Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nerve regeneration after injury is orchestrated by delicate processes in neurons, non-neural cells, and other cells at various levels from the periphery to the central nervous system.
The clinical effects of a nerve injury include not only impaired sensory and motor function, but also symptoms such as impaired dexterity, sensitivity to cold, and pain. Therefore, they have a severe impact on activities of daily living.
Correct diagnosis and classification of the injury are crucial for appropriate treatment.
The surgical approach and technique, as well as timing of surgery, are factors of detrimental importance for outcome.
The type of injury, condition of the wound, and the vascularity of the wound bed are key factors influencing surgical decision-making.
Various techniques for nerve repair are described, including epineurial, fascicular, and end-to-side repair, and techniques for nerve reconstruction.
Factors of importance for outcome, such as age, delay before repair, and level of injury. are discussed, as well as means of evaluation after surgery.
![]()
![]() Access video and video lecture content for this chapter online at Elsevier eBooks+
Access video and video lecture content for this chapter online at Elsevier eBooks+
A nerve injury may have a profound impact on the patient’s activities of daily living, with subsequent effects on professional life and leisure time. Patients may have to change profession, or be left with a permanently impaired ability to work. Nerve injuries may, therefore, generate not only costs for society within the healthcare system for treatment and rehabilitation, but also outside the healthcare system resulting from lost production. Nerve injuries most commonly affect the upper extremity. The incidence of injuries to the hand has been calculated as 7–37/1000 inhabitants/year in Europe. Similar data are available for children (incidence 2.7/1000 children/year). Most hand injuries are minor, but injuries to the nerves (that account for about 3% of hand injuries) usually impair hand function. Few reports exist about the specific incidence of nerve injuries, but the incidence in one study was reported to be 13.9/100,000 persons per year. Most of the injuries to the hand (10%) and the wrist (63%) required less than a week in hospital. However, few inpatient and outpatient data have been reported. The incidence of a digital nerve injury is estimated to 6.2/100,000 population per year. The general pattern of distribution of both hand and nerve injuries between the sexes is that it is usually young (median age 29 years) men (up to 75%) who are injured. The incidence of a radial nerve injury associated with a fracture of the shaft of the humerus is around 0.12/10,000 population per year. Based on these reports one may estimate that 70,000 and 29,000 patients in the European Community and in the US, respectively, injure a nerve trunk annually.
The costs of treatment and rehabilitation, including those for loss of production after a nerve injury, may be substantial. The costs for injuries to a median and an ulnar nerve are roughly US$70,000 and $45,000, respectively ; 87% of these costs are the result of lost production. Costs are higher for patients with coexisting tendon injuries (≥4 tendons). In addition, costs within the healthcare sector are higher for patients who had to change jobs after the injury and in patients in whom both the median and the ulnar nerves were injured. Up to 69% of patients with an ulnar or median nerve injury are back in fulltime work by 1 year after the nerve injury. Age is one important factor for outcome after nerve repair, which is probably based on better cerebral adaptation to the injury, particularly in young children. The clinical effects of a nerve injury include not only subjective and objective impaired sensory and motor functions, but also subjective complaints, such as impaired dexterity, cold sensitivity, and pain ; these injuries thus have a severe impact on activities of daily living. Altogether, nerve injuries can cause considerable problems for individual patients and costs for society. In this chapter the principles for repair and reconstruction of nerve injuries are outlined, with emphasis on the fact that such injuries should be treated promptly.
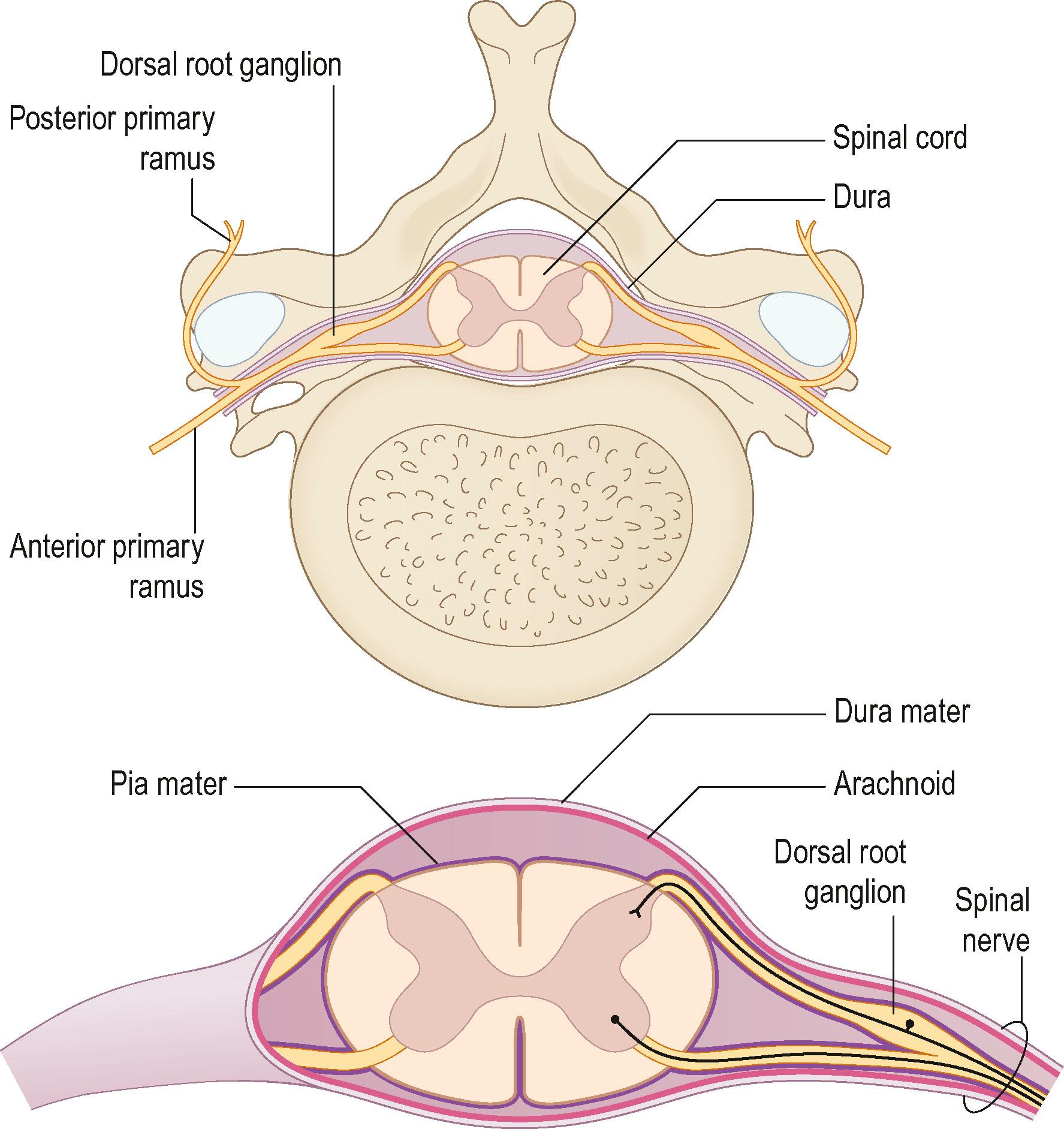
The brain and the spinal cord (central nervous system) are connected to targets by the peripheral nervous system, which consists of cranial nerves, spinal nerves with roots and rami, and peripheral nerve trunks with the peripheral components of the autonomic nervous system. The anterior and posterior nerve roots (that emerge from rootlets attached to the spinal cord) merge into the spinal nerve, where the anterior primary rami form the brachial plexus in the upper extremity ( Fig. 22.1 ). The posterior primary ramus runs posteriorly, and supplies the muscle and skin of the posterior part of the neck and trunk; it does not enter the extremity. The anterior primary rami of the cervical nerve roots C5–C8 and the ramus of T1 form the brachial plexus, from which the upper extremity receives its innervation through various branches.
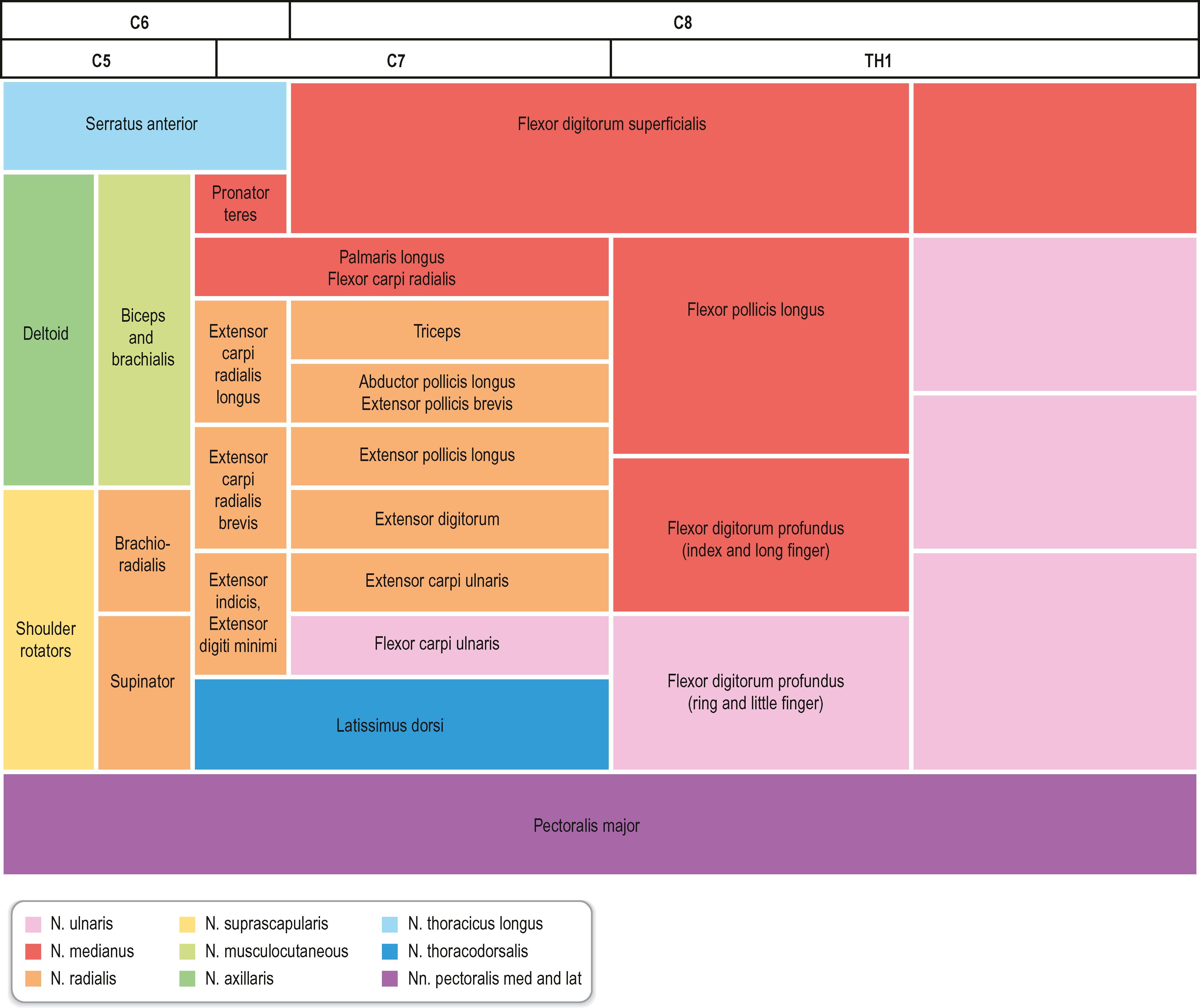
C5–C6 form the upper trunk of the brachial plexus, C7 forms the middle trunk, and C8–T1 form the inferior trunks. These trunks divide into anterior and posterior divisions in which the lateral and medial cords are formed from the anterior division, while the posterior divisions form the posterior cord, which mainly innervates the extensor muscles in the upper extremity. In contrast, the lateral and medial cords innervate the flexor muscles in the extremity. The musculocutaneous nerve originates from the lateral cord. The median nerve is formed by the joining of the lateral and medial cords, whereas the ulnar nerve branches from the medial cord together with the medial cutaneous nerves to the arm (brachial) and forearm (antebrachial). The radial and axillary nerves originate from the posterior cord. There is a segmental pattern of sensory and motor innervation ( Fig. 22.2 ).
The anatomy of the plexus and the nerve trunks may vary considerably from person to person. Variations are seen in the forearm, where a “Martin Gruber” anastomosis may be found in as many as 15%, particularly between the anterior interosseous nerve and the ulnar nerve. A similar anastomosis may also be present more distally – Riche–Cannieu branch between the motor branch of the ulnar nerve and the thenar branch of the median nerve in the thenar region in the hand. Another common and consistent variant is the palmar communicating branch according to Berrettini ( Fig. 22.3 ).
The cell bodies of the motor and sensory neurons are located in the spinal cord and in the dorsal root ganglia, respectively, with their axons extending out to their respective targets in the periphery. The sensory neuron is pseudounipolar, with one branch of the axon extending into the posterior part of the spinal cord. The transition from the central to the peripheral nervous system takes place in the rootlets (or less often, in roots of the nerves) of the transitional zone, which are almost cone-shaped. In the central part, the neurons are surrounded by oligodendrocytes and astrocyte processes, whereas in the peripheral nervous system axons are closely associated with Schwann cells. In myelinated nerve fibers, each axon is embraced by a series of continuous Schwann cells that create the myelin sheath. The border between individual and enwrapping Schwann cells is called the node of Ranvier (see Fig. 22.4 ). Each nerve fiber is enclosed in a basal lamina (or basement membrane). In contrast, several thinner axons run in troughs in one Schwann cell – nonmyelinated nerve fibers. Each Schwann cell therefore embraces several axons. The diameter of nerve fibers varies, and extends from 0.4 to 1.25 µm (nonmyelinated) and from 2 to 22 µm (myelinated). The number of nerve fibers also varies with nerve trunks. In addition, the number of nerve fibers decreases with age: as many as 26% of the nerve fibers may disappear between the second and eighth decades.
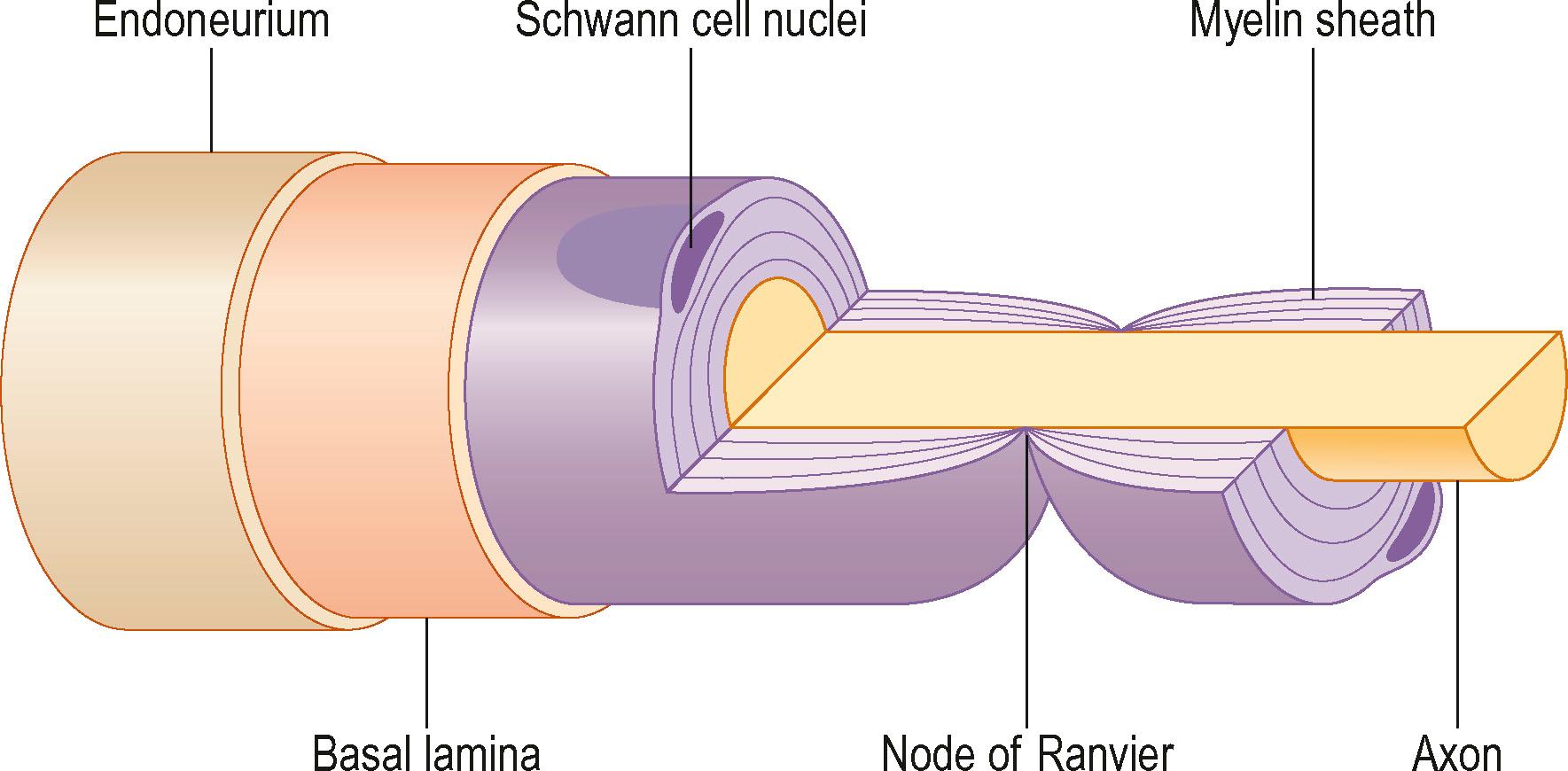
A number of nerve fibers are enclosed in bundles around which there are flattened supporting cells and layers of collagen that form the perineurium. These bundles of fibers surrounded by the perineurium are called fascicles, which are embedded in loose connective tissue (called epineurium); this is composed of collagen fibers and fibroblasts ( Figs. 22.4 & 22.5 ). The space inside the fascicles is called the endoneurial space, in which the pressure is slightly positive (endoneurial fluid pressure); it consists of collagen fibrils, and cells, such as fibroblasts and occasional macrophages and mast cells. Lymphocytes (CD4 + and CD8 + ) and macrophages are not generally found in the endoneurium (except after injury), but may be present in the epineurium. The amount of connective tissue varies among nerve trunks, and also within the same nerve trunk. More abundant connective tissue is therefore located in the nerve trunk at sites where extra protection is needed (such as joints and superficial nerve trunks). The term “mesoneurium” refers to the additional loose areolar tissue that is located superficially to the epineurium. This tissue allows for the gliding of the nerve trunk (excursion) during movements of the extremity.
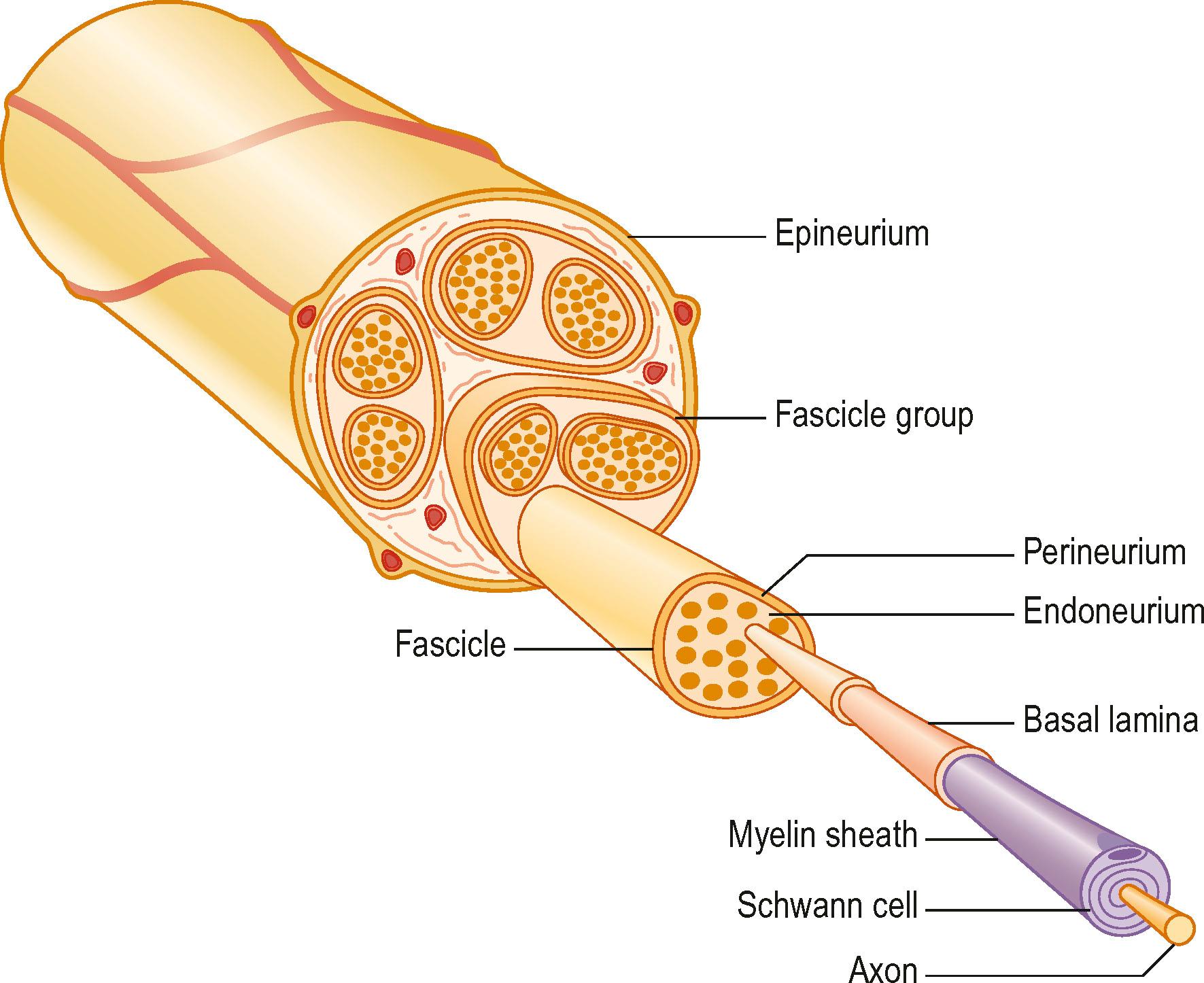
There is topographical segregation of fascicles and nerve fibers in the nerve trunks, particularly in their peripheral parts. The latter anatomical feature permits dissection of a bundle of fascicles that can be used in nerve transfers.
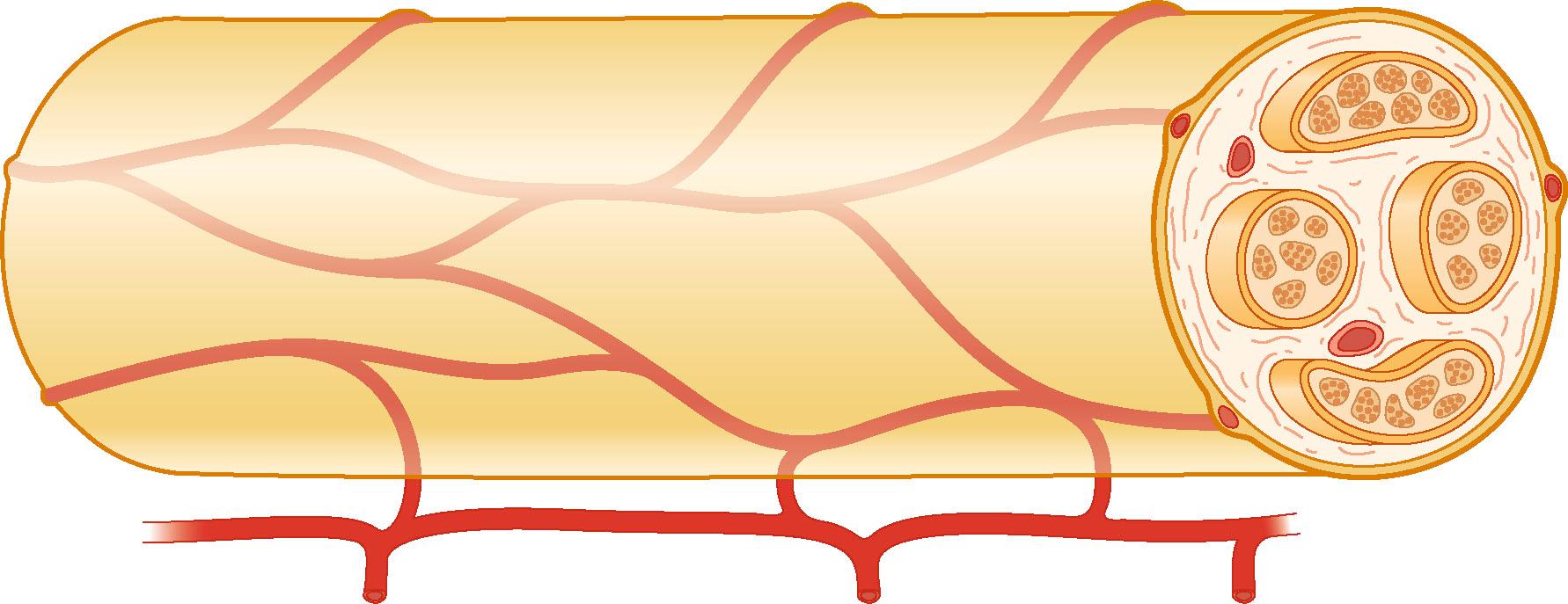
Nerve trunks receive their blood supply from segmental blood vessels ( Fig. 22.6 ), which branch into plexuses in the epineurium, perineurium, and in the endoneurial space. The blood vessels inside the fascicles consist mainly of capillaries, which are provided by circulation through blood vessels that pierce the perineurium obliquely. The epineurial blood vessels are more susceptible to trauma than the endoneurial vessels and so epineurial edema develops more easily than endoneurial edema. Because of the extensive reserve capacity of the intraneural blood supply it is possible to mobilize a nerve over an extended length without disturbing the blood supply. In addition, the blood vessels adjacent to the nerve trunks have a coiled appearance that allows excursion of the nerve trunk. In addition to the vasa nervorum, there are also nervi nervorum, such as free nerve endings in the epineurium, perineurium, and endoneurium.
To allow for propagation of the action potential, ions are exchanged between the axon and the extracellular space at the nodes of Ranvier (see Fig. 22.4 ). As this exchange occurs only at the nodes of Ranvier in myelinated nerve fibers, the conduction velocity jumps from node to node. In myelinated nerve fibers this saltatory conduction induces high conduction velocity. In contrast, the propagation of impulses is more continuous in nonmyelinated nerve fibers. After a nerve has been injured the process of Wallerian degeneration (see below) takes some time in humans, which explains why it is possible to record propagation of an impulse in an injured nerve by electrical stimulation at neurography for many days after the injury. It is generally agreed, therefore, that neurography should be done no earlier than 3–4 weeks after the injury if signs of degeneration are to be found.
The cytoskeleton of the axon contains neurofilaments and centrally located microtubules. Essential molecules are transported along the microtubules both from the cell body down to the periphery (anterograde) and from the periphery up to the cell body (retrograde) with energy-dependent axonal transport. This transport has several functions, such as recycling of materials that are not used in the periphery, provision of membrane constituents, neurotransmitters, and necessary cytoskeletal components to the axon. Transfer of important molecules is essential for survival and signaling both in the normal state and after nerve injury. After cellular injury these signals are rapidly initiated from the environment by translation into intracellular messengers or commenced in the axon at the site of injury. These changes involve profound alterations in transcription, translation, and posttranslational processes in the cells after the injury.
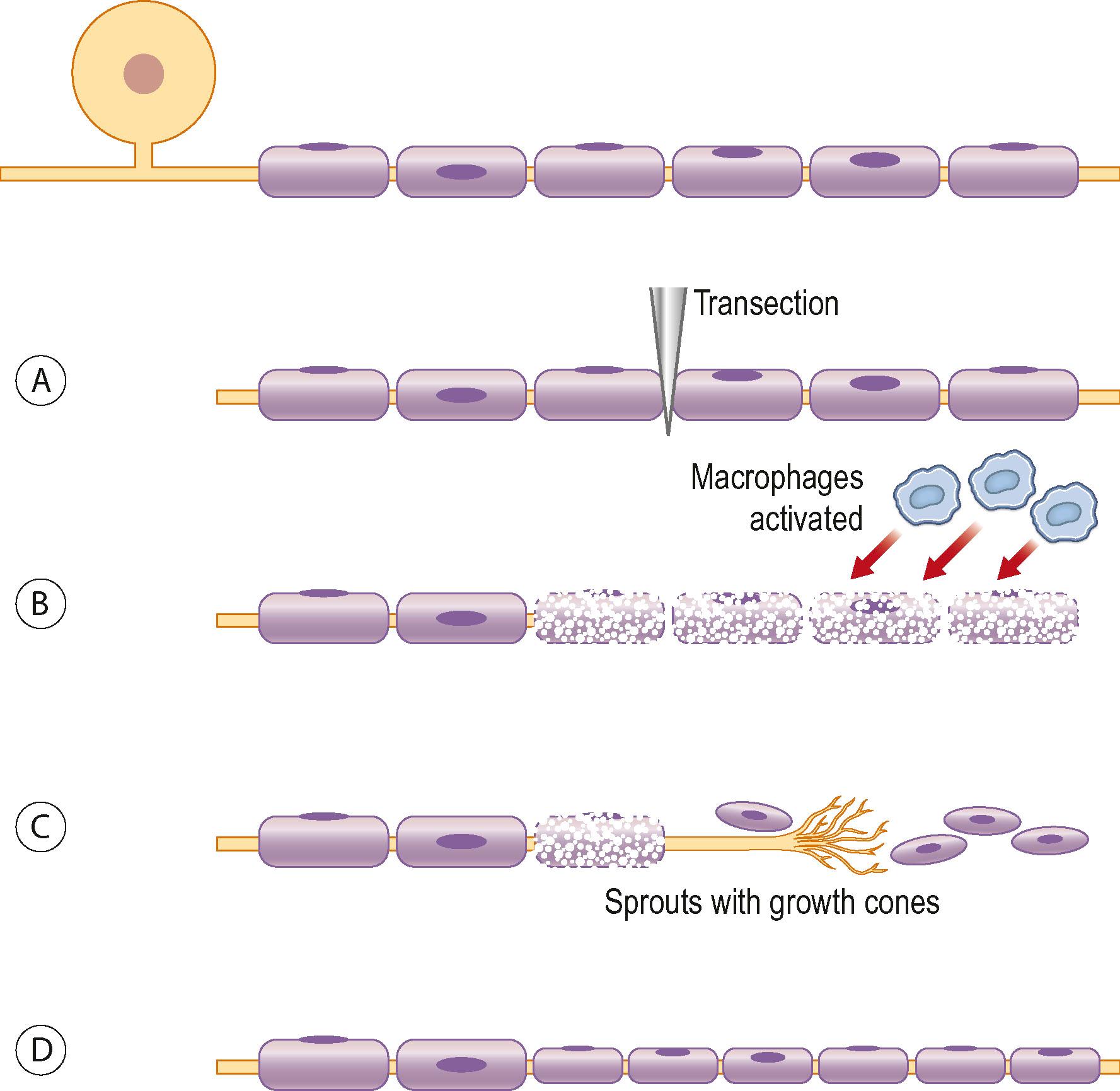
After a nerve injury, deep and intense processes are initiated in the injured neurons and their axons and in the non-neuronal cells, particularly the Schwann cells. In the cells a number of temporary and spatially orchestrated mechanisms guide survival and regeneration. In addition, there are mechanisms that lead to apoptosis of the cells. The signal transduction processes are not clarified, and this is a target of intense research. After the peripheral nerve trunk has been injured by transection or avulsion, the proximal part of the axon is resealed to form a new surface on the tip of the axon – a process that is influenced by the presence of calcium. There is usually a slight retraction of the proximal axon, which is related to the number of injured Schwann cells. At the tip of the axon, signals are formed in the signal transduction processes and transferred to the nucleus of the neuron where the functions are changed from maintenance to survival and growth.
Schwann cells are extremely important for the function of the peripheral nerve, both in the normal state and after injury. After injury there is disintegration of the axons and the myelin sheath in the distal nerve segment, the latter released by Schwann cells. It is important to remove myelin and the disintegrated axon to allow and prepare a conducive environment for the regenerating axons. Both macrophages and Schwann cells can therefore contribute to the cleaning-up mechanisms, where their relevance varies over time.
The timing of nerve repair is of crucial importance for a successful outgrowth of axons after injury, which can be related to a large number of factors that are up- and downregulated by Schwann cells after nerve injury. After the Schwann cells have been activated they proliferate along the inside of their basal lamina, and form the bands of Büngner.
Neurotrophic factors and their receptors are upregulated in Schwann cells after injury, and these may include neurotrophins (such as nerve growth factor and brain-derived neurotrophic factor), fibroblast growth factors (such as β-fibroblast growth factor), neuropoietic cytokines (such as ciliary neurotrophic factor and leukemia-inhibitory factor), and other groups of factors, such as insulin-like growth factor, transforming growth factor, and interleukins.
Apoptosis (programmed death) of various cells, such as macrophages and Schwann cells, an event that is initiated by release of proapoptotic molecules from the mitochondria (intrinsic pathway), can be activated through the death cell surface receptors (extrinsic pathway; like Fas and tumor necrosis factor receptor 1). The number of apoptotic Schwann cells in the distal nerve segment is increased if the transected nerve is repaired after a delay.
All these changes in the injured neuron and its axon and in the Schwann cells have the common goal of regrowth of the axons into the distal nerve segments down to their targets. Regrowth is a delicate process in which numerous sprouts emerge from the distal part of each axon. On the tip of each sprout there is a growth cone, which is formed as a small hand on which the “finger-like” filopodia palpate the environment with the purpose of finding guidance structures ( Fig. 22.7 ). Intricate mechanisms direct rate and direction of extension of the growth cones, where actin filaments are polymerized and organized. Microtubules are polymerized in the growth cones. The growth is a dynamic process, where the filopodia/growth cone is repulsed or attracted depending on the local mechanisms and the structure of the environment.
The extracellular matrix is extremely important for the outgrowth and advancement of the growth cone. Proteins, such as laminin, fibronectin, galactins, tenascin, and collagens, stimulate outgrowth. Laminin exerts its stimulatory effect through cell surface glycoproteins called integrins, which interact with the intracellular actin in the growth cone and in the filopodia, thus navigating outgrowth of the axon. Precise knowledge about how axons grow and how growth is directed is still limited.
Nerve injuries can be divided into two main groups: those that will temporarily block nerve conduction without loss of axonal architecture, and those with severe damage that will result in axonal degeneration distal to the injury ( Algorithm 22.1 ). This may be one useful clinical way of approaching nerve injuries, but more formal classifications have been presented to describe the complexity of the lesions further, in particular the Seddon classification, in which injuries were divided into three groups of increasing severity (neuropraxia, axonotmesis, and neurotmesis). This classification was modified by Sunderland and by Mackinnon to include six degrees of injury. In Table 22.1 we summarize the differences between the three classifications, and the specific neural changes are illustrated in Fig. 22.8 .
| Seddon | Sunderland | MacKinnon | Description |
|---|---|---|---|
| Neuropraxia | 1 | Local physiologic block with paralysis but no anatomic disturbance of the nerve. Full recovery is expected | |
| Axonotmesis | 2 | Nerve injury with degeneration of the distal segment. Intact endoneurium and perineurium. Full recovery occurs at rate of 1.5 mm/day | |
| 3 | Endoneurial damage with subsequent scarring and incomplete regeneration. Variable recovery | ||
| Neurotmesis | 4 | Nerve damaged with complete internal structural disorganization. Nerve trunk remains intact. No functional recovery unless operative intervention | |
| 5 | Nerve trunk cut completely. Early operative intervention necessary for restoration of some function | ||
| 6 | Mixed nerve injury |
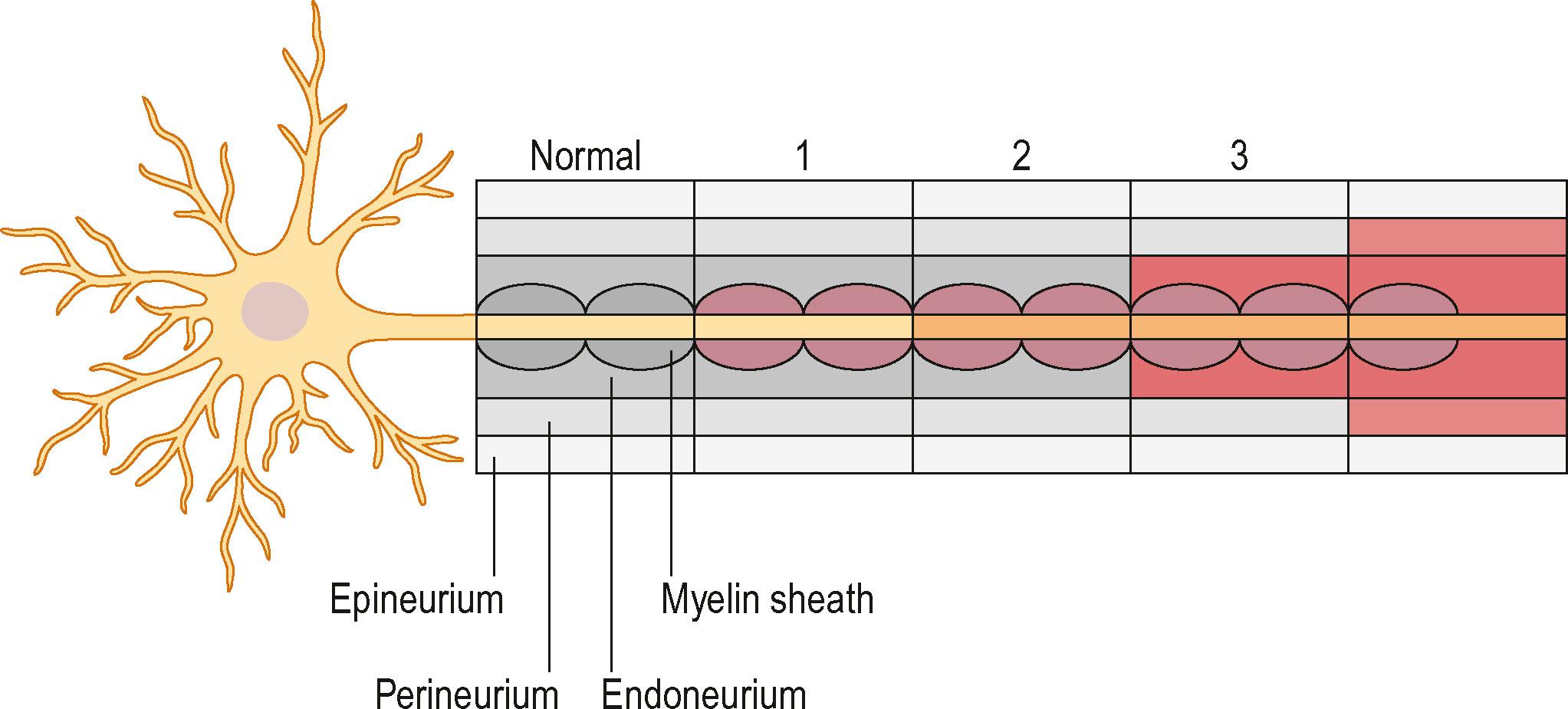
In neuropraxia there is a physiological block of conduction, but no disruption of the neural architecture at the site of injury, and there are no degenerative changes within the nerve. The pathogenesis is most often external compression, surgical traction, or local ischemia. Complete recovery is the general rule, and the healing time can vary from a few days to 3–4 months. Tinel’s sign is not present over the site of injury.
In second-degree injuries, true degeneration of axons is initiated. Axonal damage leads to distal (Wallerian) degeneration, although the basal lamina of the Schwann cells remains intact. The axons regenerate through their own endoneurial tubes by axonal contact and guidance of the regenerating growth cones at a rate of 1–3 mm/day. No topographical mismatch will occur, and the integrity of sensory and motor nerve fibers remains intact. Tinel’s sign is present, and the progress of recovery can by followed by the gradual recovery of motor or sensory function distal to the injury. Full recovery is expected.
In a third-degree injury the architecture within the perineurium is disrupted together with axonal damage. The fascicles remain intact, although progression of axonal regrowth will be with less precision as the axonal guidance is impaired and mismatch of fibers will occur. Axonal mismatch follows to a larger extent in proximally based injuries as the fascicular organization is usually of mixed motor and sensory patterns. The clinical outcome is hard to predict, mainly because of subsequent intrafascicular scarring and the failure of some axons to regenerate. Tinel’s sign is present and progresses distally with recovery. Microsurgical resection and grafting may not always be beneficial in these cases.
In a fourth-degree lesion the epineurium is intact, but all other parts of the nerve are damaged. The formation of an intraneural scar impairs both axonal regeneration and reinnervation, and typically forms a neuroma at the site of injury. A more profound retrograde neuronal effect than in a third-degree injury is expected, and this causes a higher incidence of neuronal death. Tinel’s sign is usually present over the site of the injury, but there are no signs of recovery, or proximal-to-distal progression of regeneration. The injury requires complete excision of the neuroma and microsurgical nerve repair, usually with nerve grafting.
In a fifth-degree injury the nerve is completely transected. Recovery of function is dependent on surgical repair, with or without nerve grafting. These injuries are most commonly associated with injuries that include the surrounding structures, such as tendons, muscles, bones, or blood vessels.
The sixth-degree injury was added by Mackinnon to take account of mixed injuries, for example those that occur after closed traction damage to a nerve, or gunshot or stab wounds that cause partial injuries to a nerve. This type of injury is often referred to as a neuroma-in-continuity, in which all degrees of nerve injury, from normal to neurotmesis, may coexist within the scarred nerve. A neuroma in continuity typically results from failure of the regenerating nerve growth cone to proceed beyond the injury to reach its peripheral targets. It occurs within an intact nerve with continuity in response to internally damaged axons and fascicles, resulting in a distal portion of the nerve that no longer, more or less, functions properly. Surgical exposure is often necessary and intraoperative electrodiagnostic studies can help to distinguish fascicles with signs of recovery from those without (grades IV and V).
A correct diagnosis is crucial. Confirmation of a nerve injury is usually not hard, even though every nerve injury is unique in its character, and highly variable in its symptoms. Lack of active movement, such as against resistance, because of paralysis of the muscles supplied by the nerve, can be seen immediately after the injury if the nerve is completely transected. However, when motor defects are minor or overshadowed by more striking sensory deficits, the diagnosis can sometimes be overlooked, particularly in an incoherent patient. The patient’s history and understanding of the mechanism of the injury are therefore important for the evaluation of the injury and identification of those that will be improved by operation. It is important that every patient with a dysfunctional nerve and a wound overlying the course of a nerve is regarded as having a transected nerve until proved otherwise ( Algorithm 22.2 ).
Motor and sensory function should be carefully examined and documented in all patients with injured extremities. Motor evaluation should include pinch and grip strength, and assessment of weakness and atrophy of individual muscles. Sensory function should be evaluated with the patient unable to see what the examiner is doing. Acutely, it may be easy to assess the patient’s ability to feel pain, such as that applied by forceps, in the innervation area of an individual nerve such as a digital nerve. Assessment of two-point discrimination and the patient’s ability to separate sharp and blunt objects for testing of protective sensation can also be used. Loss of sympathetic function, with vasomotor and sudomotor paralysis, gives the loss of ability to produce sweat in the innervated segment of skin, which will look red, dry, and “polished”. Tinel’s sign is present, and continuous severe pain over the region of injury usually indicates a more severe injury.
In case of confusing diagnostic signs, electrophysiological studies can help to establish the diagnosis and to identify injured nerve branches, although the results must be interpreted with caution during the first 3–4 weeks. It may take up to 4 or even 6 weeks after the injury before clearly detectable signs of nerve degeneration and muscular denervation become apparent. It is stressed that waiting for an electrophysiological examination should not delay exploration of an injured nerve if indication for surgery is strong.
Intraoperative conduction studies can be of help, particularly in the case of a previously closed injury that has failed to show satisfactory recovery at follow-up. Intraoperative examination enables a higher grade of specificity as the observed nerve or even individual nerve fascicles can be anatomically isolated, and action potentials across the scarred area can be evaluated. If electrical recovery is recorded over the area of interest the patient can be classified as Sunderland grade II or III, in which case spontaneous full recovery is expected. If no electrical recovery can be seen, the patient should be graded Sunderland grade IV and V and will most likely benefit from nerve resection and grafting. However, in the case of a neuroma-in-continuity (sixth-degree injury), the picture is more complex. Some fascicles will need resection and grafting, whereas others are actually grade II–III and will recover spontaneously. In these cases, postoperative outcomes may be uncertain and the decision to operate is hard as intervention may result in a tedious and potentially damaging dissection with internal/external neurolysis and resection of damaged sections. However, it may sometimes be possible to distinguish injured fascicles that require nerve grafting from intact healthy fascicles peroperatively. Peroperative electrophysiology is therefore warranted and extremely helpful in decision-making during the procedure.
Peripheral nerve transections rarely occur in isolation. Depending on the extent of the injury, surrounding structures may be crushed, lacerated, avulsed, or otherwise damaged. Extensive tissue damage and an unclear zone of injury together with a contaminated wound are factors that influence management and may result in delayed neural repair. Extensive debridement and cleaning of the wound are essential for a satisfactory final outcome, and to decrease the risk of a complicating infection and subsequent scarring.
Sharply transected nerves are easier to manage than crushed or avulsed ones. In a sharp transection the zone of injury is limited, and the nerve can usually be prepared and repaired at the primary operation. If, however, the nerve is seriously crushed or longitudinally avulsed, the zone of injury is harder to establish and delayed excision of the damaged nerve followed by repair may improve outcome.
Ideally, the repaired nerve should be surrounded by a well-nourished soft-tissue bed, muscle, or fat. In severe cases, this implies that the damaged soft tissues surrounding the injured nerve need to be replaced by a soft-tissue transfer to reconstitute a gliding surface either before secondary reconstruction or in connection with the primary reconstruction.
All patients with a suspected nerve injury should be judged individually as a number of factors must be considered before the decision to explore is made (see Fig. 22.8 ). When one is considering sharp lacerations, traction injuries, blast injuries, or a combination, some key factors must be considered that will be of decisive importance in the timing of operation and the approach, including the type of injury and condition of the wound, and the vascularity of the wound bed.
In cases where there are clinical signs of nerve injury, but there is no wound, the treatment should be conservative for the first 4–6 weeks, when clinical signs of recovery of function can be investigated. If at this point there has still not been a complete spontaneous recovery, electrophysiological examination can be done. This may be repeated if there is only partial, but insufficient, recovery before any operation is considered. In case of a closed injury where no recovery has been seen at all after 3 months, clinical and electrophysiological examination should be considered. If there are no signs of spontaneous recovery during this period, exploration of the injured nerve is indicated. However, if there is partial recovery during the first 3 months, regular re-evaluation may be indicated for up to another 3 months. The important point is to see if any recovery at all is present.
Nerve injuries with open wounds should always be explored. If the nerve is in continuity, it should be treated as a closed injury, and the clinical and electrophysiological function should be evaluated for up to 3 months. In contrast, a clear-cut injury with the nerve ends closely approximated is suitable for immediate repair, whereas a crush injury usually is not. In the latter case, there might be difficulties in distinguishing between viable and non viable tissue, which would call for careful debridement before any nerve repair is tried. In addition, when the patient presents with signs of an incomplete injury for which there is a chance that spontaneous recovery can yield a better outcome than immediate repair, reconstruction is preferred at a later stage only if the clinical results of conservative treatment are not satisfactory. Therefore, one of the most challenging aspects of a nerve injury will be the decision-making regarding timing of nerve exploration and reconstruction in complex cases, such as open crush injuries or gunshot wounds. In these cases, the primary goal should always be to go for careful debridement and viable tissue coverage. Nerve reconstruction can thus only be considered when the surrounding tissue provides a viable environment for the nerve (see Condition of the wound, below). In case of an open injury, where the patient presents with a sensory and/or motor function deficit, but with a macroscopically intact nerve upon surgical exposure, a close follow-up is essential for assessment of the nerve function. Such cases, which include open wound crush injuries and gunshot wounds, should, as the surrounding tissue envelope has healed, be treated the same way as closed traction injuries ( Table 22.2 ). Neurological examination is performed regularly, sometimes together with recurrently performed neurophysiological testing, and surgery is usually performed if no signs of regeneration or reinnervation occur (e.g., for some injuries, exploration after 3 or 4 months). It is, however, important to stress that all open wounds, where the patient presents with a sensory or motor loss, should be explored in the acute setting, in order to obtain the best outcome.
| Type of nerve injury | Neurography/EMG | Exploration | Revision | Repair or reconstruction |
|---|---|---|---|---|
| Sharp transection | – | Immediate | Immediate | Immediate |
| Open wound – nerve crush injury | 4–6 weeks a | Immediate | Immediate | |
| Closed traction injury | 4–6 weeks | Delayed | – | 3–4 months d |
| Open wound –gunshot wound | 4–6 weeks a | Immediate | Immediate |
a If the nerve is in continuity on exploration, neurography/EMG can be performed in the same manner as for closed injuries.
b If there is macroscopic nerve discontinuity on exploration, repair or reconstruction should be performed after repeated revision to ensure that the wound is tidy.
c If there is nerve continuity on exploration, the nerve injury should be treated as a closed injury so that repair or reconstruction is performed only when there is no sign of nerve regeneration (Tinel’s sign), e.g., for some injuries, exploration after 3–4 months.
d Repair or reconstruction is performed after 3–4 months if there are no clinical signs of nerve regeneration or reinnervation.
The condition of the wound is important. A clean wound calls for an early repair, whereas an untidy wound needs careful debridement to remove nonviable tissue before any nerve reconstruction is undertaken. In severe cases, there may be a need for multiple debridements. In these cases, the nerve repair is better planned as a secondary reconstruction that can be done when other structures have healed with better chances of satisfactory soft-tissue coverage of the reconstructed nerve. Wound coverage is preferable within 3 weeks. At the time of re-exploration, the zone of injury is likely to be better defined, and scar tissue can more easily be extracted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here