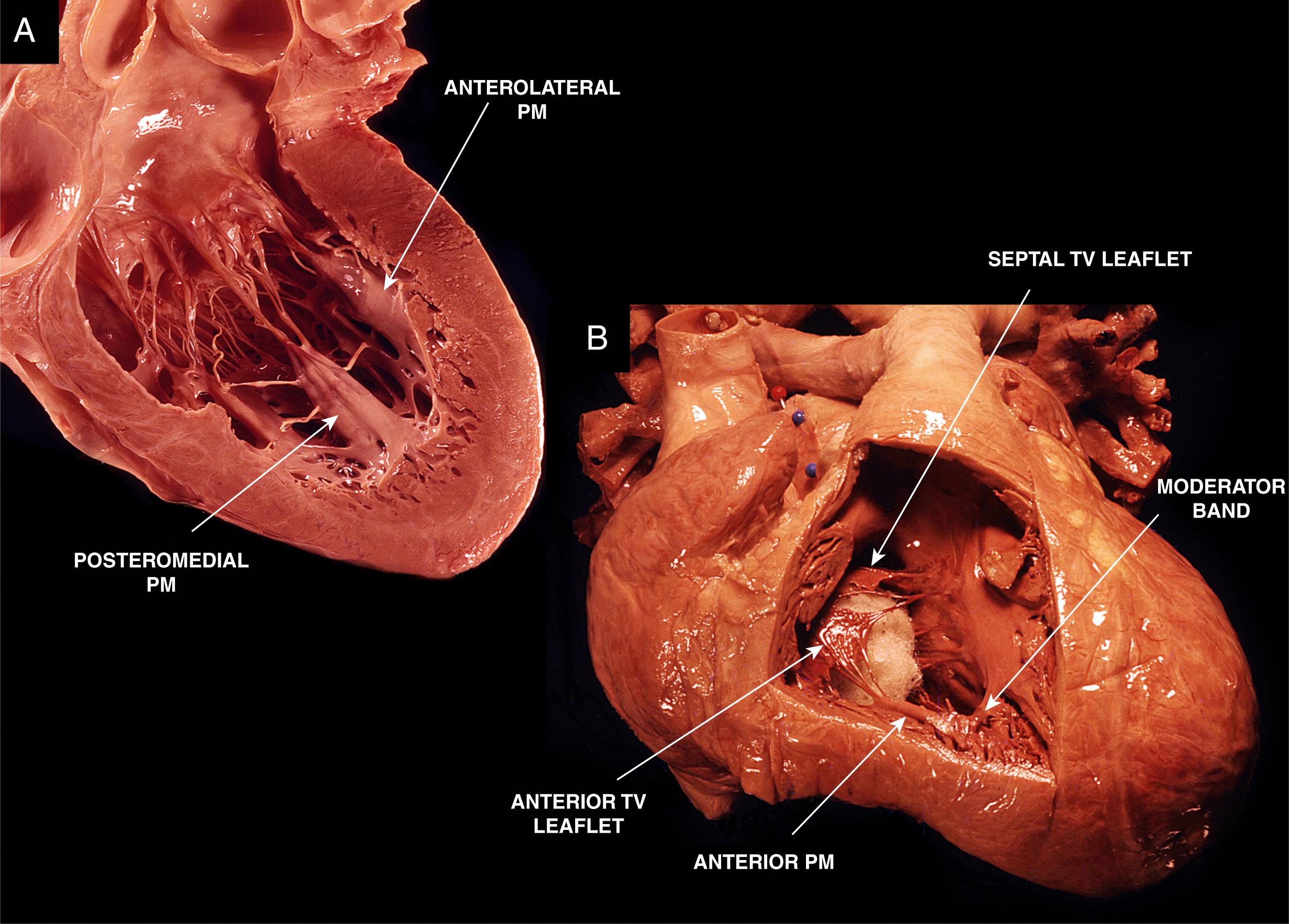
There is a wide variation in the morphology of the papillary muscles. The anterolateral papillary muscle typically has a single head or muscle group, whereas the posteromedial papillary muscle contains two or three major muscle groups. When multiple muscle groups are present, they may share a common origin or may have separate origins. The attachment of the papillary muscle to the LV wall can be finger-like, with a small focal point of attachment and few or no trabecular attachments, or tethered, with a large base of attachment and several trabecular bridges. , In the majority of cases the anterolateral papillary muscle has dual blood supply from the left anterior descending and circumflex coronary arteries, providing increased protection against ischemia. The posteromedial papillary muscle is either supplied by the right or circumflex coronary artery based on dominance. The papillary muscles play an important role in the functioning of the MV. During systole, they contract before LV wall contraction, which results in apposition of the MV leaflets, limiting the retrograde flow of blood from the LV back into the left atrium.
Histologically, the papillary muscles are composed of ventricular myocytes and a rich subendocardial network of Purkinje fibers covered by a layer of endothelium. Connections between the ventricular muscle and the Purkinje network are preferentially located near the base of the papillary muscle, and VAs are often found to originate at the Purkinje-myocardium interface.
Papillary muscle VAs have distinct electrocardiographic characteristics ( Figs. 82.2 and 82.3 ). VT/PVCs arising from the posteromedial papillary muscle exhibit a right bundle branch block (RBBB) morphology, left or right superior axis (negative or positive QRS polarity in lead I, negative QRS polarity in leads II and III), and precordial transition usually between leads V 3 and V 5 . VT/PVCs from the anterolateral papillary muscle demonstrate a RBBB morphology, right inferior axis (negative QRS polarity in lead I, positive QRS polarity in leads II and III), and transition between leads V 3 and V 5 . VAs from the anterolateral papillary muscle may also show inferior lead discordance, with negative QRS polarity in lead II and positive QRS polarity in lead III. Differences with fascicular VT include a wider QRS duration, a qR or R morphology in V 1 (compared with rsR′ for fascicular VT) and absence of q waves in leads I and aVL. , Briceño and colleagues found that papillary muscle VAs may have three distinct QRS morphologies in V 1 : Rr′ (the most common), R with a slurred downslope, or RR′; they often exhibit an initial small q wave in V 1 (75% vs. 22% in VAs from other LV locations).
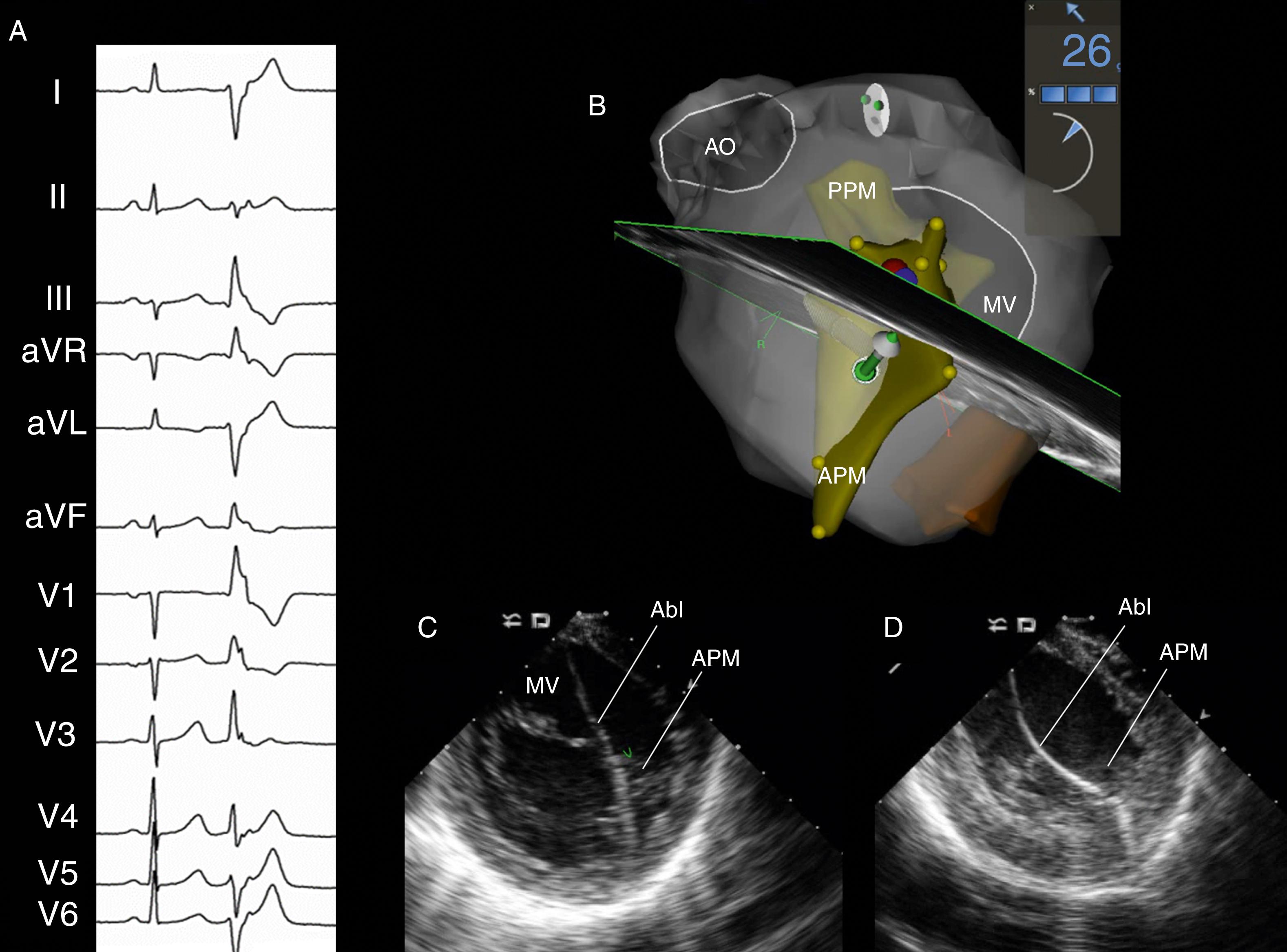
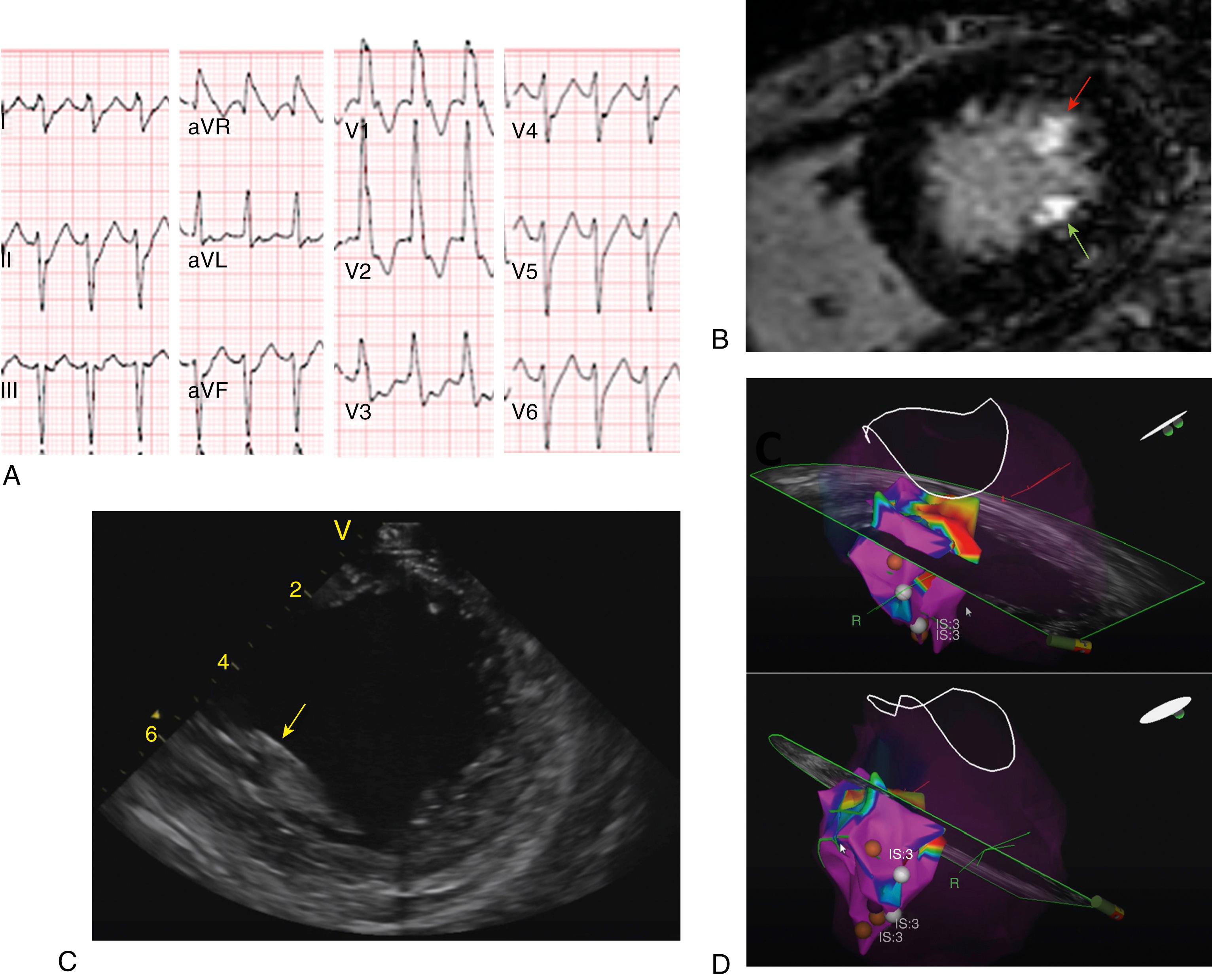
Papillary muscle VAs may exhibit variability of the QRS morphology, with slight beat-to-beat changes in axis and/or precordial transition. These variations are explained by multiple exit sites and correlate with the presence of muscular connections between both papillary muscles or between the papillary muscles and LV wall.
Mapping and ablation of papillary muscle VAs are challenging for a number of reasons : (1) they are mobile intracavitary structures, which limits catheter stability and contact; (2) their anatomy is complex, with significant patient-to-patient variation; and (3) the VA site of origin may be deep within the core of the papillary muscle.
ICE imaging is essential to ensure adequate catheter-tissue contact and correct orientation of the catheter tip during mapping and ablation. In addition, ICE imaging may identify increased echogenicity in the papillary muscle, suggesting a focal area of scar that may correspond to the site of arrhythmia origin. These echogenic areas may correlate with areas of low voltage and late potentials in sinus rhythm. To visualize the papillary muscles, the ICE catheter is first positioned in the mid right atrium to obtain a home view. From this position, the catheter is deflected anteriorly and gently advanced across the TV, followed by deflection release. On entering the RV, the inferior RV free wall comes into view. Clockwise rotation of the catheter generates a long-axis view of the LV with the posteromedial papillary muscle and the MV. Further clockwise rotation brings into view the anterolateral papillary muscle. The CARTOSOUND (Biosense Webster) module allows real-time integration of the ICE views into the mapping system anatomic reconstruction (see Fig. 82.2 ). When this feature is used, the contours of each papillary muscle can be delineated and separately incorporated into the LV anatomic shell.
Activation mapping is the preferred mapping technique, complemented by pace mapping. If spontaneous VT/PVCs are not present at baseline, induction is attempted with isoproterenol or dobutamine infusion and ventricular or atrial burst pacing; however, the resultant tachycardia from this pharmacologic intervention may further limit contact and be counterproductive. Ideal targets for ablation are sites with the earliest bipolar activation (≥30 ms pre-QRS) with a QS pattern in the unipolar electrogram. A sharp signal suggests a superficial location, whereas far-field signals indicate a deeper location within the papillary muscle core. In approximately 40% of cases, a Purkinje potential can be recorded at the successful ablation site, consistent with the role of the Purkinje system in the arrhythmogenic mechanism. , Also, in patients with evidence of papillary muscle scar the site of successful ablation may exhibit a late potential in sinus rhythm that becomes presystolic during PVC/VT, compatible with a reentrant mechanism. Pace mapping is useful, but not sufficient by itself. Sites of successful ablation usually exhibit an excellent pace map (≥11/12), but conversely, ablation at sites with perfect pace maps may fail to terminate the arrhythmia. This is likely because the exit site at the base of the papillary muscle may be located far from the site of origin, which may be higher in the body of the papillary muscle.
A retroaortic or transseptal access to the LV may be used. In our experience, the posteromedial papillary muscle and the medial aspect of the anterolateral papillary muscle are best approached with a retroaortic access, whereas the lateral aspect of the anterolateral papillary muscle is best approached in a transseptal fashion. For retroaortic access, a long SL0 or SL1 sheath (Abbott Medical) is helpful to facilitate catheter manipulation and stability. If a transseptal approach is used, an Agilis steerable sheath with a large curve is advanced into the left atrium and placed with the tip near the MV annulus. Radiofrequency application is delivered at powers of 30 to 50 W with temperature limited to 42°C, targeting an impedance drop of 10 to 15 Ω or 10% of the starting impedance value. Changes in the QRS morphology can occur during radiofrequency delivery, suggesting a change in the exit site, and several radiofrequency lesions on different parts of the papillary muscle may be required to completely eliminate the VT/PVC.
Cryoablation is an alternative to radiofrequency ablation, especially when catheter stability is an issue. Ice formation during cryoablation results in adherence of the catheter tip to the tissue, enhancing catheter stability. , We use a 6 mm Freezor Xtra catheter (Medtronic), which can fit through an Agilis or SL0 sheath. The cryocatheter is stiffer and more difficult to curve than the radiofrequency catheter and we favor a transseptal access to minimize the risk of aortic complications. However, if a retrograde approach is more convenient, we advance the long sheath into the LV using the radiofrequency ablation catheter or a pigtail, and then the cryocatheter is deployed in the LV to avoid manipulation in the ascending aorta. Lesions are delivered for up to 4 minutes, with two freeze-thaw-freeze cycles, targeting a temperature of –75°C. A retrospective study comparing both ablation strategies showed that cryoablation was associated with higher success rates and lower recurrence rates than radiofrequency ablation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here