Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The essential function of the muscles that make up the myocardium is to transfer to the arteries the volume of blood added to the ventricular chambers during diastole. This transfer must occur within the narrow limits of end-diastolic pressures and must produce a flow of materials to the organs that matches the needs of the cells. The term needs means “matching the flow of oxygen,” which is consumed by the cells at a rate greater than all other materials, to the demand for oxygen. By supplying the tissues' oxygen needs, the demand for all other substances in blood is met. An inevitable consequence of the work done during exercise is an increase in oxygen consumption, and with linear incremental increases in oxygen consumption there are linear incremental increases in cardiac output (CO) to match the increases in venous return (VR). The tight coupling of oxygen demand to CO and VR indicates a regulatory system that is able to sense the tissue oxygen needs, and to engage control mechanisms that adjust the CO. In this chapter, we are concerned with the role of the myocardium in the task of the cardiovascular system to couple oxygen demand to oxygen supply.
In accomplishing this task, the activity of the myocardium must vary over a wide range of short-term regulation (seconds, minutes, and hours) and of long-term regulation (days, weeks, and years). In the short term, during the course of a normal day as tissue oxygen demands and hence cardiac output changes from sleep to strong exercise, variations in activity of the myocardium occur by both intrinsic and extrinsic control mechanisms. The major intrinsic regulator is the Frank-Starling mechanism, in which the pressure developed by the ventricle increases as the end-diastolic volume increases. Extrinsic regulators of the myocardium include the autonomic nerves of the sympathetic and parasympathetic systems, and humoral factors including catecholamines, thyroid hormone, and insulin. In long-term regulation, the activity of the myocardium is more permanently changed in response to chronic changes in oxygen demand associated with frequent bouts of chronic exercise and altered states of the pump and vascular system associated with aging and various long-standing pathologies. In this long-term regulation, the size of the cells composing the ventricular myocardium changes (i.e., hypertrophy or atrophy, without a change in cell number). The cells are remodeled by alterations in subcellular mechanisms regulating contraction and relaxation. This long-term regulation occurs physiologically with normal development of the heart from the immature to the mature myocardium, with normal physiologic aging, and with acquired or inherited pathologies that directly or indirectly affect the function of the myocardium. In this chapter, we focus on current concepts and theories of the cellular, subcellular, and molecular mechanisms for short-term regulation of the myocardium. These mechanisms are constrained to account for the dynamic and steady-state functional properties of the heart. Changes in flow and ventricular volumes during an episode of exercise reveal these functional properties.
Figure 49-1 displays changes in heart rate (HR), CO, stroke volume (SV), and ventricular volumes of a young healthy adult during a bout of exercise on a stationary bike. The measurements were made before and after administration of propranolol, a β-adrenergic blocking agent. Note that in the control condition, CO increased with workload, but end-diastolic volume (EDV) remained rather constant even though CO nearly tripled. SV increased and end-systolic volume (ESV) decreased. These data show that the increased VR that occurs in exercise is handled by the heart largely by increases in HR and decreases in ESV. Elevations in EDV as a mechanism to increase CO are not favorable because of the increased energy cost according to Laplace's law. A decrease in ESV provides an important mechanism for matching CO to increased VR without increases in EDV. As we will show, this reduction in ESV at constant EDV can be one measure of the contractile ability of the cells of the heart—that is, the contractility or inotropic state of the heart. After blockade of adrenergic β-receptors with propranolol, there is a blunting of the ability of the sympathetic nervous system to influence the heart; however, CO still increased approximately threefold. This result demonstrates the ability of the cardiovascular system to match the CO to the increased tissue oxygen needs without sympathetic nervous system control mechanisms. However, the increase in CO in the presence of propranolol did not occur without cost. One cost was that the increase in HR was reduced. With a decrease in HR and a constant CO, the SV had to be elevated (CO = SV × HR). The increase in SV occurred largely because of an increase in EDV. Increases in EDV present a threat to the economy of contraction, and they can also stimulate hypertrophic signaling pathways as a result of cell stretch. These effects of propranolol indicate an important role of the β-receptors and the sympathetic nervous system in regulation of the ability of the heart to maintain CO with little or no change in EDV. Figure 49-2 depicts the dynamics of cardiac function with data relating time dependence of left ventricular volume before and after an episode of exercise. These data demonstrate the decrease in ESV with little change in EDV and enhanced dynamics and abbreviation of the contraction–relaxation cycle. The abbreviation of cardiac cycle time is critical for the maintenance of cardiac filling during the fast HR that occurs with exercise. Figure 49-2 also illustrates that the volume changes are associated with shortening of the cells composing the left ventricular chamber, and that the changes in cell length reflect changes in sarcomere length. Next, we discuss the molecular and cellular mechanisms responsible for maintenance of CO with elevations of VR with minimal change in EDV.
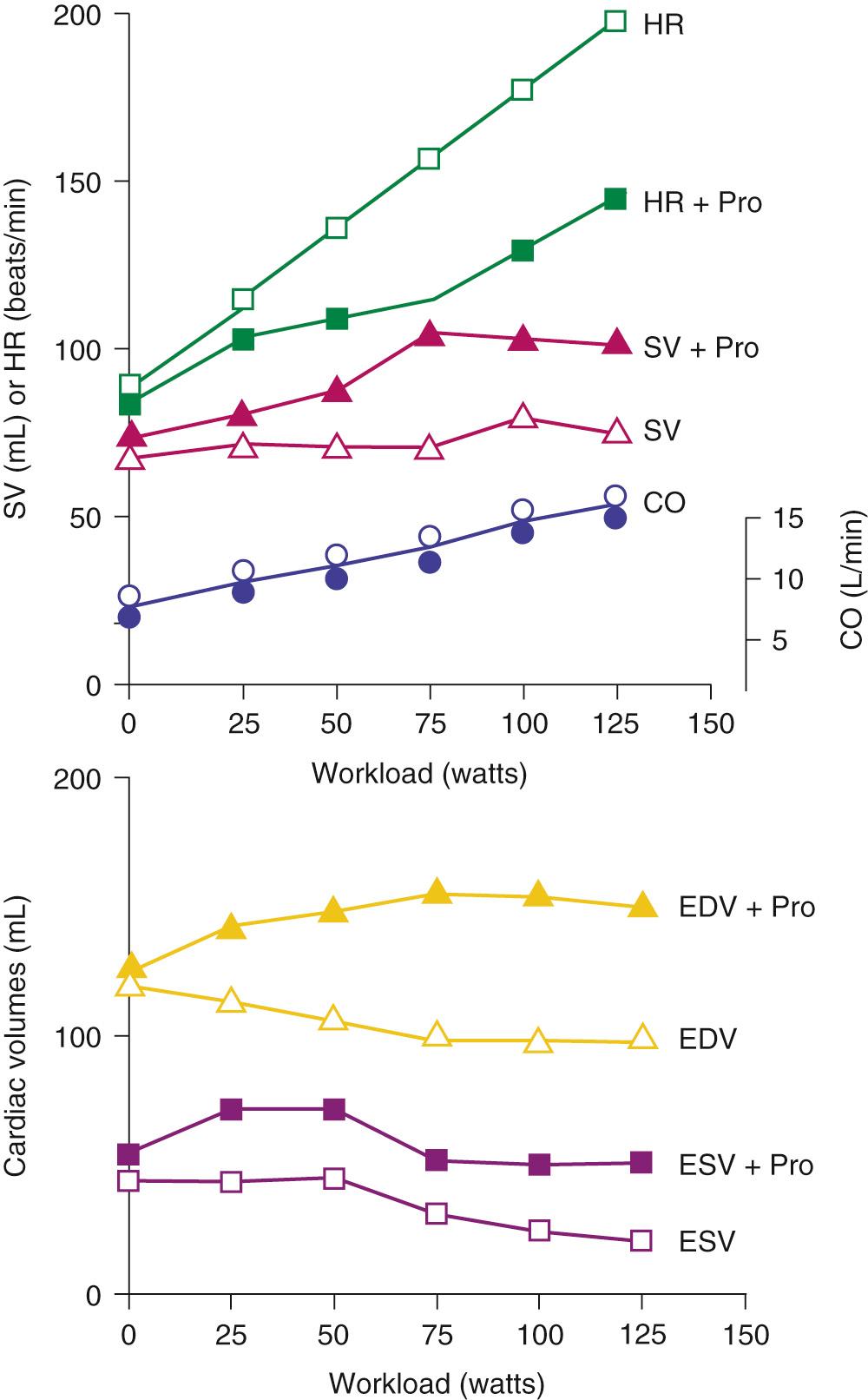
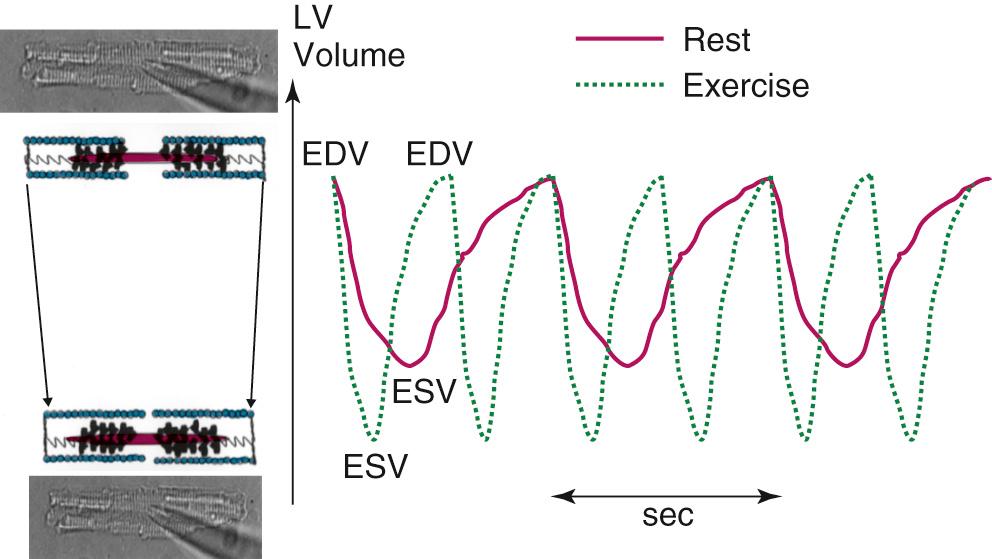
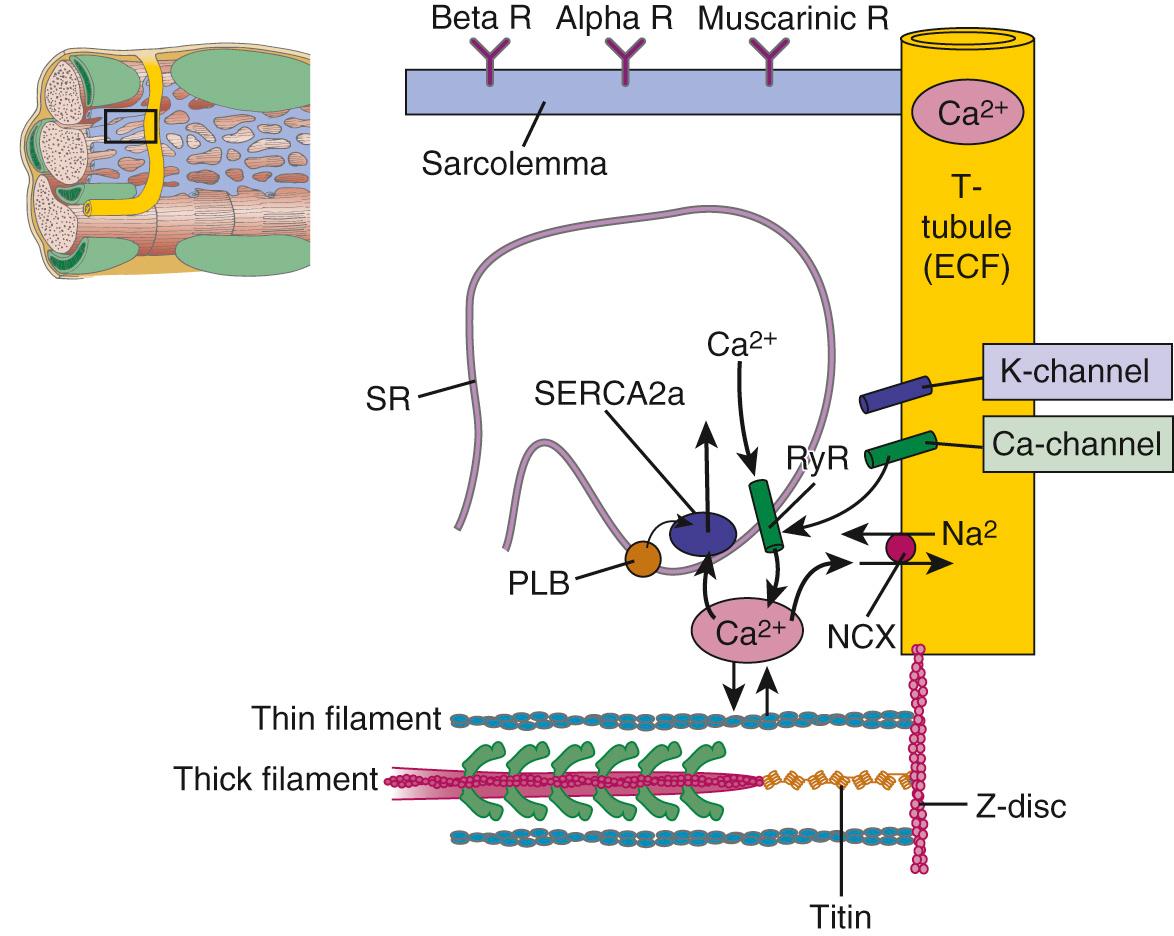
Figure 49-3 depicts cellular structures involved in excitation, contraction, and relaxation. Tight junctions of low electrical resistance connect heart cells. When one cell is activated (depolarized), all cells become activated. Therefore, unlike skeletal muscle, the heart does not recruit motor units to regulate contraction. Instead, regulation is at the level of the cells themselves. There are mechanisms that permit regulation of the activity of each cell to meet varying demands on the circulation. We will therefore concentrate now on understanding overt left ventricular cardiac function in terms of the properties of the cardiac myocytes. The objectives are to understand the following:
The molecular and cellular mechanisms responsible for the changes in wall tension and ventricular volume that occur in the transition from diastole to systole
The regulatory devices used by the heart to ensure ejection of an SV equal to the LV at optimal EDV during basal physiologic states and during exercise
The mechanisms that ensure that dynamics of the heart beat are tuned to match the prevailing frequency (i.e., the HR)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here