Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the polymerase chain reaction is the most mature and widely used nucleic acid amplification method, other methods have diagnostic applications with unique properties and advantages.
These techniques have analytic sensitivity unparalleled in laboratory medicine, creating new opportunities for the clinical laboratory to impact patient care.
Clinical applications of the technology cut across the traditional disciplines in laboratory medicine.
An understanding of the basic principles and relative strengths and limitations of the nucleic amplification methods is important for all involved in the practice of clinical pathology.
Polymerase chain reaction (PCR), transcription-mediated amplification (TMA), strand displacement amplification (SDA), loop-mediated amplification (LAMP), helicase-dependent amplification (HDA) and nicking endonuclease amplification (NEAR) are all examples of target amplification methods used in clinical laboratories. These methods share certain fundamental characteristics. They are enzyme-mediated processes in which a single enzyme or multiple enzymes synthesize copies of target nucleic acid. The amplification products in all of these techniques are defined by oligonucleotide primers that bind to complementary sequences on opposite strands of double-stranded targets. All result in the production of millions to billions of copies of the targeted sequence in a matter of hours and, in each case, the amplification products can serve as templates for subsequent rounds of amplification. Consequently, all techniques are sensitive to contamination with product molecules from previous amplifications, which can lead to false-positive reactions. However, special laboratory design, practices, and workflow have been developed to reduce the possibility of false-positive reactions to acceptable levels.
The development of PCR by Mullis and colleagues ( ; ) was a milestone in biotechnology that heralded the beginning of molecular diagnostics. PCR is a simple in vitro chemical reaction that permits the synthesis of essentially limitless quantities of a targeted nucleic acid sequence. This is accomplished through the action of a deoxyribonucleic acid (DNA) polymerase that, under the right conditions, can copy a strand of DNA. At its simplest, a PCR consists of target DNA, a molar excess of two oligonucleotide primers, a heat-stable DNA polymerase, an equimolar mixture of deoxyribonucleotide triphosphates (dATP, dCTP, dGTP, and dTTP), MgCl 2 , KCl, and a Tris-HCl buffer. The two primers flank the sequence to be amplified, are typically 18 to 30 bases long, and are complementary to opposite strands of the target.
To initiate a PCR, the reaction mixture is heated to separate the two strands of target DNA (denaturation) and then cooled to permit the primers to anneal to the target DNA in a sequence-specific manner (annealing; Fig. 69.1 ). The DNA polymerase then initiates extension of each primer at its 3′ end (extension). The primer extension products are dissociated from the target DNA by heating. Each extension product, as well as the original target, can serve as a template for subsequent rounds of primer annealing and extension.
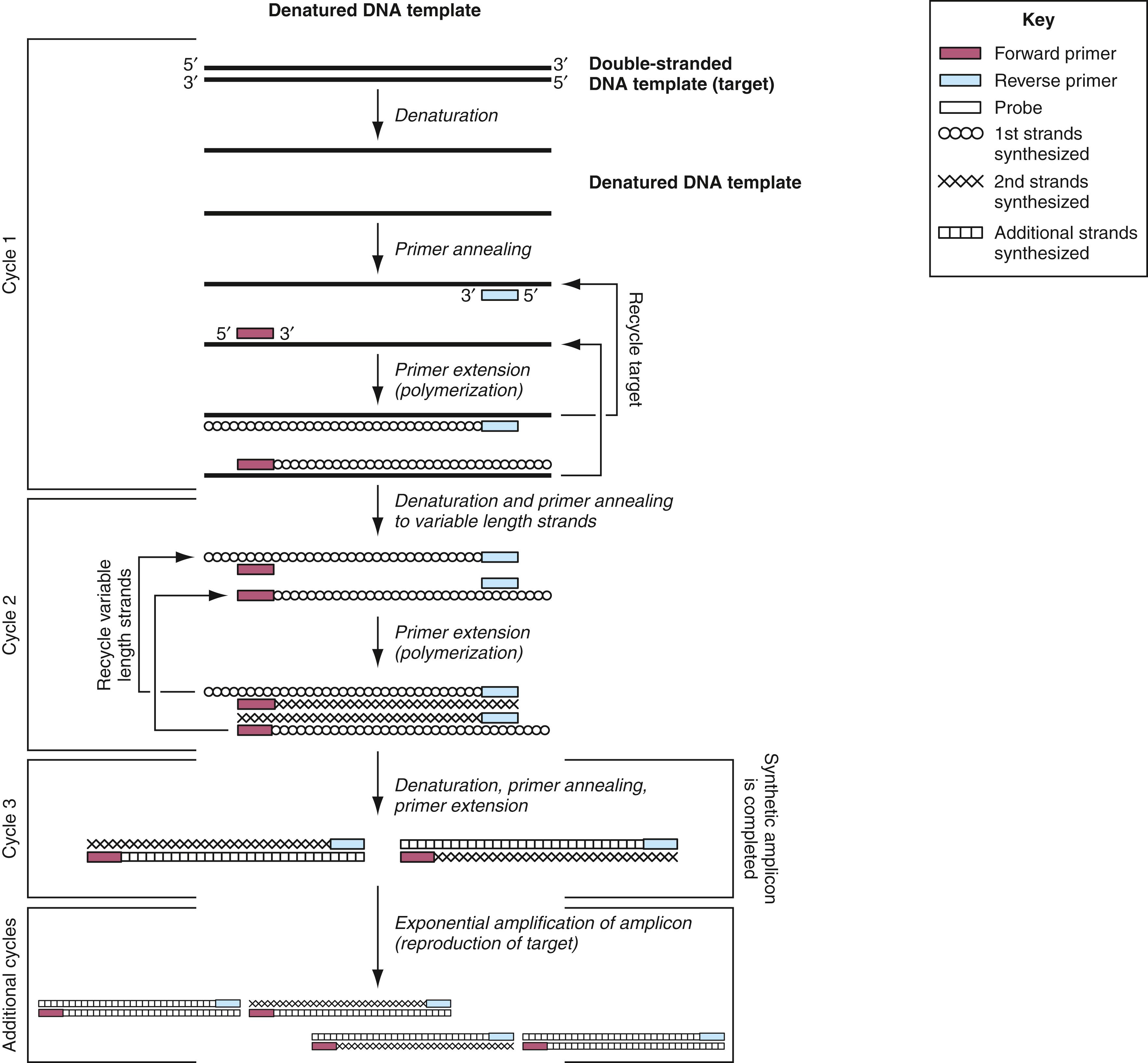
A PCR cycle typically consists of three steps at different temperatures: denaturation, annealing, and extension, although two-step cycling with a combined annealing/extension step is also common in diagnostic assays. When the annealing temperature is close enough to the extension step temperature, these can be combined into a single step to create the two-step cycling. At the end of each cycle, the PCR products are theoretically doubled. Thus after n PCR cycles, the target sequence can be amplified 2 n -fold. The whole procedure is carried out in a programmable thermal cycler that precisely controls the temperature at which the steps occur, the length of time that the reaction is held at the different temperatures, and the number of cycles. Ideally, after 20 cycles of PCR, a million-fold amplification is achieved, and after 30 cycles, a billion-fold. In practice, the amplification may not be completely efficient because of failure to optimize the reaction conditions or the presence of inhibitors of the DNA polymerase. In such cases, the total amplification is best described by the expression (1 + e) n , where e is the amplification efficiency (0 < e < 1) and n is the total number of cycles.
After amplification, the products can be detected by various methods, including sequencing or analysis. Gel electrophoresis with intercalating dye staining is a classical method that separates products by size and may be sufficient for some applications. For finer size discrimination, capillary electrophoresis can be used. PCR amplification products can also be accurately sized using mass spectrometry. Alternatively, probe hybridization methods can be used to verify or analyze the reaction product. Closed tube methods, in which the amplified products are never released to the environment, avoid contamination of future reactions with products from prior positive reactions and mitigate problems with false positives. Adding a fluorescent dye or probe before amplification to the reaction mixture allows optical monitoring to follow the accumulation of reaction product as it progresses (real-time PCR) or after the reaction is complete (melting curve analysis) with the need to process the sample for a separate analysis step.
PCR, as it was originally described, was a technique for DNA amplification. Reverse-transcriptase PCR (RT-PCR) was developed to amplify ribonucleic acid (RNA) targets. In this process, complementary DNA (cDNA) is first produced from RNA targets by reverse transcription, and then the cDNA is amplified by PCR. RT-PCR, as originally described, employed two enzymes: a heat-labile RT, such as avian myeloblastosis virus reverse transcriptase (AMV-RT), and a thermostable DNA polymerase. Because of the temperature requirements of the heat-labile enzyme, cDNA synthesis had to occur at lower temperatures. This presented problems both in terms of the nonspecific primer annealing and inefficient primer extension due to formation of RNA secondary structures. These problems have been largely overcome by the development of thermostable DNA polymerases derived from Thermus spp., which, under the proper conditions, can function efficiently as both an RT and a DNA polymerase ( ; ). RT-PCRs using these enzymes are more specific and efficient than previous protocols using conventional heat-labile RT enzymes. Commercially available kits employing this single-enzyme RT-PCR are available for detection of a variety of RNA targets in clinical specimens. RNA targets may present problems due to secondary or tertiary structure that may require special conditions or alternative target sites for efficient cDNA synthesis.
While the single enzyme RT-PCR has improved specificity and efficiency for problematic targets, there remain many applications that use a two-enzyme system. Modified heat-labile RT enzymes and optimized mixtures of reverse transcriptases with improved specificity and efficiency are now commercially available. Performing a separate RT reaction with random sequence primers allows PCR analysis of multiple targets from a single RT reaction. The RT reaction may be performed with oligo-dT primers providing cDNA of the messenger RNAs only. This scheme also allows PCR to be performed for multiple targets from a single RT reaction.
Nested PCR was developed to increase both the sensitivity and specificity of PCR ( ). It employs two pairs of amplification primers and two successive rounds of PCR. Typically, one primer pair is used in the first round of PCR of 15 to 30 cycles. The products of the first round of amplification are then subjected to a second round of amplification using the second set of primers, which anneal to a sequence internal to the sequence amplified by the first primer set. The increased sensitivity arises from the high total cycle number. The increased specificity arises from the annealing of the second primer set to sequences found only in the first-round products, verifying the identity of the first-round product. In hemi-nested PCR, one of the primers in the second PCR is identical to the first.
The major disadvantage of nested PCR is the high rate of contamination that can occur during the transfer of first-round products to the second tube for the second round of amplification. This can be avoided either by physically separating the first- and second-round amplification mixtures with a layer of wax or oil or by designing closed-tube amplification and detection protocols.
The Cepheid (Sunnyvale, CA) assay for detection of Mycobacterium tuberculosis and the rpoB gene associated with rifampin resistance ( ) and the BioFire Diagnostics FilmArray syndromic panels (see following section) use hemi-nested and nested PCR, respectively.
In multiplex PCR, two or more primer sets designed for amplification of different targets are included in the same reaction mixture ( ). With this technique, more than one target sequence in a clinical specimen can be co-amplified in a single tube. The success of a multiplex PCR reaction is dependent on the design of the specific primers used. It is preferred that primers have similar annealing temperatures and must be noncomplementary to each other to avoid primer-dimers and inefficient reactions. Multiplex PCRs are more complicated to develop and are often less sensitive than single-primer-set PCR reactions, but they allow multiple targets to be detected from a single specimen in one reaction. Multiplex PCR is analyzed by massively parallel sequencing, separating products by size by gel electrophoresis or capillary electrophoresis, by spatial separation on a surface or bead, or by probe color in real-time PCR. Real-time PCR is typically limited to 2 to 6 colors, but greater multiplexing is possible by combining color with the melting temperature of probes. Recent technological advances in decoding highly multiplex PCR reactions are described later. These innovations have led the way to development of large syndromic panels for infectious disease diagnosis ( ).
One of the first highly multiplexed platforms for PCR analysis was the xMAP system (Luminex Corp., Austin, TX). The xMAP system incorporates a proprietary process to internally dye polystyrene microspheres with two spectrally distinct fluorochromes. By using precise ratios of these fluorochromes, an array is created consisting of 100 different microsphere sets with specific spectral addresses. Each microsphere set can possess a different reactant on its surface. For nucleic acid analysis, oligonucleotide probes would be covalently bound to the microsphere surface by carbodiimide coupling. Because each microsphere set can be distinguished by its spectral address, the sets can be combined, allowing up to 100 different analytes to be measured simultaneously in a single reaction vessel. A third fluorochrome coupled to a reporter molecule quantifies the biomolecular interaction that occurs at the microsphere surface.
Microspheres are interrogated individually in a rapidly flowing liquid stream as they pass by two separate lasers in the Luminex xMAP flow cytometer. High-speed digital signal processing classifies each microsphere based on its spectral address and quantifies the reaction on its surface. Thousands of microspheres are investigated per second, resulting in an analysis system capable of analyzing and reporting up to 500 different reactions in a single reaction vessel in a few seconds.
Multiplex assays run on the Luminex platform typically consist of three major steps: nucleic acid amplification by PCR, target-specific extension, and liquid bead array decoding ( ). After PCR amplification, the amplicons are mixed with a second set of tagged primers specific for each target. If the target is present, the tagged primer will be extended through a process called target-specific extension . During this extension, a label is incorporated into the extension product. The color-coded beads are added to identify the tagged and labeled extension products. Attached to each differently colored bead is an oligonucleotide complementary to the tag sequence for each target. Samples are then placed in the Luminex xMAP flow cytometer, where the beads are read by two color lasers. One laser identifies the color of the bead; the other laser detects the presence or absence of a labeled extension product on that bead. U.S. Food and Drug Administration (FDA)–cleared assays for cystic fibrosis mutations, human leukocyte antigen (HLA) testing, respiratory virus and gastrointestinal pathogens, and pharmacogenetics are available from Luminex using this technology.
Luminex also has multiplex assays for respiratory pathogens, enteric pathogens, and identification of Gram-positive and Gram-negative bacteria from positive blood cultures based on Verigene NanoGrid technology ( ). In this closed system, nucleic acids are extracted and/or amplified, denatured, and hybridized with target-specific oligonucleotides covalently bound to a glass microarray slide inside the test cartridge. Additional steps include hybridization with mediator oligonucleotides and gold nanoparticle-labeled probes. These bound gold nanoparticle probes are then coated in silver to form silver-enhanced gold nanoparticle aggregates that scatter light with high efficiency. The silver-enhanced gold nanoparticles are detected optically at relative positions on the microarray, using the Verigene Reader.
Another technology for high-order multiplex PCR is the FilmArray developed by BioFire Diagnostics (bioMérieux, Salt Lake City, UT). It is a completely automated, integrated, and self-contained lab-in-a-pouch system ( ). The film portion of the pouch has stations for cell lysis, nucleic acid purification, reverse transcription to detect RNA targets, first-stage PCR multiplex PCR, and an array of up to 120 second-stage nested PCRs. After extracting and purifying nucleic acids from the unprocessed sample, the FilmArray performs a nested multiplex PCR that is executed in two stages. During the first-stage PCR, the FilmArray performs a single large-volume, massively multiplexed reaction. The products from the first-stage PCR are then diluted and combined with a fresh, primer-free master mix. Aliquots of this second master mix solution are then distributed to each well of the array. Each well of the array is prespotted with a single set of primers. In the second stage, small-volume PCR is performed in singleplex fashion in each well of the array. Even though this assay uses nested PCR, the entire test is performed within a sealed pouch, eliminating concerns of carryover contamination. Using amplification and melting curve data, the FilmArray software automatically generates a result for each target. FilmArray panels for detection of respiratory, gastrointestinal, and bloodstream and central nervous system pathogens have been cleared for clinical use by the FDA. In addition, a FilmArray for detection of Ebola Zaire virus, as part of a biothreat panel, was given Emergency Use Authorization by the FDA during the 2014 Ebola outbreak.
GenMark Diagnostics (Carlsbad, CA) eSensor technology couples multiplex PCR with solution hybridization to ferrocene-labeled, target-specific signal probes and a microarray of gold-plated electrodes, each coated with different capture probes specific for the targets ( ). Target DNA-signal probe complexes present in the sample hybridize to the capture probes, bringing the ferrocene label in close proximity to the gold electrode. An electrical current is applied to each electrode and a captured DNA target is analyzed by electrochemical detection using voltammetry with a signal of 3 nA or greater for a given analyte considered positive. Panels for respiratory viruses, cystic fibrosis genotyping, thrombophilia risk, and warfarin sensitivity are FDA-cleared panels available on the original semiautomated XT8 system. Other panels not FDA cleared include hepatitis C genotyping and cytochrome P450 2C19 genotyping. The ePlex system automates all aspects of nucleic acid testing, including extraction, amplification, and detection, combining electrowetting and GenMark’s eSensor technology in a single-use cartridge. It provides results in less than 2 hours, with minimal hands-on time. FDA-cleared panels for respiratory virus and blood culture identification are available on this system, with central nervous system and gastrointestinal panels in development ( ). The ePLEX RP2 cartridge can detect a multitude of respiratory pathogens in addition to COVID-19.
A linear relationship should exist between the quantity of input template and the amount of amplification product. However, because the final amount of PCR product depends on exponential amplification of the initial quantity of template, minor differences in amplification efficiency may lead to very large and unpredictable differences in the final product yield ( ). The tube-to-tube differences may depend on sample preparation and nucleic acid purification procedures, nucleic acid quality (e.g., formalin-fixed tissues), presence of inhibitors, and thermal cycler performance. For these reasons, simple quantification of product accumulated at the end of the reaction and the use of external standard curves do not provide reliable quantification of the amount of the template initially present in the sample.
A variety of PCR-based strategies have been developed to accurately quantify DNA and RNA targets in clinical specimens, but the competitive PCR (cPCR) approach has proved reliable and robust for clinical applications ( ; ). The basic concept behind cPCR is the coamplification in the same reaction tube of two different templates of equal or similar lengths and with the same primer binding sequences. Because both templates are amplified with the same primer pair, identical thermodynamics and amplification efficiency are ensured. The amount of one of the templates must be known; after amplification, products from both templates must be distinguishable.
Endpoint PCR has largely been replaced in the clinical laboratory by homogeneous kinetic (real-time) methods for product detection (see next section). These methods provide for more precise measurements of initial template concentrations because the analysis is performed early in the log phase of product accumulation and, as a result, are less prone to error resulting from differences in sample-to-sample amplification efficiency.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here