Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital masses:
Branchial cleft anomalies are congenital remnants of the branchial arches, pouches, or grooves found in the cervical region.
Dermoid cysts are present from birth and consist of both ectoderm and endoderm. They commonly arise in the head and neck. They can grow large to compress adjacent structures. Different types include nasal dermoids, intradural dermoids, extra-angular dermoids and dermoid cysts of the neck.
Thyroglossal duct cyst is a persistent embryological remnant of the descending thyroglossal duct gives rise to a cyst. This clinically presents in the first two decades of life. Treatment involves excision using the Sistrunk technique.
Vascular masses are discussed in Chapter 31 (Vascular anomalies)
Muscular masses:
Leiomyomas are benign smooth muscle tumors that have been noted all over the body. While most commonly intra-abdominal, these can arise from any visceral organ space and present as a soft-tissue mass.
Infantile myofibromas or myofibromatosis is the most common fibrous tumor of infancy. Solitary cutaneous myofibromas are diagnosed as an expansion of the dermis or a subcutaneous nodule and may appear as an ulcer, wart, or rubbery mass.
Rhabdomyosarcoma is a malignant tumor of mesenchymal origin. In the head and neck, the most common presentations are in the orbit, nasopharynx, paranasal sinuses, and middle ear. Four histologic types include embryonal, spindle-cell alveolar, and pleomorphic rhabdomyosarcoma.
Nervous system masses:
Neurofibromatoses are a group of inherited disorders with established diagnostic criteria based on symptoms of presentation. Craniofacial presentation of this disease is classified based on the surgical treatment options available.
Malignant nerve sheath tumor is the malignant degeneration of neurofibroma. Treatment with aggressive surgical debulking, radiation, and chemotherapy is recommended.
Cutaneous masses:
Pilomatrixomas are ectodermal in origin and arise from the outer root sheath cells of the hair follicle. These tumors usually occur in the head and neck region of children. Treatment is surgical excision, with radiation therapy for malignant lesions.
Lipomatous masses:
Benign lipomas comprise 66% of mesenchymal neoplasms found in the pediatric patient population and are classified as intramuscular or intermuscular.
Lipoblastomas are benign, mesenchymal tumors that arise from white fat cells. These tumors can occur during infancy or childhood and generally grow more rapidly than the child.
Liposarcomas are the most common mesenchymal neoplasms in adults, but rare in children with only 0.7% occurring in patients <16 years old. Multiple subtypes of liposarcomas exist, including well-differentiated, myxoid, round cell, and pleomorphic.
Fibroblastic masses:
Fibromatous colli is a congenital fibrous nodule of the sternocleidomastoid muscle. It presents with torticollis. Non-surgical management with physical therapy is mostly successful.
Pediatric patients with fibrosarcoma are separated into two categories: congenital/infantile fibrosarcoma (<2 years of age) and childhood fibrosarcoma (peaks at age 10–15 years). The most common sites for fibrosarcoma presentation are the extremities, and male infants are more commonly affected than females.
Dermatofibrosarcoma protuberans (DFSP) is a low-grade malignant tumor composed of fibroblastic cells. DFSPs are heterogeneous and appear as sclerotic lesions that can be either nodular or macular in form.
Synovial soft-tissue sarcomas arise from synovioblastic differentiation of pluripotent mesenchymal stem cells.
Alveolar soft-part sarcoma is a rare tumor that occurs primarily in skeletal muscles or musculofascial planes of the extremities.
Congenital masses are the product of abnormal developmental process. They are always present at birth, though depending on the size and location, they may elude diagnosis until the child is older. They can be composed of multiple tissue types and present as a solid, cystic, or vascular mass. Only in rare instances are the masses malignant, though if not treated properly there may be a potential for future malignant transformation.
In normal development, the branchial arches fuse during the first 3–6 weeks of gestation. During this complicated process, any aberrant fusion may lead to the formation of branchial cleft remnants. The remnants are named by the numbered branchial cleft that they are derived from and have a relatively consistent anatomy. Branchial cleft remnants are present at birth but can grow over the course of early childhood as the cysts expand, or present as a draining sinus, and have high rates of infection. Surgical management is the mainstay of treatment.
These structures present as cysts, internal or external sinus tracts, cartilaginous structures, or solid masses. These anomalies are often found in the anterior neck, within the anterior cervical triangle. While the pathway of these remnants is relatively consistent, the individual anomaly can be highly variable depending on the extent of the abnormal tissue left along the tract ( Fig. 33.1 ).
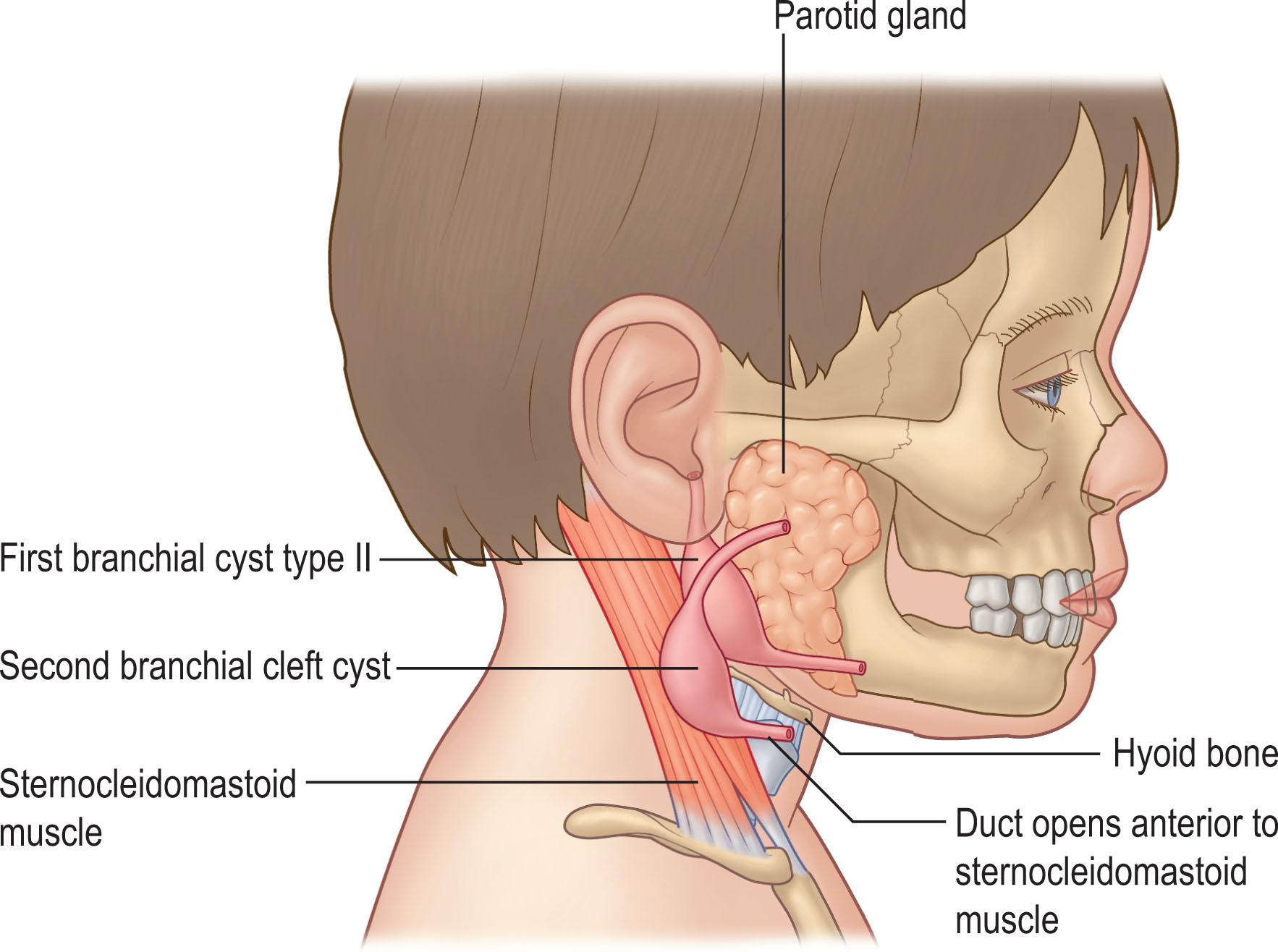
The first branchial cleft anomaly is located near the external auditory meatus with a cleft or sinus extending to the angle of the mandible and then continuing to the patient’s midline. They account for 5%–25% of all branchial cleft anomalies ( Fig. 33.2 ).
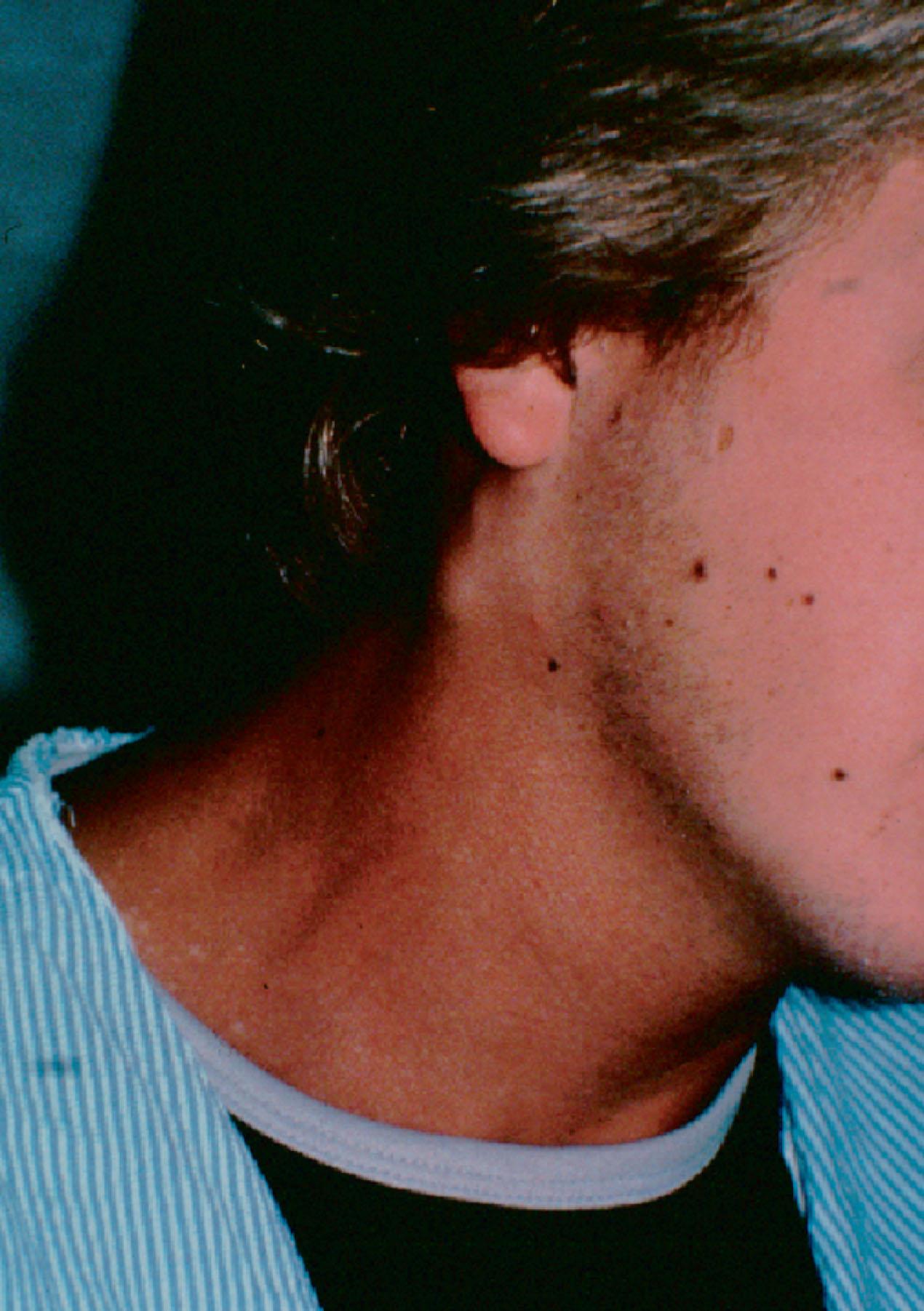
The second branchial cleft remnant originates at the sternocleidomastoid (SCM) sternal origin and continues along the anterior SCM border. It remains lateral and then superior to the hypoglossal and glossopharyngeal nerves and terminates at the tonsillar fossa. These anomalies are the most common type of remnants, accounting for 40%–95% of these anomalies ( Fig. 33.3 ).
The third branchial cleft anomaly is diagnosed as a sinus tract at the anterior border of the SCM and travels deep into the neck towards the carotid artery, passing through the thyrohyoid membrane. This sinus continues towards its origin at the inferior laryngeal pyriform sinus, passing above the superior laryngeal nerve. These account for only 2%–8% of all branchial cleft anomalies.
The fourth branchial cleft anomaly presents as a sinus at the medial lower SCM border and passes deep to the carotid artery. It then can either loop around the aortic arch (left-sided anomaly) or subclavian artery (right-sided anomaly). The recurrent laryngeal and hypoglossal nerves run deep to the sinus tract, which terminates at the laryngeal pyriform sinus apex. These are the most rare type of cleft cysts representing approximately only 1% of all masses.
While present at birth, many of these are initially small and are not diagnosed until early childhood. Physical exam demonstrates a small opening of the sinus tract, a palpable mass (cystic or solid), or remnants of cartilaginous structures. The sinus opening may have intermittent drainage and can present with infection. A CT scan or MRI is recommended for surgical planning to demonstrate the extent of the sinus.
Surgical excision is the treatment of choice for all lesions. Methylene blue may be injected into the sinus to assist with dissection. Identification of all nerves and arteries in the neck that are adjacent to the sinus is essential to preservation of function.
While surgical resection is definitive, recurrence is possible if even a small portion of the sinus tract is left behind. Previous infections may complicate the resection as tissue planes are scarred around the sinus tract or cysts.
Dermoid cysts are congenital remnants of embryonic germ cells that have been caught between two fusing layers of tissue. These abnormally located cells generate a cyst filled with an oily substance that continues to grow faster than the child does. They have a predilection for the head and neck but are seen everywhere on the body. Diagnosis and treatment of these dermoid cysts depends on their location and extent ( Fig. 33.4 ).
Dermoid cysts form as a sequestration of neuroectoderm and somatic ectoderm during embryogenesis. Histologically, they are lined with stratified epithelium. These cysts are composed of ectodermal tissue, which differentiates into sebaceous glands and epithelium. They produce sebaceous material that fills the cyst and may grow hair inside the gland as well.
For intradural dermoids, these can occur anywhere from the occiput to the sacrum. Nasal dermoids are formed between the third and eighth week of embryogenesis from the neural groove. The cyst represents a persistent connection between the foramen cecum and the fonticulus nasofrontalis. They are located anywhere between the glabella and the base of the columella. Intracranial connections have been noted. Periorbital dermoid cysts, or extra angular dermoids, are located at the superior lateral orbital brow at the level of the frontozygomatic suture. Intracranial extension at this location is extremely rare. A more medial superior orbital location of these dermoid cysts is less common but may represent a variation of a frontonasal dermoid. Additionally, dermoid cysts can be found in the neck region, generally in the midline.
Dermoid cysts present as firm, often mobile, cystic lesions. Frequent infections may be present. As the cysts are a closed space, as they fill with sebaceous material they continue to expand over childhood. Erosion of surrounding structures, including cranial bone, nasal cartilage, or soft tissue is common in larger cysts.CT scans should be used for any dermoid cysts located in the midline to rule out intracranial connections and differentiate from encephaloceles or gliomas. MRI is more useful for intracranial dermoids to evaluate connections to underlying neural structures.
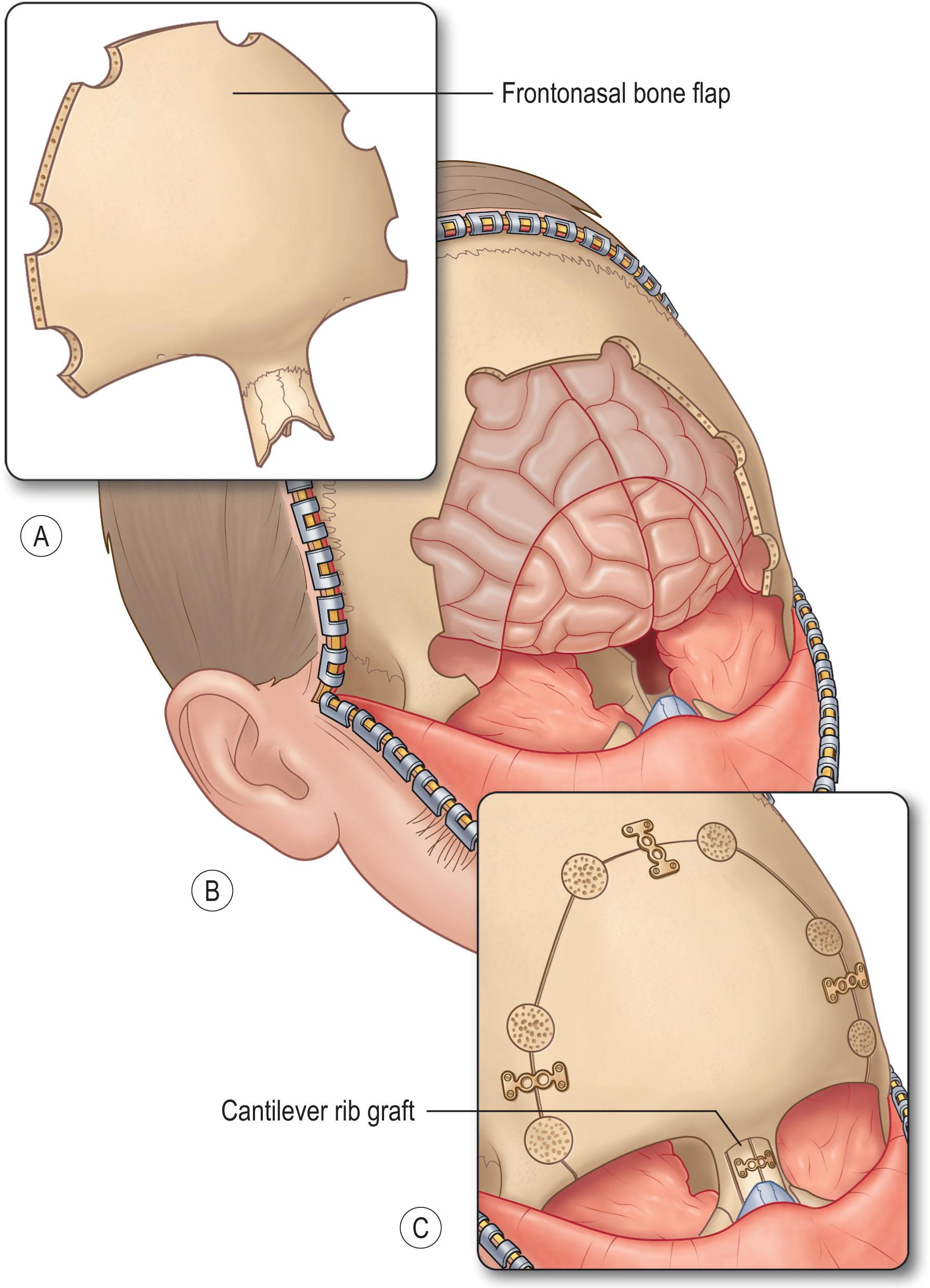
In conjunction with neurosurgery, approaches to the dermoid cyst depends on location. If the cyst is located within the dura, a craniotomy or laminectomy may be required to expose the dura depending on the location. The dermoid must be resected completely to prevent recurrence, therefore examination of the subdural space may be required. Spinal dermoids may have concurrent tethered cord anomalies which can be concurrently addressed.
Surgical planning depends on the extent of the nasal dermoid. For extracranial dermoids of the nasal tip and dorsum, an external rhinoplasty approach is preferred. Glabellar lesions may be approached either directly in a transcutaneous fashion or via a coronal incision to minimize anterior scarring ( Fig. 33.5 ). Dermoids of the nose/midline with deep extension require a more aggressive approach. Dermoids of the columella often extend along the anterior nasal spine and may bisect the septum, requiring a circumscribed removal of the sinus tract. Nasal dermoids with intracranial extension require a combined intracranial and extracranial approach to ensure all components are removed.
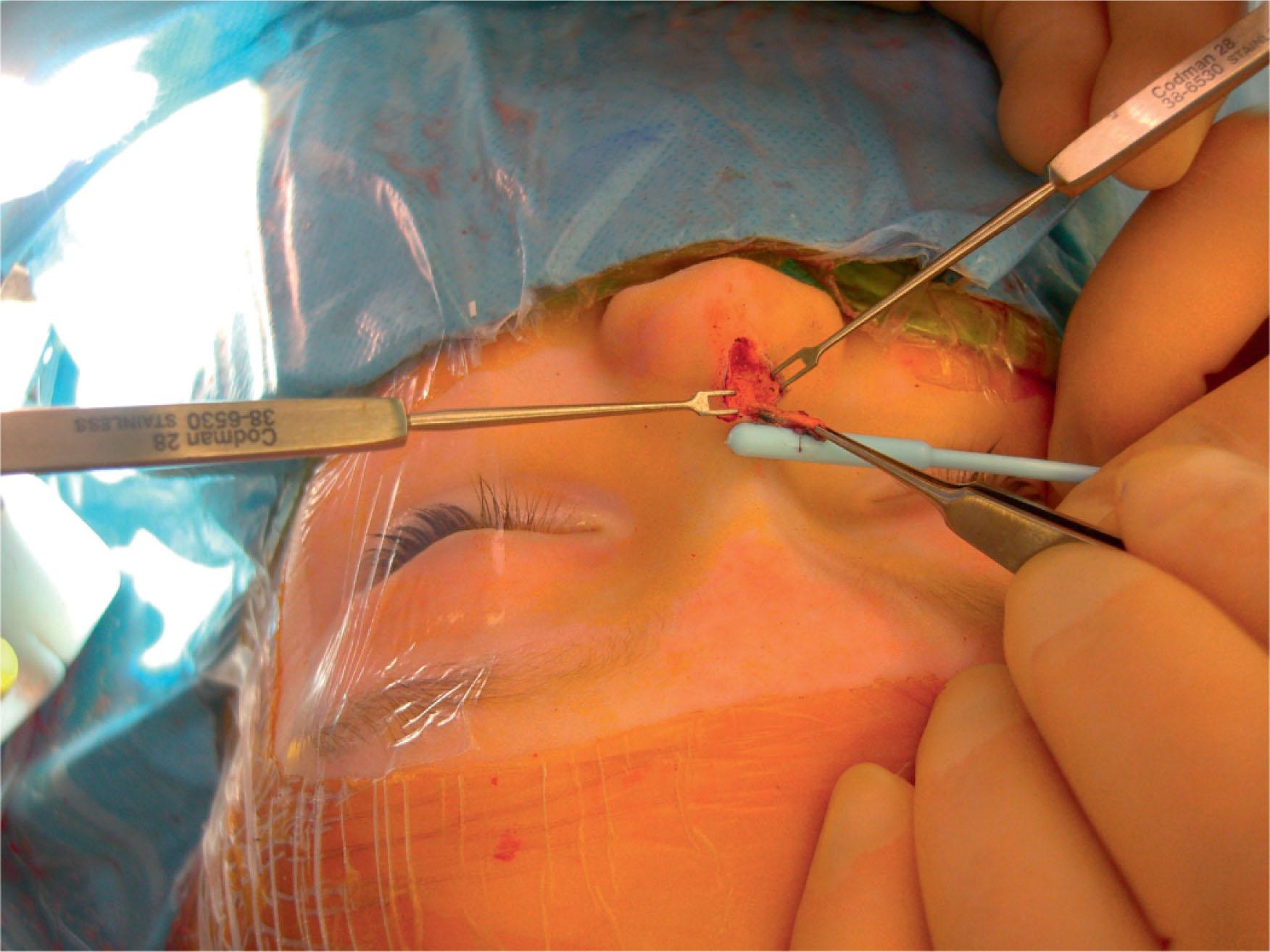
Nasal reconstruction may need to occur at the time of resection. If significant nasal cartilage is removed with the sinus, additional cartilage grafting from the rib or auricular sites may be necessary ( Fig. 33.6 ).
Most periorbital dermoids can be removed through a direct approach over the mass itself. Larger masses may have erosion through the anterior cranium so care should be taken at the base to ensure inadvertent dural injury does not occur. If the masses are at the level of the orbital rim, some may have a secondary cyst within the orbit as well and should be removed concurrently.
A direct surgical approach to the dermoids of the neck is often successful. Deeper connection or sinus tracts should be excised en bloc to prevent recurrence.
Complete surgical excision has a high rate of success. Any component of the dermoid cyst that remains after surgery has the potential to induce a recurrence. For those in the nasal and intracranial location, recurrence is associated with abscess formation, meningitis, CSF leakage and significant deformity.
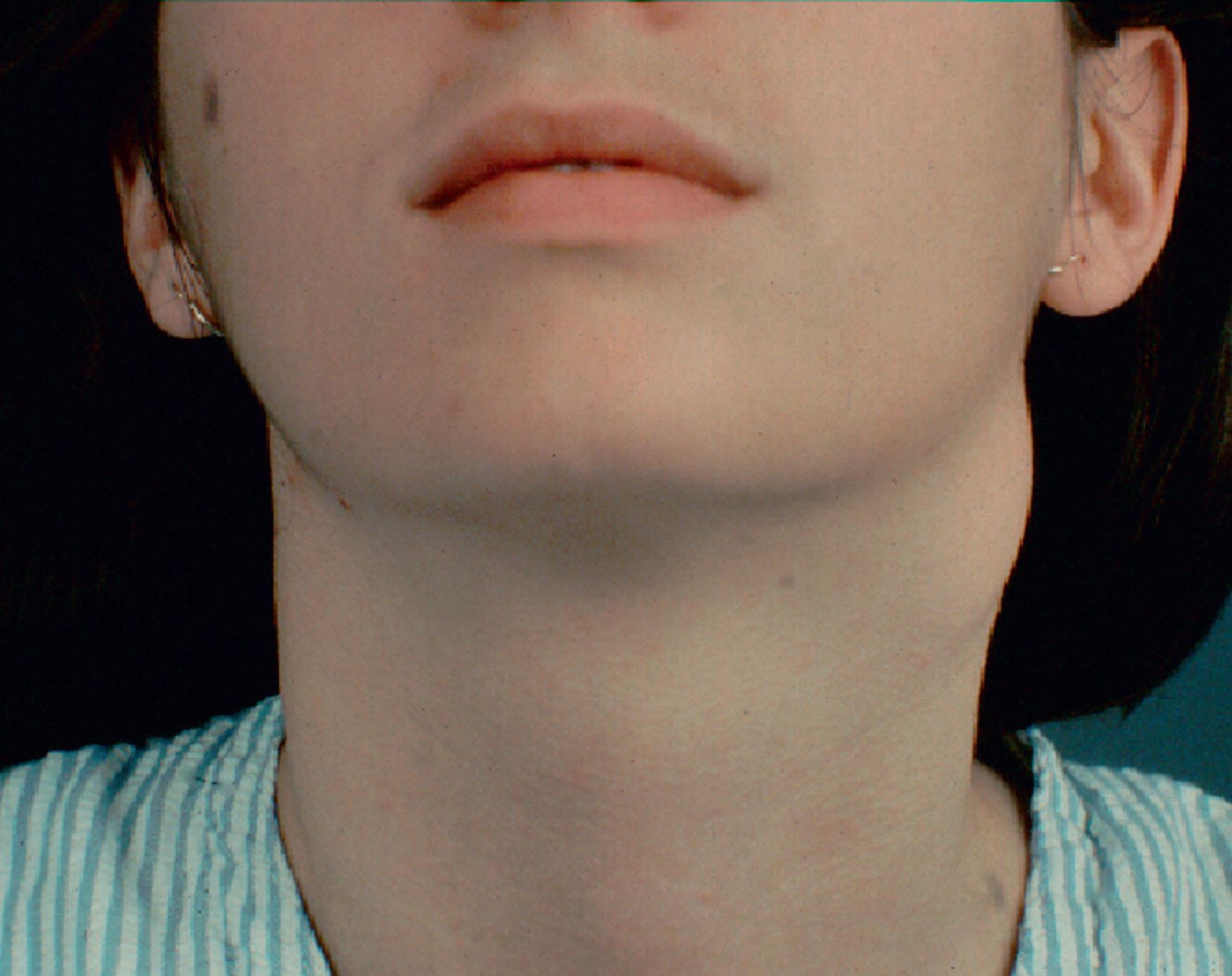
Thyroglossal duct cysts are the most common congenital tumors of the anterior cervical region. These represent the persistent embryological remnant of the descending thyroglossal duct. They are located in the midline of the neck and from the base of the tongue to the level of the hyoid.
The thyroid gland begins development at the foramen cecum during the third week of gestation. While the original position of the gland is at the base of the tongue, it descends along the pharynx until it encounters the second branchial arch. The fusion of this arch pushes the gland anteriorly where it passes the hyoid bone. Along this pathway, the ductal tissue differentiates into thyroid gland tissues. The final glandular tissues remain anterior to the hyoid bone, but the ductal tissue along this pathway should normally degenerate. Any residual glandular tissue along this pathway that does not involute will form a thyroglossal duct cyst. This tissue is lined with columnar, ciliated, or squamous epithelium.
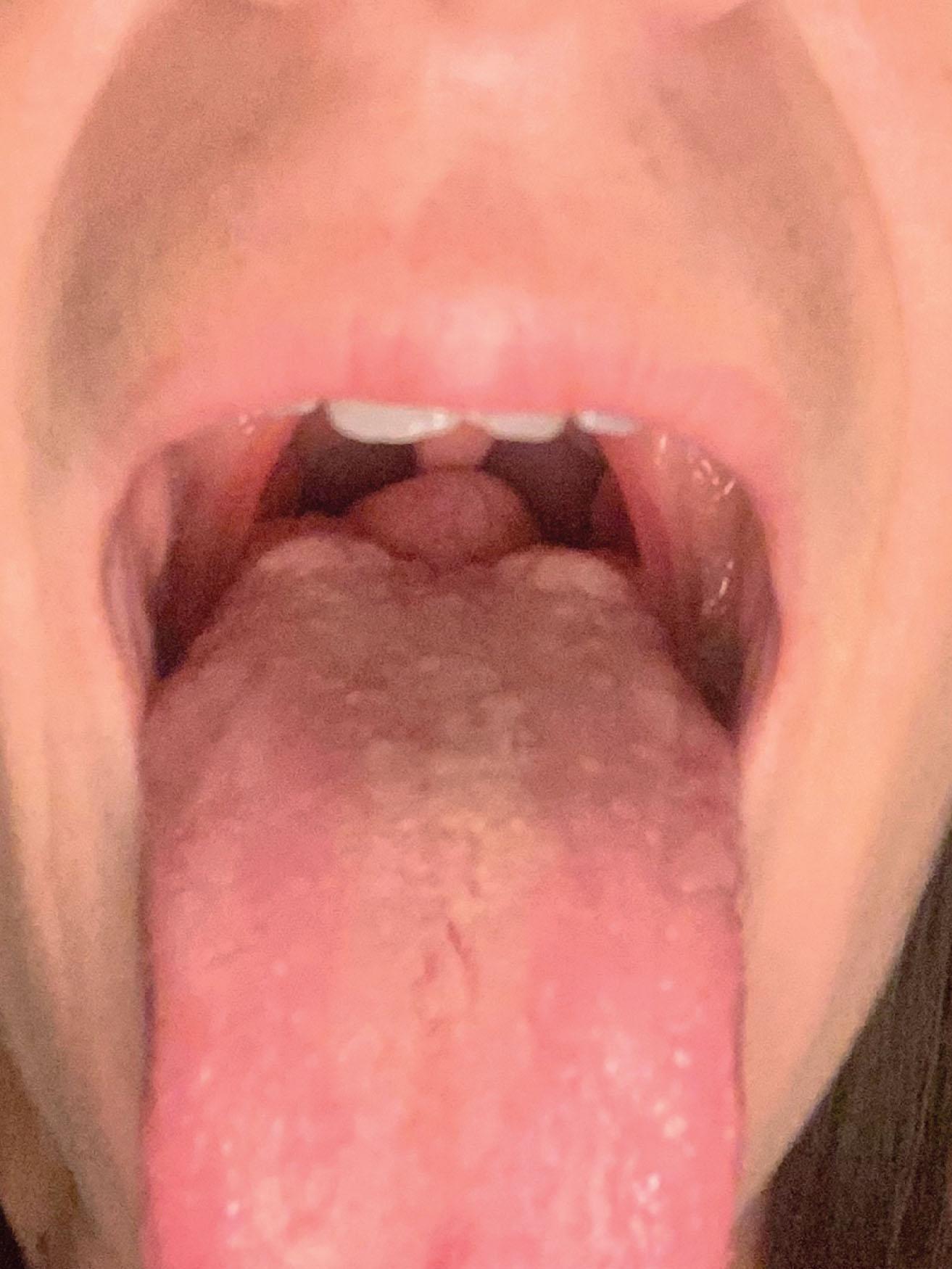
The thyroglossal duct cyst presents as a mass in the anterior neck at the level of the hyoid bone. A lingual thyroid would present as a large mass on the posterior superior tongue that may be visible during mouth opening ( Fig. 33.7 ). During swallowing, the mass moves with the bone due to its tethered location. These masses are soft, mobile, and painless. Infection may occur in these masses, which can lead to rupture and the formation of a thyroglossal duct sinus.
Surgical excision is recommended for all thyroglossal duct cysts or remnants. Pre-surgical workup includes a CT scan to evaluate the extent of the sinus. A hormone evaluation or thyroid scan should be used to demonstrate if the thyroglossal duct cyst is a functional endocrine gland so that appropriate postoperative thyroid hormones can be administered. A Sistrunk technique uses a direct incision over the cyst followed by dissection up to the foramen cecum. A segment of the hyoid bone is often removed with the ectopic gland.
With complete resection of the mass using the Sistrunk technique, recurrence rates are documented at <5%. If a portion of the mass is left behind, most often at the base of the tongue or the foramen cecum, recurrence is more likely.
Vascular masses are discussed in Chapter 31 (Vascular anomalies).
Leiomyomas are benign smooth muscle tumors that have been noted all over the body. While most commonly intra-abdominal, these can arise from any visceral organ space and present as a soft-tissue mass.
Cutaneous leiomyomas are rare and defined histologically by the presence of smooth muscle cells with calcifications and proliferations of spindle cells. These cells often have cigar-shaped nuclei and are stained for smooth muscle actin (SMA). Leiomyomas are benign but a suspicion for malignancy for any rapidly growing masses should necessitate excision. Patients with multiple cutaneous leiomyomas should be evaluated for possible Reed syndrome or hereditary leiomyomatosis with renal cell carcinoma.
Leiomyomas present as smooth, deep soft-tissue masses originating off of the tunica media of blood vessels or the arrector pili musculature of hair follicles. Smooth muscle of the genital region, including the scrotum, labia, or nipple, have also been noted. Isolated leiomyomas are solitary dermal nodules that are rarely painful. Biopsy of the lesion and histology should confirm the diagnosis. MRI may be added if larger lesions are noted or to evaluate the extent of the mass.
Surgical excision remains the gold standard. Additional treatment options for smaller lesions including CO 2 laser or cryosurgery have been described. Medical management with nifedipine, nitroglycerine, or doxazosin have been used to reduce contraction of the smooth muscle for symptomatic or pain control if surgery is not an option.
While leiomyomas are benign, surgical resection has a high rate of recurrence. Pharmacological treatment of symptoms including pain may be helpful for some patients.
Infantile myofibromas or myofibromatosis is the most common fibrous tumor of infancy. Some 88% of cases present before the age of 2 with tumors ranging in size from 2 to 7 cm.
Myofibromas present at birth and are histologically diagnosed with bundles of myofibroblasts or undifferentiated cells with irregularly branching hemangiopericytoma-like blood vessels.
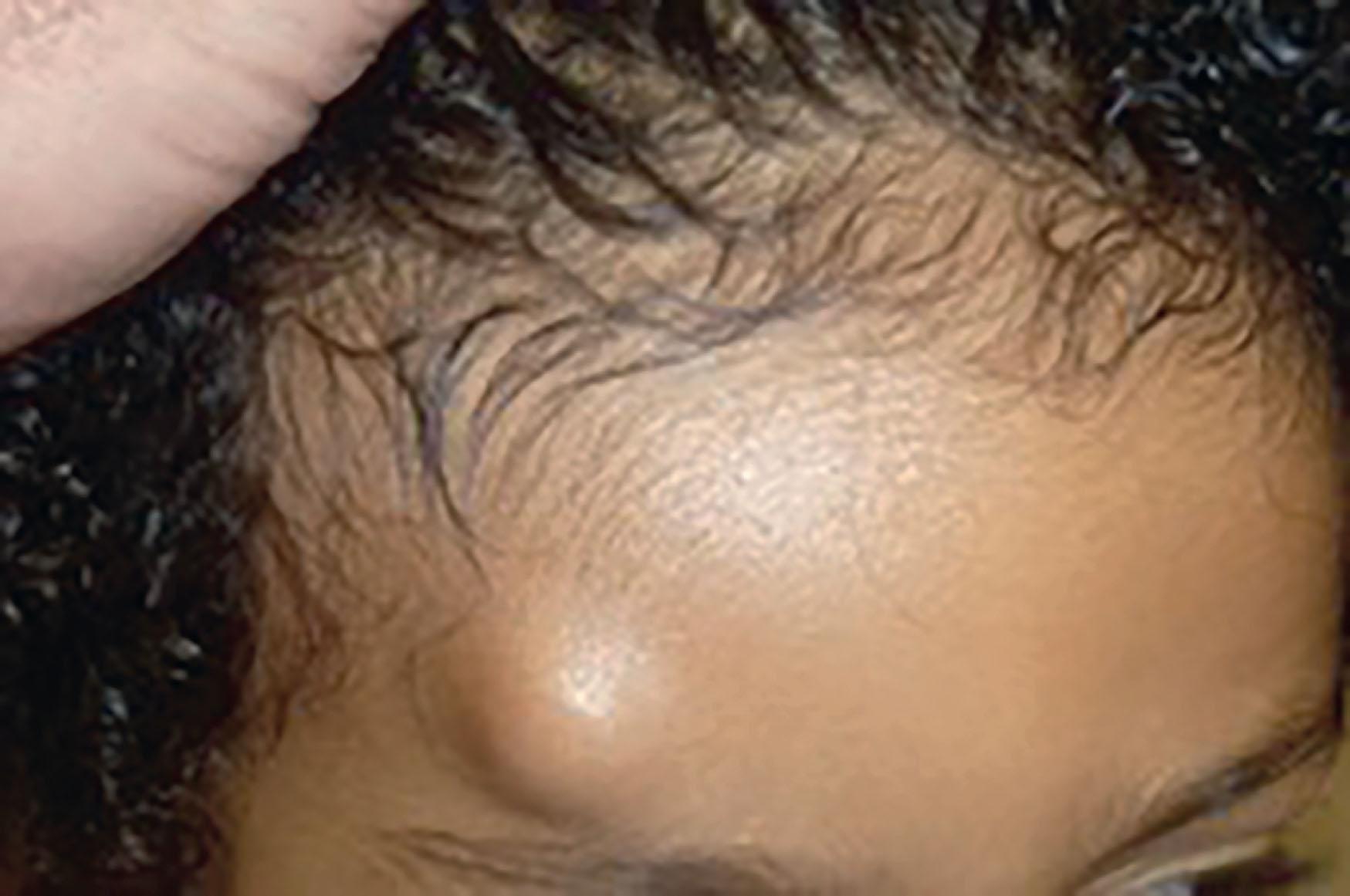
Solitary cutaneous myofibromas are diagnosed as an expansion of the dermis or a subcutaneous nodule and may appear as an ulcer, wart, or rubbery mass ( Fig. 33.8 ). Myofibromas are more often diagnosed in male infants and can involve the head, neck, or trunk. The myofibromatosis type can present anywhere in the soft tissue or viscera. Diagnosis is made with full body MRI to evaluate the extent of the lesion(s). Biopsy for histology provides confirmation.
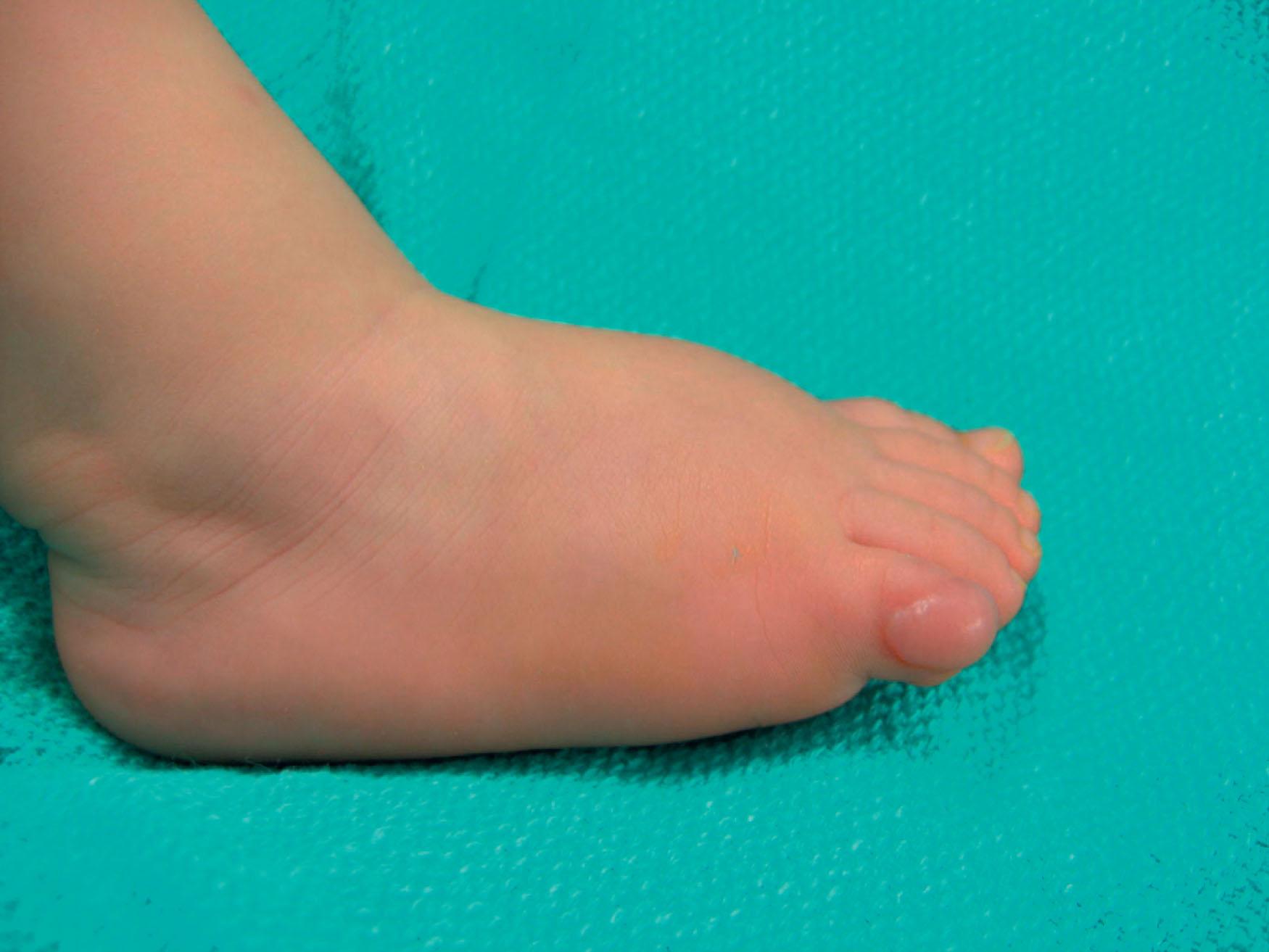
Infantile myofibromas may undergo spontaneous resolution. Larger isolated lesions or those impacting function require emergency surgical resection. Reconstruction is tailored to the site of resection and overall health of the infant. Reports of use of chemotherapeutic regimens have also demonstrated promise for multicentric disease.
Patients whose myofibroma undergoes complete resection or involution have excellent prognosis. For patients with multicentric myofibromatosis, mortality rates are noted at 33%–75%, with organ involvement (cardiac, hepatic, renal) having a worse prognosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here