Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fertilization of the human ovum usually occurs in the ampullary portion of the oviduct, although in rare instances it may take place elsewhere in the genital tract or even in the ovary. Soon after the spermatozoon enters the ovum, the male and female pronuclei fuse to form the segmentation nucleus, which rapidly divides and redivides. Segmentation, thus initiated, continues until the original fertilized ovum is transformed into a mass of cells called the morula.
At this early stage, two types of cells can be distinguished; some proliferate more rapidly, forming a sphere that encloses the aggregate of more slowly dividing cells. A semifluid substance is excreted from the outer cells and is collected in a cavity, which forms simultaneously. The sphere-shaped structure is called the blastodermic vesicle or blastocyst. One layer of ectodermal cells, the primitive trophoblast, covers it except at one pole where the rapidly dividing cells have formed the “inner cell mass,” which constitutes the beginning of the embryo.
While these changes take place, the ovum continues its passage into the uterine cavity, where it becomes implanted on the seventh or eighth day after ovulation. Various conditions may slow or obstruct the passage and cause nidation elsewhere, resulting in an ectopic pregnancy.
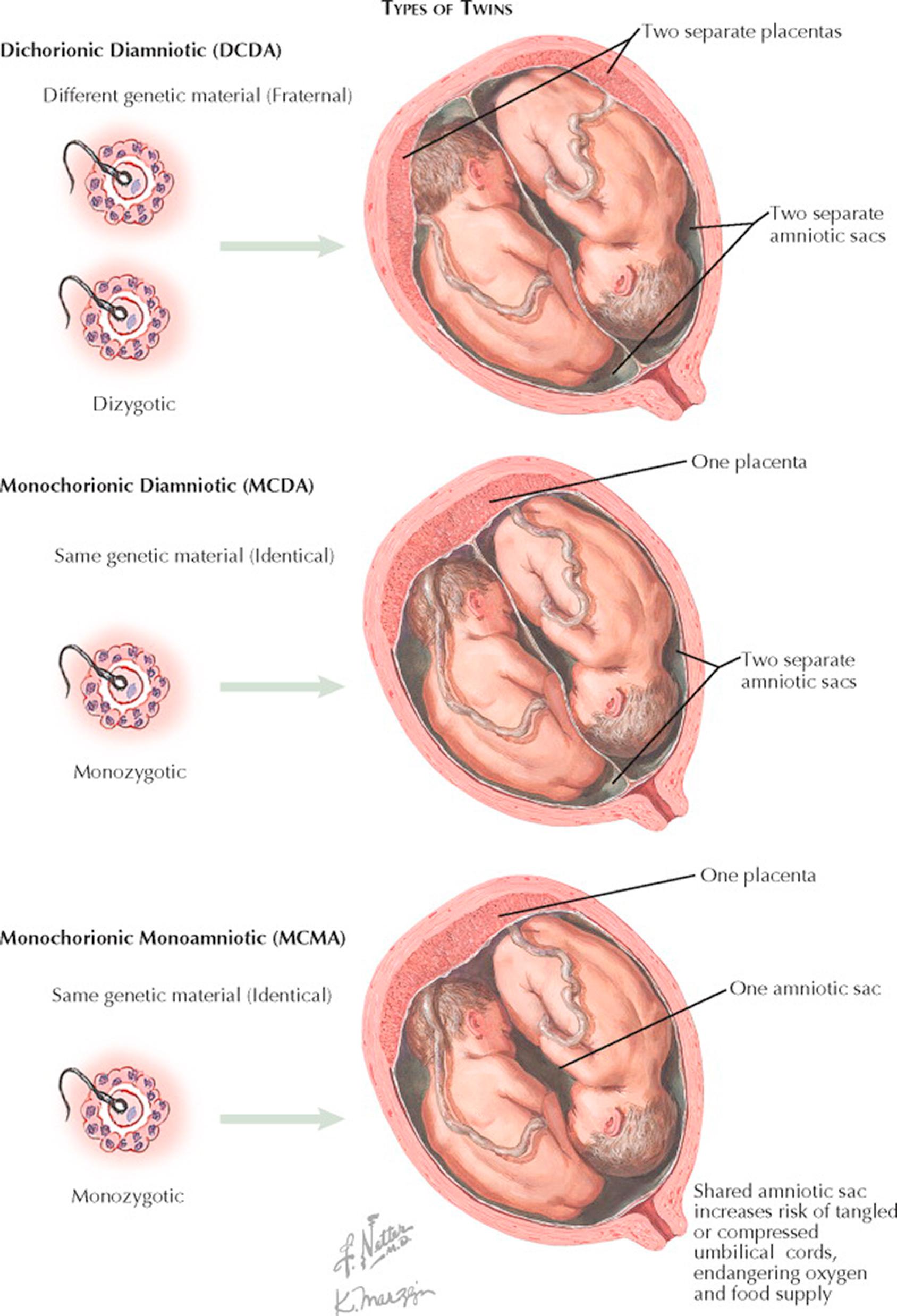
If the zygote splits very early (first 2 days after fertilization), each cell may develop separately its own placenta (chorion) and amnion (dichorionic diamniotic twins), which occurs 18% to 36% of the time. Most of the time, in monozygotic twins the zygote will split after 2 days, resulting in a shared placenta but two separate sacs (monochorionic diamniotic twins), occurring 60% to 70% of the time. In about 1% to 2% of monozygotic twinning the splitting occurs late enough to result in both a shared placenta and a shared sac (monochorionic monoamniotic twins). Later splitting of the zygote may result in conjoined twins.
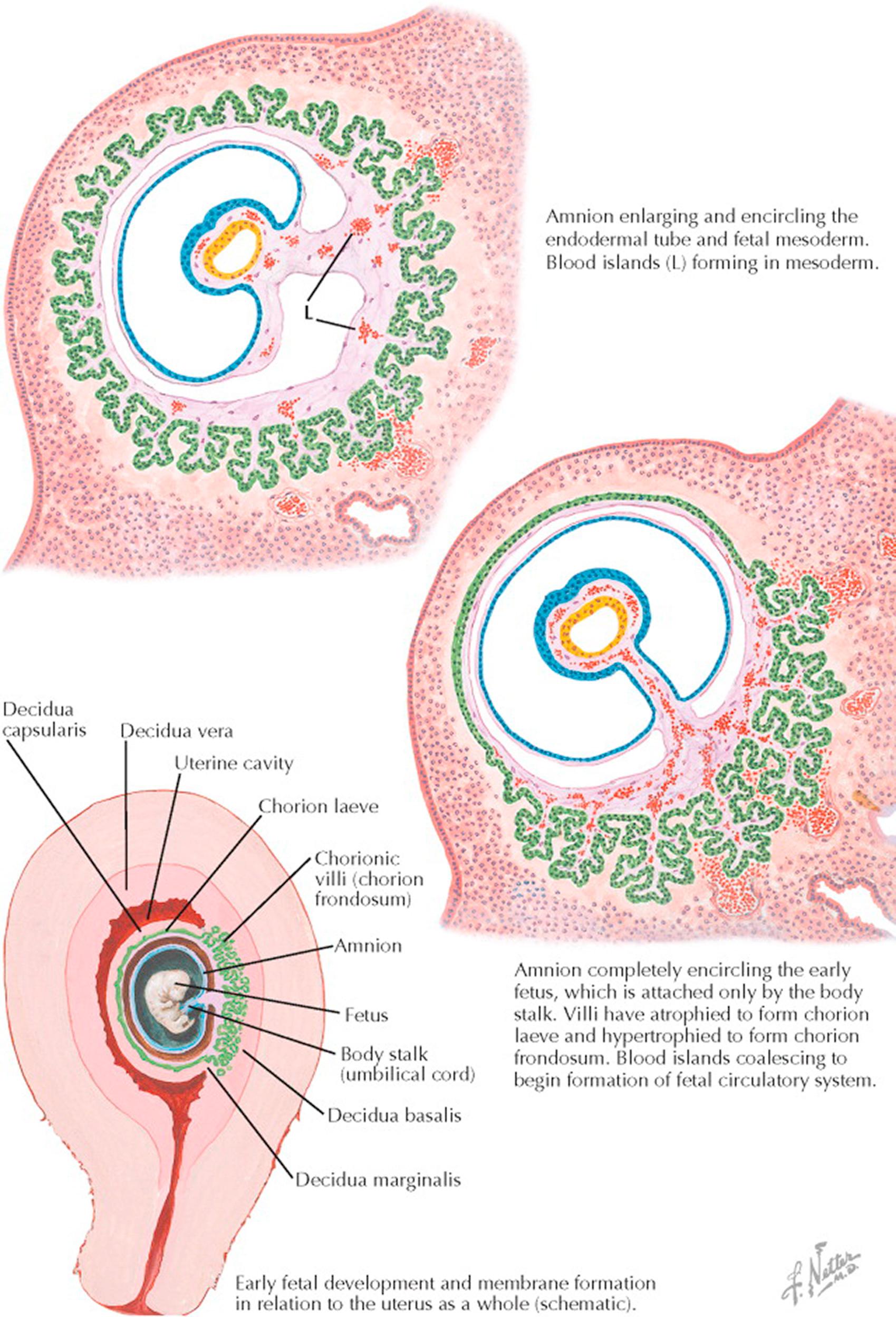
During the menstrual cycle, the ovarian hormones, estrogen and progesterone, act upon the endometrium, producing the premenstrual mucosa, which is sloughed or cast off during menstruation but remains when fertilization occurs. The pregravid endometrium gradually undergoes further changes to become the early decidua to which the blastocyst rapidly adheres once it has reached the uterus.
By the invasive capacity of its trophoblastic cells, the blastocyst sinks into the endometrium, which then closes over it and seals it from the uterine cavity, forming the decidua capsularis. The remaining decidua surrounding the blastocyst is called the decidua basalis, whereas the term decidua vera or parietalis designates the entire endometrium lining the uterus, except for the parts surrounding the blastocyst.
During the period of migration and implantation of the blastocyst, marked cellular proliferation has been taking place in the embryonic area. Three types of cells can be differentiated within the “inner cell mass.” These constitute the three primary germ layers, the ectoderm, endoderm, and mesoderm.
From the ectoderm will derive the central nervous system, the epidermis, and certain skin appendages. The endoderm will furnish the epithelial linings and the glands of the gastroenteric and respiratory tracts. The mesoderm will give rise to the epithelium of the urinary and genital systems, the linings of the serous cavities, the various supporting tissues of the body, the blood, and the cardiovascular system.
After implantation, mesodermic cells grow out beneath the primitive trophoblast, which, by proliferation, forms villous projections into the surrounding decidua. Each villus consists of a mesodermic core covered by two layers of trophoblastic cells. The outer cells have dark-staining nuclei and indefinite cell outlines. They are called syncytial trophoblasts. The more distinct cells of the inner layer are designated cytotrophoblasts or Langherans cells. These decrease in number as pregnancy progresses and are difficult to find after the third month of gestation.
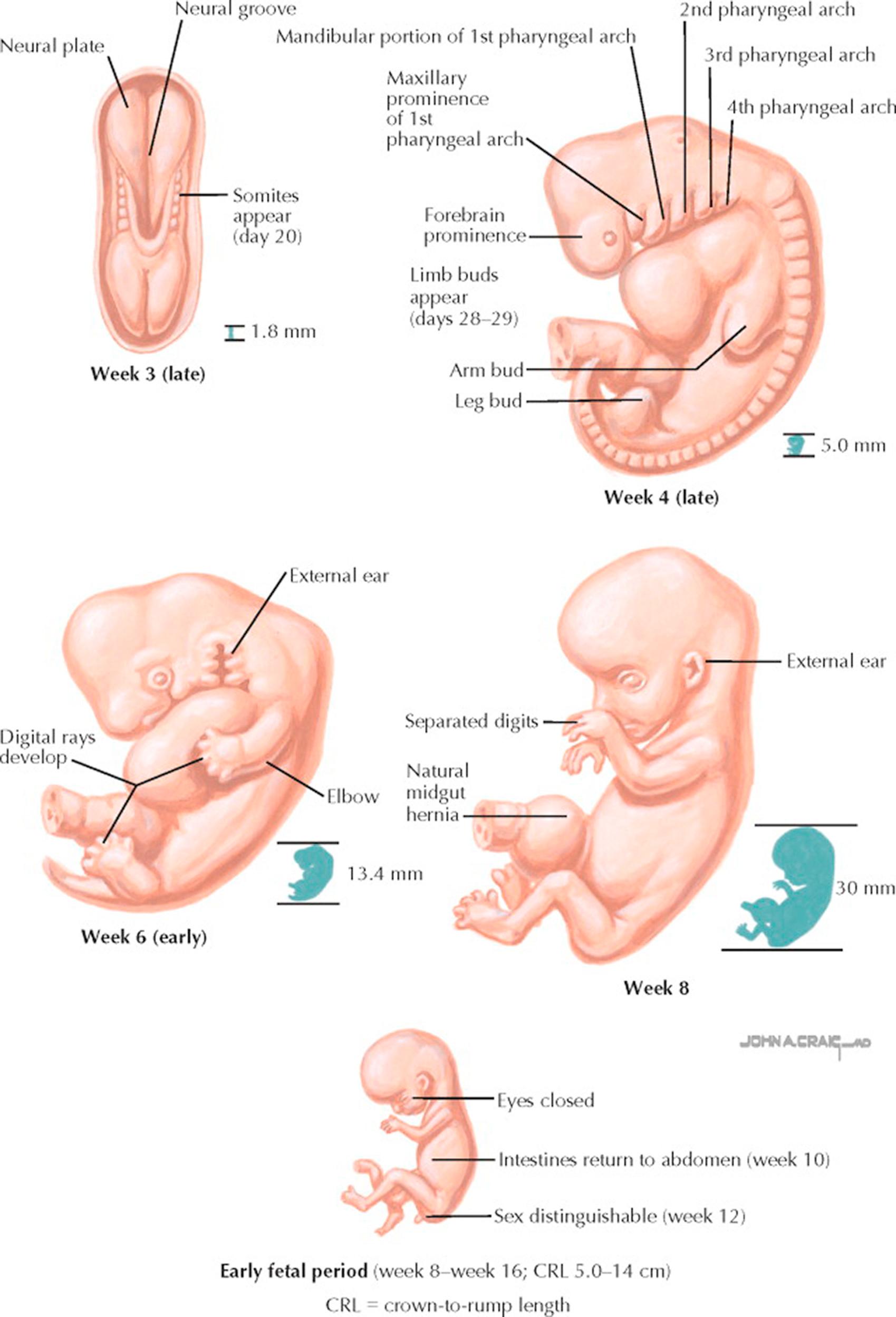
The first weeks following fertilization represent the most critical period for the success of a pregnancy. A high percentage (as high as 50% to 60%) of fertilized oocytes do not result in pregnancies completing the first trimester of gestation. Despite the dramatic changes that the conceptus undergoes in the first 14 weeks of gestation, many patients are unaware of their pregnancy or delay seeking prenatal care. Emerging evidence suggests that during this period the foundations of a successful pregnancy and even the future health of the adult individual are set.
For the fertilized egg, zygote, and embryo, a number of events must occur. During the first trimester of gestation, the developing embryo implants in the endometrium (except in the case of ectopic pregnancies), the placental attachment to the mother is created, and the major structures and organs of the body are formed. About the 12th week of gestation, the placenta takes over hormonal support for the pregnancy from the corpus luteum. If this transition does not happen smoothly, the pregnancy can be lost. When the serum level of β–human chorionic gonadotropin (β-hCG) is greater than 1500 mIU/mL, the level known as the discriminatory zone, transvaginal ultrasonography should visualize an intrauterine gestational sac in a normal, intrauterine, singleton pregnancy.
Most patients do not have any specific signs or symptoms of implantation, although it is not uncommon to experience light bleeding at implantation or cramping during the first trimester. Clinical blood and urine tests (β-hCG) can detect pregnancy soon after implantation, which is as early as 6 to 8 days after fertilization. Home urine pregnancy tests normally cannot detect a pregnancy until at least 12 to 15 days after fertilization. Morning sickness occurs in about 70% of all pregnant women and typically improves after the first trimester. Some women will experience cramping during their first trimester, though this is usually of little concern unless there is bleeding as well. Symptoms of fatigue and breast fullness may occur relatively early in the course of gestation, and abdominal distension begins later in this trimester.
For the first 2 months of growth, the conceptus is referred to as an embryo. During this phase, the developing embryo is most sensitive to exposures to toxins, medications, radiation, and the effects of maternal condition that can disrupt the development process. Errors may result in major disruptions in structure or function of the fetus, or the complete loss of the pregnancy. Later exposures to teratogens can result in a constellation of malformations related to the organ systems that are developing at that time; cardiovascular malformations tend to occur early in the embryonic period, genitourinary abnormalities tend to result from later exposures.
Embryonic development is said to be complete when the embryo attains a crown-to-rump length of 30 mm (start of the fetal stage), corresponding in most cases to day 49 after conception. At the start of the third month of gestation, the risk of miscarriage decreases sharply, all major structures including hands, feet, head, brain, and other organs are present, and they continue to grow and develop. The fetal heart can be seen beating on ultrasonography, and the fetus bends its head and also makes general and startle movements. The gender of the individual may be seen as early as the 12th week. Brainstem activity has been detected as early as 54 days after conception, and the first measurable signs of brain electroencephalographic activity occur in the 12th week of gestation. If a genetic evaluation of the fetus is indicated, chorionic villus sampling may be performed between the 10th and 12th week of gestation or amniocentesis may be done between 15 and 20 weeks.
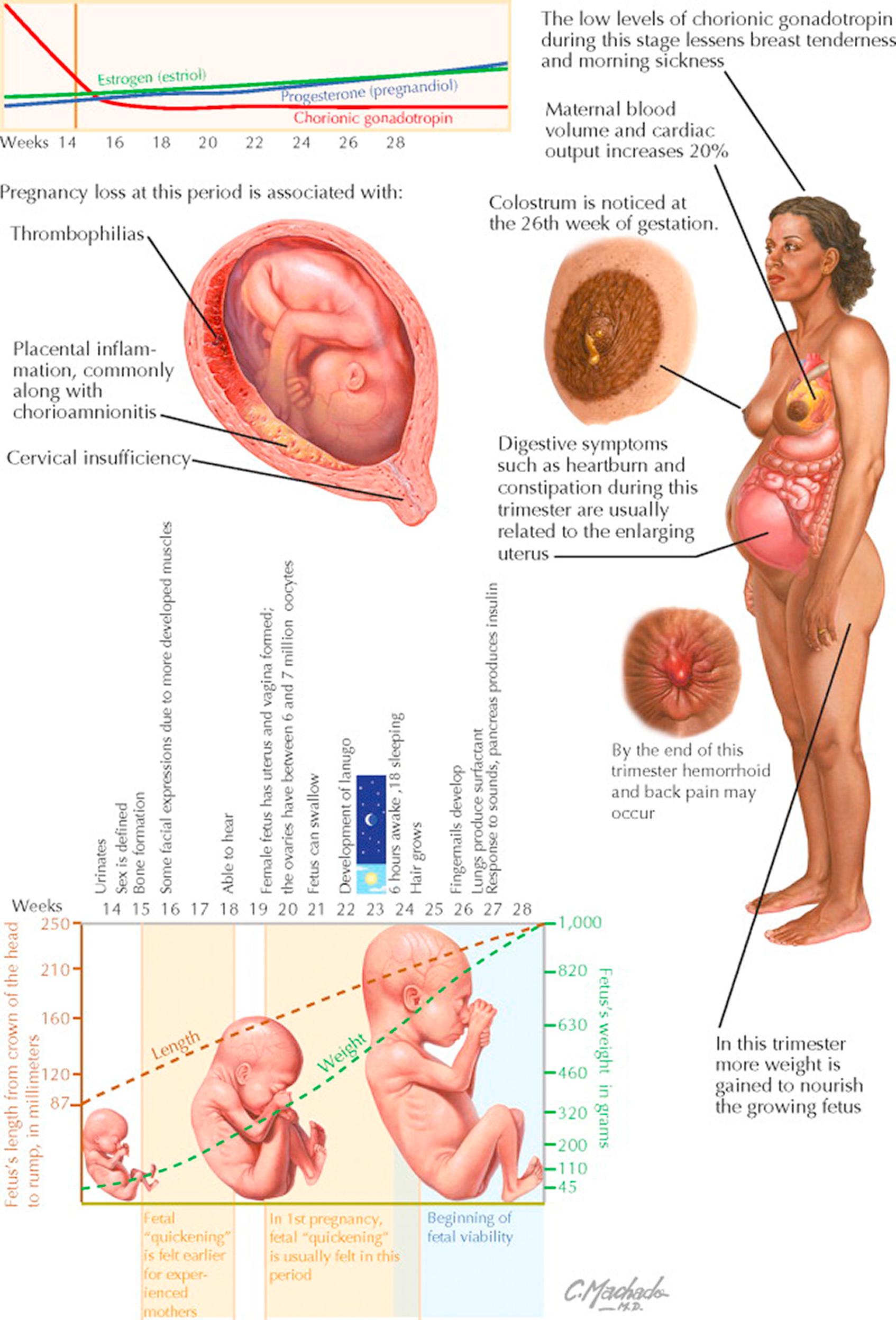
The second trimester (14 to 28 weeks) is a time of growth and refinement of function; all major structures including hands, feet, head, brain, and other organs are present, and they continue to grow and develop. During this trimester, the risk of pregnancy loss dramatically lessens and levels of human chorionic gonadotropin plateau and often decline, easing many of the early adverse symptoms of pregnancy such as breast tenderness and morning sickness, though the enlarging uterus may now precipitate heartburn and constipation. (When a second-trimester pregnancy loss occurs, it is strongly associated with placental inflammation, often related to ascending infection and/or acute chorioamnionitis, though it may also occur because of aneuploidy, by thrombophilias, by cervical insufficiency, and others.) Any weight loss experienced in the first weeks of gestation is regained and further weight is gained to provide stores needed to provide nutrition for the growing fetus. Despite the relative lack of complications during the second trimester, early signs of later problems may first appear during this phase of pregnancy.
Although the fetus begins moving during the first trimester, it is not until the second trimester that movement of the fetus (“quickening”) is felt. This typically happens around 20 to 24 weeks for first pregnancies and as early as 16 to 18 weeks for experienced mothers. Fetal waking and sleeping cycles become established and mimic those of the newborn, with the baby awake for about 6 hours a day.
Fetal and maternal changes are frequent during the second trimester: During this period, the fetus changes from a lightweight, weighing about 100 g and measuring 3 in. in length at 14 weeks, to weighing roughly 900 g (2 lb) by the end of the second trimester. By 15 weeks' gestation, the fetal heart pumps about 23 to 24 L of blood a day. Fetal viability (ability to survive apart from the mother) begins about 24 weeks, though neurologically intact survival at this stage is unlikely. There is an increase in maternal blood volume and cardiac output (20% greater) to feed the needs of the growing pregnancy. Toward the end of this trimester, maternal hemorrhoids and low back pain may make their appearance. Colostrum (the first form of breast milk) is present by 26 weeks of gestation. The placenta is fully functional, taking over the role of making estrogen and progesterone that had been performed by the corpus luteum. The fetus is making insulin and urinating, with fetal urine being a significant component of amniotic fluid. (The fetus will begin to outweigh the placenta sometime around the 16th week of gestation.) The teeth have formed inside the fetus's gums, and vernix and meconium make their first appearance. By the end of the second trimester, babies hear and respond to external sounds.
By the early portion of the second trimester, the external genitalia have formed sufficiently that they can be recognized on ultrasonographic studies, distinguishing the fetus as male or female. It is also at this point that a female fetus will have the most egg cells of any point in her life. Oocytes peak at 6 to 7 million about 16 to 20 weeks' gestation, declining to about 1 million at birth.
Screening for open neural tube and other defects (via measurement of maternal serum α-fetoprotein and other markers) is generally performed between 15 and 20 weeks. If a genetic evaluation of the fetus is indicated, an amniocentesis may be performed between the 15th and 20th weeks of gestation. It is generally during the second trimester that ultrasonographic screening for appropriate gestational age, fetal growth, and major fetal malformations is performed.
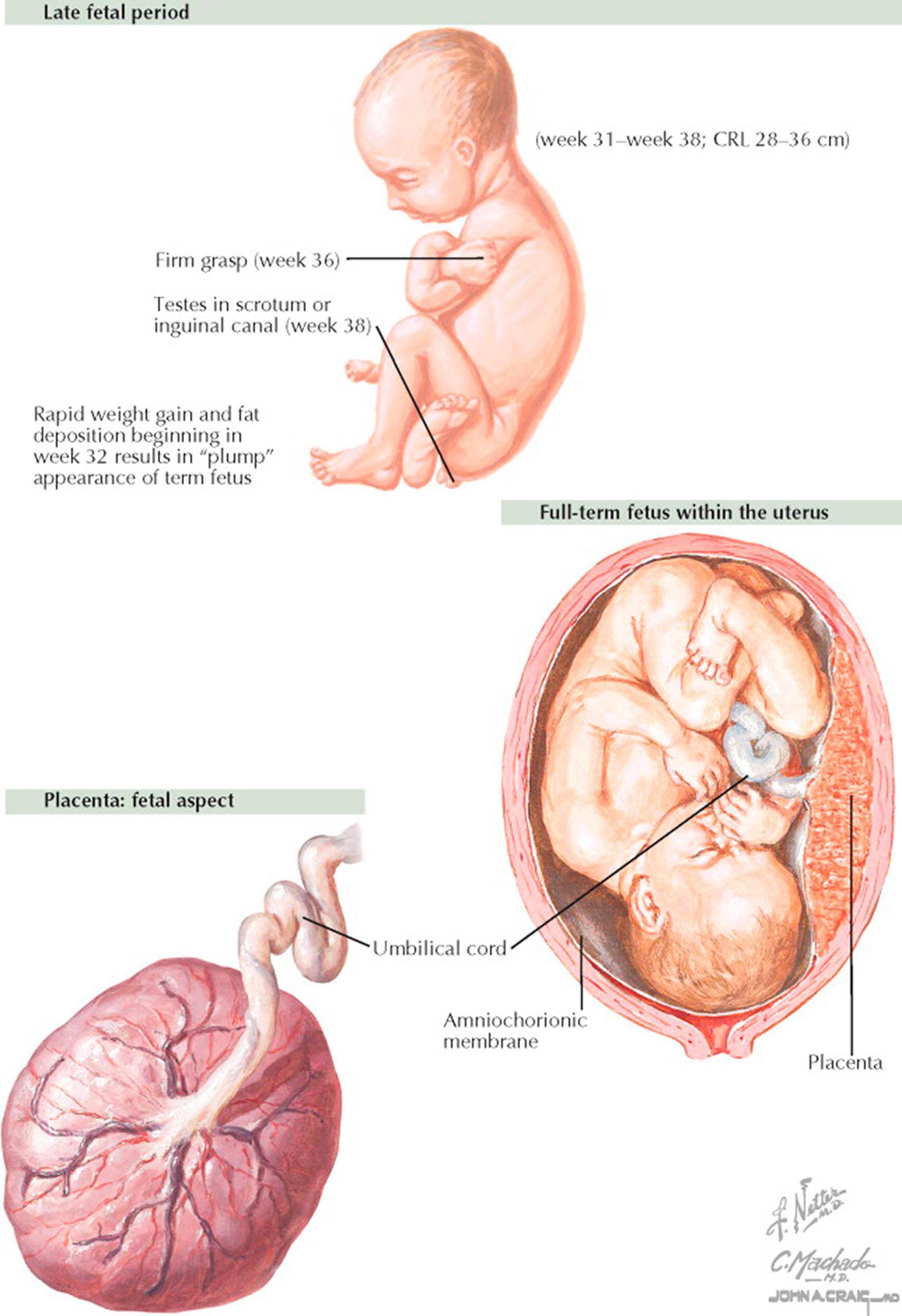
During the third trimester (27 to 40+ weeks) the fetus continues to grow and develop, and maternal physiology changes in preparation for childbirth. It is most often during this phase of pregnancy that complications such as preeclampsia, bleeding, complications of diabetes or hypertension, abnormalities of growth or amniotic fluid, and preterm labor may emerge.
During the third trimester of gestation, the dramatic growth of the fetus continues as it attains its final birth weight and its organs prepare for function as an autonomous individual. Fetal fat accumulates to provide nutrition and insulation for the first few days of independent life, accounting for about 15% of fetal weight at term. By the 29th week, the fetus has 300 bones, though eventual fusion of more than 90 of these fetal growth plates following birth will leave the adult total of 206. At the beginning of this trimester, in the male, the testes descend into the scrotum under the guidance of the gubernaculum, which in the female become the round ligaments supporting the fundus of the uterus. Most babies will settle into their final birth presentation (cephalic, breech, or transverse) by about 36 weeks' gestation.
Maternal blood volume increases by almost twice and cardiac output reaches its maximum. At term, 20% of maternal cardiac output goes to the uterus and placenta. Late in this trimester, changes in the cervix prepare for dilation and effacement during labor and delivery. It is also in the latter portion of this trimester that the number of oxytocin receptors on the uterine muscle cells increases markedly and there is an increase in the number of intercellular gap junctions. These micropores between cells provide a mechanism to facilitate the organized and effective coordinated contractions necessary for successful labor.
Uterine contractions that have been present since conception become progressively stronger and more noticeable as the trimester progresses. These are the Braxton-Hicks contractions of late pregnancy and the contractions of labor and delivery. Amniotic fluid volume peaks at about a liter at 37 weeks.
As the uterus grows, displacement of the abdominal contents results in early fullness with meals, heartburn, and constipation. The growth also results in relocation of the maternal center of gravity, causing the mother to lean backwards to compensate. This results in low back pain and the characteristic “duck waddle” of late pregnancy. When the fetal presenting part begins descent into the maternal pelvis (about 36 weeks' gestation), causing a decline in fundal height, the patient experiences improved respiratory and gastric function but at the expense of greater pelvic pressure and a reduced bladder capacity.
Planning and preparation for breastfeeding should be undertaken during this trimester. No special physical preparation is needed for successful breastfeeding, but discussion, questions, and the acquisition of needed supplies (e.g., nursing bra) are best taken care of before delivery. Normal amounts of colostrum have been present from the beginning of this trimester, and some women experience breast leakage throughout, this period.
For selected patients, “kick counts” may be used to assess the overall health of the fetus. In general, the detection of more than four fetal movements over the course of an hour indicates a healthy fetus. All patients should be encouraged to monitor their baby's activity levels and be evaluated for any prolonged reduction or absence in activity.
Because placental function declines after 40 weeks, testing of fetal and placental reserve through fetal nonstress testing, contraction stress testing, biophysical profiles, or measurements of fetal blood flow in various vessels may be indicated when there are complications of pregnancy or it extends beyond term.
As the embryo grows, it must establish an efficient means of obtaining nutrients and eliminating waste products. It does this by establishing the placenta, an efficient interface between its vascular system and that of its mother.
Trophoblastic cells have marked invasive capacities and grow into the walls of maternal blood vessels, establishing contact with the maternal bloodstream. In early pregnancy, trophoblastic cells frequently invade deep into the myometrium, but as pregnancy progresses, invasion is limited by profuse proliferation of decidual cells, which confine the trophoblastic invasion to the area just beneath the attachment of the growing placenta. In the rare instances when decidual cells fail to develop, implantation overlies an old scar or there are defects in the development of the fibrinoid layer (Nitabuch layer), invasion of the uterine wall by chorionic villi is extensive. This can result in a placenta accreta, increta, or percreta.
In the recently implanted blastocyst, the rim of trophoblastic cells, with the underlying mesodermic stroma, constitutes the primitive chorion. At the same time, the amnion first appears as a small cavitation in the mass of proliferating ectodermal cells in the embryonic area. This cavity gradually enlarges and folds around the developing embryo, so that eventually the latter is suspended by a body stalk (the umbilical cord) in a closed bag of fluid (the amniotic sac).
During the early stage of development of the amnion, another vesicle appears in the embryonic area and for a time is much larger than the amnion. This is the yolk sac (not illustrated), the function of which in mammalian development is not known. As the embryo grows, the yolk sac decreases in size, until at term only a minute remnant can be found near the site of the cord attachment to the chorionic plate.
During the first 3 weeks after implantation, a luxuriant growth of the rudimentary villi over the entire blastodermic vesicle occurs, developing into a structure called the chorion frondosum or “leafy chorion.” As the embryo, surrounded by the amnion, grows and protrudes more and more into the uterine cavity, the decidua capsularis and the underlying chorionic villi stretch and become flattened and atrophic. Most of the villi disappear from this region, which is then called the bald chorion or chorion laeve. Meanwhile the villi proliferate markedly in the highly vascular decidua basalis. Here the chorion frondosum persists and becomes a part of the fully developed placenta.
In rare cases the chorionic villi, beneath the decidua capsularis, do not undergo atrophy but establish vascular connections with the decidua vera, opposite the site of implantation, when the enlarging conceptus fills the uterine cavity. In this condition, called placenta membranacea, the entire chorion is covered with villi, and the thin placenta thus formed bleeds freely, does not separate spontaneously, and is difficult to remove manually during the third stage of labor.
The chorionic villi contain no blood vessels during the first 2 weeks of gestation, and the embryo has not yet developed a circulatory system. Nutrition is chiefly by osmosis. Toward the end of the third week, certain cells in the mesodermic stroma differentiate into blood islands, around which vascular walls soon appear. By branching and coalescence of these vessels, the entire chorion becomes vascularized. Meanwhile, a fetal heart and circulatory system have been developing. By the end of the fourth week, connections are made between the vessels of the chorion and those of the fetus, which have grown out through the body stalk, thus establishing a fetal–placental circulation.
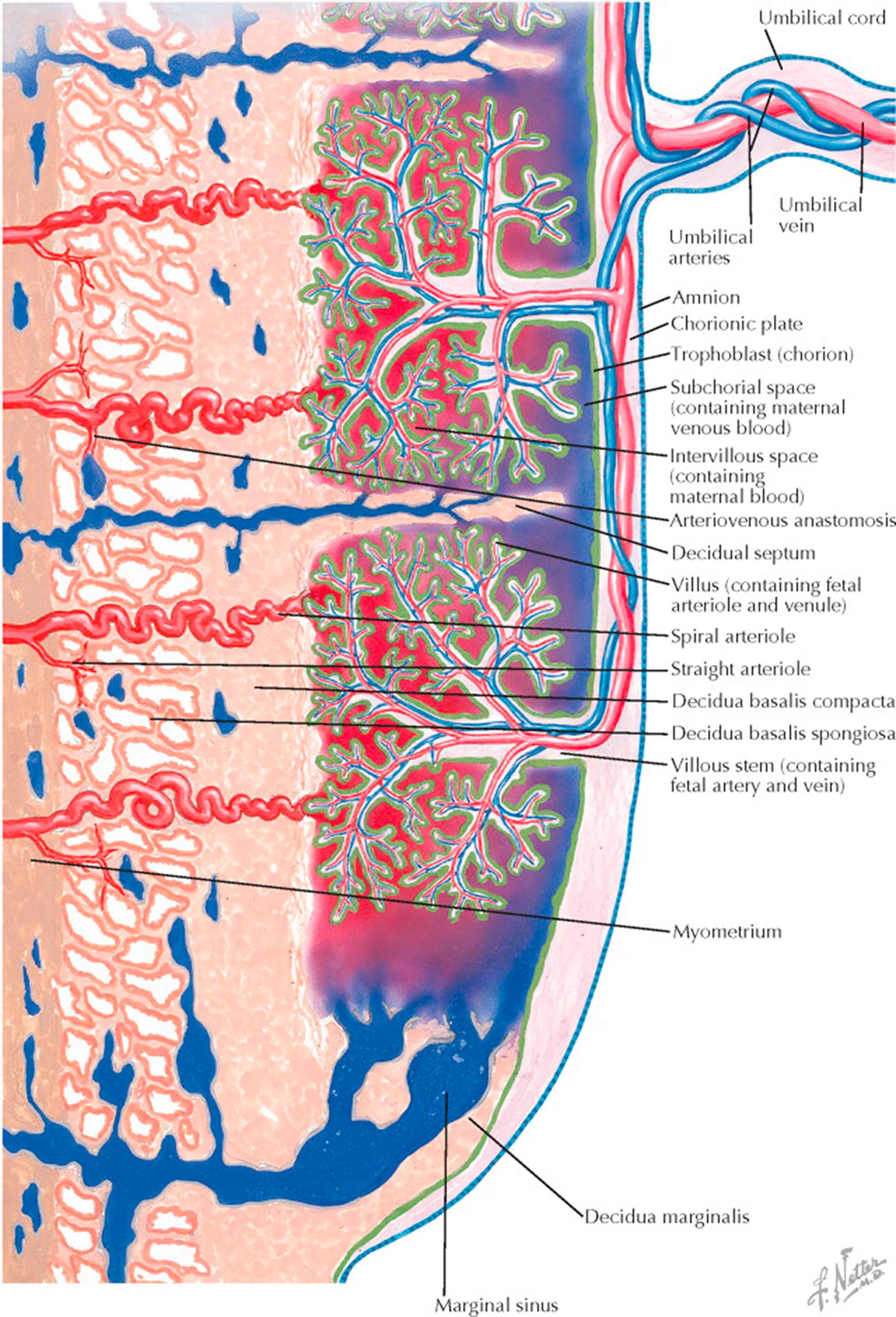
During the third week of gestation, the villi at the base of the placenta become firmly anchored to the decidua basalis. In later weeks the zone of anchoring villi and decidua becomes honeycombed with maternal vessels that communicate with the intervillous space. The spiral arteries in the decidua become less convoluted and their diameter is increased. This increases maternal blood flow to the placenta and decreases resistance. In response to the presence of the trophoblasts, the vascular endothelium is replaced by the trophoblast, and both the trophoblast and an amorphous matrix of fibrin and other constituents replace the internal elastic lamina and smooth muscle of the media. These changes are most marked in the decidual portion of the spiral arteries but extend into the myometrium as the pregnancy advances. The basal arteries are not affected.
The blood-filled lake in which the chorionic villi are suspended develops from the lacunae in the primitive trophoblast as it invades and opens up the maternal vessels of the decidua. Development of the maternal blood supply to the placenta is thought to be complete by the end of the first trimester of pregnancy (approximately 12 to 13 weeks). Abnormalities of this vascular process are found in women with fetal growth restriction and preeclampsia.
The villi absorb nutrients and oxygen from the maternal blood in the intervillous space, and these materials are transported to the growing fetus through the umbilical vein and its villous and cotyledon tributaries. Waste products for excretion into the maternal blood are brought from the fetus through two umbilical arteries, which are continuations of the fetal hypogastric arteries. These vessels terminate in the rich capillary network of the chorionic villi, where they are in close contact with the maternal bloodstream. The villi are oxygenated directly from the maternal blood and exhibit infarction whenever the maternal circulation around them ceases.
The details of the maternal blood flow through the placenta are not well understood. Observations indicate that the flow is much more rapid than was once believed and that the differences in the quality of blood in various areas of the placenta are quite marked. Currents and other dynamic factors probably cause these differences. The blood is more arterial toward the maternal aspect of the placenta, whereas in the subchorionic space it is venous in character. Although in most placentas the venous drainage is largely through the marginal sinus, part of the venous blood is returned to the uterine veins in the decidua basalis. The branching of the cotyledon stalks between the larger decidual septa divides the placenta to varying degrees into lobules, called cotyledons.
The marginal sinus is a large venous channel that courses beneath or through a gray ring of tissue formed by the membranes and the decidua marginata. It is not uncommon to find foci of obliteration, thrombosis, or rupture of the marginal sinus. This region is also the most common site for various retrograde changes in the decidua and contiguous chorionic villi. These lesions have long been considered of little or no clinical importance, though specific data are lacking.
The placenta is not only an intrauterine organ of respiration, nutrition, and excretion for the growing fetus but is also a powerful endocrine gland in the physiologic economy of both mother and fetus. Within 10 days after fertilization, trophoblastic tissue, probably the Langherans cells, has begun to produce chorionic gonadotropic hormone, and by the end of the second month the placenta is the main source for elaborating estrogen and progesterone. Other hormones include human placental lactogen (also called human chorionic somatomammotropin), insulin-like growth factor, and other growth factors.
In addition to its function as the agent of transfer of gases and nutrients, the placenta also has significant endocrine activity. It produces progesterone, which is important in maintaining the pregnancy; somatomammotropin (also known as placental lactogen), which acts to increase the amount of glucose and lipids in the maternal blood; estrogen; insulin-like growth factors; relaxin; and β–human chorionic gonadotrophin (βhCG). This hormonal activity is the main cause of the increased maternal blood glucose levels seen in pregnancy, which results in an increased transfer of glucose and lipids to the fetus.
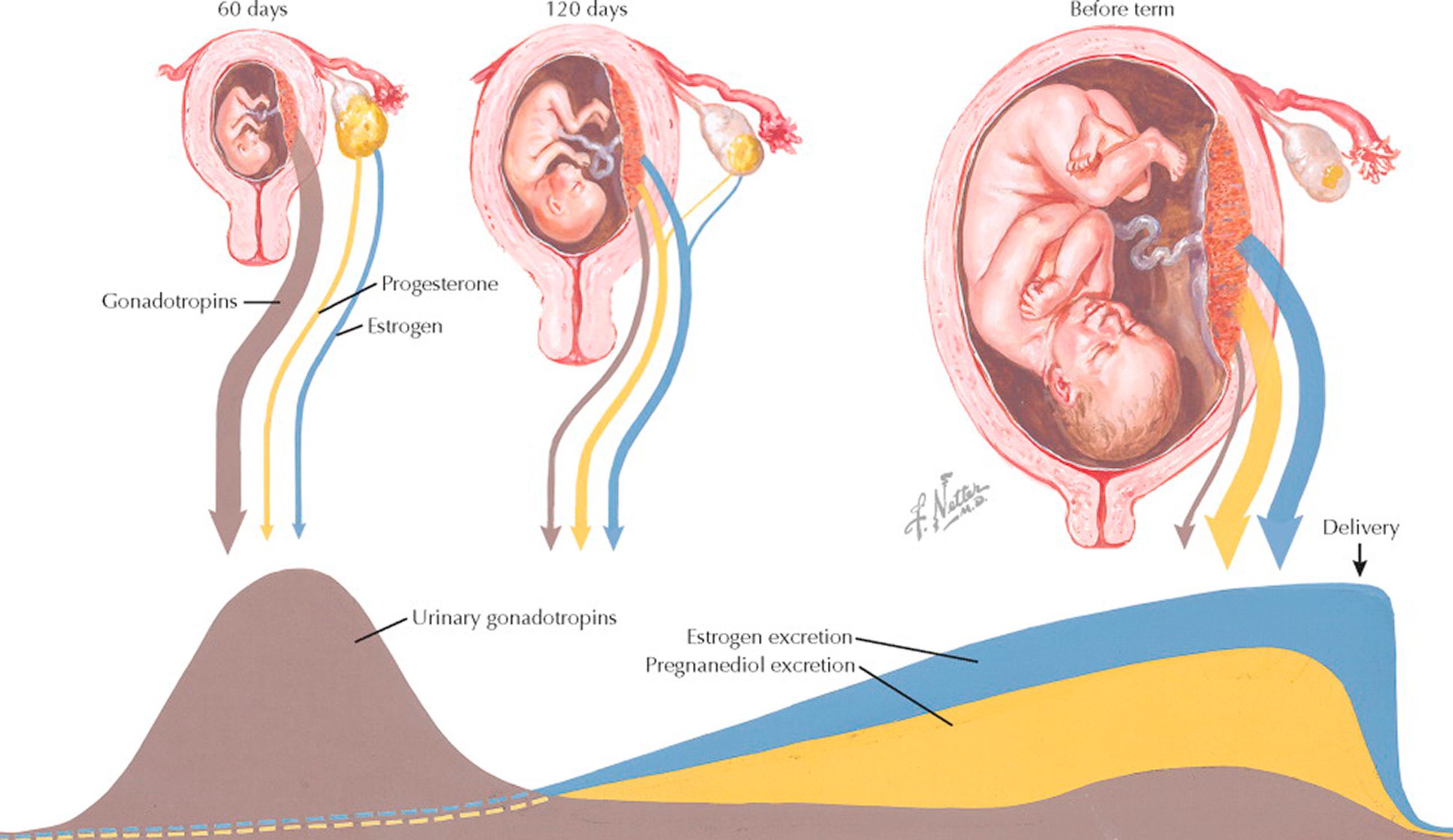
The corpus luteum of the ovary secretes estrogen and progesterone until the fourth month of gestation in amounts only slightly higher than those produced after ovulation in the second half of the regular cycle. However, not later than the 60th day of gestation, the placenta begins to secrete these hormones in progressive quantities, which reach their maximum at the end of gestation. Chorionic gonadotropin rescues the corpus luteum from programmed demise and ensures that progesterone and estrogen are secreted in early pregnancy, until about 2 months of gestation when the placenta takes on the role of producing sufficient progesterone and estrogen. Because of the marked production of estrogen and progesterone by the placenta, bilateral oophorectomy after the fourth month of pregnancy does not usually alter the course of gestation.
The site of formation of estrogen and progesterone in the placenta is the syncytial layer of the trophoblast. The production of progesterone by the trophoblast begins to decrease during the last month of gestation. This decrease is related to the cause of onset of labor, though the full nuances of the triggers of labor remain to be elucidated.
Chorionic gonadotropic hormone is secreted from the placenta soon after implantation, reaching its peak during the third month, after which its levels decrease, first sharply during the fourth and fifth months, then gradually leveling off until the end of gestation. Chorionic villi, specifically the syncytiotrophoblasts, are the site of production of this hormone. In addition to its role in promoting the continuing production of progesterone by the corpus luteum, it is thought that hCG affects the immune tolerance of the conceptus. Because of its highly negative charge, hCG may repel the immune cells of the mother, protecting the fetus during the first trimester. It has also been hypothesized that hCG may be a placental link for the development of local maternal immunotolerance. Chemically, it is a glycoprotein of a relatively large molecular size, composed of 244 amino acids with a molecular mass of 36.7 kDa. It is heterodimeric, with an α subunit identical to that of luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone, and a β subunit that is unique to hCG. A chemiluminescent or fluorometric immunoassay has been developed that can detect β-hCG levels as low as 5 mIU/mL and allows quantitation of the β-hCG concentration.
In early pregnancy, the function of this hormone seems to be aimed at keeping up the activity of the corpus luteum through interaction with the transmembrane receptor, resulting in continuing progesterone secretion, which is needed for the decidualization of the endometrium. After the fetus and placenta are well developed, the need for a corpus luteum, and therewith for this gonadotropic hormone, becomes less imperative.
The excretion rates of gonadotropins, estrogens, and pregnanediol vary to a great extent. The curves in the picture represent only an approximate graphic demonstration of the excretion changes during gestation rather than exact values at given times. For this reason, no scale has been entered. Not shown are the excretion values of adrenal cortical hormones, which are also increased during pregnancy.
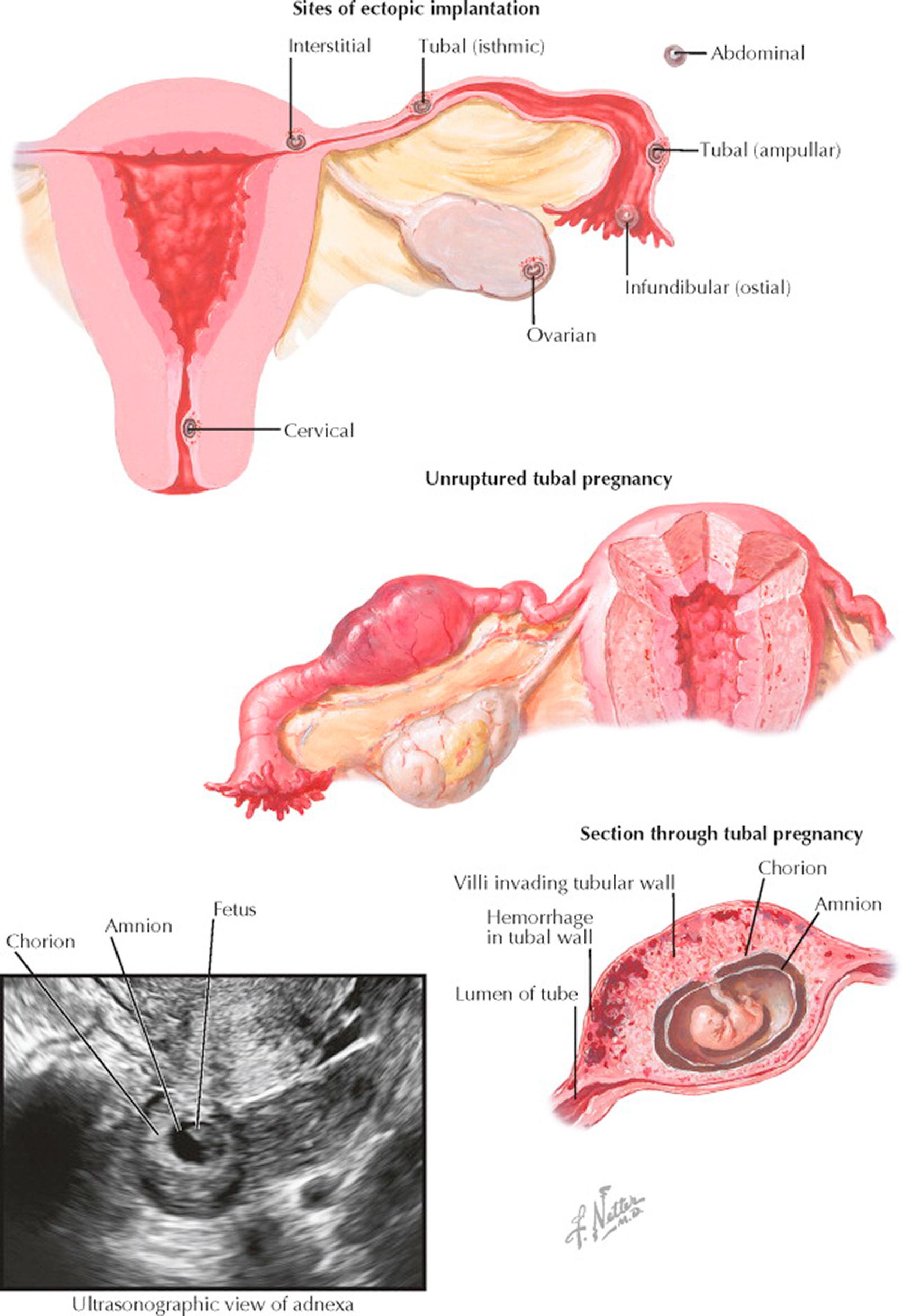
Ectopic pregnancy refers to the implantation of the embryo in any place outside the uterine cavity. According to the site of implantation, four kinds of ectopic pregnancy are distinguished: tubal, ovarian, abdominal or peritoneal, and cervical. Between 10 and 15 of every 1000 pregnancies are ectopic, with the rate varying with age, race, and geographic location (highest in Jamaica and Vietnam).
Tubal pregnancy is by far the most frequent of all ectopic pregnancies. Here again, four types are recognized, depending on the portion of the tube in which the implantation takes place: interstitial (cornual), isthmic, ampullar, and infundibular. Although ampullar implantation has the highest incidence of tubal pregnancy, it is the interstitial form that is potentially the most serious from a clinical perspective.
The most important contributors to the occurrence and development of ectopic pregnancy are tubal damage or altered motility that causes the fertilized egg to be improperly transported, resulting in implantation outside the uterine cavity. The most common cause of tubal damage is a history of acute salpingitis (50%). In the majority of the remaining patients (50%), no risk factor is apparent. Abnormal embryonic development may play a role. Pelvic infections convey a sixfold increased risk; a prior ectopic pregnancy conveys a 10-fold increased risk, followed by prior female sterilization, increasing age (age 35 to 44, 3-fold greater rate than for women aged 15 to 24), nonwhite race (1.5-fold increased risk), assisted reproduction, cigarette smoking (30+/day, 3- to 5-fold increased risk), and endometriosis. Although intrauterine contraceptive devices (IUCDs) markedly decrease the risk of ectopic pregnancy compared with use of no method of contraception, if pregnancy occurs with an IUCD in utero, it is much more likely to be ectopic.
The early development of an ectopic pregnancy is the same as of an intrauterine gestation except for its location: the trophoblast possesses the same qualities and thus secretes chorionic gonadotropin, participating in the maintenance of the corpus luteum of pregnancy. This latter, in turn, elaborates enough estrogens and progesterone to induce all the maternal changes characteristic of the early stages of pregnancy. Initially, the level of excreted chorionic gonadotropin in the urine is the same as in a normally developing pregnancy. The mother may manifest decidual transformation of the endometrium and slight enlargement and softening of the uterus, just as in uterine pregnancy.
The possibility of ectopic implantation must always be kept in mind. A good clue is the history of amenorrhea of several weeks, followed by bleeding (usually spotting) accompanied by abdominal pain, which may be slight or intense. The subjective complaints of early pregnancy may be mild or nonexistent, as in normal pregnancy. Physical examination may reveal the presence of a tumor in the adnexa or some irregular, sometimes retrouterine, growth filling the cul-de-sac. Slight enlargement of the uterus, without cervical dilation but with tenderness in the posterior region of the cul-de-sac, may be present. Signs of hemorrhagic shock and peripheral collapse are seen when the intraperitoneal hemorrhage is severe, and in that stage the diagnosis, of course, becomes less and less problematic. Transvaginal ultrasonography may document no gestational sac within the endometrial cavity and an adnexal mass. The finding of free fluid in the posterior cul-de-sac is common but not diagnostic. The finding of a fetal heartbeat in the adnexa is diagnostic.
Laboratory evaluations should include serial quantitative β-hCG levels (if patient's condition permits). (Levels lower than 3000 mIU/mL are found in about half of cases.) Normal pregnancies should demonstrate a doubling of serum β-hCG levels every 48 hours, whereas abnormal pregnancies will not. Serum progesterone (low) may be of diagnostic help if pregnancy is <6 weeks' gestation. Almost 90% of patients with an ectopic pregnancy have levels less than 30 nM/L (10 ng/mL). A hematocrit of less than 30 mL/dL is found in about one-fourth of women with ruptured ectopic pregnancy.
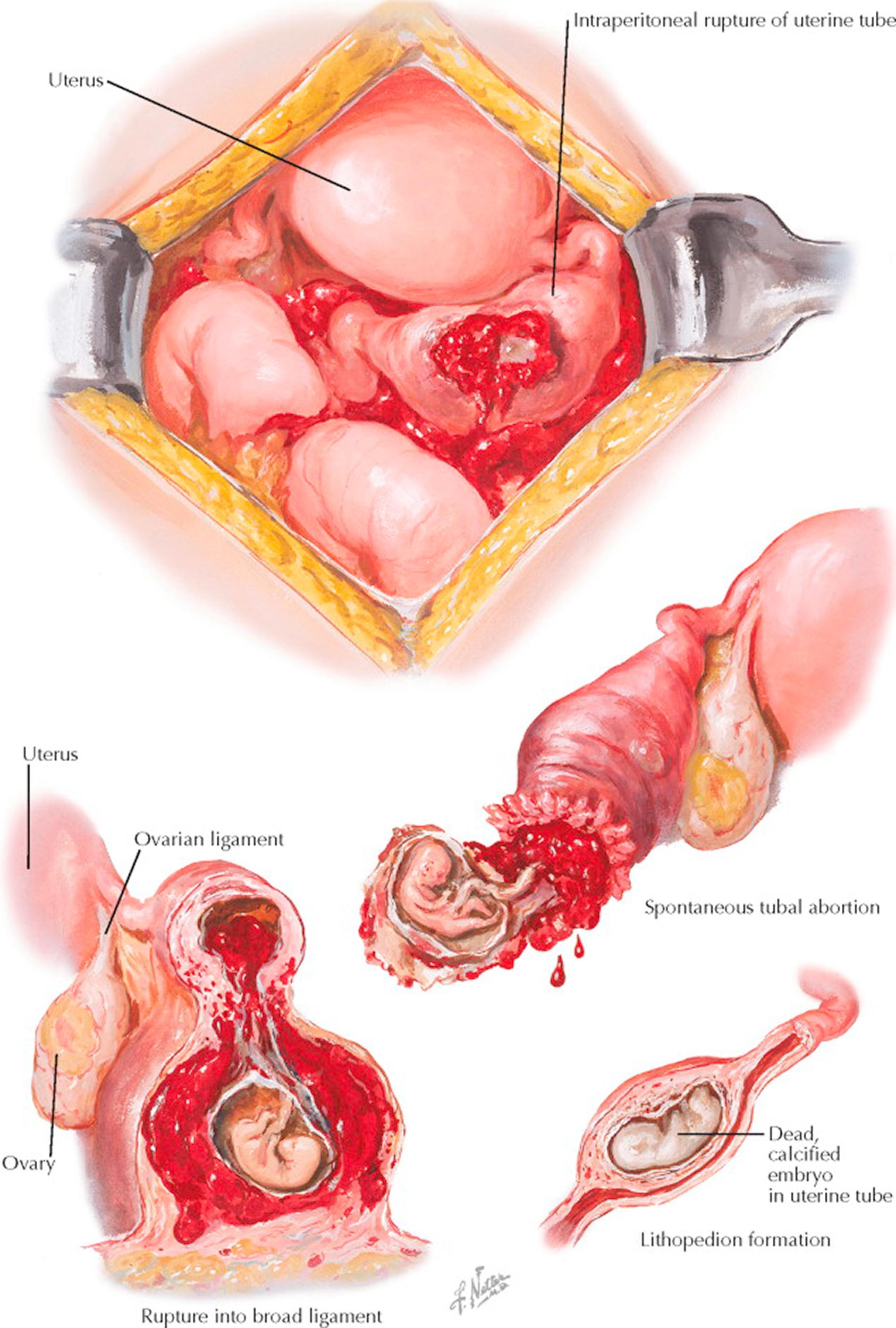
Very rarely does a tubal pregnancy develop longer than into the fourth or fifth month without symptoms and signs that ultimately lead to the diagnosis. The most frequent outcome of tubal pregnancy is abortion through the tube into the peritoneal cavity. It usually occurs between the middle of the second and the end of the third month, but it may come earlier. A partial or total separation of the trophoblast from the tubal walls occurs, leading to death of the embryo. Blood extravasation and later extrusion of the embryo with blood clots into the peritoneal cavity follow, where they may slowly be absorbed, provided the hemorrhage was slight. The uterine decidua may sometimes separate as a whole and be eliminated as a decidual cast of the uterine cavity. Passage of the decidual cast can be confused with an early spontaneous abortion, and hence the passed tissue should be carefully examined.
In many cases of tubal pregnancy, the trophoblast erodes the tubal wall. This leads to a tubal rupture, which is almost always accompanied by a serious, catastrophic clinical picture of acute shock due to extensive hemorrhage into the peritoneal cavity. The time of tubal rupture varies with the site of implantation. If the embryo develops in the interstitial portion of the tube, rupture occurs relatively late, whereas nidation in the isthmic part results in rupture in the very early weeks because of the difference in the mass of musculature in the two parts of the tube. Rupture may take place spontaneously but may also occur following defecation, coitus, and vaginal examination. The consequences of a rupture after interstitial implantation are more serious because of the major vessels in this area.
In a few cases, rupture has taken place through the lower margin of the tube, where it is not covered by peritoneum and where the two folds of the broad ligament meet only loosely. In such instances, the tubal contents empty into the connective tissue of the mesosalpinx, that is, between the two peritoneal sheets. Here hematoma may develop, and the embryo will die, or a broad ligament pregnancy, also called intraligamentary or extraperitoneal pregnancy, may continue, depending upon the degree of placental separation.
Although rupture of a tubal pregnancy or a tubal abortion with hemorrhage is a surgical emergency treated by laparoscopy or laparotomy, when an ectopic pregnancy is diagnosed early, medical therapy may be appropriate. Medical therapy may be considered for asymptomatic or mildly symptomatic patients. Methotrexate is generally used for chemical management of these patients. Methotrexate should not be used if the β-hCG level is higher than 15,000 mIU/mL, the adnexal mass is greater than 3 cm, or the patient's hemodynamic status is unstable. Patients with a history of active hepatic or renal disease, fetal cardiac activity demonstrated in the ectopic gestation, active ulcer disease, or significant alterations in blood count (white blood cell count <3000, platelet count of <100,000) are not candidates for this therapy.
All Rh-negative, unsensitized women with ectopic pregnancies should receive Rh immunoglobulin at a dosage of 50 μg if the gestation is of less than 12 weeks' duration and 300 μg if it is beyond 12 weeks.
Termination of tubal pregnancy by death of the embryo and its transformation into a lithopedion is a very rare event. Such a process may go on completely asymptomatically, with slow dehydration and mummification. This “missed tubal abortion,” as it has been called, may be found only incidentally during laparotomy.
Hydatid mole formation and choriocarcinoma development have been observed in ectopic pregnancy but are extremely rare.
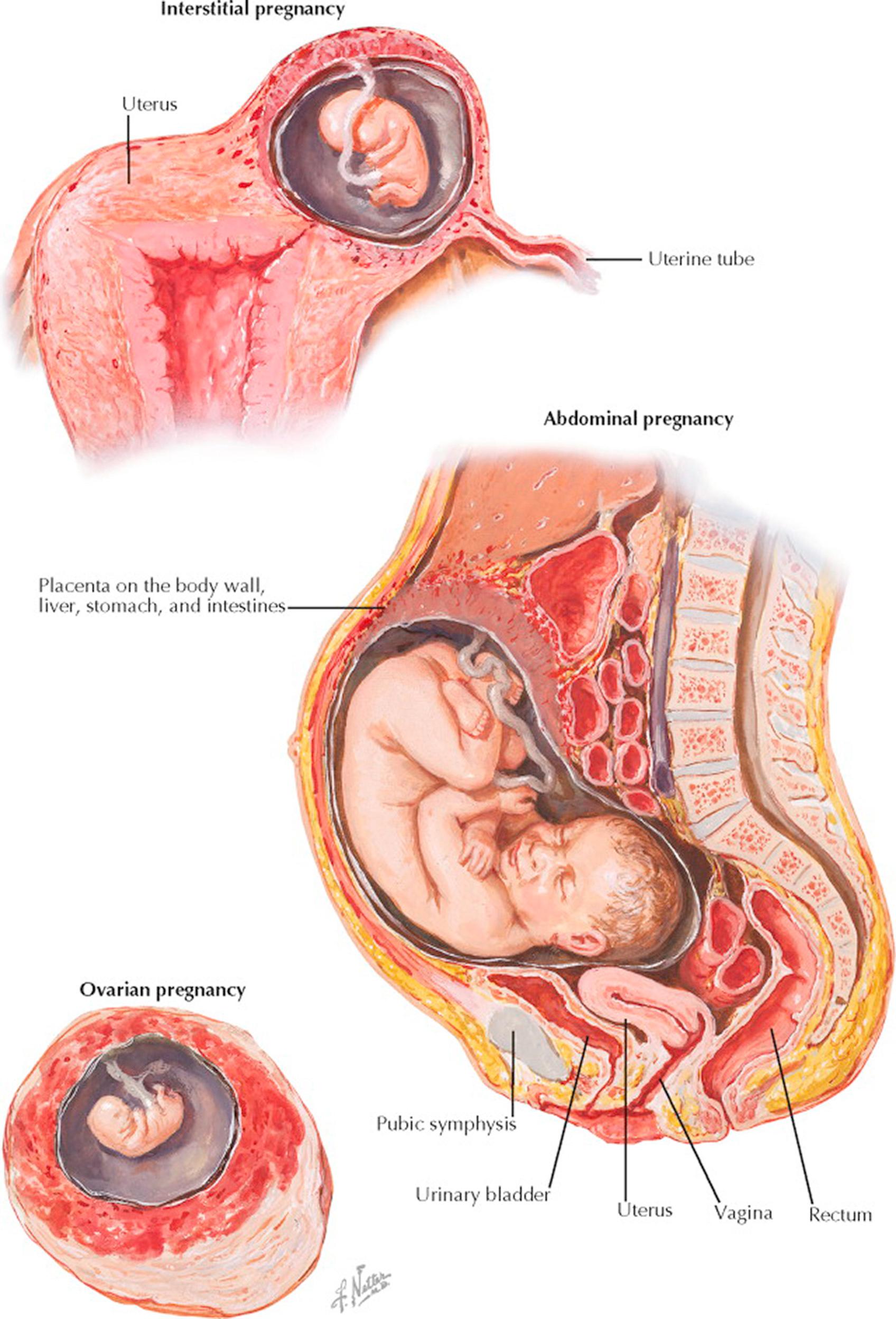
When, during the process of abortion or rupture, the trophoblast, after total separation, implants itself again somewhere in the peritoneum, as happens on rare occasions, it may grow and develop into a secondary abdominal pregnancy. The embryo in such cases may have remained in its original amniotic sac, or a new sac may have formed from the surrounding tissues. A secondary abdominal pregnancy may also result from a beginning tubal implantation that ruptured and became inserted between the leaves of the broad ligament. If the latter should rupture again, the embryo in the fetal sac may extrude into the peritoneal cavity, with the placenta remaining in the extraperitoneal position between the broad ligament sheets. In still more exceptional cases, the fertilized ovum may escape through the open end of the tube, attaching itself to the parietal or visceral peritoneum or the omentum, developing into a primary abdominal pregnancy. It has even been reported that an abdominal pregnancy has originated from a defect in the uterine wall, which had been filled and closed up by the omentum during the healing period after cesarean section. The remarkable feature of these abdominal pregnancies is that they may continue to near term before an occasion for diagnosis may even arise, even in the face of repeated ultrasonographic studies. The incidence of abdominal pregnancy is estimated to be roughly 1 in 10,000 live births.
Fetal salvage in these cases is the exception, though survival rates of more than 50% have been reported if the pregnancy progresses beyond 30 weeks' gestation. When survival does occur, there is a much increased rate of fetal malformations, including facial or cranial asymmetry, limb defects, and central nervous system anomalies. Delivery must be by laparotomy, but such surgeries are associated with massive hemorrhage even when care is taken not to disturb the placenta.
Ovarian pregnancy is the rarest form of ectopic pregnancy. Although full-term ovarian pregnancies are on record, they more often eventuate in encapsulation and degeneration of the fetal mass. The diagnosis can be made only by finding ovarian structures around the amniotic sac upon microscopic study of the removed ovary. In a primary ovarian pregnancy, the oviduct and the broad ligament should not be involved.
In a low percentage of tubal implantations, the fertilized ovum may settle in the uterine end of the tube—its intramural or interstitial segment. In an interstitial pregnancy, owing to the greater muscular mass and vascularity, fetal growth may continue longer without rupture than in other types of tubal pregnancy. The danger resulting from rupture, however, is also greater, because the hemorrhage may be so profuse that it is fatal within a very short time. Furthermore, the diagnosis of ectopic gestation in cases of interstitial pregnancies is more difficult in view of the lack of a mass in the tube by palpation or ultrasonography and an asymmetric uterine enlargement, which may be interpreted as a seemingly normal pregnancy.
Cervical pregnancy (not illustrated) has been observed in only a few cases. The cervical endothelium, not undergoing the typical progestational changes, is not adequately prepared to receive the trophoblast or permit nidation. The placenta is attached to the cervical myometrium, and gestation advances not longer than into the third month, when abortion occurs. Some authors do not classify this condition with the ectopic pregnancies, but one should bear in mind that it shares with these anomalies all the dangers connected with the difficulty of removing the placenta without serious hemorrhage. The low contractility of this portion of the uterus and the proximity of the uterine vascular supply increase the risk of hemorrhage even during curettage.
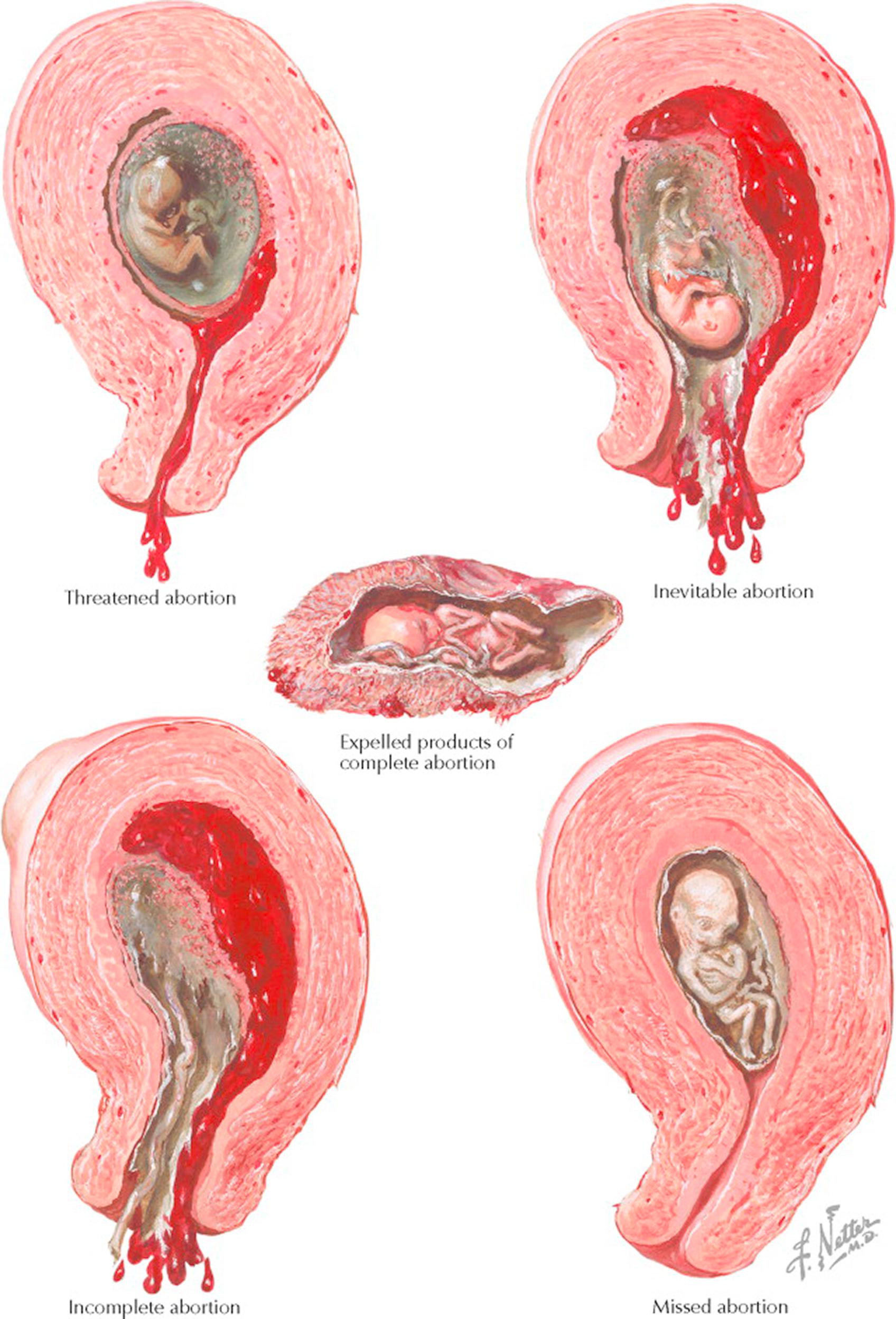
Abortion is the loss or failure of an early pregnancy and it is defined in several forms: complete, incomplete, inevitable, missed, septic, and threatened. A complete abortion is the termination of a pregnancy before the age of viability, typically defined as occurring at less than 20 weeks from the first day of the last normal menstrual period or involving a fetus of weight less than 500 g. Most complete abortions generally occur before 6 weeks or after 14 weeks of gestation. An incomplete abortion is the spontaneous passage of some, but not all, of the products of conception. A pregnancy in which rupture of the membranes and/or cervical dilation takes place during the first half of pregnancy is labeled an inevitable abortion. Uterine contractions typically follow, ending in spontaneous loss of the pregnancy for most patients. A missed abortion is the retention of a failed intrauterine pregnancy for an extended period. A septic abortion is a variant of an incomplete abortion in which infection of the uterus and its contents has occurred. A threatened abortion is a pregnancy that is at risk for some reason. Most often, this applies to any pregnancy in which vaginal bleeding or uterine cramping takes place but no cervical changes have occurred.
Estimates for the frequency of complete abortions are as high as 50% to 60% of all conceptions and between 10% and 20% of known pregnancies. Less than 2% of fetal losses are missed abortions. Septic abortions occur in 0.40 to 0.6 of 100,000 spontaneous pregnancy losses. Threatened abortions occur in 30% to 40% of pregnant women.
Abortion may be initiated by the death of the embryo or fetus, followed shortly thereafter by gradual involution of the placenta, which leads to its partial or total separation. Another possibility is that the placental separation may precede the death of the fetus. In either event, the clinical signs and symptoms of abortion manifest themselves with vaginal bleeding followed by expulsive uterine contractions and cervical dilation.
Clinically, a distinction is made between threatened abortion and inevitable abortion. In the former, slight vaginal bleeding is seen, with or without feeble uterine contractions. The characteristic finding of this type of abortion is the absence of cervical dilation. Inevitable abortion is characterized by cervical dilation together with more severe vaginal bleeding and uterine contractions. The distinction between threatened and inevitable is of some prognostic importance, because in a fair number of cases of threatened abortion, pregnancy can proceed until full viability.
In inevitable abortion, uterine contractions become stronger as time progresses, bleeding becomes more severe, and the process ends by expulsion of the uterine contents. Abortion is called complete when the entire fetus, placenta, and membranes are eliminated. It is called incomplete when the fetus is expelled and all or part of the placenta remains inside the uterus. In the latter case, vaginal bleeding may continue as long as the placental parts are not removed spontaneously or by intervention.
In missed abortion, the fetus (if present) dies but the placenta is not detached from the uterine walls. In such cases the amniotic fluid is reabsorbed, and the fetus undergoes a process of dehydration and mummification.
Ultrasonography is useful in establishing the presence of a living embryo. The established tests for pregnancy, determining the presence of chorionic gonadotropins, are usually positive as long as any part of the placental tissue remains in contact with the maternal circulation, and, after complete abortion, until the circulating chorionic gonadotropic hormones are completely eliminated. Although the presence of a living embryo is reassuring, it does not guarantee the successful outcome of the pregnancy.
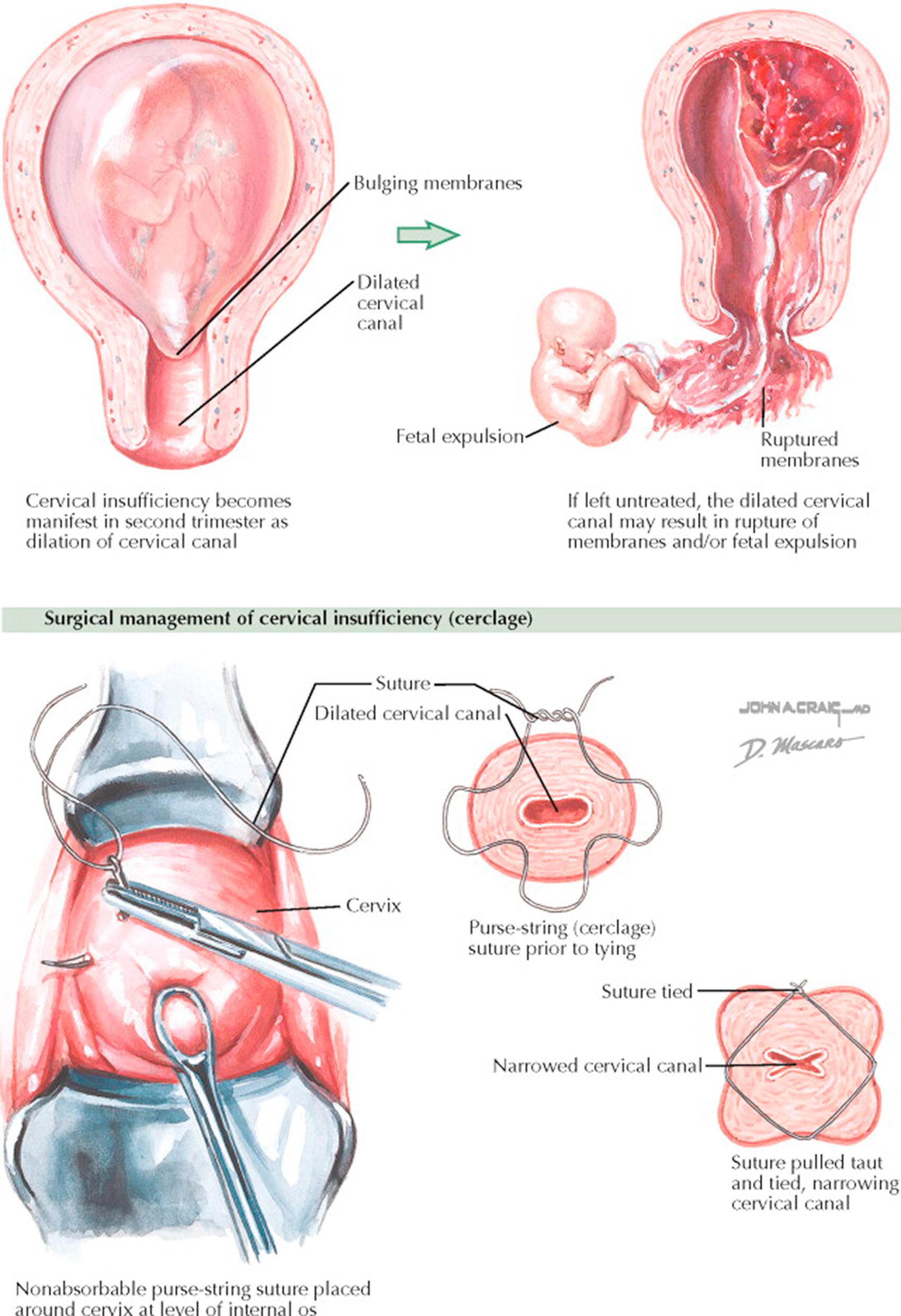
Cervical insufficiency is characterized by asymptomatic dilation of the internal os during pregnancy. This generally leads to dilation of the entire cervical canal during the second trimester with subsequent risk of rupture of the membranes and/or expulsion of the fetus. This affects 1/54 to 1/1842 pregnancies (resulting from uncertain diagnostic criteria). Though uncommon, it is thought to be involved with as many as 20% to 25% of all second-trimester pregnancy losses.
Cervical insufficiency may come from iatrogenic sources, most often damage from cervical dilation at the time of dilation and curettage or other manipulation, or damage caused by surgery (conization). Other possible causes include congenital tissue defect, uterine anomalies (uterus didelphys), prior obstetric lacerations, and in utero exposure to diethylstilbestrol.
Generally cervical insufficiency is suggested by a history of second-trimester pregnancy loss accompanied by spontaneous rupture of the membranes without labor, or rapid, painless preterm labor. The finding of prolapse and ballooning of the fetal membranes into the vagina without labor would strongly suggest cervical insufficiency. Cervical insufficiency must be differentiated from the presence of uterine anomalies, chorioamnionitis, and other sources of midpregnancy loss.
When the patient is at high risk for cervical insufficiency (generally by history) or cervical change is suspected, ultrasonography should be used to assess cervical length. Ultrasonography must also be performed before cervical cerclage to assess for abnormal fetal development. Although cervical length can be measured by ultrasonography, routine use of this has not proven to be an effective screening tool except in the face of a high-risk history. (Normal cervical length is approximately 4.1 cm [±1.02 cm] between 14 and 28 weeks and gradually decreases in length to 40 weeks, when it averages between 2.5 and 3.2 cm.) Signs of cervical funneling and cervical shortening are associated with an increased risk of preterm delivery, but management in the absence of other risk factors is unclear.
Currently the best screening technique remains frequent vaginal examinations beginning around the time of previous cervical change or the second trimester, whichever is earlier. Attempts to define or identify cervical insufficiency by hysterosonography, pull-through techniques with inflated catheter balloons, measurement of cervical resistance to cervical dilators, magnetic resonance imaging, and others have not gained clinical acceptance.
Treatment of cervical insufficiency is by cervical cerclage (placement of a concentric nonabsorbable suture close to the level of the internal cervical os) generally performed between 10 and 14 weeks of gestation. When the suture is placed vaginally, it is generally removed at 38 weeks of gestation. If labor occurs before this point and cannot be stopped, the suture should be removed immediately because of the risk of uterine rupture with an obstructed outlet. Cervical cerclage is occasionally performed transabdominally. When placed in this manner, these sutures are intended to remain permanently and they preclude vaginal delivery. The use of lever pessaries (such as the Smith-Hodge) has been reported to be associated with outcomes similar to those obtained by cerclage, but this modality is infrequently used. Bleeding, uterine contractions, obvious infection, or rupture of the membranes are contraindications to cerclage. Because of scarring after cerclage, some patients require cesarean delivery. With correct diagnosis and cervical cerclage, fetal survival increases from 20% to 80%.
Restriction of activity is often suggested, but evidence that this alters the outcome of pregnancy is lacking. After 24 weeks of pregnancy, bed rest may be the only therapy available because the risk of cerclage to trigger labor may outweigh the potential benefit. Prophylactic antibiotics and β-mimetics (tocolytics) have not been shown to be effective in prophylactic cerclage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here