Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pericardiocentesis is a catheter-based procedure in which fluid is aspirated from the pericardium. It is indicated in patients with cardiac tamponade, for large pericardial effusions (usually >2 cm), for moderate effusions (10 to 20 mm) in symptomatic patients, or if the fluid is needed for diagnostic purposes; it is also used to drain purulent, tubercular, or neoplastic effusions in patients who are resistant to treatment.
The pericardial space can be safely entered with a blunt-tipped needle using a subxiphoid approach under fluoroscopic guidance even in the absence of a significant pericardial effusion.
Percutaneous balloon pericardiotomy (PBP) is a less invasive alternative to surgical pericardial window and is an effective therapy for recurrent, free-flowing, and hemodynamically significant pericardial effusions especially if associated with neoplastic disease.
PBP consists of creating a parietal pericardial window with a balloon catheter under fluoroscopic guidance in the cardiac catheterization laboratory.
Catheter-based diagnostic and interventional techniques in the pericardial space have become increasingly common and include but are not limited to epicardial mapping and ablation, intrapericardial delivery of therapies, intrapericardial echocardiography, and pericardioscopy.
The clinical presentation of patients with pericardial effusion varies. Some are completely asymptomatic, but others develop pericardial tamponade and cardiovascular collapse. Pericardiocentesis is a catheter-based technique that uses a needle to aspirate the pericardial fluid, usually under fluoroscopic or echocardiographic guidance.
Percutaneous balloon pericardiotomy (PBP) is a relatively novel catheter-based technique that is gradually replacing the more invasive surgical pericardial window procedure. The improved techniques for percutaneous access to the pericardial space and the adjunctive use of pericardioscopy provide additional opportunities for the use of this space in diagnostic and interventional techniques. As a result, novel pericardial interventions—such as epicardial mapping and ablation, percutaneous pericardial biopsy (PPB), intrapericardial therapy, and intrapericardial echocardiography—are rapidly evolving.
The normal pericardium is a fibroelastic sac composed of visceral and parietal layers separated by a thin layer (25 to 50 mL) of straw-colored fluid. The normal pericardium has a steep pressure-volume curve; it is distensible when the intrapericardial volume is small but gradually becomes inextensible when the volume increases. The intrapericardial pressure depends on the relationship between the absolute volume of a pericardial effusion, speed of fluid accumulation, and pericardial elasticity. The clinical presentation is related not only to the size of the effusion but also to the rapidity of its accumulation.
Pericardial effusion may result from a variety of clinical conditions ( Table 59.1 ). Among medical patients, malignant disease is the most common cause of pericardial effusion with tamponade. Pericardial tamponade is a clinical syndrome with defined hemodynamic and echocardiographic abnormalities that result from the accumulation of intrapericardial fluid and impairment of ventricular diastolic filling. The ultimate mechanism of hemodynamic compromise is the compression of cardiac chambers due to increased intrapericardial pressure.
| Idiopathic Cases |
|
|
|
|
|
|
|
|
| Collagen and Other Autoimmune Disorders |
|
|
|
|
|
| Neoplastic Disorders |
|
|
|
|
|
| Miscellaneous Disorders |
|
|
|
|
|
|
In all cases of cardiac tamponade, initial treatment consists of removing pericardial fluid by prompt pericardiocentesis and drainage.
Autopsy and surgical studies have shown that myocardial or pericardial metastases are found in approximately 50% of patients who have cardiac tamponade due to malignancy. Although the short-term survival of patients with cardiac tamponade depends primarily on its early diagnosis and relief, long-term survival depends on the prognosis of the primary illness regardless of the intervention performed.
Pericardiocentesis is the technique of catheter-based aspiration of pericardial fluid. It is used to diagnose and treat patients with pericarditis complicated with pericardial effusion, cardiac tamponade, and effusive-constrictive pericarditis.
Many asymptomatic patients with large effusions but no hemodynamic compromise do not require pericardiocentesis unless there is a diagnostic need for fluid analysis. In a prospective study with long-term follow-up of patients with large, idiopathic, chronic pericardial effusions, Sagrista-Sauleda and colleagues concluded that the pericardial effusions were usually well tolerated for long periods by most patients with severe tamponade; however, they can develop unexpectedly at any time. Although pericardiocentesis was effective in resolving these effusions, recurrences were common, prompting the study authors to recommend referral of patients with recurrences for pericardiectomy.
When cardiac tamponade occurs, the emergency drainage of pericardial fluid by pericardiocentesis is lifesaving therapy for a patient who would otherwise develop pulseless electrical activity and cardiac arrest. Pericardiocentesis can relieve tamponade, obtain fluid for appropriate analysis, and assess hemodynamics before and after pericardial fluid evacuation to exclude effusive-constrictive pericardial effusion. In one series of 205 consecutive patients undergoing pericardiocentesis at Mayo Clinic, 16% were found to have effusive-constrictive pericarditis.
Pericardiocentesis is an essential technique to be mastered by interventional cardiologists as it may be needed when coronary artery perforation (CAP) occurs during percutaneous coronary interventions (PCIs). In a case series of 150 CAPs from a single center (2005 to 2016), pericardiocentesis for tamponade was required for 48.0%, predominantly for Ellis type 3 CAP. Coronary perforation can occur with higher frequency as a complication of chronic total occlusion (CTO) PCI. In a contemporary dataset of 2097 CTO PCIs performed in 2049 patients (2012 to 2017), CAP occurred in 85 patients (4.1%), of whom 12 (14%) experienced tamponade requiring pericardiocentesis.
Elective pericardiocentesis is contraindicated for patients receiving anticoagulation, those with bleeding disorders, or cases of thrombocytopenia with platelet counts less than 50,000/μL. Pericardiocentesis is also ill-advised when the effusion is very small or loculated. Preoperative controlled pericardiocentesis is safe and effective in patients with acute type A aortic dissection who have critical cardiac tamponade.
Pericardiocentesis was traditionally performed using a subxiphoid approach under electrocardiographic (ECG) and fluoroscopic guidance ( Fig. 59.1A ). Traditionally, pericardiocentesis has been performed in the cardiac catheterization laboratory. Fluoroscopic guidance is vastly enhanced by using a 90-degree true lateral projection (right lateral is preferred to reduce operator radiation exposure). The use of contrast and simultaneous right heart catheterization and hemodynamic monitoring has been proposed by some authors to enhance the efficacy of the procedure and also in the early diagnosis of effusive and constrictive pericarditis. Echocardiography-guided pericardiocentesis was introduced at the Mayo Clinic in 1979, and the use of ultrasound guidance has now become the standard of care. The procedure is now also performed in the noninvasive laboratory, intensive care unit, or at bedside under echocardiographic guidance.
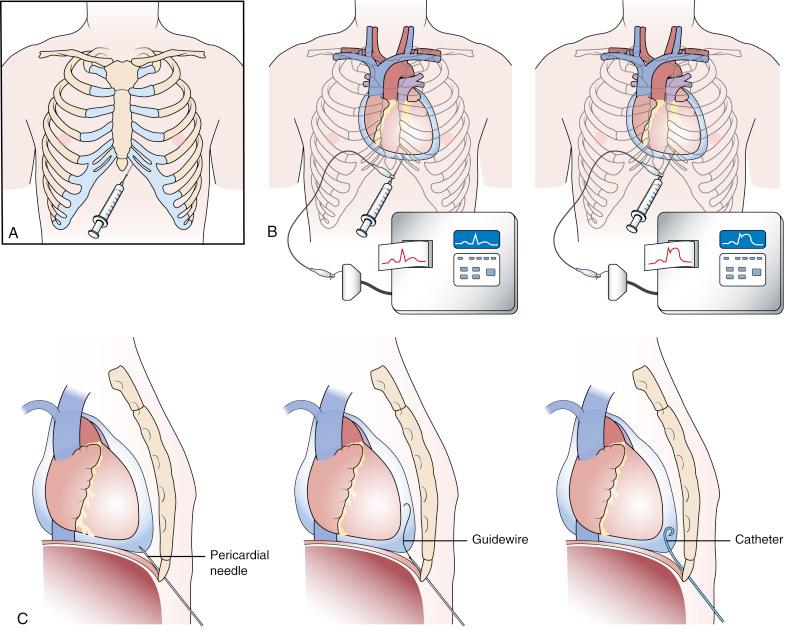
Pericardiocentesis is based on the Seldinger technique of percutaneous catheter insertion. After local anesthesia has been administered to the skin and deeper tissues of the left xiphocostal area, the pericardial needle is connected to an ECG lead. The needle is advanced from the left of the subxiphoid area while aiming toward the left shoulder. This is usually done under fluoroscopic or echocardiographic guidance, but blinded procedures can be undertaken in emergencies. Often, a discrete pop is felt as the needle enters the pericardial space. When ST-segment elevation is observed on the ECG lead tracing, it signifies that the needle has touched the epicardium and should be withdrawn slightly until the ST-segment elevation disappears (see Fig. 59.1B ). After the pericardial space has been entered, a stiff guidewire is introduced into the pericardial space through the needle, which is then withdrawn. A catheter is then inserted into the pericardial sac over the guidewire (see Fig. 59.1C ). The drainage catheter typically has an end hole and multiple side holes. Intrapericardial pressure is measured by connecting a pressure transducer system to the intrapericardial catheter. Pericardial fluid is then removed, and samples of pericardial fluid are sent for appropriate biochemical, cytologic, bacteriologic, and immunologic analyses for diagnostic purposes. The first sample is usually reserved for microbiologic studies.
In cases of pericardial tamponade, aspiration of fluid should be continued until clinical and hemodynamic improvement occurs. The catheter is frequently left in place for continuous drainage and as a route to instill sclerosing or chemotherapeutic agents when needed. The catheter is secured to the skin using sterile sutures and covered with a sterile dressing. The success rate of pericardiocentesis is greater and the incidence of complications improves with increasing size of the effusion.
With the increasing need to access the pericardial space (discussed later), especially in patients with no pericardial effusion (“dry taps”), variations in the technique have been developed. For example, a microneedle or a 17-gauge Tuohy needle (Pajunk Medical Systems, Norcross, GA) containing a curved tip can be introduced using the subxiyphoid approach while observing its track fluoroscopically in the lateral projection. This reduces the risk of inadvertent right ventricular puncture.
Potential complications of pericardiocentesis include a heart or coronary vessel laceration, sometimes causing fatal consequences. Puncture of the right atrium or ventricle with hemopericardial fluid accumulation, arrhythmias, air embolism, pneumothorax as well as puncture of the peritoneal cavity or abdominal viscera have been reported. Acute pulmonary edema may infrequently occur when the pericardial tamponade is decompressed too rapidly.
The right xiphocostal, apical, right-sided, and parasternal approaches are also used for pericardiocentesis. The right xiphocostal approach is associated with a higher incidence of right atrial and inferior vena cava injury. Puncture of the left pleura and the lingula occurs more frequently with the apical approach, and puncture of the left anterior descending artery and the internal mammary artery is more common with the parasternal approach. Echocardiographically guided pericardiocentesis is a safe and effective technique. In a cohort of 1127 therapeutic echocardiographically guided pericardiocenteses performed in 977 patients at the Mayo Clinic (1979 through 1998), the procedural success rate was 97% overall, with a total complication rate of 4.7%. Echocardiography may be especially useful for patients with loculated effusions; unlike pericardiocenteses performed in the cardiac catheterization laboratory, the left chest wall is often used with echocardiographically guided pericardiocenteses. Novel safer techniques are emerging, including percardiocentesis using continuous ultrasonographic guidance with a 7-cm micropuncture needle or computed tomography-guided pericardiocentesis for high-risk patients (e.g., presence of pericardial adhesions, temporary pacing wires, or vascular conduits).
Pericardiocentesis may not completely evacuate the effusion in most cases because active secretion and bleeding into the pericardial space may continue. The pericardial catheter should be left in place for 24 to 72 hours after initial fluid evacuation until the total daily drainage is 50 mL or less.
The patient is admitted for continuous monitoring and assessment of the rate of pericardial drainage. The pericardial space should be drained every 8 hours and the catheter flushed with heparinized saline. Systemic antibiotics—usually first-generation cephalosporin for empiric coverage of Gram-positive bacteria—are administered for the duration of the catheter stay. Based on the cause of the effusion, the patient’s clinical and hemodynamic condition, and the amount of fluid drained, the pericardial catheter is usually removed within 72 hours or decisions for additional therapy are then contemplated.
Echocardiography can be used to monitor the resolution of the pericardial effusion and signs of cardiac compression before catheter removal. Patients who continue to drain more than 50 mL/24 hours after 3 days of standard catheter drainage should be considered for more aggressive therapy. Reaccumulation of the pericardial fluid is particularly common in patients with malignant pericardial effusions. Additional therapeutic approaches to prevent pericardial fluid reaccumulation include intrapericardial instillation of sclerosing agents and the use of chemotherapy, radiotherapy, PBP, and surgical pericardial window. Reaccumulation of fluid with recurrence of cardiac tamponade is considered an absolute indication for a pericardial window.
For many patients with a pericardial effusion and tamponade, standard percutaneous pericardial drainage with an indwelling pericardial catheter is sufficient to avoid recurrence. Recurrences after catheter drainage have been reported for 14% to 50% of patients with pericardial effusion and tamponade. Patients who continue to drain more than 50 mL/24 hours after 3 to 5 days of standard catheter drainage are considered for more aggressive therapy. Several approaches are available to prevent the reaccumulation of pericardial fluid, including intrapericardial instillation of sclerosing agents, chemotherapy, and radiation therapy. A surgically created pericardial window may provide an alternative for the treatment of pericardial effusions, but morbidity and late recurrences are common.
The use of a subxiphoid surgical pericardial window has been advocated as primary therapy for malignant pericardial tamponade based on the high initial success in relieving tamponade and an acceptable recurrence rate. However, it is associated with high morbidity rates. Patients with advanced malignancy and cardiac tamponade are often poor candidates for surgical therapy. Because life expectancy is already limited, the increased length of hospital stay associated with a surgical procedure may compromise the quality of these patients’ remaining lives. The malnutrition and chemotherapy associated with advanced malignancy increase the risk of infection and other perioperative complications. It is preferable to offer a less invasive alternative.
Palacios and colleagues proposed PBP as a less invasive alternative to the surgical pericardial window procedure. With this technique, a pericardial window and adequate drainage of pericardial effusion can be done percutaneously with a balloon catheter ( Fig. 59.2 ). Since their initial report of eight patients, the multicenter PBP registry investigators have reported data on more than 130 patients.
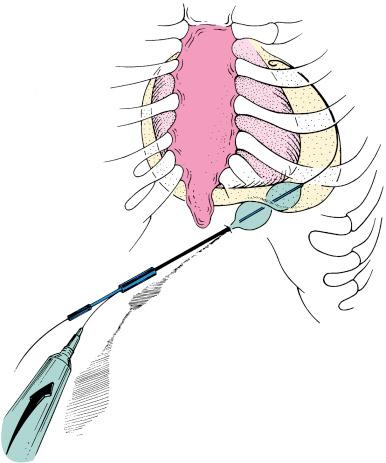
The PBP technique is relatively simple and safe. It is performed in the catheterization laboratory with the patient under local anesthesia and mild sedation with intravenous narcotics and a short-acting benzodiazepine. There is minimal discomfort. Patients may be candidates for PBP if they have undergone prior pericardiocentesis and have persistent catheter drainage. PBP also may be done as a primary therapy at the time of initial pericardiocentesis. For those who have previously undergone standard pericardiocentesis using the subxiphoid approach, a pigtail catheter has typically been left in the pericardial space for drainage. For patients who continue to drain more than 50 mL/24 hours after 3 to 5 days, PBP is offered as an alternative to a surgical procedure.
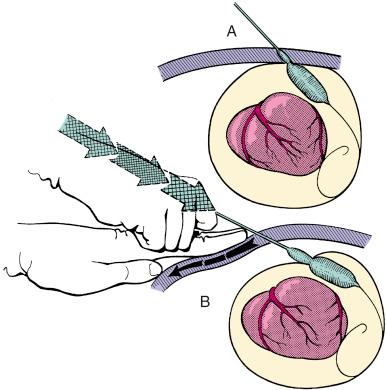
The subxiphoid area around the indwelling pigtail pericardial catheter is infiltrated with 1% lidocaine. A 0.038-inch guidewire with a preshaped curve at the tip is advanced through the pigtail catheter into the pericardial space ( Fig. 59.3A ). The catheter is then removed, leaving the guidewire in the pericardial space. The location of the wire should be confirmed by its looping within the pericardium. After predilation along the track of the wire with a 10-Fr dilator, a 20-mm-diameter 3-cm-long balloon dilation catheter is advanced over the guidewire and positioned to straddle the parietal pericardium. Care should be taken to advance the proximal end of the balloon beyond the skin and subcutaneous tissue. Precise localization of the balloon is accomplished by gentle inflation to identify the waist at the pericardial margin. The balloon is inflated manually until the waist produced by the parietal pericardium disappears (see Fig. 59.3B and C ). If the pericardium is apposed to the chest wall, as indicated by failure of the proximal portion of the balloon to expand, a countertraction technique should be used in which the catheter is withdrawn slightly and then gently advanced while the skin and soft tissues are pulled manually in the opposite direction. This maneuver isolates the pericardium for dilation ( Fig. 59.4 ).
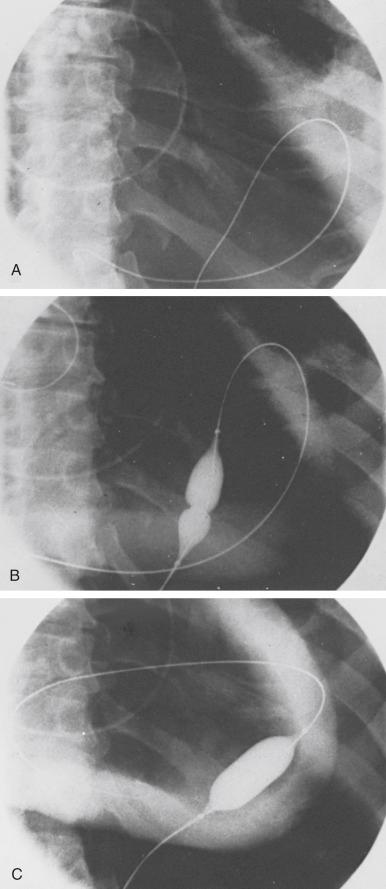
Fluoroscopic imaging using multiple views (preferably biplane fluoroscopy) helps to ensure correct positioning of the balloon, which should be straddling the parietal pericardium ( Fig. 59.5 ). At the operator’s discretion, 5 to 10 mL of radiographic contrast material may be instilled into the pericardial space to help identify the pericardial margin. Two or three balloon inflations are then performed to ensure the creation of an adequate opening in the pericardium. Although transthoracic and transesophageal echocardiography may provide additional guidance to some aspects of the procedure, it is our experience that the balloon cannot be imaged adequately with echocardiography to identify the waist at the site of the pericardial margin.
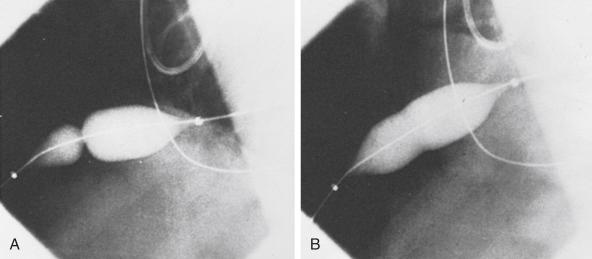
The balloon dilation catheter is then removed, leaving the 0.038-inch guidewire in the pericardial space. A new pigtail catheter is then advanced over this guidewire and placed in the pericardial space. If PBP is being performed at the time of primary pericardiocentesis, the pericardium is entered using a standard subxiphoid approach and a drainage catheter is inserted into the pericardial space. After the pericardial pressure has been measured, most of the pericardial fluid should be withdrawn, which reduces the volume remaining to pass into the pleural space.
Technical variations of the subxiphoid technique have included dilation of two adjacent pericardial sites, use of the apical approach, use of an Inoue balloon catheter, use of double balloons, use of a combination of one long and one short balloon, and use of an 18-mm dilating balloon to facilitate introduction of a 16-Fr chest tube into the pericardial space. Other investigators have attempted laparoscopic pericardial fenestration, used a cutting pericardiotome, or implanted a pericardioperitoneal shunt. Thoracoscopic techniques have been developed to create a larger pericardial window, with low morbidity rates compared with those for open surgical techniques. With this technique, adequate long-term drainage may be provided and specimens for pathologic study obtained.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here