Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human skin is a complex structure with unusual functional diversity. , Topologically, the skin is continuous with the lung and intestinal epithelia. The lung and gut are generally viewed as exchange surfaces for gases and nutrients; the skin is more commonly considered a barrier. , , The concept of an integumental barrier emphasizes the role of the skin as a protective boundary between an organism and a potentially hostile environment. This protective role is evident at birth as the fetus abruptly transitions from a warm, wet, sterile, protected sphere to a cold, dry, microbe-laden world filled with physical, chemical, and mechanical dangers. Focusing only on the barrier properties of the skin, however, minimizes the critical role of the skin in social communication, perception, and behavioral interactions. The skin, as the surface of an organism, is both a cellular and a molecular structure, as well as a perceptual and psychological interface. This dual functionality befits a true boundary and must be kept in mind to fully appreciate the dynamic organization of the skin and its close kinship with the nervous system.
The skin also provides the physical scaffold that defines the form of an animal. A wide variety of strategies have been devised by animals to cope with different habitats ( Table 44.1 ). Arthropods have a largely inflexible body surface, covered with an exoskeleton. Many vertebrates, such as amphibians, live on land but are confined to humid or wet microhabitats. Reptiles and fish have a skin surface covered predominantly with scales; birds have evolved feathers; and most mammals are covered with a protective mantle of fur. Among primates, humans are unique in possessing a non-furred skin with a thick stratified interfollicular epidermis and a well-developed stratum corneum.
| Type | Biologic Example |
|---|---|
| Simple membrane | Earthworm |
| Simple stratified epidermis, often mucus secreting , 259 | Frog, toad |
| Exoskeleton | Insects, crustaceans |
| Scales | Reptiles, fish |
| Feathers | Birds |
| Fur | Most mammals (including all primates except humans) |
| Nonfurred Complex Stratified Epidermis | |
| Thick interfollicular epidermis with separate functional strata and well-developed stratum corneum | Humans |
| Lipid droplets in stratum corneum | Dolphins |
The question of the presumptive advantage of losing a protective and insulating coat of fur has long intrigued evolutionary biologists and physical anthropologists. Three of the most distinctive physical features distinguishing humans are (1) a non-furred skin surface, (2) a large, versatile, highly complex brain, and (3) opposable thumbs. The close embryologic connection between the epidermis and the brain (both are ectodermal derivatives) supports the contention that these peculiar structural aspects of human development have coevolved. We often overlook the direct participation of the skin in higher-level functions, such as perception and behavioral interactions. Cutaneous attributes form the basis for many readily observed biologic distinctions (age, race, sex), as well as multiple, overlapping sociocultural characteristics (tattooing, cosmetics, tanning). During recovery from illness, the skin forms a critical interface linking a patient with caregivers and a nurturing environment.
In this chapter, specific aspects of the physiologic development of fetal and neonatal skin are highlighted, focusing particularly on the development of the epidermal barrier and the functions subserved by the outermost layer of the skin, the stratum corneum. Where relevant, new areas of skin biology pertinent to a fetus and a newborn are emphasized. Areas of active research and unanswered questions will be identified in the hope of spurring new investigation of this highly accessible but extraordinarily complex interface.
Human epidermis consists of several renewing structures, including the interfollicular epidermis, the hair follicle, the sweat gland, and the sebaceous gland. , The major component is the dermis, composed of collagen and elastin fibers embedded in a hydrated glycosaminoglycan matrix and containing blood vessels and most of the cutaneous nerve endings. Dermal and subcutaneous fat cells (e.g., fibroblasts and adipocytes) derive from embryonic mesoderm. Epidermal appendages (i.e., hair follicles, sweat glands, sebaceous glands, and the interfollicular epidermis) derive from embryonic ectoderm.
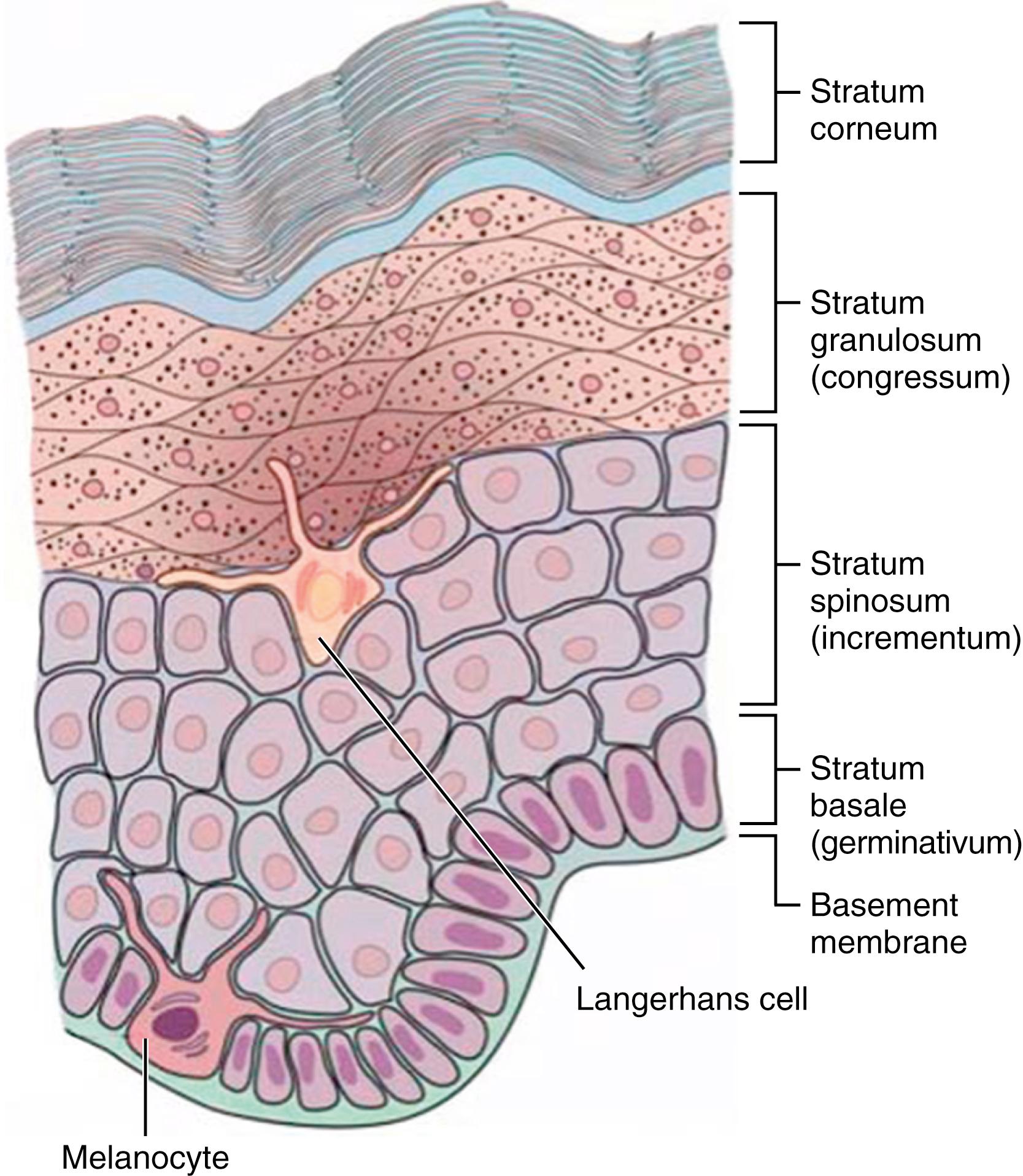
The epidermis consists of multiple cell types, including keratinocytes, melanocytes, Merkel cells, and antigen-presenting Langerhans cells derived from mesoderm. , The epidermis is traditionally segmented into four separate structural and functional compartments ( Fig. 44.1 ): (1) stratum basale , responsible for keratinocyte proliferation and epidermal renewal; (2) stratum spinosum , with tightly packed keratinocytes linked via desmosomal connections; (3) stratum granulosum , responsible for barrier lipid synthesis and corneocyte production via programmed cell death; and (4) stratum corneum (SC), the anucleated outermost layer, forming the physical environmental interface. , As discussed later, the SC is markedly deficient in preterm infants. Protective functions of the SC are listed in Table 44.2 .
| Functions | Structural Basis | Biochemical Mechanisms |
|---|---|---|
| Mechanical integrity/resilience | Cornified envelope, cytosolic filaments | Crosslinked peptides (e.g., loricrin, keratin filaments) |
| Xenobiote defense | Lamellar bilayers, extracellular matrix | Acidic pH; free fatty acids; antimicrobial peptides |
| Antioxidant defense | Corneocytes and extracellular matrix | Keratins; sebaceous gland–derived vitamin E and other antioxidants |
| Cytokine signaling | Corneocyte cytosol | Storage and release of interleukins; serine proteases |
| Permeability barrier | Lamellar bilayers | Hydrophobic lipids |
| Hydration | Lamellar bilayers, corneocyte cytosolic matrix | Sebaceous gland–derived glycerol; filaggrin breakdown products (natural moisturizing factors) |
| Waterproofing/repellency | Lamellar bilayers | Keratinocyte and sebum-derived lipids |
| Cohesion/desquamation | Corneodesmosomes | Acidic pH serine proteases |
| UV protection | Corneocyte cytosol | Structural proteins; urocanic acid; light scattering/absorption |
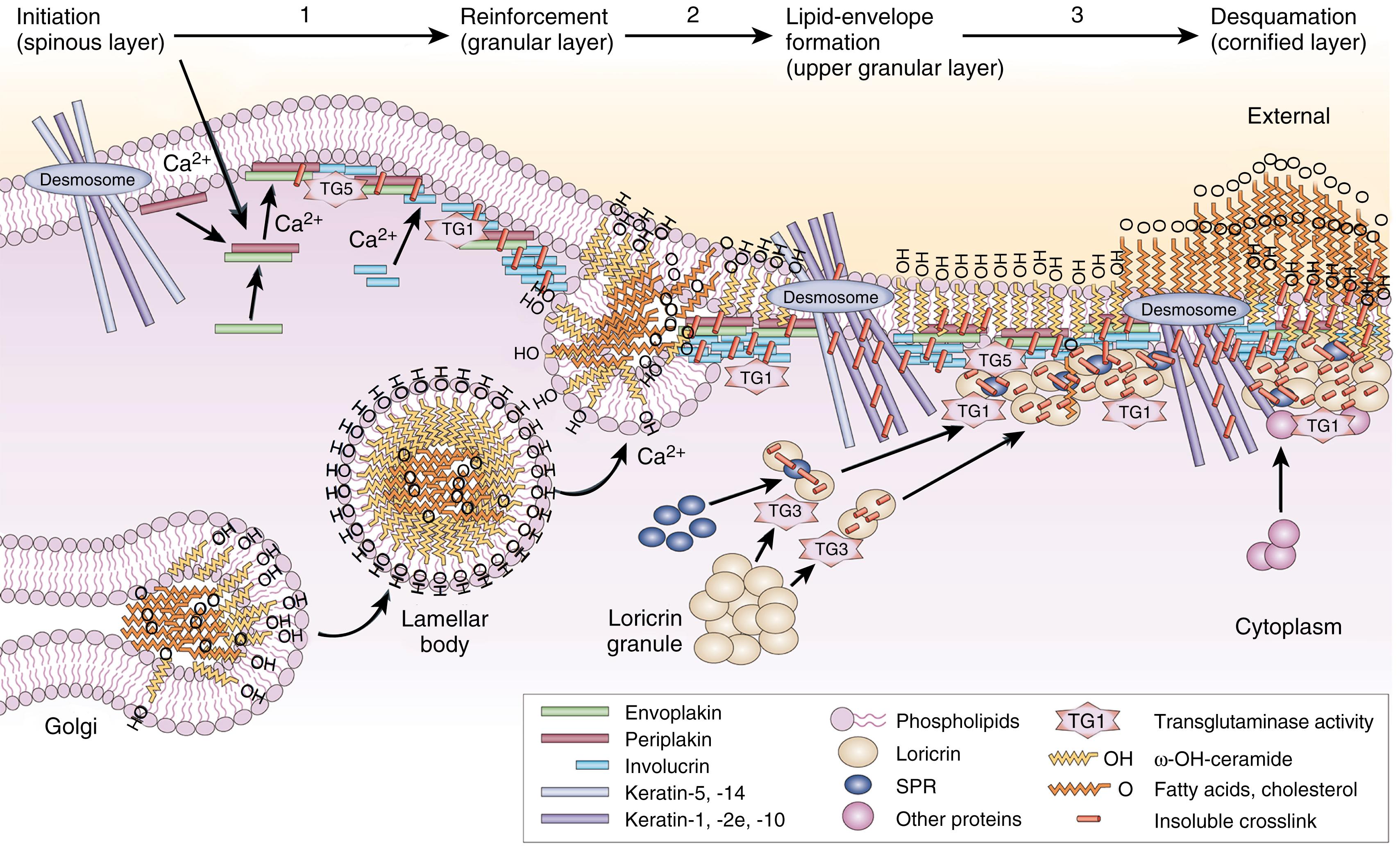
During later gestation, epithelial surfaces at environmental interfaces undergo structural and functional changes, including complex proteolipid synthesis. These highly coordinated events can be influenced by gender and prenatal hormone exposure (rat model), for example, maternal glucocorticoids. The three stages of fetal skin development are stratification, follicular keratinization, and interfollicular epidermal keratinization. Interfollicular keratinization occurs in a rostrocaudal and dorsoventral fashion, beginning at 23 to 24 weeks of GA, coinciding roughly with the time of postnatal viability. The formation of a barrier to water loss and infection is essential for extrauterine survival. Fig. 44.2 illustrates the SC cornified envelope assembly. Synthesis and secretion of epidermal barrier lipids occurs as lamellar bodies, similar to a parallel process in the developing lung ( Table 44.3 ). The intracellular lamellar body fuses with the plasma membrane and extrudes barrier lipids, consisting of free fatty acids, cholesterol, and ceramides, to form the primary barrier to transepidermal water loss (TEWL); the barrier lipids form a regular, bilayer structure that further ensures impermeability to outside agents (see Table 44.3 ). Covalent cross-linking of structural proteins (e.g., keratins) by intracellular transglutaminases and ceramides forms the insoluble, cornified SC envelope, , essentially a huge macromolecule. , This cross-linked assembly forms the scaffolding for the highly ordered lamellar lipid matrix (see Chapter 43 ). The cells within the stratum spinosum and stratum granulosum are attached to each other in all directions by desmosomes, protein-based materials that provide mechanical integrity (see Fig. 44.2 ).
| Epidermis | Lung | |
|---|---|---|
| Cell type | Spinous and granular keratinocytes | Type II alveolar cell |
| Size/structure | Ovoid (0.25–0.5 μm) | Spheroid (1.2–1.6 μm) |
| Disklike lamellations | Lamellated sacs with amorphous core | |
| Lipid content | 40% phospholipids | 85% phospholipids |
| 20% glycosphingolipids | 10% free sterols | |
| 20% free sterols | 5% other neutral lipids | |
| 20% other neutral lipids | ||
| Protein content | Acid phosphatase | Acid phosphatase |
| Glycosidases | Proteases | |
| Proteases | Glycosidases | |
| Lipases | Surfactant apoproteins | |
| Function | Delivery of lipids to form extracellular matrix of stratum corneum | Delivery of surfactant lipid to alveolar spaces |
| Skin permeability barrier | Lower alveolar surface tension |
Scientifically, the development of epidermal barrier function has many similarities to surfactant production and lung development (see Table 44.3 ). Both epidermal keratinocyte and type II alveolar cells are lipid-synthesizing cells that secrete barrier lipids as lamellar bodies. Both ultimately interface with a gaseous environment and are under similar hormonal control at similar periods of development. The mechanisms by which the lung develops a functionally mature epithelial surface ready for air adaptation and skin surface matures under total aqueous conditions for terrestrial adaptation to a dry environment are analogous. An unanswered question, however, is by which mechanism the epidermal barrier forms under total aqueous immersion. Prolonged exposure of the skin to water in adults is harmful. Vernix caseosa, a complex proteolipid cellular cream, forms during the last trimester, interacts with the epidermis, and facilitates the SC formation ( Fig. 44.3 ).
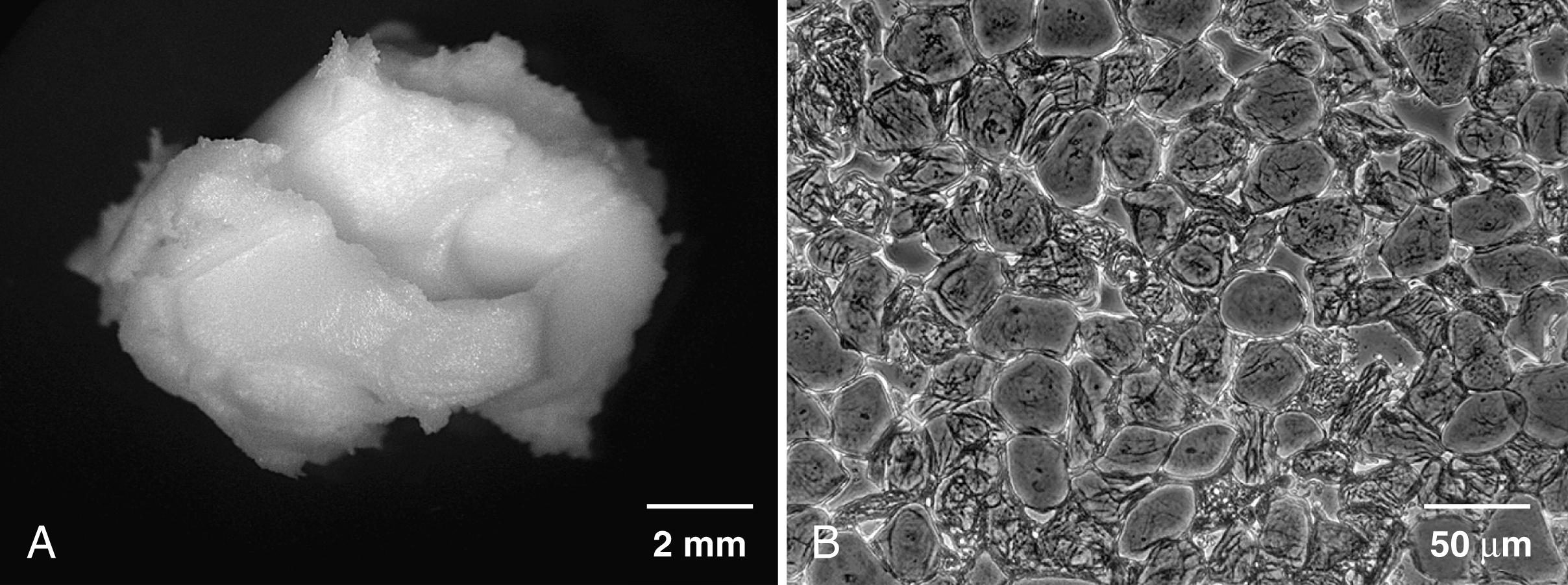
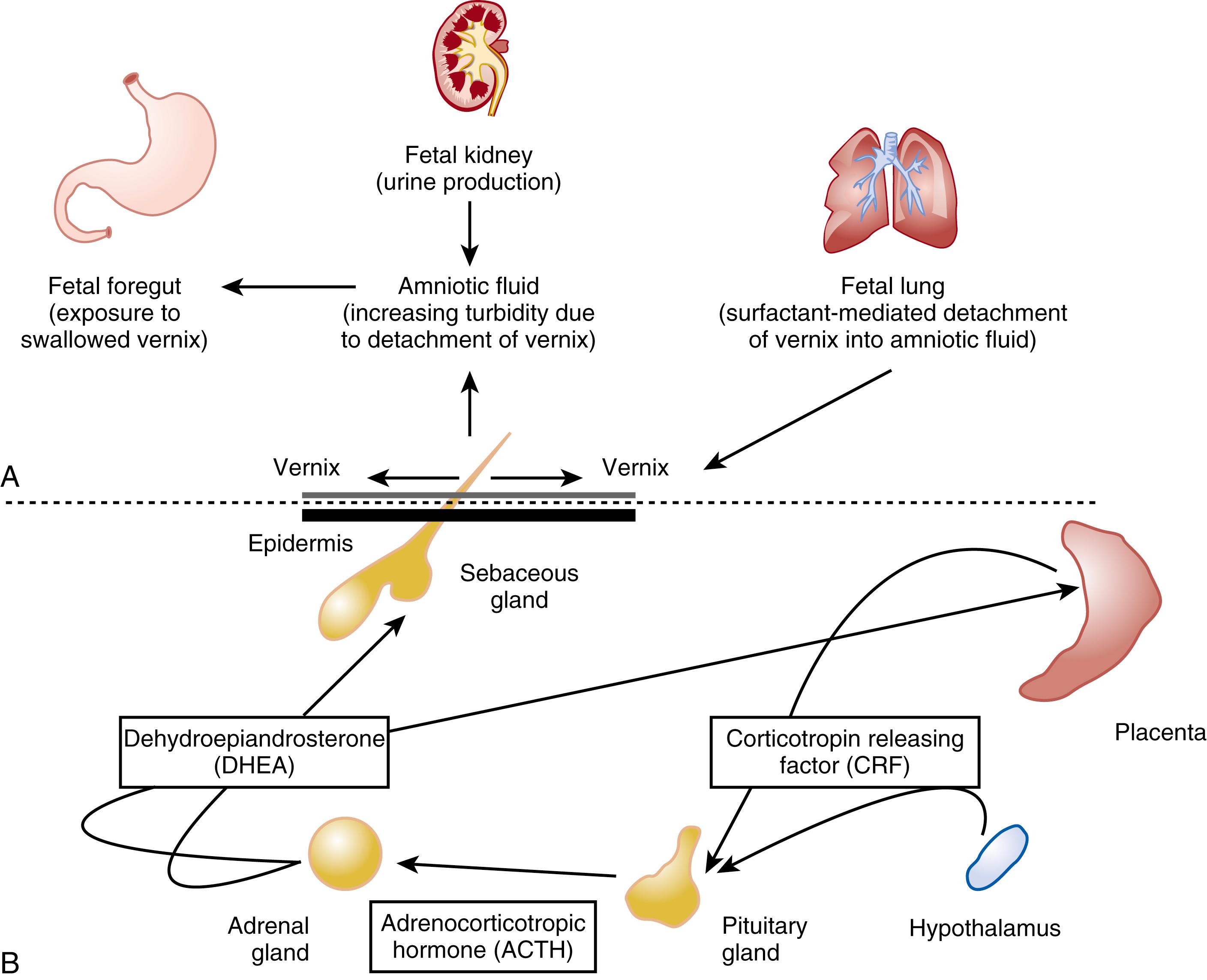
The barrier forms initially in the region of the pilosebaceous apparatus. , This finding strongly supports the hypothesis that vernix caseosa (partly from sebaceous secretions) participates in “waterproofing” the skin surface, allowing cornification initially in the hair follicles and then over the interfollicular skin, perhaps analogous to skin culture systems raised to an air-liquid interface for cornification. Sebaceous glands excrete sebum, a complex nonpolar lipid mixture synthesized de novo by the glands. Sebaceous glands are multiacinar, holocrine-secreting structures that occur over the skin. Their development is closely related to hair follicle differentiation. In neonates, sebaceous glands are hyperplastic and visible in areas such as the nose. The timing of vernix production and the developing pilosebaceous apparatus supports a mechanism by which the surge in sebaceous gland activity leads to production of vernix overlying the developing SC. Sebaceous gland hyperplasia in term infants is putatively secondary to androgenic stimulation from hyperplastic fetal adrenal glands, providing a testable model integrating hyperplasia of sebaceous and adrenal glands during later gestation ( Fig. 44.4 ). This hypothesis explains other clinical observations, such as turbidity of amniotic fluid during the last trimester of pregnancy. In vitro, the addition of physiologically relevant amounts of pulmonary surfactant leads to emulsification and release of immobilized vernix (i.e., a test tube coated with native vernix). This finding is consistent with a mechanism by which the outermost portion of the vernix coating is “removed” from the fetal skin surface by lung-derived surfactant contained in the amniotic fluid (see Fig. 44.4 ). The fetus subsequently swallows the detached vernix (see Fig. 44.4 ). Vernix contains branched chain fatty acids (BCFA), which are trophic and protective factors for the developing gut. , A diet enriched with vernix-like BCFA significantly reduced necrotizing enterocolitis in the neonatal rat model and BCFA-enriched vernix-monoacylglycerol decreased inflammatory markers in human enterocytes. Vernix contains antimicrobial agents, including lactoferrin and lysozyme, and antioxidants, providing additional protection at birth. When vernix is retained on the skin surface at birth, the skin is more hydrated and has a lower surface acidity than when it is removed.
Few events are as physiologically abrupt as birth. The skin must immediately perform multiple functions vital to the survival ( Box 44.1 ). Epidermal physiologic mechanisms in the epidermis activate eccrine sweating, important for thermoregulation and bacterial homeostasis and sebum production. The neutral pH rapidly develops an acid mantle. , The epidermis and the SC remain in balance via cornification and desquamation. , Transepidermal water flux regulates DNA and lipid synthesis. SC hydration is necessary for normal desquamation. ,
Barrier to water loss
Thermoregulation
Infection control
Immunosurveillance
Acid mantle formation
Antioxidant function
Ultraviolet light photoprotection
Barrier to chemicals
Tactile discrimination
Attraction to caregiver
Postnatally, lipid synthesis and metabolism subserve multiple functions, including SC structure formation, sebum, and adipose tissue for biomechanical support ( Table 44.4 ). The epidermis and the brain contain unusually high concentrations of ceramides, supporting the embryologic and functional connection between two ectodermal derivatives.
| Source | Composition | Functions |
|---|---|---|
| Stratum corneum | Ceramides, cholesterol sulfate, neutral lipids (free and esterified sterols, free fatty acids, triglycerides) | Permeability barrier, cohesion/desquamation, antibacterial |
| Sebaceous glands | Triglyceride, wax/sterol esters/squalene | Antibacterial, moisturizing |
| Adipose tissue | Triglyceride | Systemic energy reservoir |
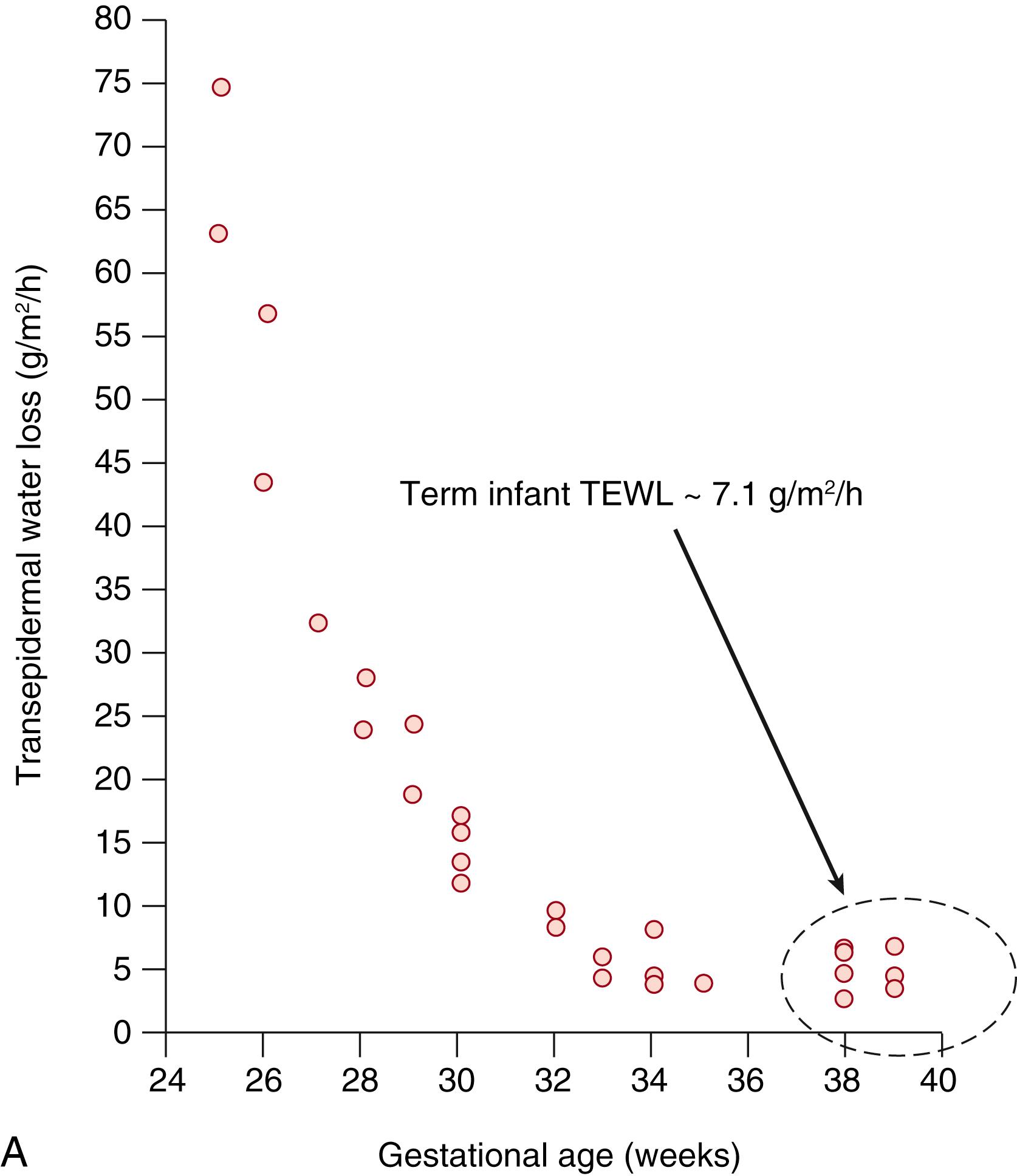
A dry environment necessitates protection against dehydration. Barrier formation against TEWL is a function of gestational age ( Fig. 44.5 ), , decreasing dramatically and approximating adult values near term. This critical function resides almost entirely in the outermost 20 μm of the skin surface—in the SC. Heat exchange occurs between infant skin and the environment via evaporation, radiation, conduction, and convection. Evaporative heat loss is the primary mode of temperature instability in not only in very low-birth-weight preterm but in all infants and is the primary mode of heat loss at birth. It is important to distinguish between evaporative heat loss from standing water or amniotic fluid on the skin surface and evaporative heat loss secondary to a poorly developed epidermal barrier, which occurs after delivery in very low-birth-weight preterm infants. Evaporative heat and fluid loss are ongoing and are exacerbated in non-humidified, radiant-heated environments.
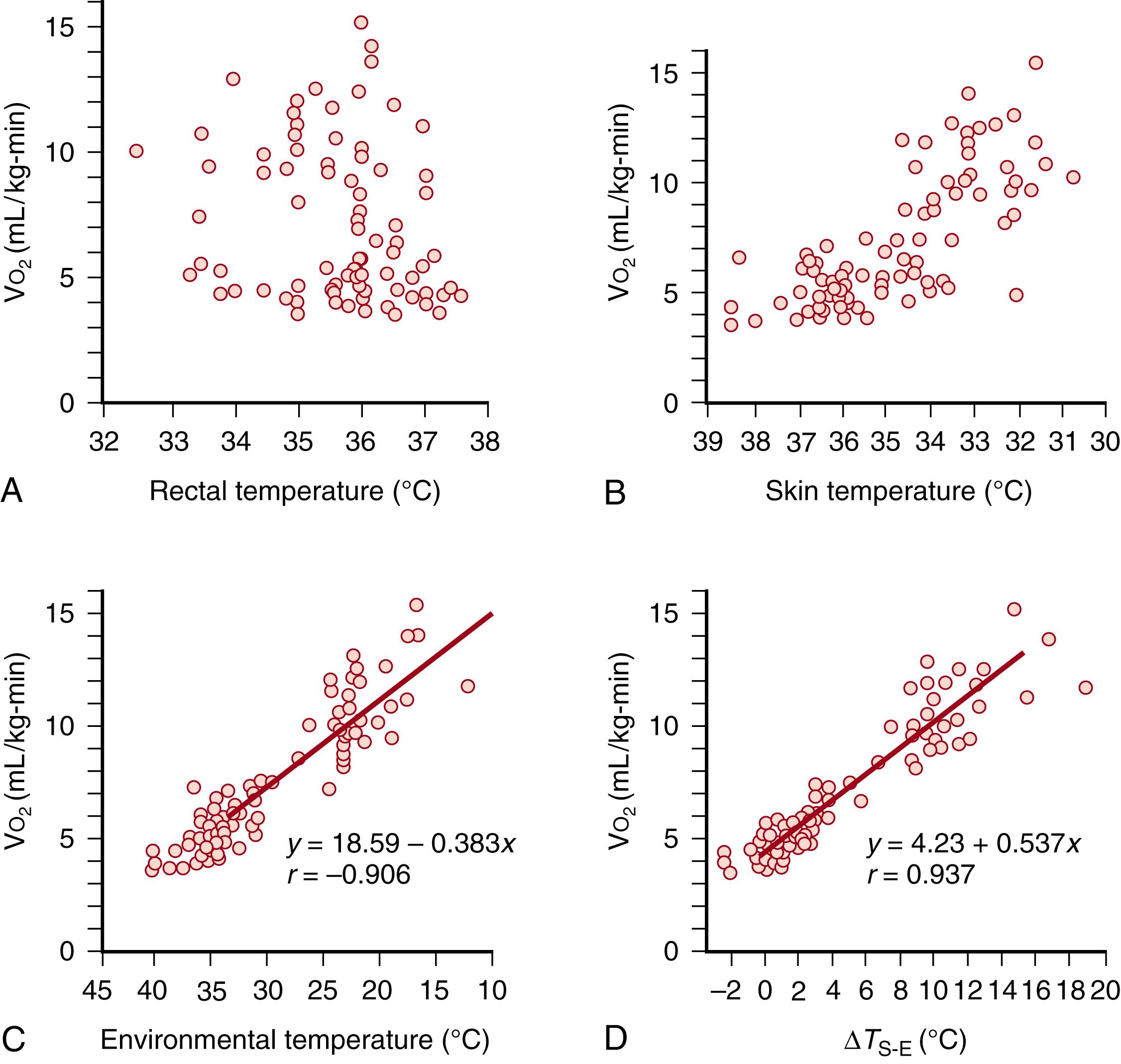
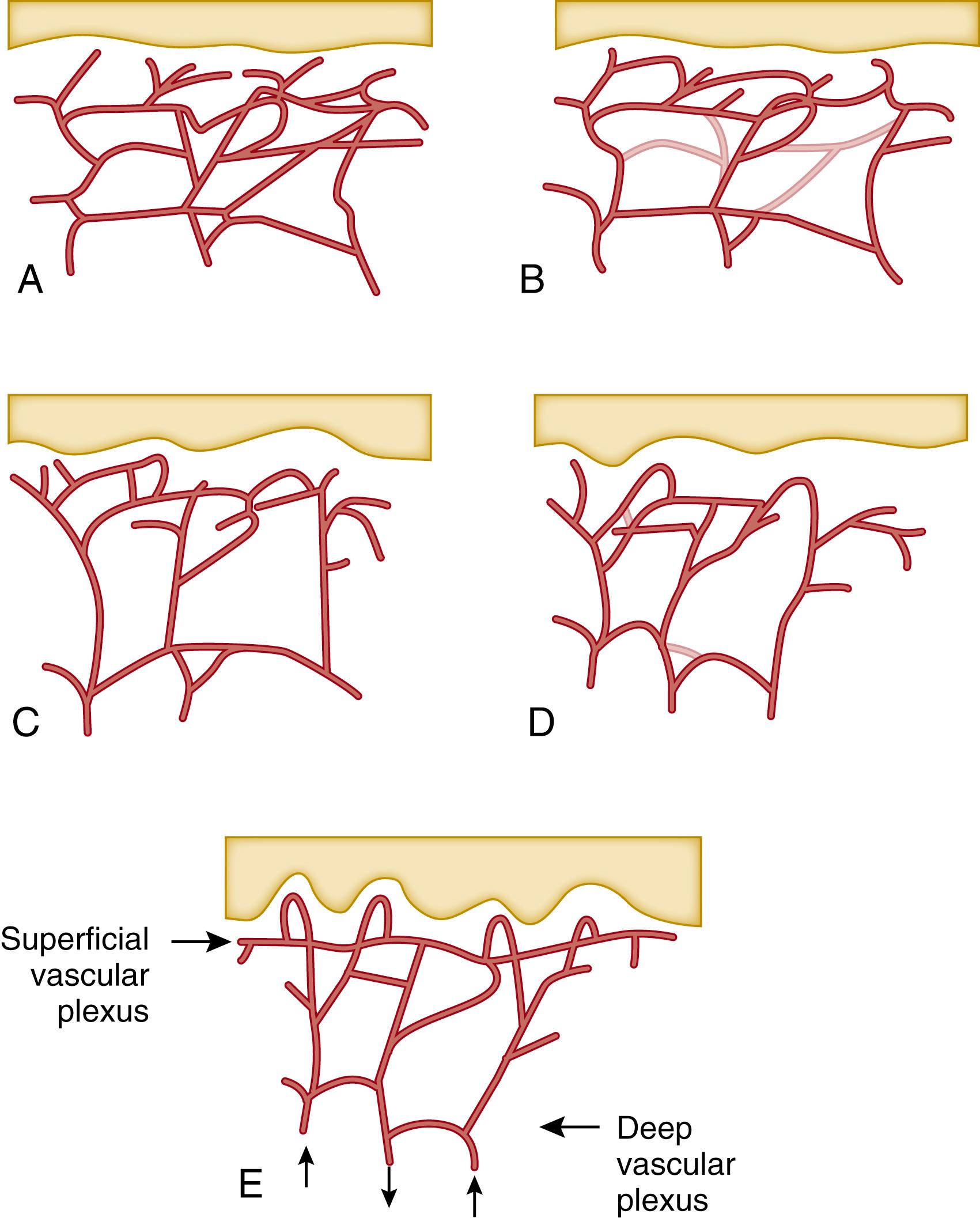
The periphery is important in temperature control in newborn infants ( Fig. 44.6 ). Given the critical importance of limiting evaporative water and heat loss in temperature control, it is surprising how little attention is given to the role of the SC in thermoregulation. The strategic position of the SC is taken for granted, and focus is placed on more central mechanisms for maintaining body temperature, yet it is essential in organizing, maintaining, and guiding central nervous system development. At 4 hours of age, oxygen consumption is not simply a function of central body temperature, as might be expected with warm-blooded endothermic organisms, but is largely a function of heat exchange at the skin-environment interface, as central homeothermic mechanisms are not well developed at birth. Preterm infants are more vulnerable to environmental heat loss due to a higher surface area to mass ratio, lower endogenous heat generation (e.g., brown adipose tissue), an incompetent epidermal barrier, and inability to self-regulate with flexural positioning. Central control of body temperature requires autonomic mechanisms such as eccrine sweating and vasodilatation/vasoconstriction, which are not well-established at birth. Newborn infants may exhibit vasoconstriction of the extremities (acrocyanosis) and have widely variable hematocrits and blood volumes. Peripheral cooling results in increased blood viscosity and decreased blood flow. In the first 3 months, the cutaneous vascular bed undergoes reorganization, with development of a subpapillary plexus ( Fig. 44.7 ). The formation of a superficial vascular plexus is associated with increased convolution in the epidermis (rete peg formation) a hallmark of the mature epidermis. Abnormal cutaneous vascular development and the role of associated growth factors have been reviewed elsewhere. ,
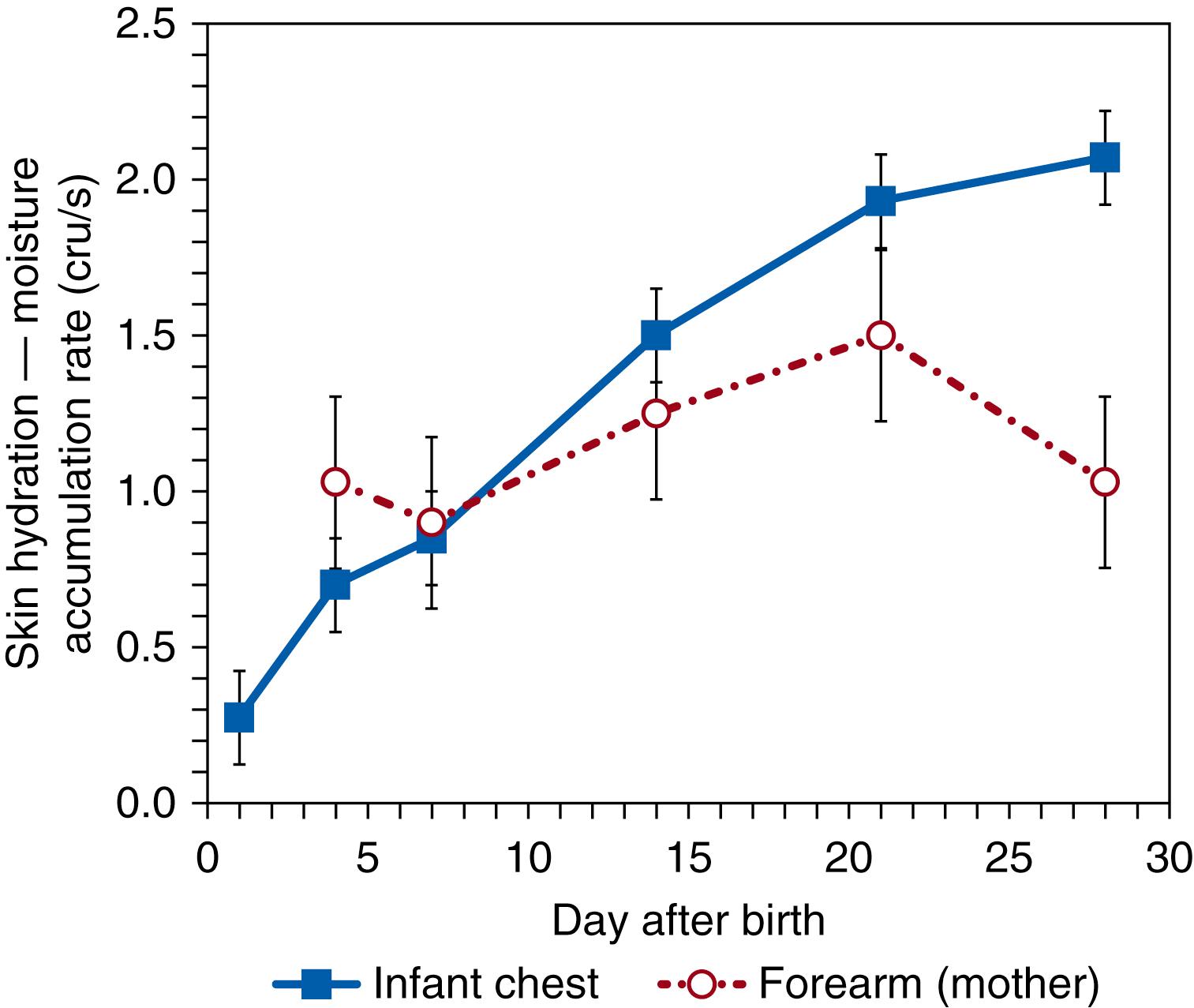
At birth, full-term skin is well-developed and protective, despite having been in a wet environment during gestation. An appropriate level of moisture (hydration) is necessary for flexibility with movement, desquamation of the outer layers during SC self-renewal, and other functions. The level of skin hydration depends upon presence or vernix, environmental temperature, and body site. , Hydration decreases rapidly during postnatal day one then increases significantly over the next 2 weeks ( Fig. 44.8 ), but the skin remains drier than older infants and their mothers. Low hydration is likely caused by a delay in the production of water-binding materials, including amino acids, from enzymatic proteolysis of filaggrin in the outer SC layers. These processes are delayed under high humidity in utero and are activated in the drier extrauterine environment.
Without the prevention of water and heat loss provided by the SC, life in a terrestrial environment is impossible. Very premature neonates have an underdeveloped epidermal barrier with few SC layers, , increasing their risk for water loss, electrolyte imbalance, thermal instability, exposure to toxins, increased permeability, delayed skin maturation, and infection. The dermis is also deficient and susceptible to skin stripping and tears/splits. The SC is nearly absent at 23 weeks GA with TEWL of 75 g/m 2 /h (see Fig. 44.5A ). Even at one month of age, TEWL is higher in preterms than full-terms (see Fig. 44.5B ). By week 26, a few cornified layers have formed (TEWL of ∼ 45 g/m 2 /h), corresponding essentially to wounded skin. , At 29 weeks, TEWL is 17 g/m 2 /h, still markedly higher than full-term values. The time of completion of SC maturation is largely unknown, but may be as late as 9 weeks postnatal age (see Fig. 44.5C ). , Exposure to the dry environment promotes rapid SC maturation ( Fig. 44.9 ). , The sticky, wet, translucent skin of a very low birth-weight infant rapidly transitions to the dry, opaque stratum of corneum, and excessive scaling/desquamation often occurs.
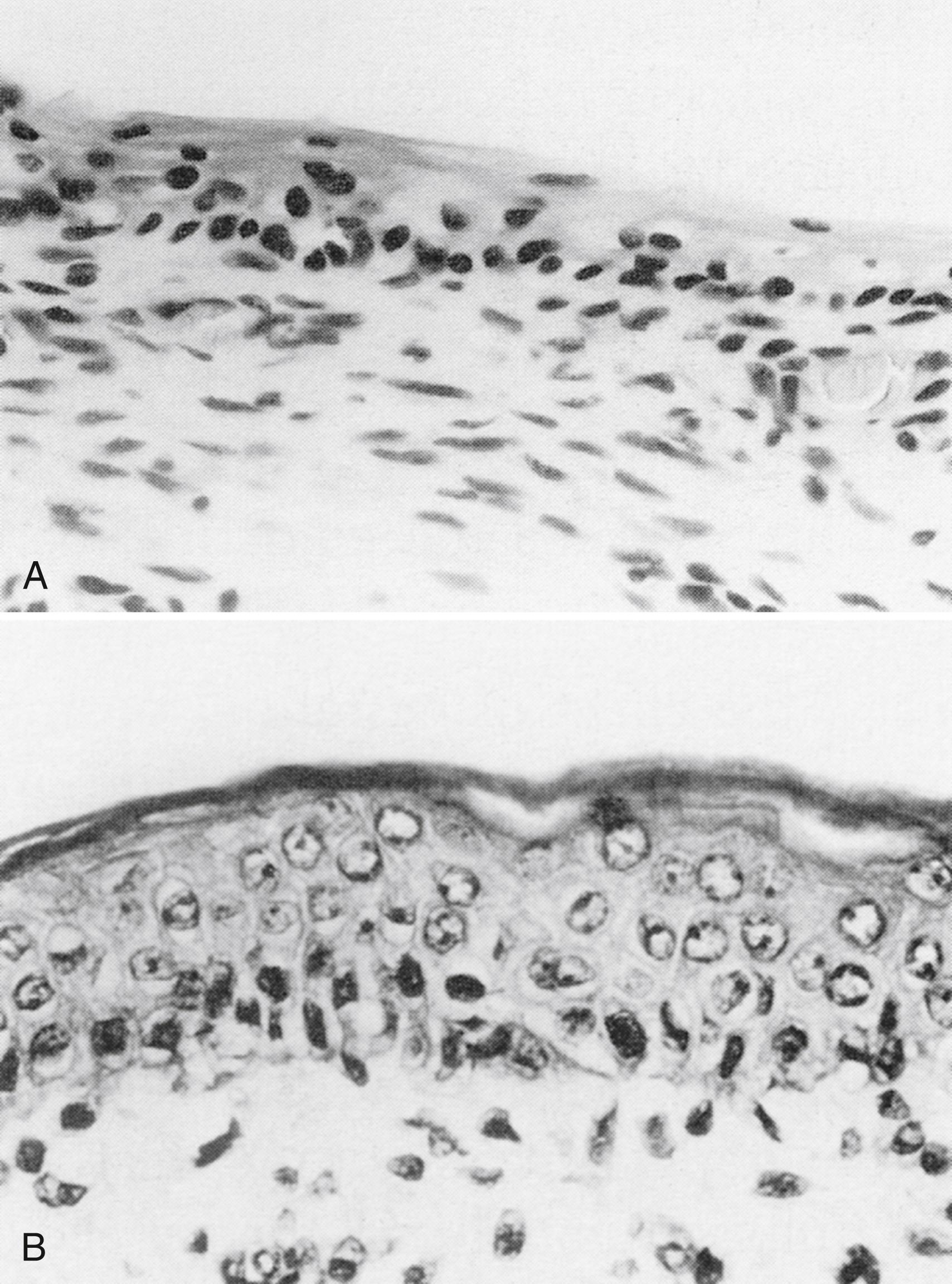
New SC sampling methods and analytical techniques facilitate investigations of neonatal skin development. Selected biomarkers of innate immunity differed for premature versus full-terms and adults. Infants 32 weeks GA or younger had higher levels of involucrin, albumin, and proinflammatory cytokines (interleukin [IL]1β, IL6, MCP1, and IL8) than full-terms and adults. All infants had higher IL1α concentrations and lower keratin 1,10,11 and tumor necrosis factor-α than adults. Involucrin was higher in full-terms than adults and inversely related to GA.
Immature preterm skin is permeable, and care is required to avoid toxic transdermal exposures. , The permeable barrier allows the efflux of carbon dioxide and the influx of oxygen in a manner similar to that of water vapor. Gaseous movement across the skin surface, facilitated by local skin heating, is the basis for the use of transcutaneous gas electrodes in clinical practice.
A number of physiologic protective, skin-based mechanisms are triggered at birth, as they were unnecessary during gestation. The skin surface of full-term infants has a neutral pH at birth. However, an acid skin surface, known as the acid mantle, is necessary for effective barrier function. Multiple mechanisms are responsible for acid mantle development. The lamellar bodies in the stratum granulosum (see Fig. 44.1 ) contain phospholipids that are hydrolyzed to free fatty acids that lower skin pH. Activity of the sodium-hydrogen antiporter NHE-1, located at the stratum granulosum/SC interface, is responsible for pH reduction. The protein filaggrin aggregates keratin proteins in the stratum granulosum and SC and plays a key role in skin barrier function. Filaggrin is hydrolyzed by skin enzymes to form lower molecular weight compounds, collectively known as natural moisturizing factor (NMF). The components of NMF (1) bind water in the upper SC to hydrate and plasticize the tissue and (2) lower the skin surface pH. An acidic surface pH enhances SC formation, for example, lipid metabolism and structure, and SC integrity, cohesion, desquamation, and homeostasis. An acidic pH facilitates skin colonization with appropriate bacteria, that is, S. epidermidis attachment to the skin, antimicrobial peptide activity, microbiome diversity, and inhibition of pathogenic bacterial such as S. aureus . In contrast, increased skin pH activates enzymes that damage SC integrity, enhances susceptibility to mechanical trauma, and increases the amount of pathologic flora.
Full-term skin pH is neutral at birth, decreases markedly over days 1 to 4 and continues to decline for as long as 3 months after birth. , , For example, NMF levels were very low at birth in full-term infants but increased over the first postnatal month, , contributing to the acid mantle. Vernix plays a role in this process. Full-term skin pH was lower when vernix was retained at delivery than when vernix was removed at 4 or 24 hours after birth.
In low-birth-weight infants, skin pH decreased rapidly over days 1 to 3, more slowly over days 4 to 7, and declined further up to day 28. Individually, GA and day of life did not influence the skin pH but significantly impacted the reduction in combination. A rapid decrease in skin pH occurred in infants 24 to 34 weeks GA during postnatal week 1 then was more gradual. Infants weighing less than 1000 g had a higher skin pH for longer than infants weighing more than 1000 g. Unlike full-term neonates, premature infants less than ∼29 weeks GA have little vernix on the skin surface at birth. Consequently, the premature skin barrier may not experience the benefits of reduced pH and increased hydration observed in full-term infants.
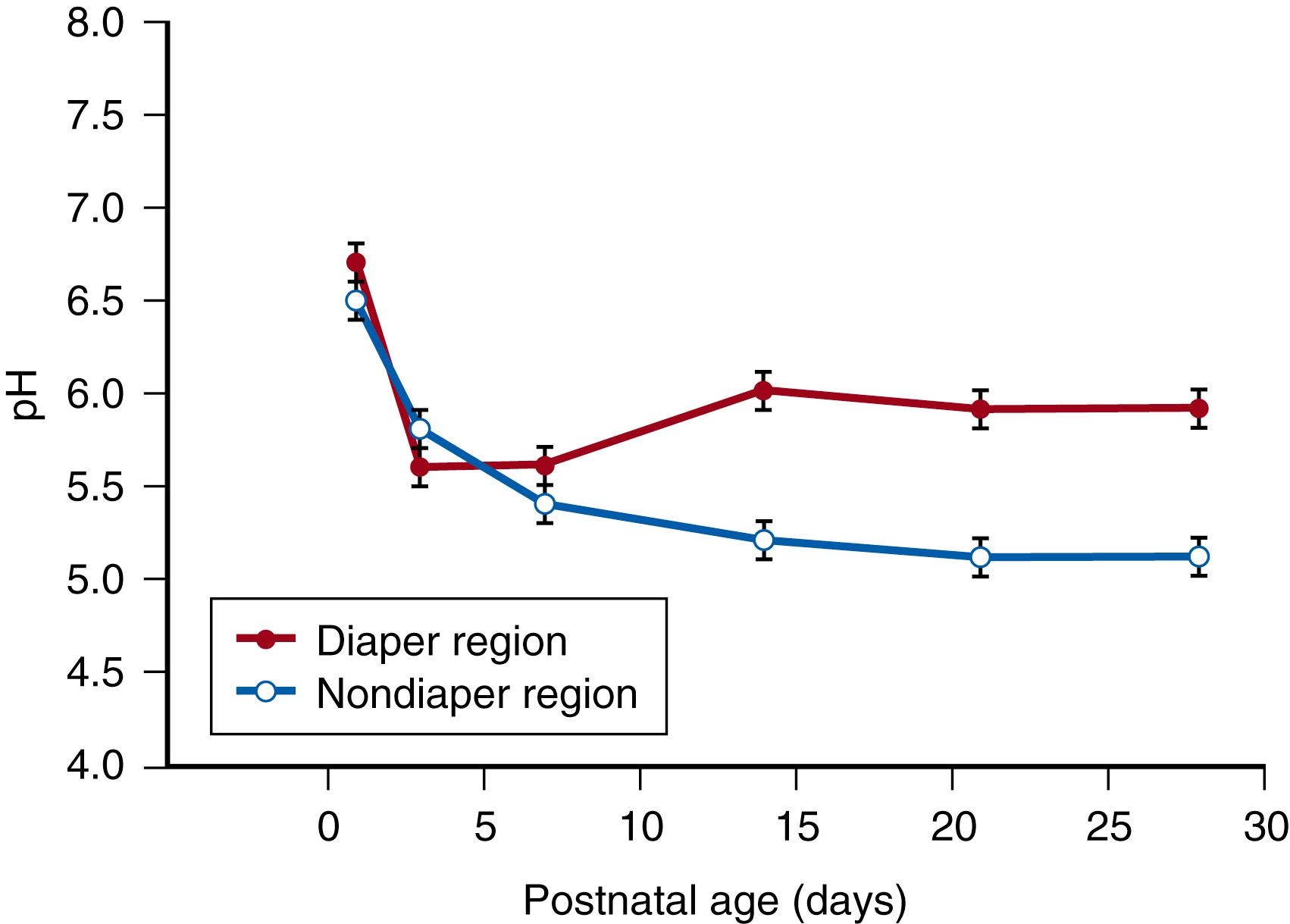
The skin pH can be altered by topical products and items. For example, the pH of skin under the diaper was higher than a non-diapered site in neonates and older infants ( Fig. 44.10 ). , Higher diaper skin pH was related to greater irritant contact dermatitis and hydration (wetness). Higher pH may increase the risk of mechanical damage in vulnerable infants (e.g., friction, cleaning) and increase skin permeability to toxins. Higher skin pH may predispose development of inflammatory skin diseases of infancy, including atopic dermatitis and seborrheic dermatitis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here