Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pneumocystis was discovered in 1909 in Brazil by Carlos Chagas, who mistakenly interpreted the organism as a trypanosome. In 1912 Pierre and Eugénie Delanöe, in Paris, identified Pneumocystis as a separate genus and species and named the organism in honor of Antonio Carini, another researcher. Pneumocystis first came to medical attention when in the 1940s–50s it was identified as the cause of interstitial plasma cell pneumonia in institutionalized and debilitated infants in central and eastern Europe. In the 1960s Pneumocystis became widely appreciated as an important cause of pneumonia (now termed Pneumocystis pneumonia, or PCP) in immunocompromised hosts; however, with the development of safe and effective antimicrobial drugs, interest in the organism waned. The dramatic rise in the incidence of PCP, associated with human immunodeficiency virus (HIV) infection in the 1980s, rekindled interest in Pneumocystis as a major medical and public health problem. During the 1990s advances in the treatment of HIV reduced the frequency of PCP and other complications. Nevertheless, Pneumocystis remains a leading cause of opportunistic infection, morbidity, and mortality in these patients. It is estimated that there are more than 400,000 annual cases of PCP worldwide, with more than 52,000 deaths per year. Interest in the organism has also been spurred by studies of Pneumocystis colonization and its association with infant sudden unexpected death, chronic obstructive pulmonary disease (COPD), and asthma ; and also the observed increase in cases of PCP among the non–HIV-infected population; status post–organ transplantation; those with hematologic or rheumatologic conditions and in receipt of biologic disease-modifying agents ; continuing outbreaks of PCP in health care settings; and sequencing of the human Pneumocystis genome.
Pneumocystis describes a genus of closely related unicellular fungi of low virulence found in the lungs of humans and a variety of mammals. The taxonomic status of the genus was resolved in the late 1980s, when analysis of the ribosomal RNA (rRNA) gene suggested that the organism is more closely related to fungi than to protozoa. This conclusion has been confirmed at every molecular locus analyzed and by whole-genome sequence analysis. Phylogenetic studies place the organisms as ascomycetes on a deep basal branch among the archiascomycetes; however, Pneumocystis is unusual among fungi in that the organism lacks ergosterol in its plasma membranes and is insensitive to available antifungal drugs that target ergosterol biosynthesis.
Species within the genus demonstrate genotypic and phenotypic differences manifested by antigenic differences, ultrastructural morphologic differences, and host specificity. Genetic studies have demonstrated differences between Pneumocystis spp. at a karyotypic level, in the organization and structure of gene families within specific genomes, and at a sequence level within individual genes. Whole-genome sequence analysis of Pneumocystis carinii, Pneumocystis murina, and Pneumocystis jirovecii confirm these differences and provide novel reagents to further characterize differences. Structural genomic differences exist in the mitochondrial genomes in addition to those found in the nuclear genomes; the mitochondrial genome of P. jirovecii is circular, whereas those of P. carinii and P. murina are linear. Not only are there genetic differences in Pneumocystis among different animal hosts, but there are also species or strain differences, or both, in organisms from the same host. Ultrastructural morphologic differences are evident only at the level of electron microscopy, whereas other phenotypic differences between species require specialized reagents to determine antigenic characterization or multilocus enzyme electrophoresis.
Experimental models have demonstrated that Pneumocystis taken from a given host species appears unable to proliferate in other host species. Associated with a better understanding of the host specificity and genetic differences among members of the genus Pneumocystis, a need has arisen to define individual species within the genus. In recognition that the organisms described by the Delanöes were isolated from infected rats, a formal taxonomic description of rat-derived species was made, retaining the name of P. carinii. Pneumocystis isolated from humans was formally described as P. jirovecii in recognition of Otto Jirovec, whose group first identified Pneumocystis as a human pathogen and the causative agent of interstitial plasma cell pneumonia. A second species identified in rats has been named Pneumocystis wakefieldiae, whereas P. murina and Pneumocystis oryctolagi have been identified in mice and rabbits, respectively.
Despite the strenuous efforts by many investigators, the lack of a reliable Pneumocystis in vitro cultivation system remains an intractable problem. Limited (up to 10-fold) replication of rat-derived organisms has been achieved in different cell lines and in axenic media. A continuous culture system for rat- and human-derived Pneumocystis has been described but has proved difficult to reproduce and maintain. Short-term culture has been used to study Pneumocystis metabolism and susceptibility to antimicrobial drugs, but standardization and reproducibility among laboratories have not yet been achieved.
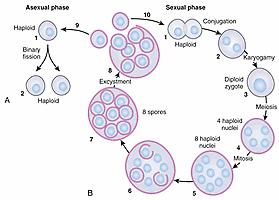
Studies of the life cycle of Pneumocystis have been based mainly on light and electron microscopic analysis of forms seen in infected lungs or in short-term culture ( Fig. 269.1 ). Three developmental stages of the organism are commonly seen in conjunction with additional intermediate forms. The trophic form is small (1–4 µm) and pleomorphic and commonly exists in clusters; this stage can be identified on Giemsa stain by its reddish nucleus and blue cytoplasm ( Fig. 269.2A ). In the asexual phase of the life cycle the trophic forms multiply by binary fission, although trophic binary fission is rarely visualized or documented. In the sexual phase the haploid trophic forms are postulated to conjugate to form a diploid zygote that becomes a 4- to 6-µm sporocyte (precyst); this form is difficult to distinguish from the other developmental stages at the light microscopic level. The sporocyte undergoes meiosis followed by mitosis, leading to the formation of the ascus (cyst), which contains eight haploid ascospores. The expression of meiosis-specific genes, such as the functionally conserved meiotic control kinase Ran1, the meiotic activator Mei2, and the meiosis-specific recombinase Dcm1, have been confirmed in the lungs of infected mammalian hosts, suggesting sexual replication occurs in the lungs of infected mammalian hosts. The finding that the genomes of P. jirovecii , P. murina, and P. carinii all contain a single region with only three genes involved in mating-type differentiation supports the presence of a homothallic (self-fertile) sexual cycle. The 5- to 8-µm ascus has a thick cell wall that stains well with stains such as methenamine silver or toluidine blue O (see Fig. 269.2B ). The ascospores or intracystic bodies are formed by compartmentalization of nuclei and cytoplasmic organelles, exhibit different shapes, and appear to be released through a rent in the cell wall. Studies using echinocandin inhibitors of β-1,3-glucan (BG) synthase suggest that the cystic form is an integral part of the life cycle and critical for transmission.

Biochemical and metabolic studies of Pneumocystis have been limited by the problems of culturing the organism. Functional analyses of the P. carinii and P. jirovecii genomes are consistent with the lifestyles of obligate parasites with a reduced repertoire of amino-acid synthase genes and an abundance of transporter genes, suggesting limitations in the ability to synthesize essential nutrients and a necessity to scavenge these from their mammalian host. The surface of Pneumocystis is rich in glucose and mannose, N -acetylglucosamine, and galactose N -acetylgalactosamine residues. The cell walls of cysts and trophic forms contain a number of immunogenic glycoproteins that may be part of a large complex (see Fig. 269.2 ). BGs are a major component of the cell wall, whereas little or no chitin has been detected, although a chitin synthase has been detected, and upregulation of host chitinase activity is noted in the lung during infection. Lipids have received considerable attention because of their relationship with antifungal therapy. Cholesterol is the dominant sterol present in Pneumocystis . Instead of ergosterol, the organism synthesizes distinct Δ C24 alkylated sterols. Coenzyme Q10 (CoQ10) is the major ubiquinone homologue synthesized by the organism; CoQ10 homologues, such as 8-aminoquinolones and hydroxynaphthoquinones, have shown good activity against the organism. A variety of enzymes and metabolic pathways have been characterized as potential therapeutic targets.
Several major groups of Pneumocystis antigens have been identified. The 95- to 140-kilodalton (kDa) major surface glycoprotein (Msg), or gpA, is highly immunogenic, exhibits shared and species-specific antigenic determinants, and contains protective B- and T-cell epitopes. Immunization with Msg also elicits a protective response in some, but not all, animal models. Msg also facilitates interaction with host cells by adherence to the extracellular matrix proteins fibronectin, vitronectin, possibly laminin, the surfactant proteins A and D, and the mannose receptor. Msg is composed of up to 10% N-linked carbohydrates, particularly mannose, which participate in the binding to these proteins.
Msg actually represents a complex family of proteins encoded by multiple genes that are arranged in clusters at the ends of chromosomes. Transcription of Msg genes occurs at a single expression site, termed the upstream conserved sequence (UCS), which is thought to result in only one Msg isoform being expressed on the surface of Pneumocystis at a time. Changing the Msg gene at the UCS, by recombination and gene conversion, changes the surface Msg, resulting in antigenic variation. This ability to undergo antigenic variation may be an important mechanism whereby Pneumocystis evades the host immune response. Studies suggest that antigenic variation and evasion of the host immune response may occur at both the cellular immune level and the humoral level. Recombinant Msg preparations have overcome the previous limitations of crude Pneumocystis antigen preparations and offer promise to be helpful serologic tools. Variants of the recombinant Msg antigens have been used to better characterize the reactivity of the humoral responses in a number of studies.
A second family of surface antigens was identified during studies characterizing Msg. A subtilisin-like serine protease encoded by the PRT1 gene, also known as the Kex multigene family, was localized to the surface of rat-derived Pneumocystis . In P. murina and P. jirovecii only a single key gene has been identified. In other fungi these proteases are involved in the processing of preproteins as they make their way to the cell surface and may play a role in antigenic variation in Pneumocystis. There is a considerable body of evidence that Kex not only contains protective T-cell epitopes but also has protective B-cell epitopes. Immunization of CD4 + cell–depleted mice with a Kex vaccine protects against the development of PCP and is protective in acquisition of Pneumocystis infection in immunosuppressed nonhuman primates.
The third major antigen complex is a glycoprotein that migrates as a broad band of 45 to 55 and 35 to 45 kDa in rat and human Pneumocystis, respectively. The gene encoding a rat Pneumocystis 45- to 55-kDa antigen (p55) has been cloned and sequenced; the 3′ end of the molecule stimulates a host immune response. Immunization with recombinant p55 antigen or DNA p55 encoding DNA afforded partial protection to subsequent infection. Gene variation has also been demonstrated in the p55 gene, with up to five variants identified within the Pneumocystis genome. The predicted amino-acid sequence of p55 shows a repeated motif rich in glutamic acid residues that, in other microbes, such as Plasmodium, has been suggested as a mechanism to divert the host immune response. The 35- to 45-kDa band of human Pneumocystis is frequently found in respiratory tract specimens and is also recognized by serum antibodies.
Pneumocystis infection is mainly acquired by inhalation, and recent evidence suggests that the infective form is the cystic form or ascus. In addition, there are reports of trans -placental or vertical transmission. Primary infection is acquired early in life, and infants probably serve as the natural host. This infection can either be asymptomatic or manifested by mild (usually upper) respiratory illness. Serologic studies in different geographic locations have shown that most (≈80%) healthy children have been exposed to Pneumocystis by 2 to 3 years of age. Cross-sectional studies by investigators in Chile, who used the polymerase chain reaction (PCR) amplification of the P. jirovecii mtLSU rRNA gene, found the prevalence of P. jirovecii colonization to be 52% in infants and 65% in adults who died and were autopsied in Santiago.
Over the past decade there has been significant interest in P. jirovecii colonization. Risk factors for colonization include immunosuppressive conditions (HIV, low CD4 cell count, cancer, autoimmune diseases, organ transplantation); immunosuppressive drugs (corticosteroids, tumor necrosis factor-α [TNF-α] inhibitors); COPD and other chronic lung disorders; other conditions (pregnancy, cigarette smoking); treatment with ibrutinib or idelalisib; and lack of surfactant protein A in mice. Colonization also varies in different geographic areas. The prevalence of colonization in healthy subjects is estimated to be about 20%. The duration of colonization is variable. A study of a nonhuman primate colony revealed that all of the animals became colonized over a 2-year period, and the average duration of colonization was about 2 months.
P. jirovecii colonization can have several consequences. Colonization is thought to precede the development of PCP but does not necessarily lead to PCP. When PCP does develop, the P. jirovecii genotype associated with pneumonia is often the same as in the genotype in colonization. Patients colonized with P. jirovecii can transmit the infection to others, and when recipients are immunosuppressed, PCP can result. Colonization is an independent risk factor for the development of airway obstruction, more severe PCP, or both; increased levels of matrix metalloprotease 12, an important contributor to the development of COPD; and an increased systemic inflammatory response. Nonhuman primates infected with simian-human immunodeficiency virus (SHIV) that became colonized with Pneumocystis spontaneously developed progressive COPD, whereas SHIV-infected primates not colonized with Pneumocystis did not.
PCP in HIV patients was first reported in 1981, and its incidence increased dramatically in high-income countries throughout the 1980s. The number of cases were several orders of magnitude greater than in non-HIV patients. With widespread use of PCP chemoprophylaxis and combination antiretroviral therapy (ART), the incidence has steadily fallen since the early 1990s. PCP now mainly occurs in individuals who are unaware that they are HIV positive, lack access to medical care, or who are noncompliant with medication. Yet PCP still remains a leading opportunistic infection in HIV-positive patients in North America, Europe, and Australasia. PCP is increasingly recognized in low- and middle-income countries.
Risk factors for PCP in HIV-positive patients include low absolute and percentage CD4 + cell count, oral candidiasis, fevers, nonadherence with medication, polymorphism in the FcγIIIa receptor, and possibly P. jirovecii colonization.
By contrast with HIV-positive patients, the incidence of PCP in non-HIV patients is rising. The likely reasons for the increase of PCP in non-HIV patients include broader use of new and standard regimens of immunosuppressive drugs. New agents include immunomodulators, such as TNF-α inhibitors, and B-cell modifiers. Conditions requiring the use of these compounds are cancer (solid tumors, hematologic malignancies), organ transplantation, rheumatologic and connective tissue disorders, and inflammatory bowel disease. Evidence to guide clinicians in prevention of PCP in non-HIV patients are available ( Table 269.1 ).
| HIV/AIDS | SOLID-ORGAN TRANSPLANT | HEMATOLOGIC MALIGNANCY | CONNECTIVE TISSUE DISEASE | |
|---|---|---|---|---|
| Risk factor for developing PCP |
|
|
|
|
| Prophylaxis given to patients |
|
|
|
|
| Duration of prophylaxis |
|
|
|
|
| Prophylaxis regimen | First choice: TMP-SMX, 1 double-strength (DS) tablet OD or 1 single-strength (SS) tablet OD Alternatives: Dapsone or dapsone plus pyrimethamine plus leucovorin, Aerosolized pentamidine (via Respirgard II nebulizer) Atovaquone |
First choice: TMP-SMX, 1 SS tablet OD or 1 DS tablet OD (or three times per week) Alternatives: Dapsone Aerosolized pentamidine Atovaquone Clindamycin plus primaquine |
First choice: TMP-SMX, 1 SS tablet OD or 1 DS tablet OD (or three times/wk) Alternatives: Dapsone Aerosolized pentamidine |
First choice: TMP-SMX, 1 DS tablet OD or 1 SS tablet OD Alternatives: Dapsone, or dapsone plus pyrimethamine plus leucovorin Aerosolized pentamidine Atovaquone |
| Notes |
|
There is an ongoing debate about the mechanisms by which PCP develops. The older theory is reactivation of latent infection. Pneumocystis infection is acquired in early childhood, becomes part of the host's resident microbial flora, and remains dormant for long periods of time. With a decline in the host's immune function, active replication of the organism occurs and results in PCP. The alternative view, which is held by most investigators, is that exposure to Pneumocystis is transient and that individuals are frequently exposed to disparate environmental sources of the organism throughout their lives. These theories have different implications when developing infection control procedures for PCP in health care facilities. If P. jirovecii is communicable, patients with PCP should not have direct contact with other immunocompromised hosts.
Several studies have raised the possibility of environmental sources of Pneumocystis . Pneumocystis DNA has been detected in the air and in pond water. Outdoor activities, such as gardening and hiking, have been shown to be independent risk factors for PCP. Geographic clusters of PCP in HIV-positive patients have been found in the United States in San Francisco and Cincinnati and in the United Kingdom. Cases of PCP were mainly found in the more affluent areas of the US cities where there was more green space. These clusters suggested the possibility of either exposure to a common environmental source of Pneumocystis or to a specific location where many at-risk individuals gathered and spread of P. jirovecii occurred.
Studies of environmental factors affecting the occurrence of PCP have mainly focused on climatologic factors. The most frequent association has been the occurrence of PCP during the warmer parts of the year. Another report found a significant monthly and seasonal variation in the occurrence of specific P. jirovecii genotypes. In addition to season, air-pollution factors are related to PCP; increased ambient sulfur dioxide is associated with risk of hospitalization with PCP, but its effects are modified by increased levels of carbon monoxide.
There is considerable evidence that Pneumocystis is communicable. Experimental transmission of Pneumocystis infection has been shown by close contact or the airborne route in immunodeficient, immunosuppressed, and normal mice, rats, and other animals. There have been outbreaks of PCP in immunodeficient rodent colonies, as well as Pneumocystis colonization and transmission of Pneumocystis infection in commercial rodent colonies under strict barrier conditions.
Outbreaks of PCP in humans occurred in crowded orphanages during World War II and in immunosuppressed patients in the 1960s and 1970s. PCP outbreaks in immunosuppressed patients over the past several decades have occurred mainly in renal transplant recipients. A systematic review that examined 16 outbreaks involving about 200 patients over the past 3 decades revealed common problems, such as frequent interpatient contact, a lack of chemoprophylaxis, and a lack of adherence to isolation procedures. No environmental source or seasonal occurrence of PCP was found. There was considerable mortality, but it decreased over time. Pneumocystis genotyping showed that most outbreaks were caused by a single or dominant strain of P. jirovecii, and that there was person-to-person transmission. Recommendations included isolation of PCP patients and use of chemoprophylaxis. Subsequent publications have emphasized the importance of P. jirovecii colonization and the need for chemoprophylaxis of these patients, revised chemoprophylaxis guidelines, and the importance of PCP in other immunosuppressed patient populations.
Procedures to collect and quantify viable Pneumocystis organisms in the air of patients with PCP have been developed. Viable P. jirovecii was detected in 80% of samples at 1 m from the bedside, in 33% of the samples at 8 m from the bedside, but not in the air at other locations inside or outside the hospital. These data support the idea that P. jirovecii infection can be spread from person-to-person by close contact and support the idea that patients with active PCP should be isolated from other immunocompromised (and thus at-risk) patients.
Serologic studies using crude antigen preparations have established that P. jirovecii infection is acquired early in life, so by age 2 or 3 years of age most (≈80%) children have been exposed to the organism ; however, these tests have lacked the sensitivity and specificity to be of help in other epidemiologic, clinical, or diagnostic studies. The development of recombinant Msg fragments has helped investigators to overcome these problems. A sequential serologic study that followed up healthy infants during the first 2 years of life showed that the force or rate of infection was 8% to 10% per month, that a proportion of subjects developed recurrent episodes of P. jirovecii colonization, and that there were seasonal changes in these responses. An analysis of health care workers showed that people who had clinical contact with patients have significantly higher antibody levels to Pneumocystis Msg fragments than workers who do not ( Fig. 269.3 ). This observation is important because it raises questions about whether health care workers might contribute to the spread of P. jirovecii in hospitals.
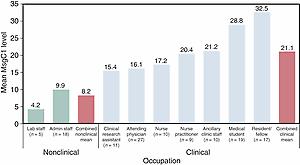
Other serologic studies have shown that P. jirovecii infection has worldwide distribution, but factors such as geography, smoking, and previous episodes of PCP independently affect antibody levels; that serology has promise as a method of diagnosing PCP; and that antibody levels to specific Msg fragments and recombinant Kex have prognostic value.
After inhalation, the cystic stage of Pneumocystis releases the trophic forms, which initiate the infection by adhering to epithelial cells of the respiratory tract. Our current concepts of how this occurs are almost entirely based on studies of alveolar epithelial cells. The trophic form preferentially attaches to the alveolar type I cell, with close apposition of the cell surfaces but no fusion of the membranes. Although the type I cell does not replicate, in vitro studies have shown that Pneumocystis attachment to cultured alveolar type II cells increased differentiation into a type I cell–like phenotype. Other studies have shown that the adherence of Pneumocystis also occurs via extracellular matrix glycoproteins, resulting in altered gene expression, morphologic changes, and organism proliferation.
The attachment for Pneumocystis to lung epithelial cells requires an intact cytoskeleton and results in changes in both the organism and host. Pneumocystis maintains an extracellular existence within alveoli and probably obtains essential nutrients from the alveolar fluid or living cells. This is supported by the finding of overexpression of multiple transporters found in the Pneumocystis genome and the corresponding absence of important synthetic genes. In HIV-positive patients Pneumocystis also enhances HIV proliferation. Another effect of the attachment is to suppress the growth of lung epithelial cells through cyclin-dependent kinase regulatory pathways. Other reports have shown that the organism alters lung guanosine triphosphate–binding regulatory proteins and induces expression of intracellular adhesion molecule 1 and fibrinogen. These properties may influence both the lung damage and host inflammatory and reparative responses in PCP.
Host defenses against Pneumocystis include innate immunity and adaptive or acquired immunity. The innate immune system, which is the first level of defense, is composed of alveolar macrophages, surfactant protein (SP)-A, SP-D, and other factors. Because alveolar macrophages have early and late actions in the infection, they are discussed later in this chapter. SP-A is a pulmonary collectin that functions in host defense (e.g., as a nonimmune opsonin) and as an immune modulator. Immunosuppressed SP-A knockout mice develop higher infection levels of Pneumocystis and an increased host inflammatory response compared with wild-type mice. The role of SP-D in host defenses is less clear, but it appears to modulate the inflammatory response in conditions such as the immune reconstitution inflammatory syndrome (IRIS).
There is accumulating evidence that B cells and humoral immunity play an important part in host defenses against Pneumocystis infection. Natural immunoglobulin M (IgM) antibodies that recognize common fungal carbohydrate antigens have been discovered and may provide protection against early Pneumocystis infection. PCP has been reported in patients and mice with B-cell defects or given medication (e.g., rituximab) that acts on B cells. Mice depleted of CD4 T-cells after recovery from a primary infection are still protected from a secondary fungal challenge. Depletion of CD8 T-cells or macrophages, which can mediate opsonic phagocytosis, abrogated protection in a secondary challenge model. Studies in SHIV-positive primates have shown a correlation of acquired antibody levels to Kexin and susceptibility or resistance to Pneumocystis colonization. A positive therapeutic effect has been found with the passive administration of hyperimmune serum or monoclonal antibodies to Msg and other antigens in experimental models of PCP. Immunization with live Pneumocystis or Kex DNA with dendritic cells engineered to express CD40L, as a genetic adjuvant, protected CD4 cell–depleted mice from organism challenge and appeared to be mediated by antibodies via helper T cell (Th)1- or Th2-type responses. A subsequent report showed that interleukin (IL)-23 produced by these dendritic cells played a critical role in eliciting recall antibody responses and that IL-23 might be of value as a potential adjuvant. Conserved antigens, such as the recently described PCA1 that is homologous between murine and human Pneumocystis spp., may serve as better vaccine targets. B cells function as not only the source of antibodies but also as antigen-presenting cells and in helping the development of memory CD4 cells. Antibodies contribute to host defenses against Pneumocystis by acting as opsonins.
Analysis of the role of antibodies in humans has been difficult because of the high prevalence of serum antibodies in the population and the lack of information about which antigen epitopes are protective. HIV induces abnormalities in B-cell number and function, which results in impaired antibody responses to vaccines or microbial antigens. In a South African cohort of children hospitalized with PCP, humoral responded to Msg antigens compared with HIV-uninfected PCP cases. Recombinant Msg antigen fragments have shown promise in seroepidemiologic studies. Recent reports have suggested that low antibody levels in HIV patients to Kex are a risk factor for developing PCP, and antibody levels to Msg are predictors of survival in hospitalized patients. Although local bronchoalveolar lavage fluid (BALF) antibodies have received only limited attention, there is evidence of decreased antibody responses in HIV patients and in SHIV-positive nonhuman primates.
Impaired cellular immunity has long been considered an important predisposing factor in the development of PCP. Naturally occurring outbreaks of PCP have occurred in immunodeficient animals, particularly colonies of severe combined immunodeficiency disease (SCID) mice and athymic nude mice and rats. The central role of CD4 + cells in host defenses against this organism has been shown by cell depletion and reconstitution experiments and by knockout mice. Other contributors to these defenses include T-cell costimulatory molecules, such as CD28 and CD2; the CD40 to CD40L pathway, which facilitates the interaction of T cells with B cells; and CD8 cells, which function as cytotoxic cells or by secreting cytokines. The role of γδ T cells in host defenses against Pneumocystis is unclear, but it is thought that these cells might help recruit CD8 cells and modulate their effects. Natural cells also play a role in host defenses against Pneumocystis but require CD4 + cells to become activated.
PCP can be induced in normal rodents by the administration of corticosteroids, and these models have been used for more than 3 decades. Protein malnutrition and an immature immune system also impair host defenses against Pneumocystis, although the defect in neonatal mice is related more to factors in the lung milieu than to T cells. The clearest evidence of the role of defective cell-mediated immunity in the development of PCP in humans comes from persons infected with HIV (see Table 269.1 ). The risk of developing PCP in adult HIV patients increases greatly when circulating CD4 cells fall below 200 cells/mm 3 . Because CD4 counts are much higher in young children than in adults, different criteria must be used. The presence of other clinical complications of HIV (e.g., fever and oral candidiasis) increases the risk of PCP independent of the CD4 count. Cases of PCP associated with CD4 counts <200 cells/mm 3 have been encountered in cancer patients receiving cytotoxic drugs, in adults with idiopathic CD4 lymphopenia, and in otherwise healthy individuals with subtle T-cell defects (see Table 269.1 ). In general, non-HIV patients have higher peripheral blood and BALF CD4 cells than HIV patients; however this patient group is often receiving corticosteroids and other immunosuppressive drugs that can lower CD4 + cell counts. There are clear guidelines based on CD4 + counts and other risk factors for initiating PCP chemoprophylaxis in non-HIV patients.
The occurrence of PCP in other patient populations with impaired cellular immunity include premature debilitated infants and children with primary immunodeficiency diseases, particularly SCID, which involves both T- and B-cell defects, and the hyper-IgM syndrome, which involves disruption of the CD40-CD40L pathway, and patients receiving immunosuppressive drugs for the treatment of a variety of conditions (see Table 269.1 ). The principal immunocompromised hosts at risk for PCP include patients with hematologic malignancies and solid tumors (e.g., brain tumors), solid-organ and bone marrow transplant recipients, and collagen vascular disorders such as granulomatosis with polyangiitis. The number of these individuals has grown over the years with better survival and the more widespread use of cytotoxic and immunosuppressive therapies. Corticosteroids, used alone or in combination with other agents, such as infliximab, etanercept, and calcineurin inhibitors, remain the most common immunosuppressive drugs implicated in the development of PCP. The relationship of corticosteroids to Pneumocystis has been emphasized by the occurrence of PCP in patients with Cushing syndrome but also in children receiving corticosteroids for diseases such as asthma, which are not known to predispose to opportunistic infections. Cases of PCP in patients on chemotherapy regimens without corticosteroids are described. Protein malnutrition is an important risk factor for the development of PCP, both by itself and as a complication of the patient's underlying disease or chemotherapy. Lung factors (e.g., radiotherapy and fibrosis) have been suggested as additional predisposing factors in non-HIV patients.
Alveolar macrophages are the first line of defense against Pneumocystis and the principal effector cell in clearing the organism from the lung. Rodents depleted of macrophages are susceptible to Pneumocystis infection; however, activated macrophages in the absence of CD4 cells are unable to control P. jirovecii infection. Recognition and adherence of Pneumocystis to macrophages occur by multiple pathways involving Msg and BG in the organism; extracellular matrix and surfactant proteins; and mannose, dectin-1, and Fc receptors. Toll-like receptor 2 (TLR2) and TLR4 help regulate host cytokine responses. Macrophages ingest, degrade, and kill Pneumocystis, releasing cytokines, such as TNF-α, eicosanoids, nitric acid, and reactive oxidants. Macrophages also undergo apoptosis, which is mediated by polyamines.
Both the life cycle form of Pneumocystis organisms and the type of macrophage response seems critical in organism clearance. The cystic form, which is rich in BG, is recognized by alveolar macrophages, dendritic cells, and lung epithelial cells and primes a robust T-cell–mediated response. Trophic forms lacking BG elicit reduced CD4 T-cell recruitment and interferon (IFN)-γ production. In addition, the trophic forms actively suppress the proinflammatory response initiated by cystic forms, in that trophic form–loaded dendritic cells had reduced capacity to stimulate CD4 T-cell proliferation and polarization, potentially promoting trophic form survival. Recent studies demonstrated that classic macrophages (M1) predominate in the innate response to Pneumocystis infection in the absence of an intact immune system, such as seen in the immunosuppressed rat model, and were defective in clearing P. carinii. Immunocompetent animals had more prominent alternative macrophage (M2) responses with clearance of infection. Treatment of immunosuppressed rats with M2 cells reverted the animals to a protected phenotype. Compared with alveolar macrophages from adult mice, macrophages from newborn mice are intrinsically unresponsive to Pneumocystis infection. On the other hand, Pneumocystis infection of adult mice downregulates the transcription factor PU.1, which leads to decreased expression of dectin-1. Recently, an intracellular isoform of osteopontin, a protein expressed by many cells, has been shown to play an important role in innate immunity in Pneumocystis infection.
Alveolar macrophage function is impaired in HIV-positive patients and other immunosuppressed populations. HIV downregulates mannose receptor expression, which results in decreased binding and uptake of Pneumocystis; impairs MD88-associated TLR4 signaling; and changes the macrophage cytokine response. Pneumocystis itself impairs phagocytosis by promoting shedding of the mannose receptor and enhances the replication of HIV.
The BG in Pneumocystis cysts elicits inflammatory responses in macrophages and other types of lung cells that contribute to the lung damage in PCP. Alveolar epithelial cells secrete neutrophil chemoattractant proteins, TNF-α, IL-8 in humans and MIP-2 in rats, and other inflammatory mediators. The process involves signal transduction and lactosylceramide, a prominent constituent of the alveolar epithelial cell microdomains. BG also stimulates dendritic cells, which are antigen-presenting cells, to secrete IL-23 and IL-6; these latter cytokines then stimulate Th17 phenotype.
Exposure to Pneumocystis or its antigens stimulates production of a multitude of cytokines and chemokines. Two proinflammatory cytokines, TNF-α and IL-1, have been shown to be important in host defenses against the organism, particularly in the early stages of the infections. TNF helps recruit lymphocytes and monocytes, which contribute to Pneumocystis clearance, and chemokines (e.g., IL-8) from alveolar epithelial cells as part of the inflammatory response. IL-6, another proinflammatory cytokine, has been produced in response to Pneumocystis, but its contributions to host resistance to the organism are unclear. IFN-γ and granulocyte-macrophage colony-stimulating factor are important contributors to host defense by macrophage activation or in cooperation with TNF-α. The role of IFN-γ is particularly complex, and the results obtained can vary, depending on the experimental design. Recent studies have shown that IL-12 and IL-23 also contribute to host defenses to Pneumocystis . IL-10 has also been shown to modulate the host inflammatory response, but no role in the host defense against Pneumocystis has been found for IL-4 or granulocyte-colony stimulating factor.
The pathologic changes that occur during the development of PCP in animal models and in humans are similar. As the host defenses become compromised, Pneumocystis organisms begin to proliferate and gradually fill alveolar lumens. In the corticosteroid-treated rat model, the organism number increases from 10 5 per lung, or fewer, to 10 9 to 10 10 per lung after 8 to 10 weeks of corticosteroid administration. The principal histologic finding is the formation of a foamy, eosinophilic alveolar exudate ( Fig. 269.4 ); as the PCP increases in severity, there may also be hyaline membrane formation, along with interstitial fibrosis and edema. The host inflammatory response is usually inconspicuous and is characterized by type II cell proliferation (a typical reparative response) and scanty mononuclear cell infiltrate. SCID mice exhibit cytokine production only late in the course of PCP, when elevated levels of TNF-α and IL-1 are found in the lungs. Several studies have shown that rats with corticosteroid-induced PCP, as well as HIV and non-HIV patients with the disease, have elevated levels of proinflammatory cytokines in their respiratory tract but not in the peripheral blood. Some patients exhibit atypical findings, such as lack of the alveolar exudate or the development of cavitary lesions, granulomas, or microcalcifications. On electron microscopy, there is increased alveolar-capillary permeability, followed by evidence of damage to the type I cell. Physiologic changes include hypoxemia with an increased alveolar-arterial oxygen (PAO 2 -PaO 2 ) gradient and respiratory alkalosis; impaired diffusing capacity, suggesting alveolar-capillary block; and alterations in lung compliance, total lung capacity, and vital capacity. The resulting picture suggests diffuse lung damage similar to that seen in the acute respiratory distress syndrome.
The pathophysiologic changes described are caused not only by the effects of Pneumocystis on the type I cell but also alterations in the surfactant system and host inflammatory response. There is a fall in surfactant phospholipids (mainly phosphatidylcholine) that is caused by inhibition of phospholipid secretion mediated by Msg and other organism constituents. Changes in the surfactant proteins include a decline in SP-B and SP-C and rise in SP-A and SP-D levels. Fractionation of the surfactant has shown that most of the increased SP-A and SP-D levels are localized mainly in the small aggregate compartment. There is now clear evidence that the host's immune inflammatory response to Pneumocystis can have harmful and helpful effects on the host lung. These effects are complex and depend to some degree on the experimental model being used. Immune reconstitution and cell depletion studies using SCID mice have shown that the clearance of PCP is associated with a hyperinflammatory response composed of increased proinflammatory cytokines and chemokines and reduced oxygenation and compliance. These effects involve not only the complex interaction of CD4 cells and CD8 cells but also subsets of CD4 cells (CD25 + , CD25 − ) and CD8 cells (TCI, TC2), which have effector or immune-modulatory functions. In contrast to humans, neutrophils are a marker of inflammation and lung damage in mice but do not cause lung damage. The administration of a large number of splenocytes or CD4 cells sensitized to Msg in rats with corticosteroid-induced PCP results in clinical illness and a cytokine cascade, along with a reduction in organism burden. The presence of steroids has no apparent effect on these events. The contribution of the host inflammatory response to lung damage in HIV patients with PCP has been suggested by studies that have correlated increased numbers of neutrophils and levels of IL-8 in BALF with more severe pneumonia and worse prognosis. IL-8 functions as a potent chemoattractant, its interaction with P. jirovecii is mediated by Msg, and its interaction with alveolar macrophages requires the coexpression of the mannose receptor and TLR2. Alterations in eicosanoids, TNF-α, IL-1, other cytokines, and inflammatory mediators have also been noted in these and other studies; however, the pathogenic significance of these changes is unclear. HIV patients with PCP also frequently experience a worsening of respiratory function soon after receiving antimicrobial drugs. Corticosteroids, if given promptly, can ameliorate or prevent this outcome and improve survival. It is thought that the beneficial effects of corticosteroids are attributable to their antiinflammatory properties or their effects on surfactant components; however, studies examining these issues have produced inconsistent results.
The presenting symptoms of PCP in the immunocompromised host are nonspecific and are mimicked by a wide variety of infectious and noninfectious etiologies. Presentation is typically with progressive exertional dyspnea, fever, and a nonproductive cough, which is often associated with an inability to make a maximal inspiration (not due to chest pain).
On occasion, sputum is produced; hemoptysis is not a feature. Patients receiving immunosuppressive drugs frequently develop these clinical manifestations after the corticosteroid/immunosuppressive therapy agent dose has been tapered and are typically increasingly symptomatic for about 1 to 2 weeks before seeking medical attention. Among HIV-infected persons, symptoms are usually of longer duration than among medically immunosuppressed patients. HIV-infected patients frequently have prolonged prodromal periods with subtle clinical manifestations developing over 3 to 8 weeks; however, some individuals present with a fulminant worsening of symptoms over 7 to 10 days, or less; the intrapulmonary organism burden is higher, but lung damage is less severe. Studies have also compared the clinical features of PCP in adult HIV patients by age and underlying risk group. In both HIV-infected and non–HIV-infected patients, however, the clinical picture is variable; for example, lung allograft recipients who develop PCP are frequently asymptomatic at the time of diagnosis.
On physical examination, varying degrees of respiratory distress may be evident (manifest as tachycardia, central cyanosis, use of accessory muscles of respiration), as well as stigmata of immunodeficiency/treatment-induced immune suppression (e.g., after receipt of chemotherapy or immunosuppressive medication posttransplantation), including molluscum contagiosum, seborrheic dermatitis, cutaneous Kaposi sarcoma, oral Candida, and oral hairy leukoplakia. Children may additionally demonstrate central cyanosis, flaring of the nasal alae, and intercostal retractions. Auscultation of the chest is usually normal; fine end-inspiratory crackles may be heard in about one-third of adults.
The spread of P. jirovecii beyond the lungs is rarely encountered and occurs mainly in patients with advanced HIV infection who are taking no prophylaxis or only aerosolized pentamidine. The actual incidence of extrapulmonary pneumocystosis is unclear as the diagnosis is made by histologic examination of sites where there are clinical manifestations (e.g., lymph nodes, spleen, liver, bone marrow, gastrointestinal [GI] tract, eyes, thyroid, adrenal glands, and kidneys), or at autopsy. Manifestations of extrapulmonary pneumocystosis include thyroid mass, pancytopenia from bone marrow necrosis, retinal cotton wool spots, and multiple hypodense splenic lesions on ultrasound or computed tomography (CT) scan. Biopsy or fine-needle aspiration shows areas of necrosis filled with foamy material. Grocott-Gomori methenamine silver (GMS) or fluorescent monoclonal antibody stain reveals numerous organisms.
Another clinical problem is the immune reconstitution inflammatory syndrome (IRIS) that occurs in HIV-infected patients days to months after starting ART, as the patient's immune system begins to recover with a rise in CD4 + cells and is manifested as worsening of a known condition or a new condition. IRIS occurs in up to 25% of HIV patients starting ART. The inciting agent can be a viable subclinical pathogen or a residual antigen from a pathogen treated months or years previously. This distinction is important because antimicrobial drugs are administered for infection, whereas antiinflammatory agents are needed for inflammation. In the context of PCP, clinical manifestations include shortness of breath, cough, and progressive pulmonary infiltrates, often mimicking an early relapse of PCP.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here