Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neural function is essential to human existence. Thus, loss of any neural element in the course of a critical illness represents a major loss to a given individual. Neurons or supporting elements may be lost in a small, virtually unnoticeable manner, perhaps manifest as cognitive or behavioral deficit, or there may be widespread selective neuronal loss or tissue infarction with more apparent and disabling deficits. Based on the notion that neural function is the essence of acceptable survival from critical illness, it is crucial for perioperative management to include considerations of neural viability and the impact and interactions of the primary diseases and therapeutics on the nervous system.
There are numerous perioperative scenarios where a patient may present with neurologic dysfunction. In a general sense, these scenarios often involve ischemia, trauma, or neuroexcitation. Each of these, as they progressively worsen, at some point typically involve a period of decreased cerebral perfusion pressure (CPP), usually resulting from elevated intracranial pressure, eventually compromising cerebral blood flow sufficiently to produce permanent neuronal loss, infarction, and possibly brain death. A variety of biochemical pathways play a major role. In this chapter we review the important physiologic factors, and intracranial pressure (ICP) considerations and therapeutic options critical to contemporary perioperative care of the patient with injury to the central nervous system (CNS).
The neurologic examination of the patient with an acute neurologic condition is a process that requires the physician to have a specialized anatomic and physiologic knowledge of the nervous system and the anesthesiologist should be familiar with the most basic components of the neurologic examination to identify promptly life-threatening conditions that may require urgent action. The majority of the information necessary to localize a lesion in the patient with neurologic disease can be obtained through a careful history and physical examination. Although a detailed description of neurologic examination is beyond the scope of this chapter, a rapid 5-min neurologic assessment may be sufficient to establish baseline conditions and to detect subsequent changes in neurologic status. Therefore, for the nonneurologist it is important to focus on the components of the neurologic examination described in the following sections.
Certain findings of the mental status examination can only be interpreted by knowing the patient’s ability to perform fundamental daily living tasks. Mental status should be reported starting with the level of alertness, followed by language function and memory. The rest of the mental status examination can be reported in any order and includes attention and concentration, memory, visual spatial recognition, praxis, calculations, etc.
Mental status is assessed by orientation to person, place, and time. General mental status is determined by assessing general information (i.e., the name of the president, obtaining a description of some current events, assessing spelling ability for simple words, and asking the patient to add or subtract serial threes or sevens). In addition, the patient can be asked to recall three objects several minutes after the examiner mentions them. Attention is tested by asking the patient to recall a series of numbers or to spell “world” backward.
Cranial nerves can be assessed as follows:
CN I (olfactory): Test each nose nare with a mild agent such as soap or tobacco.
CN II (optic): Near and far visual acuity and gross visual fields are assessed. Ophthalmoscopic examination is performed.
CN III, IV, VI (oculomotor, trochlear, abducens): Pupillary light response. Lateral and vertical gaze are assessed.
CN V (trigeminal): Assessment of touch sensation in both sides of the face is performed in all three trigeminal divisions. In addition, corneal blink reflex can also be assessed.
CN VII (facial): Symmetrical smile assessment is performed. In addition, brow wrinkling can be assessed to determine central versus peripheral seventh nerve function.
CN VIII (auditory): Ability to hear fingertips moving is assessed. Lateralizing the sound of a tuning fork placed over the mid-forehead can localize a hearing deficit.
CN IX, X (glossopharyngeal, vagus): Gag reflex is assessed with a tongue blade or tonsil tip suction device.
CN XI (accessory): Bilateral shoulder elevation (trapezius) and head-turning (sternocleidomastoid) strength is assessed.
CN XII (hypoglossal): The patient is asked to extrude his/her tongue. If there is weakness on one side of the tongue, the tongue will deviate to that side.
Motor examination is performed by assessing pronator drift of the upper extremity. A unilateral pronator drift in one arm suggests an upper motor neuron lesion affecting that arm. Hand grasps, toe and foot dorsiflexion, and the major flexors and extensors of the upper and lower extremities are assessed. In addition, individual muscles can also be assessed and graded ( Table 24.1 ).
| Muscle strength | Reflexes |
|---|---|
| 0 = no contraction | 0 = absent |
| 1 = visible muscle twitch but no movement of the joint | 1 = reduced (hypoactive) |
| 2 = weak contraction insufficient to overcome gravity | 2 = normal |
| 3 = weak contraction able to overcome gravity but no additional resistance | 3 = increased (hyperactive) |
| 4 = weak contraction able to overcome some resistance but no full resistance | 4 = clonus |
| 5 = normal |
Sensory examination involves testing the primary afferent sensory pathways with modalities such as light touch, pain and temperature, vibration and joint position, and then areas that test the ability of sensory and association cortices to interpret sensory input. These areas test sensory functions such as stereognosis, graphesthesia, point localization, etc. A brief sensory examination can be performed by stimulation with a pin on hands and feet to determine the patient's ability to distinguish between sharp or dull sensation. The examination should be done in a repetitive manner because patients can report both stimuli as sharp bilaterally and then some 10 seconds later report to only one side as sharp, indicating a subtle deficit on the dull side. A similar maneuver is performed on the cheeks for cranial nerve V and the dorsum of the feet. It is important to note that this examination technique has been associated with the spread of infectious diseases and thus, a new pin or other sharp device should be used for each assessment with every effort made not to cause bleeding. Light touch sensation is tested in a similar manner using the touch of a finger in the place of a pin.
Coordination is assessed by finger-to-nose and heel-to-shin testing and assessment of rapid alternating movements of the hand and or foot. This aspect of the examination tests cerebellar and basal ganglia function because these structures play an important role in coordination. Deep tendon reflexes are assessed in the biceps, triceps, patella, and Achilles tendon (see Table 24.1 ). The Babinski reflexes are assessed by stroking the lateral foot. In addition to the test for meningeal irritation, the Kernig (elevation of straightened lower extremity) and Brudzinski (head flexion by examiner) tests are performed.
The neurologic examination in a comatose patient represents a different challenge. Procedures performed in evaluation of the comatose patient include determination of viable vital signs and assessment of hand drop over the head when nonphysiologic coma is suspected. Pupil size and response to light is assessed. Pinpoint pupils imply a pontine lesion or drug effect whereas large pupils can suggest a structural lesion, hypoxia, or a drug effect. Uncal herniation will produce a unilateral pupillary enlargement with ptosis and inferior–lateral position resulting from third cranial nerve lesion. Eye movements are assessed by first evaluating eye position. In destructive cerebral lesions, the eyes deviate toward the side of the lesion. Assessment of the oculocephalic (doll's eye) reflex is performed by turning the head suddenly to one side. Eyes tend to lag behind when the patient has lethargy or is semi-comatose, but they move with the head in the awake state and when brainstem centers are impaired. Further indication of brainstem impairment is present when the corneal and gag responses are absent and when there is extensor or flexor posturing. Response to noxious stimulus is assessed peripherally by stimulating the sternum or nail beds or centrally by supraorbital pressure. The Babinski reflex is assessed.
Hourly evaluation of neurologic and mental status is recommended as part of the neuromonitoring protocol. Function of pyramidal and extrapyramidal systems, cranial nerves, cerebellum, and spinal cord whenever possible, and any trend in change of neurologic status should be recorded for every patient. In critically ill patients, however, such a complete neurologic evaluation can sometimes be unreliable or impossible owing to the use of sedatives and the need for intubation and ventilatory support as part of the medical treatment of the neurologic problem. Along with the neurologic examination, information about vital sign trends and key laboratory values should be available at all times. The Glasgow Coma Scale (GCS) ( Table 24.2 ) is used as a standardized scale for recording neurologic status in the ICU. GCS has been the standard outcome tool for neurocritical care for many years. Newer assessment tools such as the Neurological Outcome Scale for TBI (NOS-TBI) have demonstrated adequate concurrent and predictive validity as well as sensitivity to change, compared with the gold-standard outcome measure. The NOS-TBI may enhance prediction of outcome in clinical practice and measurement of outcome in TBI research.
| Type of response | Score |
|---|---|
| Motor response | |
|
6 |
|
5 |
|
4 |
|
3 |
|
2 |
|
1 |
| Verbal response | |
|
5 |
|
4 |
|
3 |
|
2 |
|
1 |
| Eye opening | |
|
4 |
|
3 |
|
2 |
|
1 |
Pupillary assessment is vital to determine critical neurologic conditions that may require urgent intervention. However, observer variability exists and it is subject to human error. A handheld pupilometer is a new technology that may reduce observer variability in the neurologic examination. Infrared quantitative pupillometry can produce accurate, reproducible pupillary measurements that are clearly superior to those obtained manually at the patient’s bedside by even an experienced nurse or physician. A recent study using this device reported good reliability when correlating the pupillary constriction velocity as a predictor of ICP elevation in neurosurgical patients. Neuro-ICUs are quickly adopting this type of monitoring, because evidence has shown that there is a high inter-device reliability of automated pupillometers. An important limitation of this device is that assessment is quite challenging in patients with altered mental status, in patients with periorbital or scleral edema, and in uncooperative patients. Ambient light and physiologic factors may also affect the measured pupillary characteristics.
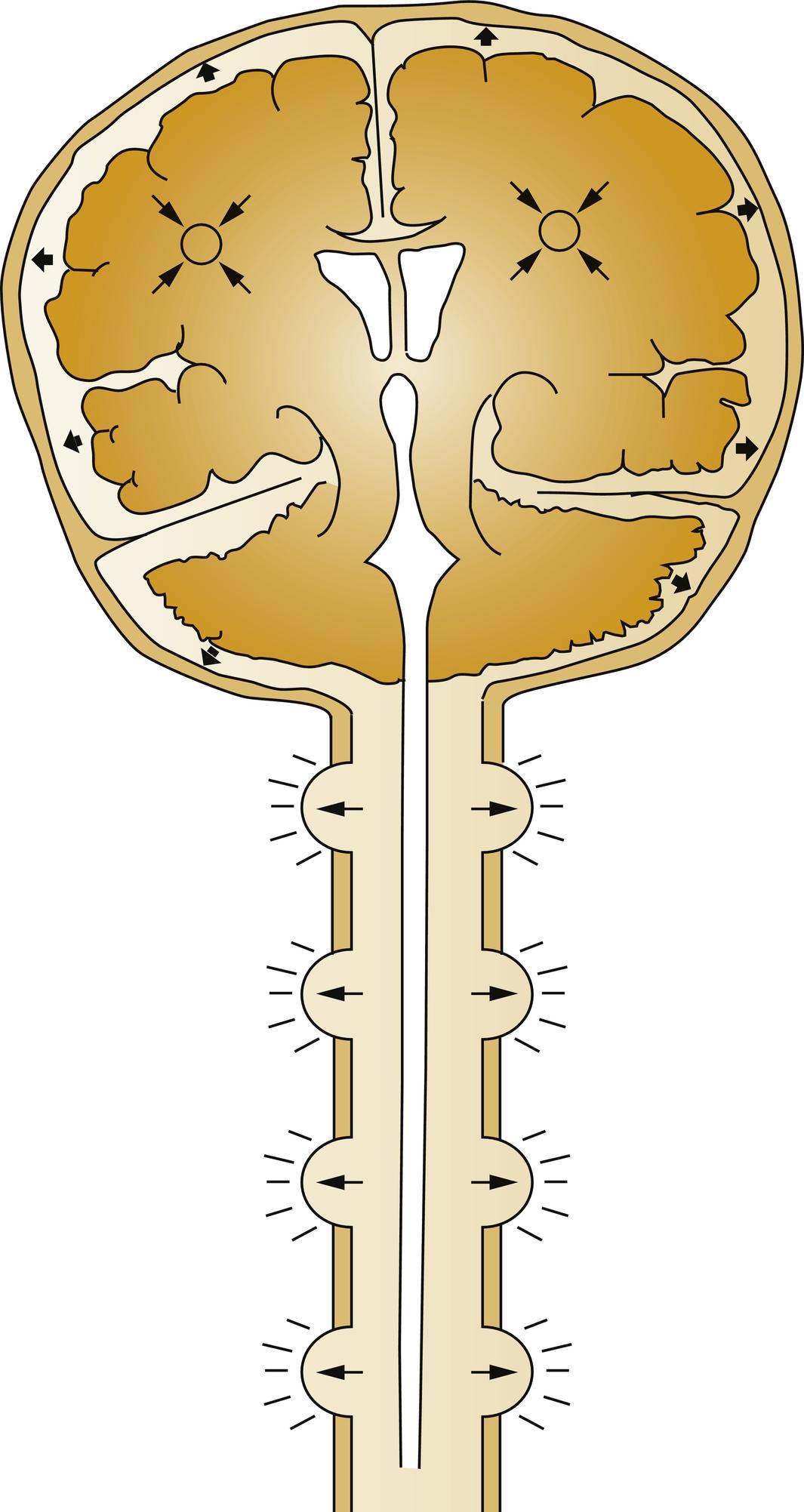
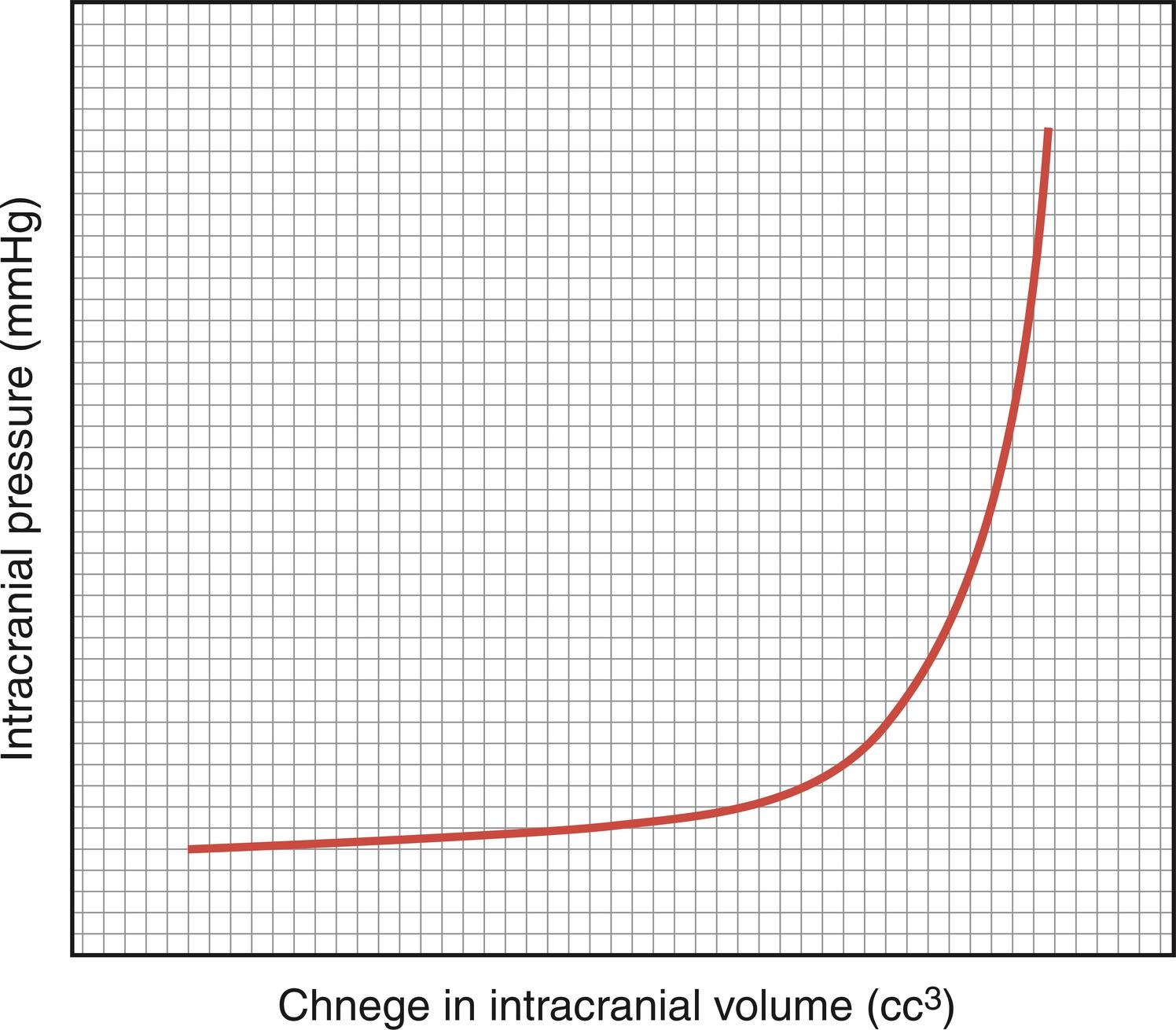
The brain, spinal cord, cerebrospinal fluid (CSF), and blood are encased in the skull and vertebral canal, thus constituting a nearly incompressible system ( Fig. 24.1 ). In a totally incompressible system pressure would vary linearly with increased volume. However, there is capacitance in the system, thought to be provided by the intervertebral spaces and the vasculature. Once this capacitance is exhausted, the ICP increases dramatically with increased intracranial volume ( Fig. 24.2 ).
Based on the relation:
where CBF is cerebral blood flow, MAP is mean arterial pressure, and CVR is cerebrovascular resistance.
The concern arises that increasing ICP is necessarily associated with decrements in CBF. However, the effect of increasing ICP on CBF is not straightforward as MAP may increase with ICP elevations, and CVR adjusts with decreasing CPP (increasing cerebral blood volume) to maintain CBF until maximal vasodilatation occurs. This is thought to occur at CPP < 50 mmHg although considerable interindividual heterogeneity in this value exists and the lower limit has been nicely critiqued by Drummond. Thus, increasing ICP is often associated with cerebral vasodilatation and/or reflexly increased MAP to maintain CBF.
Normal ICP is < 10 mmHg. ICP > 20 mmHg is generally associated with escalation of ICP-reducing therapy. However, this is an epidemiologically derived number. Head trauma studies have indicated that patients with ICP > 20 mmHg generally do not do well. However, physiologically, simply elevating ICP to > 20 mmHg is not necessarily associated with decrements in CBF, provided the above-noted compensatory mechanisms occur. Recent guidelines recommend treatment of intracranial pressure when it is > 22 mmHg because values above this level are associated with increased mortality.
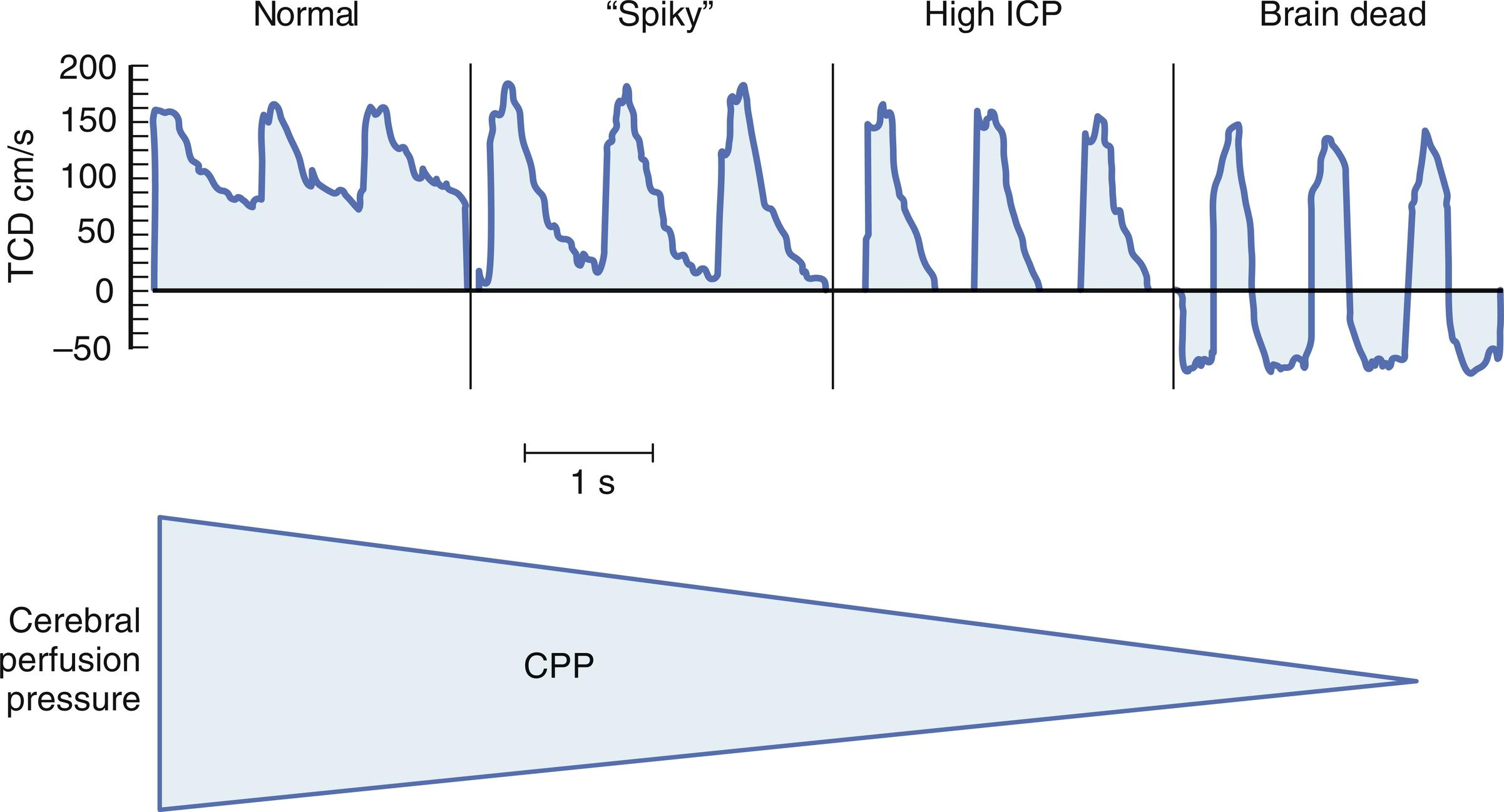
Nonetheless, increasing ICP because of mass lesions or obstruction of CSF outflow can exhaust compensatory mechanisms. When this occurs, compromise of CBF does eventually occur. Initially, abnormality arises in distal runoff of the cerebral circulation. As the process continues, compromise of diastolic perfusion arises. With this the normally continuous (through systole and diastole) cerebral perfusion becomes discontinuous ( Fig. 24.3 ). Further compromise of cerebral perfusion pressure results in anaerobic metabolism, exacerbation of edema, and ultimately intracranial circulatory arrest. Thus, when ICP increases it is important to detect it and ascertain whether this lethal sequence of events may be occurring.
Under normal conditions, the intracranial volume is occupied by the brain parenchyma (80%), CSF (10%) and blood (10%). Pathologic structures, including masses caused by blood (hematoma), neoplasia, or abscesses, may also occupy space. Since the volume of the intracranial vault is somehow fixed, when the volume of one or more of these compartments enlarges sufficiently to exhaust normal capacitive compensatory mechanisms, ICP begins to rise. Further increases in volume of one or more of these compartments then leads to the sequence of events associated with increasing ICP described previously.
In a general sense, there are two types of intracranial hypertension, categorized according to CBF as hyperemic or oligemic ( Fig. 24.4 ). Although conceptualized here as a dichotomous process, undoubtedly the real physiology is more of a continuum between the two.
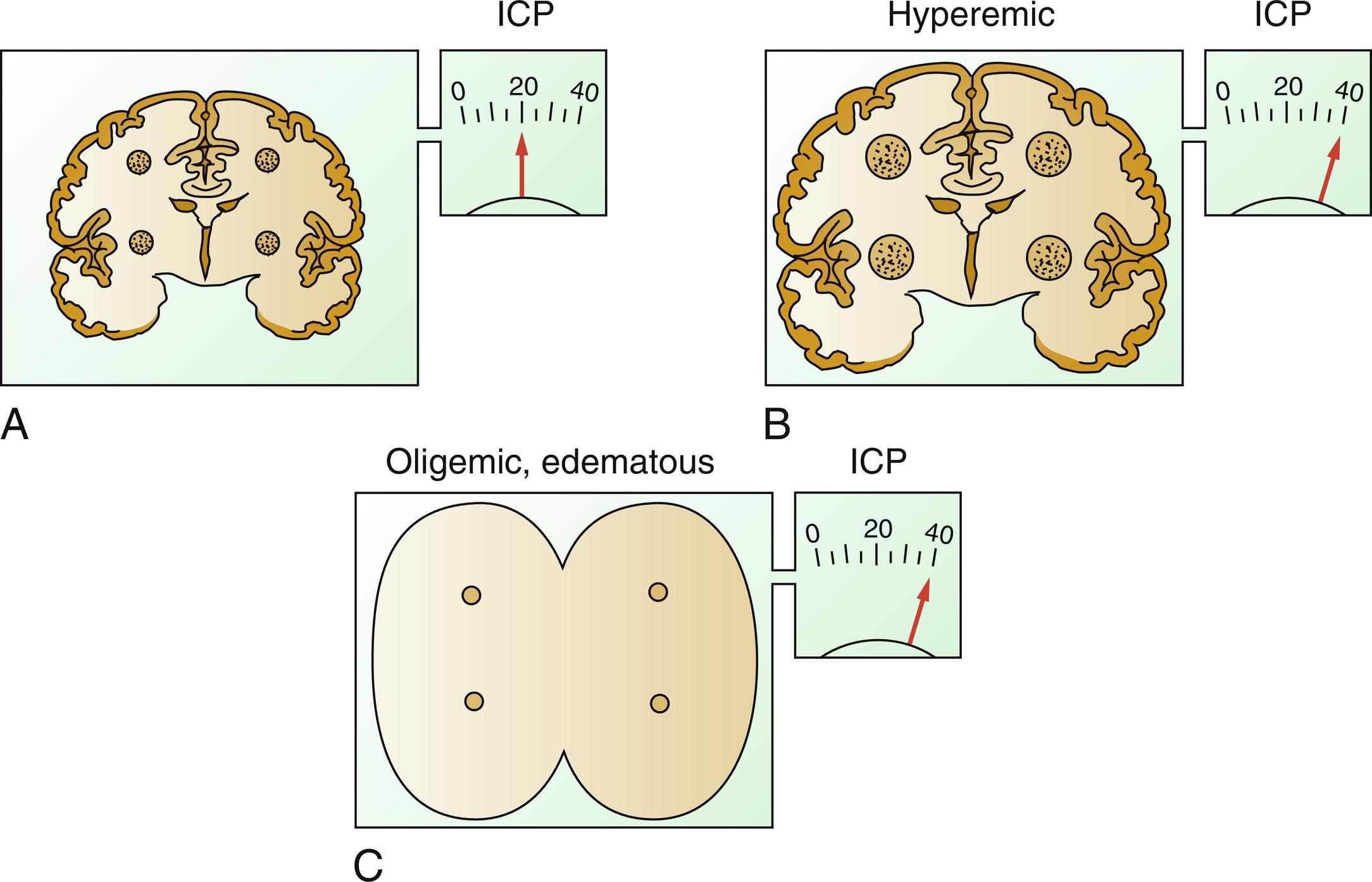
In the normal state, increases in CBF are not associated with increased ICP, as the normal capacitive mechanisms absorb the increased intracranial blood volume. However, in the situation of disordered intracranial compliance, small increases in intracranial volume resulting from increased CBF produce increases in ICP. When cerebral blood volume (CBV) increases, intracranial contents increase hence increasing ICP in a noncompliant system.
This, however, raises questions about an important issue. Elevated ICP has traditionally been considered to be a concern because it indicates that cerebral perfusion might be jeopardized. It is, however, unclear whether it is appropriate to be concerned about high ICP inducing intracranial oligemia when the cause of the high ICP is intracranial hyperemia. There have been no detailed examinations of this question although there have been some studies that allow reasonable inferences about the significance of hyperemic intracranial hypertension.
For many years, it has been known that brief noxious stimuli briefly increase ICP in the setting of decreased intracranial compliance. Studies have reported that such situations are associated with hyperemia, strongly suggesting that hyperemic intracranial hypertension is not a dangerous situation. However, it is reasonable to be concerned, at least theoretically, about such hyperemia for three reasons. First, elevated ICP caused by hyperemia in one portion of the brain may increase ICP to compromise CBF in other areas of the brain in which rCBF is marginal. Second, increased pressure in one area of the brain may produce gradients that might lead to a herniation syndrome. Third, there is theoretical concern that inappropriate hyperemia may predispose the brain to worsened edema or hemorrhage as occurs with other hyperperfusion syndromes. Thus, hyperemic intracranial hypertension has a theoretical potential to be deleterious but this has yet to be conclusively demonstrated. For brief periods, as may occur during intubation or other limited noxious stimuli, it is suggested that it might not be problematic.
In contrast, oligemic intracranial hypertension is associated with compromised cerebral perfusion. This is supported by the high mortality observed in head trauma patients in whom ICP rises as a result of brain edema after head injury with decrements in CBF. Transcranial Doppler (TCD) and CBF studies on these patients have demonstrated that CBF is low and perfusion is discontinuous during the cardiac cycle (see Fig. 24.3 ). Moreover, jugular venous bulb data indicates that O 2 extraction is markedly increased, suggesting anaerobic metabolism. In this setting, noxious stimuli can further increase the ICP, thus producing the situation of hyperemic or oligemic intracranial hypertension. Presumably in this setting the hyperemic rise in ICP acts to compromise rCBF further in areas of brain edema.
Although frequently linked, intracranial hypertension is not a synonym of brain herniation. These events can occur both independently or in association. Intracranial hypertension is currently defined as a sustained elevation for more than 5 minutes of ICP > 22 mmHg, and its accurate identification requires placement of an invasive intracranial monitoring. However, intracranial monitors are usually absent during the acute phase of the traumatized brain management, therefore, anesthesiologists should be familiar with clinical signs and symptoms suggestive of elevated ICP. These signs and symptoms include headache, nausea, or vomiting, altered mental status, and the Cushing triad (bradycardia, hypertension, and irregular respirations or apnea).
On the other hand, herniation syndromes are the result of differences in pressure gradients across the intracranial compartments ultimately leading to the shifting or compression of vital neural and vascular structures. Common sites of brain herniation are the medial temporal lobe (uncal), cingulum (subfalcine), and inferior cerebellum (tonsillar herniation). The classic signs of uncal herniation include loss of consciousness associated with ipsilateral pupillary dilatation and contralateral hemiparesis resultant from compression of the ascending arousal pathways, oculomotor nerve (CN III), and corticospinal tract ( Table 24.3 ).
| Syndrome | Clinical signs and symptoms | Pathophysiology |
|---|---|---|
| Uncal |
|
|
| Central, transtentorial |
|
|
| Tonsillar |
|
|
| Subfalcine |
|
|
| Transtentorial ascending |
|
|
The purpose of monitoring ICP is to improve reliably the clinician’s ability to maintain adequate CPP and cerebral oxygenation. The only way to determine CPP reliably is to monitor both ICP and MAP continuously. This combined approach has been associated with the potential to improve patients’ outcomes after closed head injury.
Despite its widespread application, supporting evidence from randomized clinical trials (RCTs) is lacking or insufficient and indications outside TBI management are somewhat ill-defined. In 2012, Chestnut et al. conducted a multicenter, controlled trial in 324 patients who suffered from severe TBI. Patients were randomly assigned to one of two specific protocols: guidelines-based management of ICP in which a parenchymal monitor was used or a treatment protocol based on clinical and imaging findings. The primary outcome was survival time, impaired consciousness, and functional status at 3 and 6 months after initial injury. Intracranial ICP monitoring failed to show superiority to care based on imaging and clinical findings. A large body of level II evidence exist with regard to ICP monitoring in TBI. Management of severe TBI patients using information from ICP monitoring is recommended to reduce in-hospital and 2-week postinjury mortality. Thus, the current recommendations are to monitor ICP in all salvageable patients with a TBI (GCS 3–8 after resuscitation) and an abnormal CT scan that reveals hematomas, contusions, swelling, herniation, or compressed basal cisterns. Also, ICP monitoring is indicated in patients with severe TBI with a normal CT scan if more than two of the following features are noted at admission: age > 40 years, unilateral or bilateral motor posturing, or SBP < 90 mmHg.
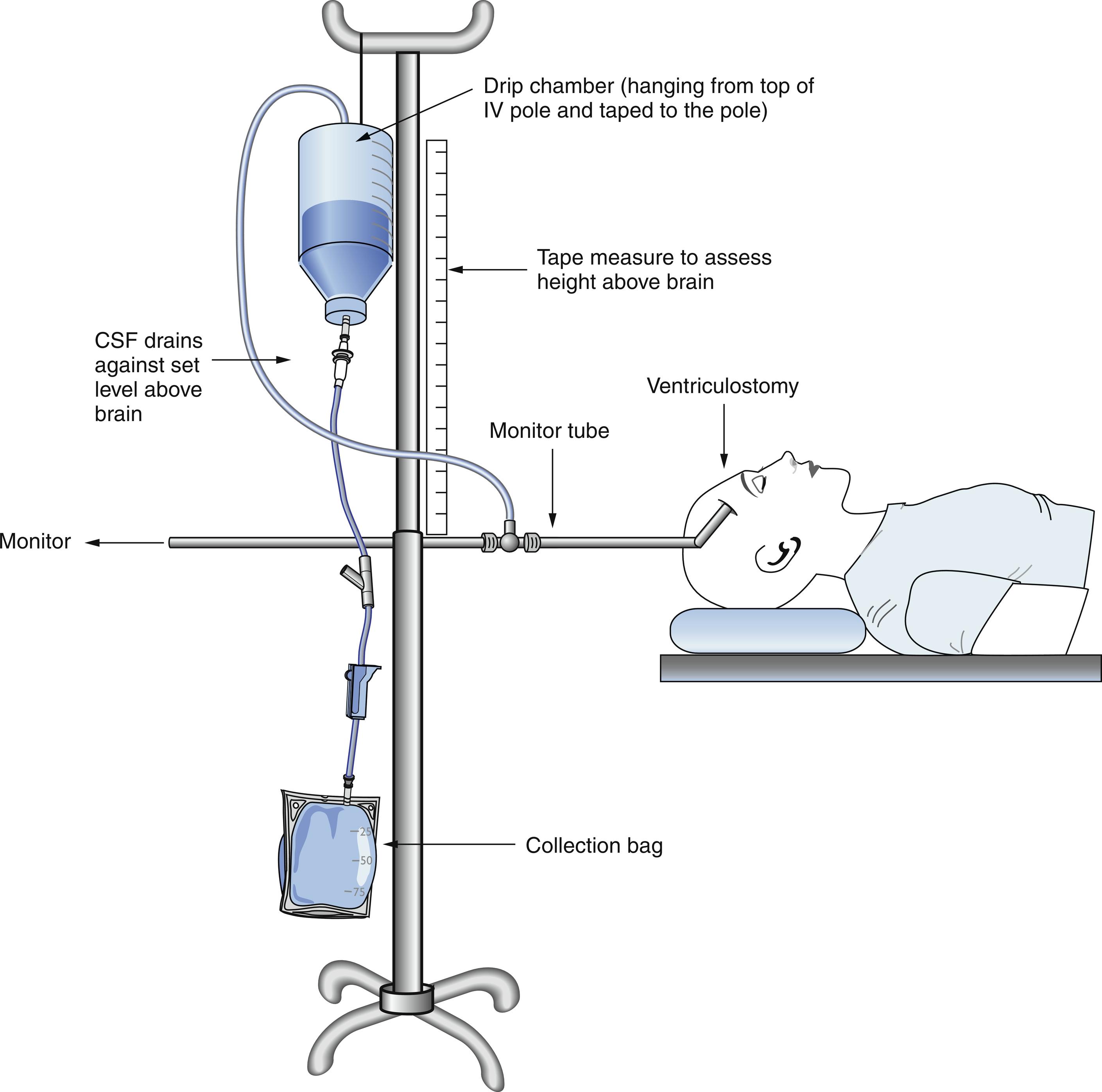
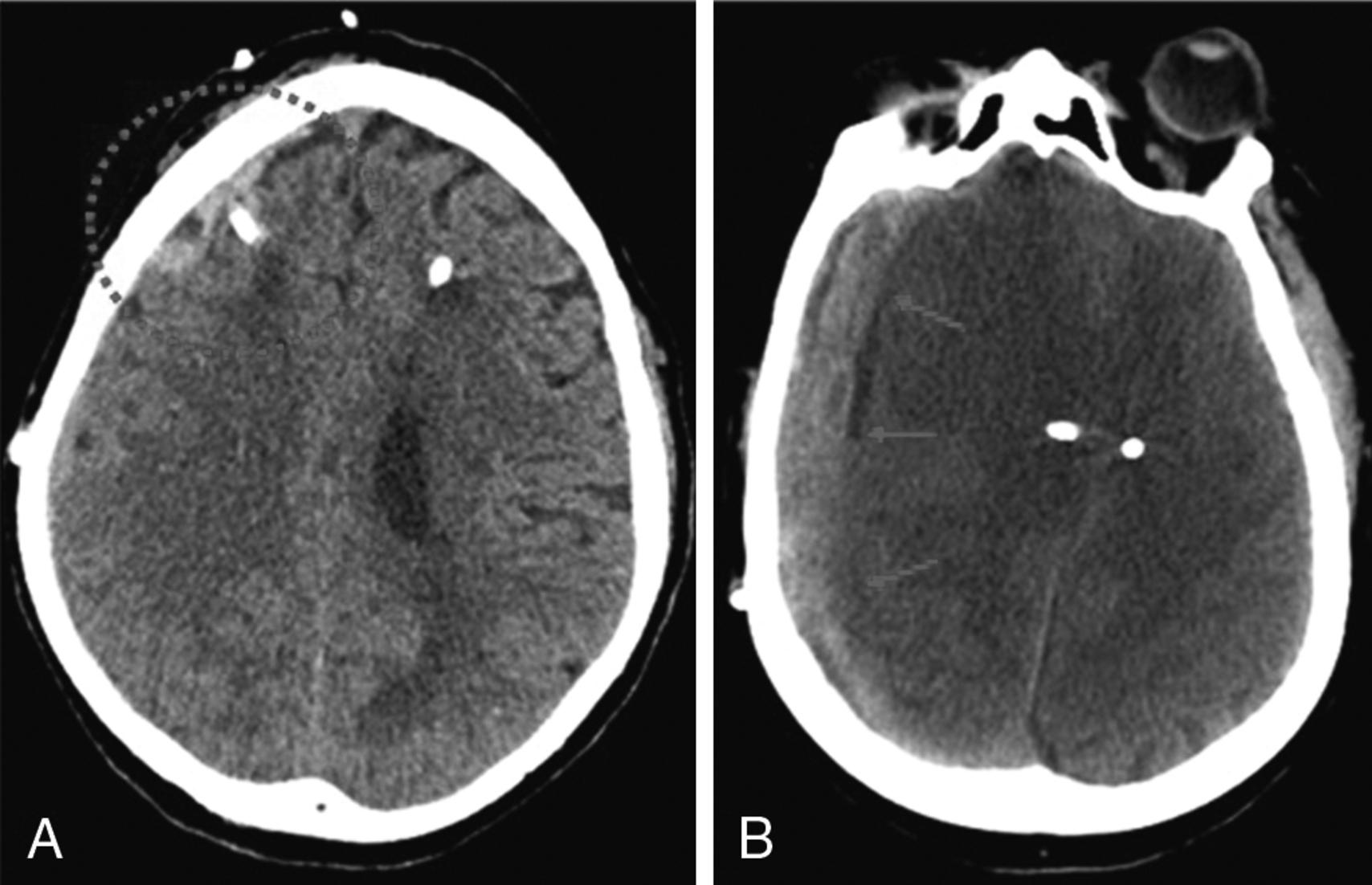
The current gold standard for ICP monitoring is the ventriculostomy catheter or external ventricular drain (EVD), where a catheter is inserted in either lateral ventricle, usually via a small right frontal burr hole. This ventricular catheter is connected to a standard pressure transducer via fluid-filled tubing ( Fig. 24.5 ). The external transducer must be maintained at a specific level to ensure adequate CPP. The reference point for ICP is the foramen of Monro, although in practical terms, the external auditory meatus is often used as a landmark ( Fig. 24.6 ). EVDs allow the clinician to measure global ICP and have some useful advantages over other ICP monitors, including the ability to perform periodic in vivo external calibration and therapeutic CSF drainage and sampling. The primary disadvantage of intraventricular catheters is the associated increased rate of infection and accidental overdrainage. This infectious risk increases the longer the EVD is in place, and prophylactic catheter changes do not appear to reduce the risk of infection. Another potential disadvantage of intraventricular systems includes a small risk of hemorrhage during placement; this risk is greater in patients taking anticoagulants or antiplatelets and misplacement ( Fig. 24.7 ). In addition, when intracranial mass lesions or ventricular effacement caused by swelling are present, EVD placement may be difficult even for the most experienced neurosurgeon.
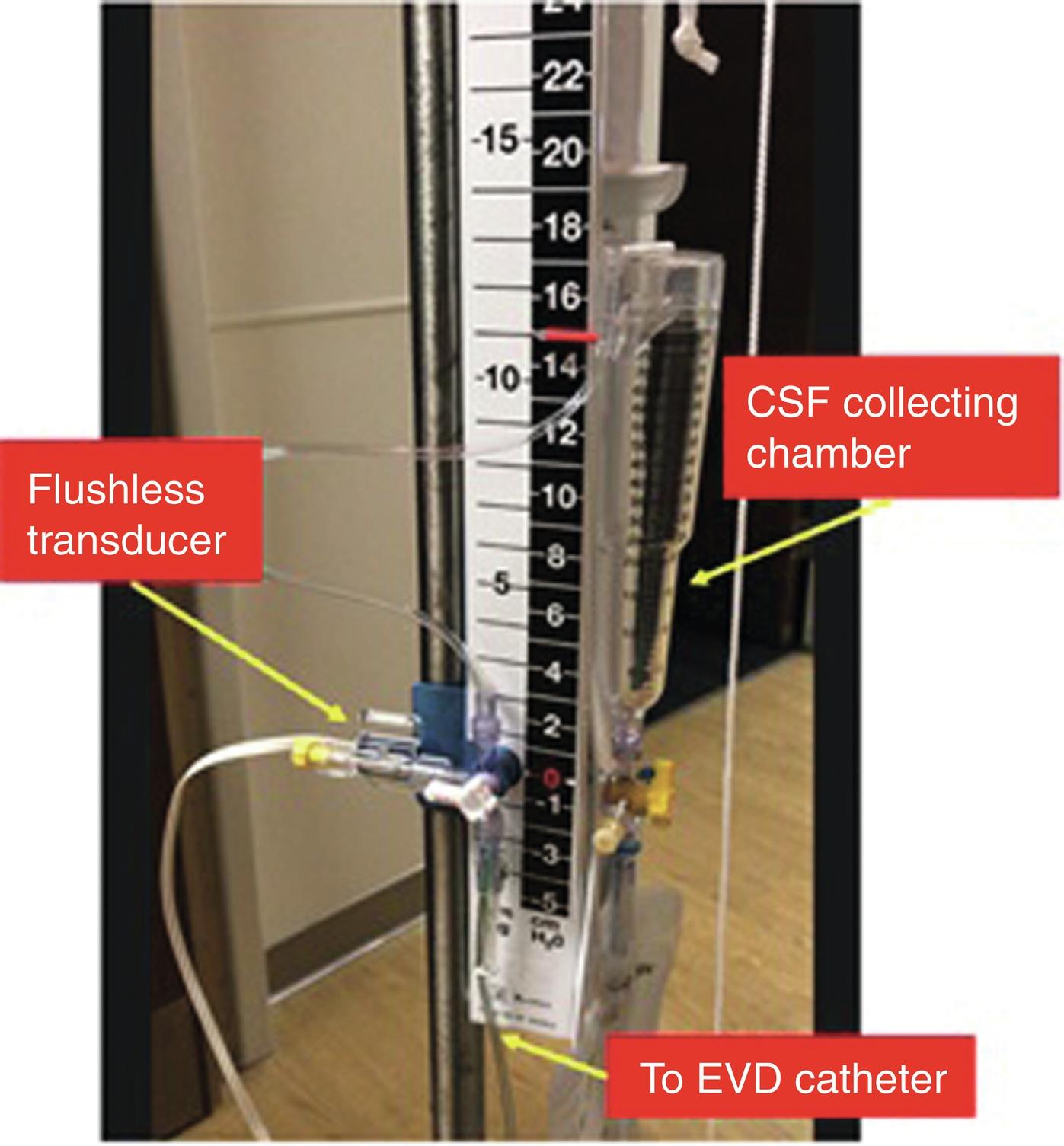
In addition to intraventricular catheters, there is intraparenchymal, subarachnoid, and epidural ICP monitoring catheters. Intraparenchymal catheters consist of a thin wire with a fiberoptic transducer at the tip (fiberoptic Camino system, Natus Medical Inc., Middleton, WI), inserted into the brain parenchyma via a small skull burr hole. They have a lower risk of infection and hemorrhage than EVDs. Disadvantages include the inability to drain CSF for therapeutic or diagnostic purposes and the potential to lose accuracy or development of “zero drift” (degree of difference relative to zero atmosphere) over the course of days.
Noninvasive ICP monitoring devices have been developed to reduce the complications associated with invasive devices. Tympanic membrane displacement, TCD pulsatility index, intraocular pressure, optic nerve sheath diameter, and magnetic resonance imaging (MRI) of the optic nerve sheath have been used to provide a noninvasive estimate of ICP. So far, none of these methods has provided accuracy sufficient to replace invasive ICP monitors.
Normal ICP waveform consists of three arterial components superimposed on the respiratory rhythm. The first arterial wave is the percussion wave (P1), which reflects the ejection of blood from the heart transmitted through the choroid plexus in the ventricles. The second wave is the tidal wave (P2), which reflects brain compliance; and finally, the third wave is the dicrotic wave (P3), which reflects aortic valve closure. Under physiologic conditions, the percussion wave is the tallest, with the tidal and dicrotic waves having progressively smaller amplitudes. When intracranial hypertension is present, cerebral compliance is diminished. This is reflected by an increase in the peak of the tidal and dicrotic waves exceeding that of the percussion wave ( Fig. 24.8 ).
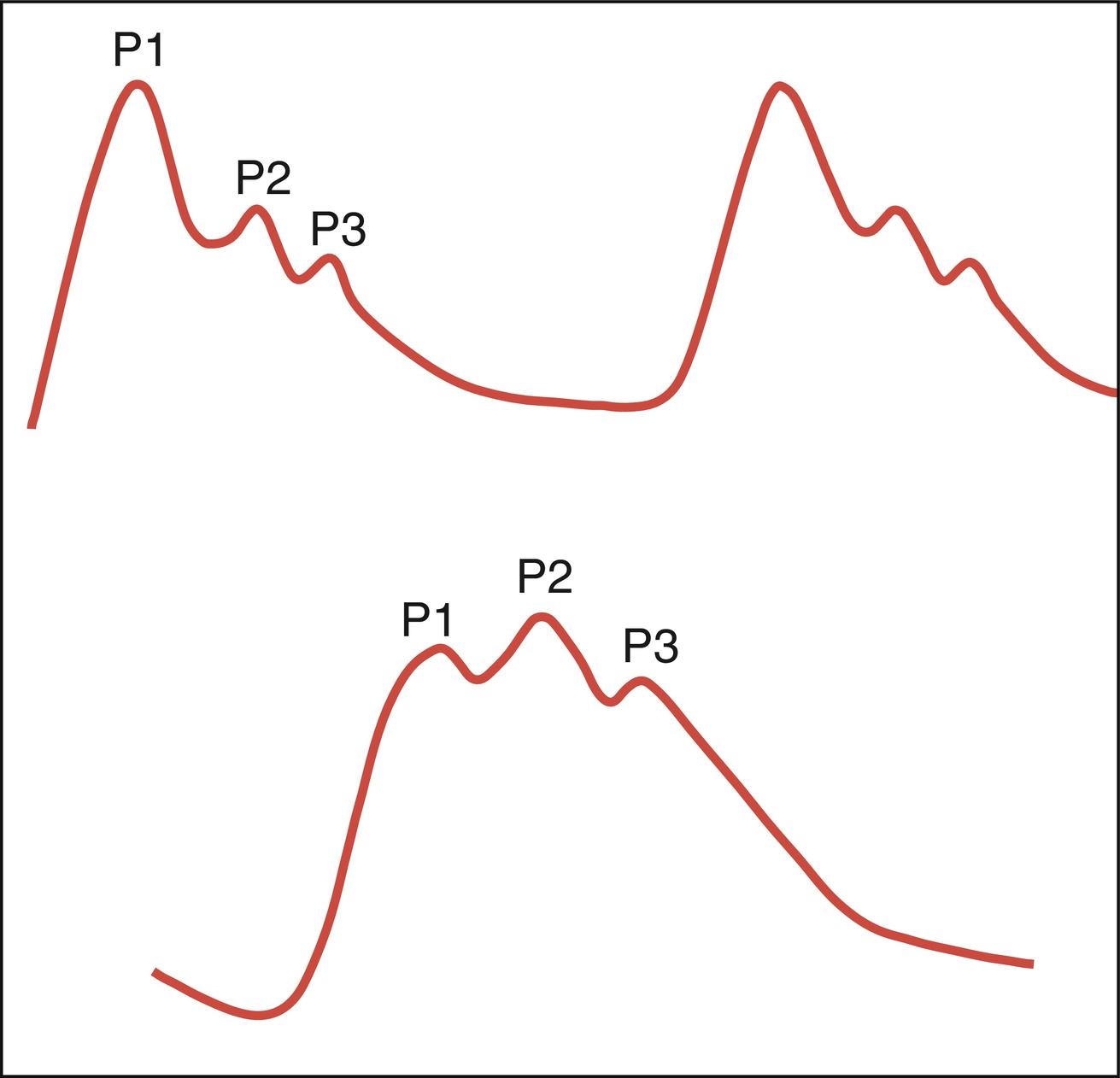
Pathologic waveforms include A, B, and C described by Lundberg in the 1960s. Pathologic A or plateau waves are abrupt, marked elevations in ICP of 50 to 100 mmHg, which usually last for 5–20 minutes and represent the loss of intracranial compliance and autoregulatory mechanisms. Moreover, they can lead to development of a positive feedback cycle that eventually leads to continuous reduction in CPP with subsequent cerebral ischemia. Thus, the presence of A waves should suggest the need for urgent intervention to help control ICP. Type B waves occur at a frequency of 0.5 to 2 waves/min with elevations of ICP of 20–30 mmHg, reflecting change in vascular tone that occurs when the CPP is at the lower limit of normal autoregulation. C waves occur at a frequency of 4–8/min; they reflect changes in systemic vasomotor tone and have little pathologic significance.
The main goal in treating intracranial hypertension is to prevent the development of secondary injuries by maintaining adequate CPP, oxygenation, and glucose supply (without hyperglycemia) to promote adequate oxygen and nutrient supplies. The best clinical strategy is to diagnose and to treat the underlying cause(s), avoid exacerbating factors, and reduce ICP ( Table 24.4 ). Current management of acute brain injury is aimed to maintain ICP < 22 mmHg, CPP between 60 and 70 mmHg, and SBP at ≥ 100 mmHg for patients 50 to 69 years old or at ≥ 110 mmHg for patients 15 to 49 or > 70 years. CPP augmentation to higher perfusing pressures may elevate the risk of systemic complications, including the development of acute respiratory distress syndrome (ARDS). More recent evidence has failed to show such association. This can be explained in part to the development of better lung-protective strategies to treat ARDS. In addition, as the Lund group has suggested in the past, hypertension-induced exacerbation of brain edema can further increase ICP. This increase in ICP leads to reduced venous outflow and increased venous pressure, which in turn act to worsen the brain edema, constituting a positive feedback cycle initially started by arterial hypertension.
| Focal lesions |
|
|
|
|
| Extra-axial fluid collections |
|
|
|
|
|
| Diffuse lesions |
|
|
|
|
|
Therapy of intracranial hypertension is primarily directed at removing the cause as far as possible. This is not always possible. In such cases, therapy is aimed at controlling ICP, hoping that the primary cause of the intracranial hypertension will resolve. Controlling ICP is thus a supportive maneuver, intended to preserve viable neuronal tissue until the high ICP situation resolves. In general, therapeutic maneuvers to reduce ICP involve one of six classes of therapy: (1) decrease cerebral blood volume, (2) decrease CSF volume, (3) induce serum hyperosmolarity, (4) resect dead or injured brain tissue or resect viable but less important brain tissue (e.g., anterior temporal lobe), (5) resect non-neural masses or hematomas, and (6) remove the calvarium to permit unopposed outward brain swelling (decompressive craniectomy). For purposes of this chapter, we will categorize interventions based on current tiered therapy algorithms ( Fig. 24.9 ). Recent advances in the use of brain tissue oxygen monitors occasionally affect the manner in which these maneuvers are used to ensure continued optimal PbrO 2 . However, their use is somewhat limited in the operating room given the invasive nature and obstruction of the surgical field.
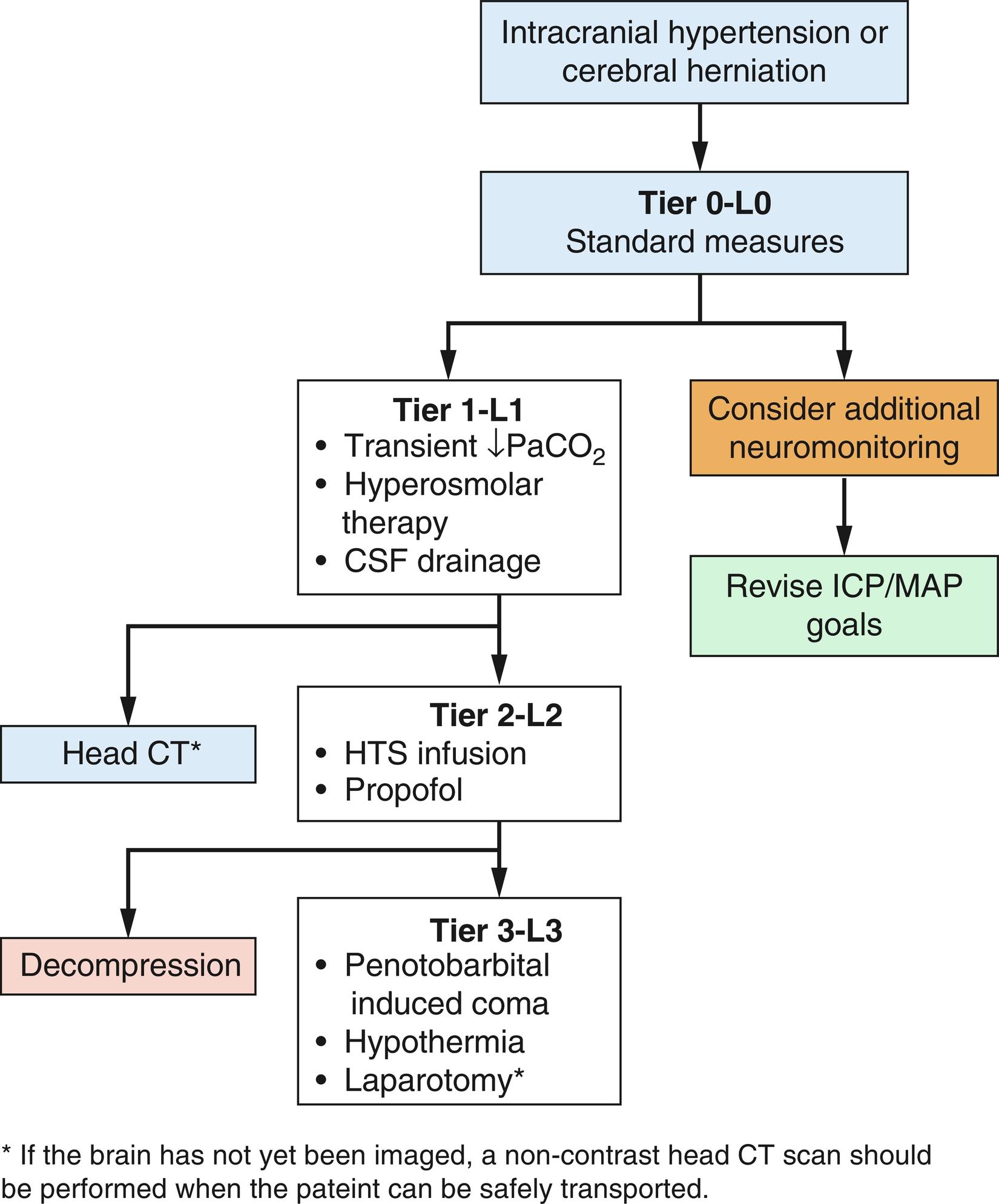
ABCs
Head of bed elevation
Steroids
Antipyretics and antishivering drugs
CT scan
The urgent assessment of airway patency, oxygenation, ventilation, and adequate circulation are particularly important in TBI to minimize development of secondary insults such as hypotension and hypoxia. If emergent intubation is required, care should be taken to minimize further elevations in ICP during intubation through careful positioning, appropriate choice of paralytic agents (if required), and adequate sedation (but not drug-induced coma). Pretreatment with lidocaine has been recommended as a useful intervention to decrease the acute elevation in ICP associated with laryngoscopy; however, good clinical evidence supporting this approach is limited.
Although the beneficial effect of head of bed elevation (HBE) is unclear given the lack of consistency among existent trials, HBE should be 30 degrees when possible and kept in a neutral position to promote adequate cerebral blood venous drainage. Reverse Trendelenburg position can be utilized in the operating room if head elevation is not possible. Venous return improvement and CSF redistribution are some of the proposed benefits of HBE. The main routes for cerebral venous drainage include the deep and superficial sinus system and the internal and external jugular veins. If a cervical collar is present, ensure it is not too tight thereby limiting blood venous drainage. Providers should minimize ICP raising maneuvers (suctioning, coughing, pain). HBE should be considered when calculating CPP.
Fever increases cerebral metabolism, thereby increasing metabolic demand and CBF, which ultimately leads to ICP elevation. Experimental studies have associated fever with increased brain injury. A recent study of 355 TBI patients evaluated the fever burden as an independent predictor for prognosis of TBI. They found that early fever might be an independent risk factor for poor prognosis in TBI.
In patients with TBI, the use of steroids is not recommended for improving outcome or reducing ICP. High-dose methylprednisolone was associated with increased mortality and is contraindicated. Moreover, glucocorticoids are not considered to be useful in the management of cerebral infarction or cerebral edema secondary to intracranial hemorrhage.
High-dose corticosteroids (dexamethasone) should be initiated if there is concern for vasogenic edema derived from brain tumors, meningoencephalitis, or abscesses. Reduction of intracranial pressure and improvement of symptoms usually occur within hours of steroid administration, and MRI changes indicating edema improvement are usually seen within 48–72 hours. Dexamethasone increases the clearance of peritumoral edema, upregulates angiopoietin-1, which is a strong stabilizing factor in the blood–brain barrier (BBB), and downregulates vascular endothelial growth factor in astrocytes. The usual initial dose of dexamethasone is 10 mg as a loading dose, followed by 4 mg every 6 hours. Patients with steroid-refractory intracranial hypertension may benefit from a carbonic anhydrase inhibitor.
In the setting of a neurologic emergency, a head CT scan should be obtained without delay to identify a process that may require immediate medical or surgical treatment. Resuscitation measures and stabilization of the patient must take place before transporting the patient to the CT scanner. Because of its rapid availability and ease to perform, CT scan is preferred over MRI in the initial setting, although MRI may be required later in the course of treatment.
Hyperosmolar therapy including mannitol and hypertonic saline
Transient hyperventilation
External ventricular drain placement
NOTE: If L1 interventions do not successfully achieve adequate ICP control and/or there are impending signs of herniation, emergency decompressive surgery should be considered.
Hyperventilation is a quick and efficient method to lower ICP transiently in acute emergencies. In normal adults, CBV is 3–4 mL per 100 g of cerebral tissue and 70% of the total volume corresponds to venous blood. Veins and capillaries do not react to fluctuations in PaCO 2; therefore, hyperventilation-related changes in CBV are a result of arterial reactivity to changes in PaCO 2 . Hyperventilation has been utilized for almost a century and the earliest documented use was reported by Lundberg in 1959. Because CBF is largely dependent on PaCO 2 , as PaCO 2 decreases with hyperventilation, there is an associated cerebral vasoconstriction leading to a subsequent reduction in cerebral blood volume and ICP. CBF decreases by 3% for every 1 mmHg reduction in PaCO 2 and this effect is mediated by extracellular fluid (ECF) pH changes. Thus, PaCO 2 levels between 20 and 25 mmHg are associated with a reduction of CBF up to 40%–50%. However, CBF returns to its original state over 6–24 hours as the brain adapts by adjusting the bicarbonate levels in the ECF to normalize the pH. At the level of the proximal renal tubule, bicarbonate reabsorption is inhibited and excretion of H + is stimulated. These responses initiate minutes after the onset of alkalosis and persist for hours or days allowing normalization of CSF and perivascular pH. It is important to recognize that acute discontinuation of hyperventilation can cause a rebound increase in CBF leading to an ICP crisis and hyperventilation should therefore be discontinued gradually.
Hyperventilation is performed in the intubated patient by increasing tidal volume or rate, and it is commonly used to facilitate neurosurgical exposure as it provides “brain relaxation” in the surgical field. A prior multicenter randomized trial showed that hyperventilation to PaCO 2 = 25 ± 2 mmHg) was effective at reducing ICP and improving the surgical exposure during craniotomy. However, current consensus is to maintain normocapnia during intracranial surgery and to consider transient hyperventilation as a temporary measure when the “tight brain” is resistant to other means of treatment.
Multiple cerebral and systemic effects have been identified that can affect all organ systems, not only the cerebral vasculature. It decreases perfusion to kidneys, gastrointestinal tissue, skin, and muscle. Respiratory effects include hypocapnia-induced bronchoconstriction, reduced hypoxic pulmonary vasoconstriction, and increased permeability of the alveolo-arterial membrane. Moreover, respiratory alkalosis complicates tissue hypoxia by shifting the oxygen-dissociation curve to the left and compromises coronary blood flow, thus increasing the risk of coronary spasm. Hyperventilation has also been found to increase extracellular lactate and glutamate levels, which may contribute to secondary brain injury.
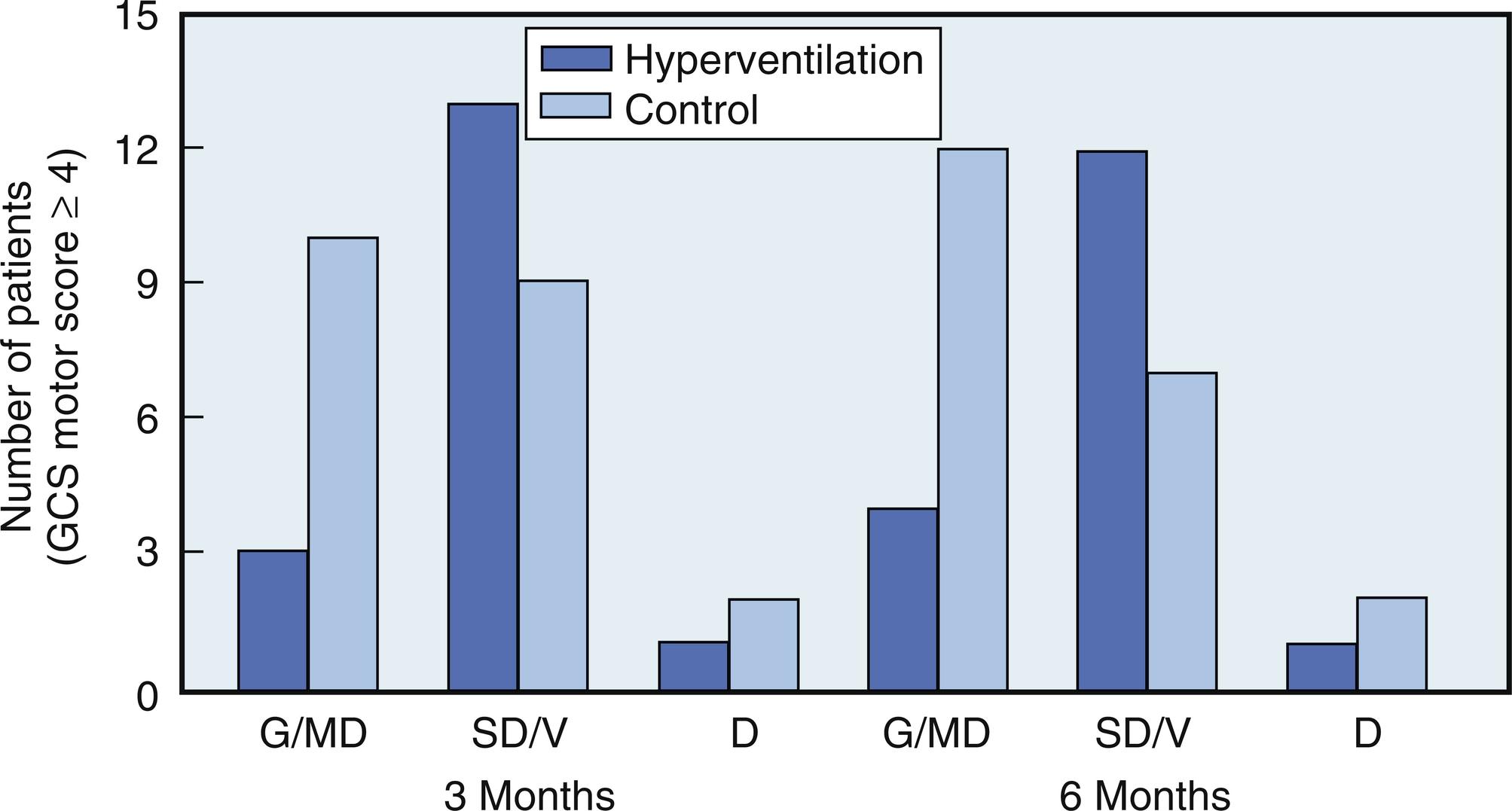
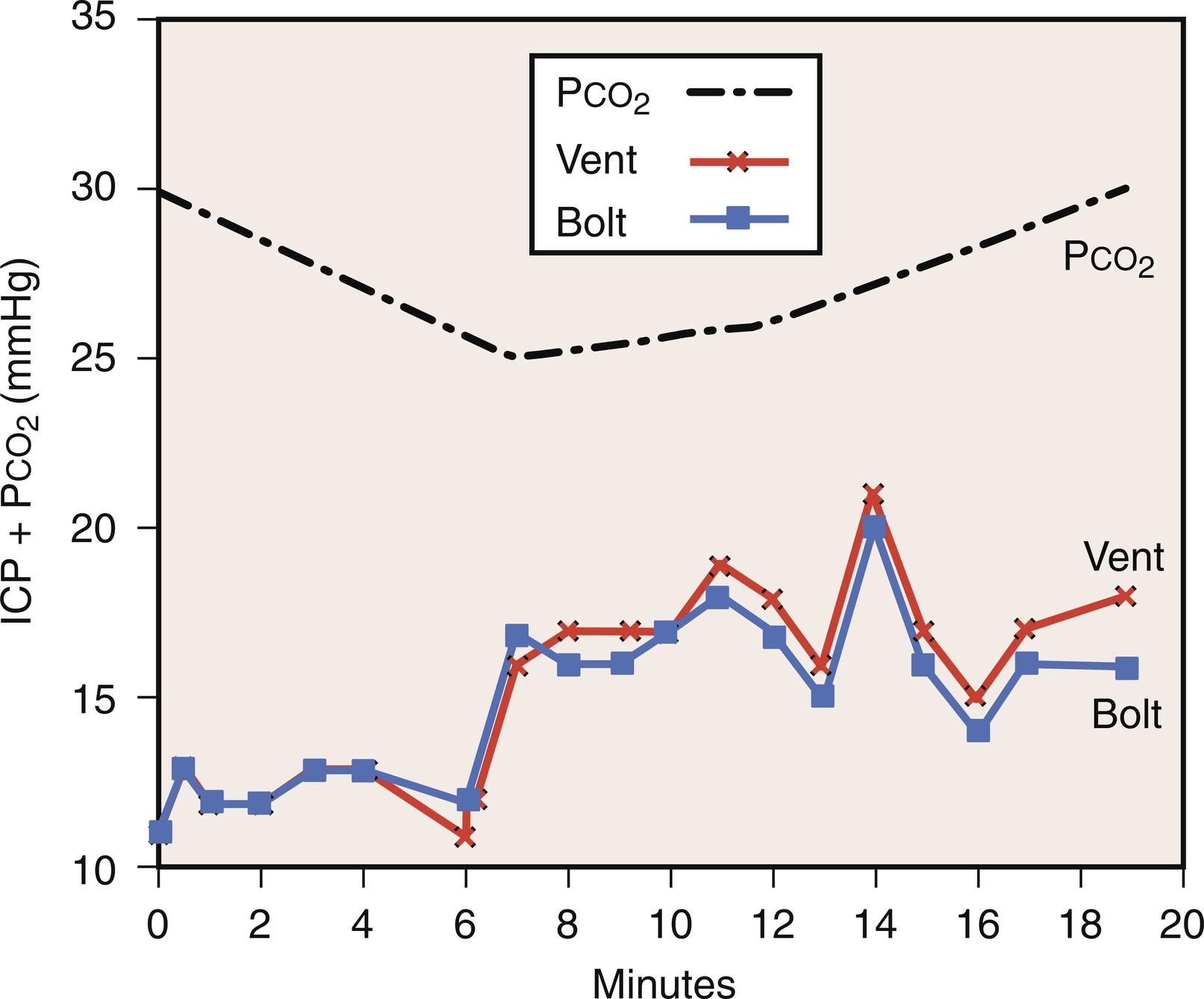
Hyperventilation introduces a risk of decreasing CBF to a dangerous level ( Fig. 24.10 ). A study in trauma patients showed that routine hyperventilation was associated with worse neurologic outcome at 3 and 6 months after the injury ( Fig. 24.11 ). The reason for this is uncertain as no-one has demonstrated that anaerobic metabolism occurs with hyperventilation in this setting. Possible causes are: (a) HV produces alkalemia and increased affinity of oxygen for hemoglobin, (b) it decreases seizure threshold, (c) the potential exists for hyperventilation to produce only a transient effect or to produce a paradoxical increase in ICP ( Fig. 24.12 ), and (d) upon discontinuation of hyperventilation, a paradoxical CSF acidosis can occur.
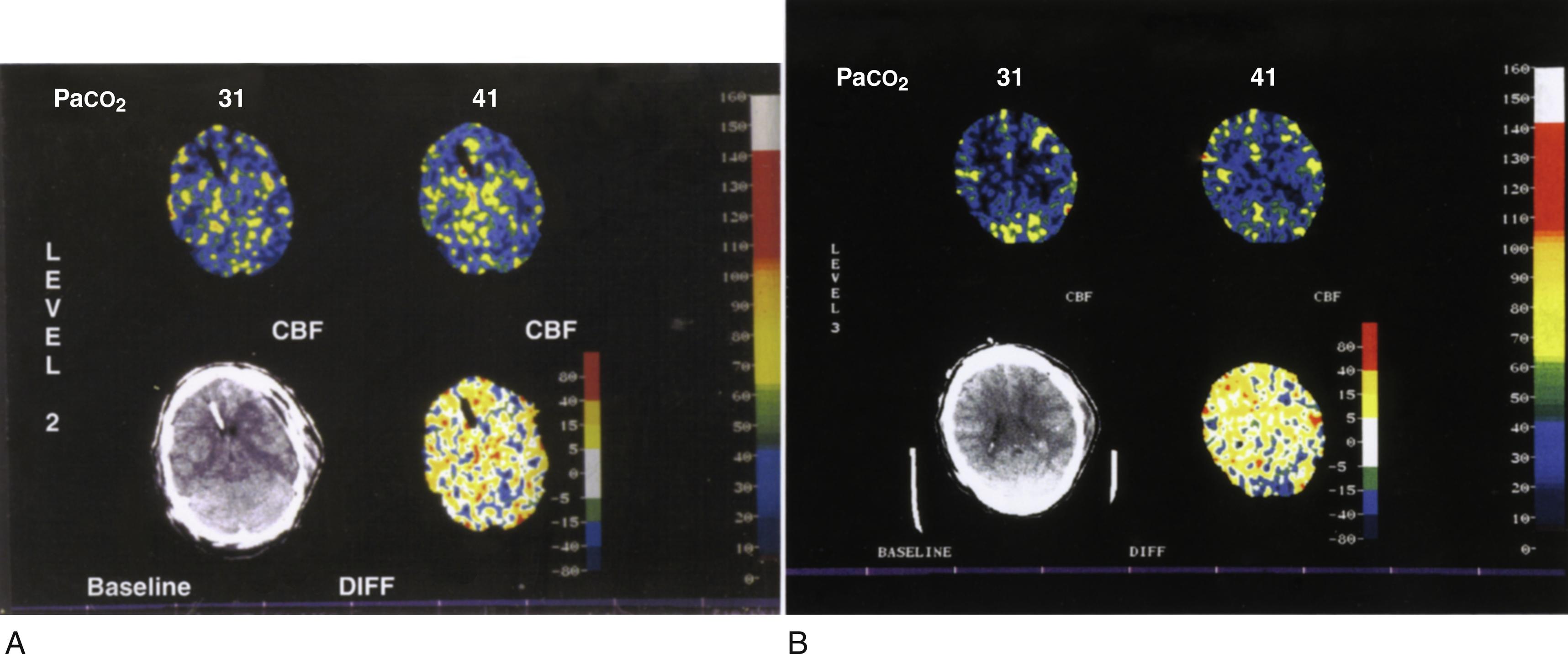
Multiple clinical studies in TBI have confirmed that hyperventilation can cause significant reductions in CBF. Jugular bulb venous oxygen saturation (SjvO 2 ) and partial brain tissue oxygen pressure (PbtO 2 ) values can be dramatically reduced by continuous hyperventilation. Hypocapnia increases the likelihood of detecting brain tissue hypoxia when using PbtO 2 monitors. This effect is more pronounced during the first few days post-TBI and it is associated with poor outcomes. Using a modified PET scan, Coles et al. evaluated CBF, CBV and oxygen extraction factor (OEF) in patients within 10 days of trauma. Hypocapnia was found to cause a decrease in CBF, and an increase in volume of ischemic areas and OEF.
The presence of hyperemia can be determined by the use of direct brain CBF determination or via jugular bulb oximetry. Brain tissue PbrO 2 may be helpful in this determination but this remains to be definitively demonstrated. High ICP associated with a low A-VDO 2 across the brain (3–4 vol%) is thought to indicate that hyperventilation can be safely used. In an emergency situation, even if the nature of the high ICP (oligemic vs hyperemic) is not known, acutely administered hyperventilation should be used to keep ICP down or to reverse a herniation syndrome or plateau wave until more definitive diagnosis or therapy can be performed.
A post-traumatic brain is extremely sensitive to ischemic damage. The autoregulatory pressure and the CO 2 reactivity mechanisms are usually exacerbated during the acute phase of TBI, especially in penumbral areas adjacent to cerebral contusions or hematomas. Loss of pressure autoregulation and CO 2 reactivity is usually associated with poor TBI outcomes. For these reasons, it is vital to maintain CPP while avoiding hypotension and hypocapnia-induced vasoconstriction.
No recent studies have established the direct association of hyperventilation and clinical outcome after TBI. In 1971, Gordon described a large retrospective series of 251 patients treated with prolonged hyperventilation, 51 of whom were hyperventilated to a PaCO 2 between 25 and 30 mmHg for a time period that varied between 6 hours and 41 days (mean 10 days). The hyperventilation group had a lower mortality (9.8% vs 32.8%); however, survivors had increased severe neurological sequelae. Among patients who experienced a complete recovery, there was no difference between groups. Another prior prospective, randomized study evaluated TBI outcome in patients who were treated with and without hyperventilation for 5 days. Favorable outcomes toward the hyperventilation group were seen at 3 and 6 months; however, after a year the differences were no longer significant.
Despite the fact that there is insufficient evidence to establish clearly whether hyperventilation is beneficial or deleterious after TBI, current guidelines recommend against its prophylactic use.
In emergency situations, a short period of 10–15 minutes of hyperventilation to a PaCO 2 of 30–35 mmHg is recommended to treat acute intracranial hypertension.
Targeted PaCO 2 during normoventilation is 35–40 mmHg with a pulse oxygen saturation of ≥ 95% and/or PaO 2 of ≥ 80 mmHg.
Hyperventilation should be avoided during the first 24 hours after injury when CBF often is reduced critically and the risk of ischemia is higher.
If hyperventilation is used, SjvO 2 or PbtO 2 measurements are recommended to monitor oxygen delivery.
Do not stop hyperventilation suddenly because it may cause rebound ICP elevation.
If CBF is known and observed to be hyperemic, contributing to intracranial hypertension, there is a theoretical basis for hyperventilation used in this personalized manner, but with continued CBF monitoring. Such monitoring is not currently widely available but may be an element of future routine care.
Hyperventilation is recommended as a temporary measure to reduce high levels of ICP in the following situations :
Herniation syndromes as described previously
Life-threatening elevations of ICP. For example, type A plateau waves, while investigating triggers and expecting the effect of osmotherapy.
Refractory intracerebral hemorrhage (ICH). Hyperventilation is used in conjunction with other tier-3 level measures, such as decompressive craniectomy, hypothermia, or high doses of barbiturates.
To facilitate brain relaxation during neurosurgical interventions only when other measures have failed.
The intravascular administration of hyperosmolar agents such as mannitol and/or hypertonic saline (HTS) creates an osmolar gradient that facilitates water transport across the BBB into the circulation, where it is excreted by the kidneys. The net effect is a reduction of the interstitial fluid and a decrease in ICP. For acute elevation in ICP, therapy with either mannitol or HTS have shown similar efficacy in decreasing ICP.
Mannitol remains a commonly used hyperosmolar agent. It is an osmotic diuretic freely filtered by the renal glomerulus and does not undergo tubular reabsorption. It lowers ICP through two main effects; first, through hypoviscosity-mediated autoregulatory vasoconstriction, also called the “rheological effect,” and second, it may induce a further decrease in ICP through brain dehydration in areas with intact blood brain barrier. However, this may be limited through generation of intracellular idiogenic osmoles equalizing transmembrane osmolar gradients.
Mannitol is administered as 0.5–1 g/kg as intravenous (IV) bolus through either peripheral or central line and can be repeated every 4–6 hours. Reduction in ICP is usually achieved within minutes, peaks at about 1 hour, and last 4 to 6 hours. Fluid balance, renal function, and plasma osmolality must be monitored because no therapeutic effect is seen with osmolality > 320 mOsm/kg. If plasma osmolality is > 320 mOsm/kg, osmolar gap (measured osm – calculated osm) should be calculated and if < 20 mOsm/kg, mannitol should be administered. If > 20 mOsm/kg, HTS should be considered, because the risk of renal dysfunction is higher. Patients with known renal disease may be poor candidates for osmotic diuresis. Another frequent complication related to mannitol is hypotension because of its diuretic effect. Acute hypotension is most commonly seen after rapid infusions and can be minimized by prolonged infusions (15–30 minutes).
Unfortunately, mannitol can have delayed effects to increase ICP. This can occur by four mechanisms. First, as a potent osmotic diuretic, mannitol can have a secondary effect to decrease systemic blood volume, thus decreasing cardiac output and blood pressure. This can result in normal reflex autoregulatory increases in CBV, which can increase ICP. Second, the increased urine output, if not replaced with commensurate IV fluid therapy, can elevate hematocrit, thus opposing the initial mannitol-induced decrease in viscosity. Third, mannitol can cross the BBB in an unpredictable manner with the possibility introduced of rebound increase in ICP, similar to that observed with urea. This is partly related to the reflection coefficient of 0.9 indicating that it can even slowly diffuse into the normal brain. Fourth, there is a theoretical possibility of generation of increased intracellular osmolarity, via so-called idiogenic osmoles, which may predispose to rebound increase in brain volume with discontinuation of mannitol. These complications of mannitol are probably lessened if urine output is replaced with balanced crystalloid infusion and if, once blood osmolarity is increased, it is not allowed to decrease rapidly to the prior level unless clinical improvement indicates that weaning of ICP-reducing therapy is appropriate. This certainly should be considered in any patient demonstrating periodic abrupt increases in ICP.
HTS has increasingly been used for treatment of acute elevated ICP, replacing mannitol as a first-line agent in multiple institutions. With a reflection coefficient of 1.0 and no potential to produce deleterious diuresis and undesired hypovolemia, it has properties that made it the most attractive hyperosmolar agent.
The ability of HTS to lower ICP was initially described in 1919 by McKesson and it is available in different concentrations ranging from 2% to 23.4%. HTS can be given as a bolus alone or in addition to mannitol. HTS 2% and 3% boluses can be given via a peripheral line and concentrations > 5% should be given via a central venous catheter because of elevated osmolarity ( Table 24.5 ). However, in acute emergencies 3%–7.5% HTS should be administered regardless of the presence of central venous access. Two prior prehospital trials using HTS 3% and 7.5% via peripheral lines did not show any negative effects. Administration of HTS through intraosseous access should be done carefully and with concentrations of < 7% because of the potential risk of myonecrosis.
| Fluid | Na concentration |
Cl concentration |
Osmolarity (mOsm/L) |
Dose |
|---|---|---|---|---|
| Mannitol 20% | N/A | 1098 | 0.5–1 g/kg | |
| Mannitol 25% | N/A | 1375 | 0.5–1 g/kg | |
| HTS 3% | 513 | 513 | 1027 | 150 mL |
| HTS 5% | 856 | 856 | 1711 | 150 mL |
| HTS 23.4% | 4004 | 8008 | 30 mL | |
| Normal saline 0.9% | 154 | 154 | 308 | N/A |
The mechanism of action behind HTS involves many theories, with the most common involving the creation of an osmotic shift of fluid from the intracellular space to the intravascular and interstitial space. In addition, HTS shrinks red blood cells, making them more deformable and enhancing their passage across capillaries. A recent study shows that HTS can reverse brain oxygenation and metabolism dysfunction through vasodilatory, mitochondrial, and anti-edema effects. The combination of these effects result in a biphasic reduction in ICP by improving rheologic properties and then by osmotic activity through aquaporins across the BBB. Aquaporins are water channels present in the brain responsible for CSF production and water distribution; its downregulation after TBI is associated with development of cerebral edema. In addition, prior evidence suggests that HTS modulates the innate immune response by diminishing the neutrophil and endothelial cell activation leading to a reduction in microvascular injury and permeability.
HTS has been studied in animal models of ICH, subarachnoid hemorrhage (SAH), and TBI. In a notable prior related canine ICH study, HTS was associated with decreased intraparenchymal pressure throughout the brain, including the perihematomal region. More recently, Schreibman et al. described an experimental ICH model in which hyperosmolar therapy shows reduction of neuroinflammation by causing a reduced activation of microglia/macrophages in perihematomal tissues. Yin et al. suggest that HTS ameliorates TBI-induced cerebral edema by suppressing brain edema, proinflammatory cytokine expression and apoptosis via downregulation of aquaporin 4 in a rat TBI model. In dogs with ICH treated with either mannitol or HTS, both drugs effectively decreased ICP although HTS duration of action was longer.
In humans, the effect of HTS has been reported in ischemic stroke, ICH, SAH, TBI, and hepatic encephalopathy. All studies show that HTS effectively and reproducibly reduces ICP with concomitant improvement in CPP. Indeed, Suarez et al. showed one important effect of 23.4% HTS in eight patients as a therapy that effectively decreases ICP when all other medical therapies do not work ( Fig. 24.13 ). Tseng et al. reported that in 10 patients with poor grade SAH, ICP was found to decrease by around 75% and CBF was enhanced in the face of decreased cerebrovascular resistance, indicating potential rheologic and cerebral vasodilatory of HTS 23.5%. Al-Rawi et al. demonstrated that an increase in CBF is followed by improvement in tissue oxygenation and metabolism when poor-grade SAH patients are treated with 23.5%. More importantly, this increment in tissue oxygenation was found to correlate with improved neurologic outcomes.
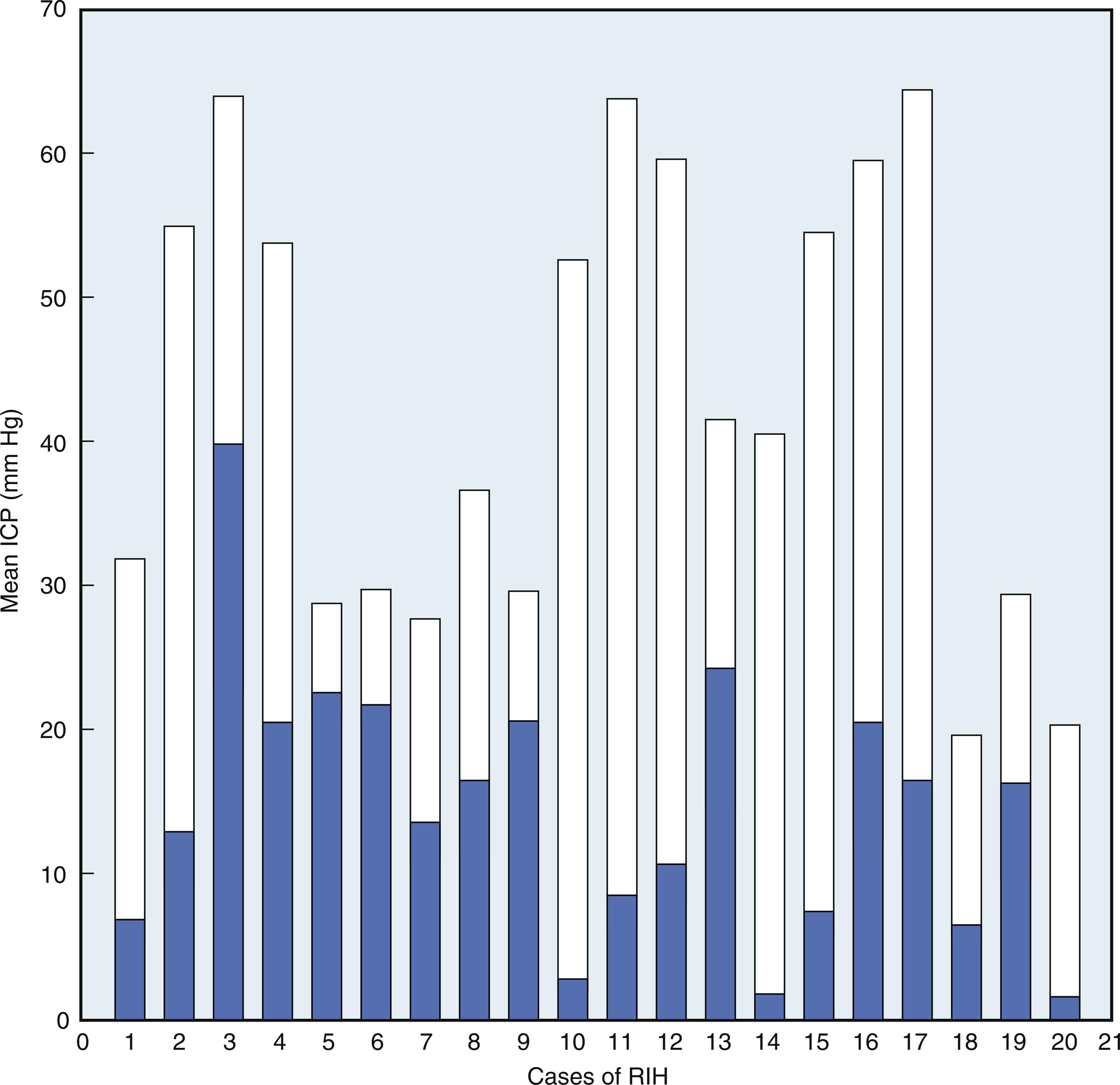
Ware et al. assessed the effects of 23.4% HTS in TBI patients with elevated ICP refractory to mannitol. They found HTS to have a longer effect on ICP reduction than mannitol and to be effective in patients with Na > 150 mEq/L. Paredes-Andrade et al. studied HTS 23.4% on ICP in the setting of different serum and CSF osmolarities. HTS was found to decrease ICP irrespective of serum or CSF osmolarity, possibly indicating a nonosmotic mechanism of action. More recently, a 30% HTS concentration was tested in TBI and no associated harmful or hematologic abnormalities were observed. In 2013, Eskandari et al. tested HTS 14.6% after TBI and showed that patients who exhibited refractory intracranial hypertension can be treated effectively and safely with repeated boluses of 14.6% with no significant change in renal function or hemodynamics. Oxidative stress processes play an important role in secondary brain injury. In a study of 33 adults with TBI, HTS showed antioxidant effects by decreasing the amount of serum total antioxidant power, reactive oxygen species and nitric oxide. Diringer et al. studied the effects of HTS 23.4% and mannitol 20% on CBF, CBV, OEF, and cerebral metabolic rate (CMR) O 2 in nine patients who developed cerebral edema with midline shift from acute ischemic stroke. They found that increased CBF on the contralateral unaffected hemisphere was dependent on mean arterial pressure. No effect on CBV was observed, arguing against the compensatory cerebral vasoconstriction previously proposed as a mechanism for ICP reduction.
Several small studies in humans have assessed the ability of HTS to reduce ICP. A retrospective study by Qureshi et al. showed that HTS decreases ICP in head trauma in postoperative brain edema but not nontraumatic ICH or ischemic stroke. Schatzman et al. performed a prospective nonrandomized evaluation of HTS in severe head injury and found that it effectively reduced ICP. A subsequent prospective randomized study by Vialet et al. in TBI patients found better ICP control compared with mannitol. Another prospective randomized study did not show better ICP control with HTS compared with standard therapy. However, in this study the sample size was relatively small and the HTS patients were sicker on entry into the study. Three studies report that HTS can be safely and effectively used in children to decrease ICP after TBI. Suarez et al. studying severe SAH patients reported that HTS therapy effectively decreased ICP while concomitantly increasing CBF (see Fig. 24.13 ). Schwartz et al. evaluated HTS/hetastarch therapy in ischemic stroke patients with high ICP, compared with mannitol. Both therapies decreased ICP, but the HTS group had better control.
Patients with intracranial hypertension associated with hepatic encephalopathy have also been demonstrated by Murphy et al. to sustain ICP decrements after HTS. Moreover, HTS and mannitol have been reported as a useful bridge therapy while awaiting liver transplant in patients who suffer from cerebral edema.
Although many studies show the efficacy of HTS in improving ICP and other parameters, the effect of this intervention on long-term clinical outcomes remains unclear. In 1991, Vassar et al. evaluated this hypothesis showing a trend for better outcomes in HTS-treated patients. One controlled trial randomly assigned 226 patients with TBI to prehospital resuscitation with 250 mL HTS 7.5% or the same volume of Ringer lactate. Survival until hospital discharge, 6-month survival, and neurologic function 6 months after injury were not different. This study did not assess the impact of systematic use of HTS throughout the hospital course. Koenig et al. evaluated the effect of 23.4% HTS on reversal of transtentorial herniation in 68 patients who suffered from various intracranial pathologies. HTS reversed transtentorial herniation quickly and reduced ICP, but clinical outcomes remained poor.
Overall, mannitol and HTS have been compared in some randomized trials of patients with intracranial hypertension derived from a variety of causes (traumatic brain injury, stroke, tumors). Meta-analyses of these trials have found that HTS appears to have greater efficacy in managing elevated ICP, but clinical outcomes have not been systematically examined. Further clinical trials are required to clarify the appropriate role of hypertonic saline infusion versus mannitol in the acute management of intracranial hypertension.
HTS clearly has the potential to exert a positive impact in the management of intracranial hypertension. However, there are concerns expressed about possible deleterious effects. Perhaps one of the most worrisome concerns is renal failure. This was mentioned in a report by Peterson wherein 2 of 10 children developed reversible renal failure, also, however, related to multiorgan failure and sepsis. Nonetheless, the issues were further underscored in editorials by Valadka et al. and Dominquez et al. but with disagreement about this as a significant risk expressed by Bratton et al. Other potential adverse effects were reviewed by Suarez and include:
Rebound edema.
Disruption of the BBB (“osmotic opening”).
Excess neuronal death. Postulated after continuous infusion of 7.5% saline in a rat model worsened outcome after transient ischemia. This has not been found to be clinically relevant.
Alterations in the level of consciousness associated with hypernatremia.
Central pontine myelinolysis. This is typically associated with too-rapid correction of (chronic usually) hyponatremia. It has not been associated with the use of HTS in humans from a normal sodium concentration.
Congestive heart failure.
Transient hypotension. This has been reported inanimals after rapid intravenous hypertonic fluid infusions.
Decreased platelet aggregation and coagulation factorabnormalities of HTS. However more recent evidence failed to show this effect in coagulation using rotational thromboelastometry (ROTEM) to assess coagulation and platelet function. Patients receiving HTS or mannitol for ICP control did not exhibit impaired coagulation function.
Hypokalemia and hyperchloremic metabolic acidosiswith large quantities of HTS.
Phlebitis. Infusion should be done via a central venouscatheter.
Renal failure. As discussed previously, the relationship to HTS was not clear in the report in which this was described. There are no reports of renal failure in animals or humans in the absence of more common ICU etiologies of renal failure, such as sepsis and organ failure.
CSF volume is reduced by removal via ventricular drain (see Figs. 24.5 and 24.6 ). This can be affected by setting the drainage at a prescribed level above the midbrain or prescribing that the drain be opened anytime ICP exceeds 20–25 mmHg. Leaving a drain open risks excessive CSF drainage when the patient coughs or with drain manipulation in the course of routine nursing procedures and can contribute to collapse of the ventricles or subdural hemorrhage. Excessive and abrupt decrease in local pressure around the drain can produce intracranial gradients, leading to a herniation syndrome. Leaving the drain clamped and monitored, however, risks the development of untreated intracranial hypertension.
Hypertonic saline infusion: If HTS has been initiated,sodium goal should be established. In general, a sodium level > 160 mEq/L is unlikely to provide any extra benefit. In order for HTS to be an effective ICP control measure, an intact BBB and a sodium gradient (Na serum -Na brain ) must be present to promote the escape of water from the brain tissue. If adequate ICP control is achieved with HTS infusion, the serum sodium concentration at which ICP was controlled should be maintained until cerebral edema has subsided. (See Tier 1 section on hyperosmolar therapy for detailed information.)
Cerebral blood volume reducing drugs: propofol, etomidate, lidocaine.
CBF decreasing (and therefore CBV decreasing) drugs that can decrease ICP include barbiturates, benzodiazepines, etomidate, and propofol. Notably, all are CNS depressants and become ineffective once the EEG become isoelectric. Thus, their use indicates acceptance on the part of the clinician to lose any reliable neurologic examination. Unlike hyperventilation, these agents decrease CBF coupled to CMR. Thus, the CBF decreases should not provide a milieu for anaerobic metabolism. Lidocaine also decreases CBF and CMR to decrease ICP although with a less pronounced decrement in neurologic function. Mannitol’s immediate effects are also thought to be mediated by reduction in cerebral blood volume, although this is undoubtedly minor and temporary as a mechanism. Barbiturates are discussed as a tier-3 therapy in the next section.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here