Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Brain tumors are the most common solid pediatric tumors and are the leading cause of death in children from solid tumors. The incidence rate of all childhood primary brain and central nervous system (CNS) tumors is estimated to be 5.47 cases per 100,000 person-years. An estimated 4830 new cases of childhood primary malignant and nonmalignant brain and other CNS tumors are expected to be diagnosed in the United States in 2017. The incidence of brain tumors is highest in infants (<1 year of age), followed by children ages 1 to 4 years. The most common site of childhood brain tumors is the cerebellum (18.7%), followed by the frontal, temporal, parietal, and occipital lobes (15.7%).
The most recent World Health Organization (WHO) classification of central nervous system tumors represents a major restructuring of the diffuse gliomas, medulloblastomas, and other embryonal tumors, and incorporates new entities defined by both histology and molecular features. Of particular note, the embryonal tumors have also undergone substantial changes in their classification, most notably with removal of the term primitive neuroectodermal tumor (PNET) from the diagnostic lexicon. The following description tries to incorporate some of these more recent changes while acknowledging the previously accepted terminology that is still currently used in clinical discussions.
The etiology of pediatric brain tumors is an area of continuing research beyond the scope of this chapter, requiring understanding of genetic alterations, signaling systems, and molecular genetics and pathways. No specific risk factor explains more than a small proportion of childhood brain tumors. Known risk factors for childhood brain tumors include therapeutic doses of ionizing radiation to the head for brain tumors and radiation for leukemia, and certain genetic syndromes. Some genetic inherited syndromes have an association with brain tumors including neurofibromatosis types 1 and 2, Gorlin syndrome (basal cell nevus syndrome), tuberous sclerosis, Turcot syndrome, von Hippel–Lindau syndrome, and Li–Fraumeni syndrome.
Wide availability of computed tomography (CT) has resulted in its use for diagnosis for patients with brain tumors presenting acutely. Its advantages include the ability to detect a mass, to identify lesion mass effect, to check for ventricular effacement and/or enlargement, lesional hemorrhage, calcification, and osseous involvement.
Magnetic resonance imaging (MRI) with contrast is the modality of choice at diagnosis to determine lesion size, location, and characterization due to its superior soft tissue resolution, multiplanar capability, and lack of ionizing radiation. Presence of contrast enhancement reflects disruption of the blood-brain barrier. The degree of contrast enhancement does not always correlate with tumor grade. For example, benign tumors such as choroid plexus papillomas and pilocytic astrocytomas can enhance avidly, whereas anaplastic astrocytomas may not enhance at all.
The definition of lesion margins and accurate staging of the tumor by confirming the presence or absence of tumor spread through the neural axis are essential for treatment planning. In addition, diffusion tractography and functional MRI can be used to show the relationship of the tumor to eloquent brain and other vital structures to minimize treatment damage to these regions. Intraoperative MRI systems are being increasingly used to guide tumor resection and stereotactic and minimally invasive tumor resection.
MRI can be used to assess tumor response and progression and to monitor treatment effects. After surgical resection, MRI is used to determine the presence of residual tumor, to look for postoperative complications, and for routine follow-up. Advanced techniques (discussed later in this chapter) are used to complement structural imaging and provide insight into tumor physiology.
Contrast on diffusion-weighted imaging (DWI) reflects the mean distance travelled by free water protons in tissue due to Brownian motion. Diffusivity of tissues is indicated by the apparent diffusion coefficient (ADC), which is a scalar parameter with units of mm 2 /sec. Diffusion occurs freely in the direction of tract orientation and is restricted in orthogonal planes.
Diffusion tensor imaging (DTI) is an adaptation of DWI, and is performed by acquiring diffusion data in six or more directions, enabling determination of the direction and magnitude of water diffusion. The degree of anisotropy is expressed by a variety of quantitative measures, the most common of which is fractional anisotropy (FA), which reflects the presence of myelinated white matter tracts and related processes such as axonal transport in tissues. Connecting the directions of diffusion in each voxel to those of neighboring voxels using a variety of mathematical algorithms enables creation of a three-dimensional white matter tract map, termed tractography.
DWI can help assess cellularity of a lesion, as markedly decreased ADC usually correlates well with the histologic measures of tumor cellularity in brain neoplasms. The ADC value depends on the complexity of the cytoarchitecture and the rapid exchange of protons between different compartments. The cytoarchitecture of the tumor can inhibit random Brownian motion and thus causes water diffusion to deviate from strict Gaussian behavior. Generally, highly cellular tumors have reduced diffusivity, whereas vasogenic edema and necrosis show increased diffusion. However, overlap between tumor grades makes it imperative that the ADC values are interpreted in conjunction with the structural MRI sequences.
DWI can be extremely useful in the postoperative period, when low ADC values at the surgical margins or within the resection cavity may be indicative of ischemia, residual cellular tumor, or abscess, and in conjunction with conventional MRI helps exclude artifact from hematoma. ADC values can also be used to assess treatment response, detect tumor recurrence, and may help differentiate tumor recurrence from pseudoprogression.
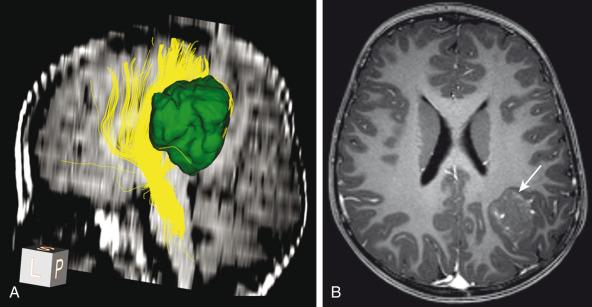
DTI can help identify the patterns of tumor interaction with white matter fiber tracts: deviation, edema, infiltration, and destruction. DTI in conjunction with volumetric data can guide surgical resection and predict possible postoperative deficits resulting from white matter tract damage ( e-Fig. 35.1 ). Reduction in FA has been found in the white matter of medulloblastoma patients, even in the absence of abnormalities on structural sequences. Deceased FA values correlate with the age at which radiation was administered and with worse school performance, and may be a biomarker of the effects of radiotherapy.
Other applications include ADC histogram technique in diffuse midline tumors of the pons, which has demonstrated significant correlation between diffusion metrics and survival, with lower diffusion values (increased cellularity), and increased enhancement associated with shorter survival. Functional diffusion mapping (fDM) has been suggested as a tool for early detection of tumor treatment efficacy, although interpretation requires careful correlation with structural sequences and the underlying biology of both tumor and healthy tissue. Serial parametric response mapping of ADC, performed at multiple time points of therapy, has the potential to distinguish pseudoprogression from true progression of tumors.
Magnetic resonance spectroscopy (MRS) enables detection and quantification of normal and abnormal metabolites in the brain. MRS can help identify tumor tissue, differentiate tumor types, and separate active tumor from radiation necrosis or scar formation. MRS can be performed on most standard MRI scanners, typically incorporating either the point resolved spin echo (PRESS) or stimulated echo acquisition mode (STEAM) techniques. Simultaneous acquisition of MRS from multiple voxels increases spatial resolution and is called chemical shift imaging (CSI) or magnetic resonance spectroscopic imaging (MRSI).
Normal metabolites detected in the brain include N-acetylaspartate (NAA), a neuronal marker; choline (Cho), a membrane marker; and creatine (Cr), a marker of energy metabolism. Myoinositol (mI), a glial marker, can be better resolved with short echo-time MRS techniques. Concentrations of metabolites are usually expressed in the form of metabolite ratios.
Most brain tumors are characterized by the presence of increased Cho/Cr and decreased NAA/Cr ratios, indicating loss of neuroaxonal integrity and increased cell membrane turnover. Presence of lactate, an indicator of anaerobic metabolism, indicates impaired energy metabolism.
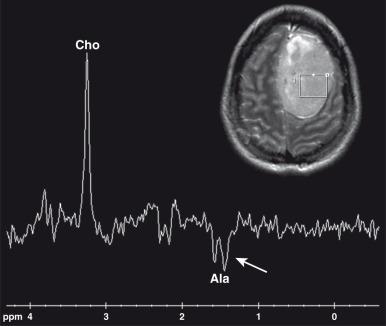
Tumor characterization has been attempted using MRS. In general, high-grade tumors have higher Cho/Cr and lower NAA/Cr ratios than low-grade ones. One exception is juvenile pilocytic astrocytoma, in which the choline peak can be markedly elevated. Necrotic areas in rapidly growing malignant tumors such as medulloblastomas and atypical teratoid rhabdoid tumors may contain lipid resonances. In adults, lactate is typically associated with high-grade gliomas. In children, lactate is frequently paradoxically elevated in pilocytic astrocytomas, a low-grade tumor. The presence of specific metabolites such as alanine in meningiomas (an inverted doublet at 1.44 parts per million [ppm]), and taurine (peak at 3.3–3.4 ppm) in medulloblastoma may help in narrowing the differential diagnosis ( e-Fig. 35.2 ). Citrate has been described in pediatric brain tumors and can be particularly high in pontine gliomas and in Grade 2 astrocytomas that have progressed.
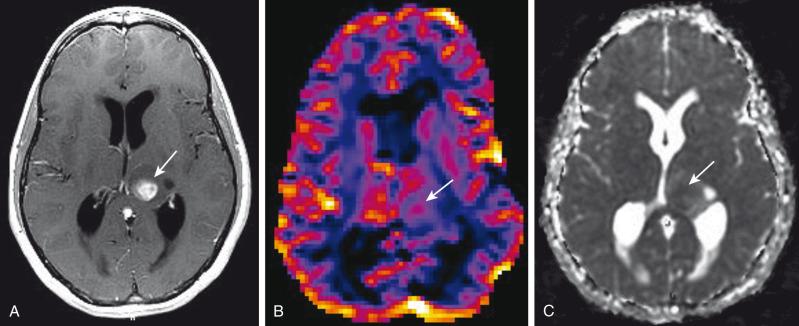
Perfusion-weighted imaging (PWI) measures cerebral hemodynamics at the microcirculation level ( e-Fig. 35.3 ). Measured parameters include cerebral blood volume (CBV), cerebral blood flow (CBF), and mean transit time (MTT). CBV is defined as the volume of blood in an area of brain tissue expressed in mL/100 g, and is the most commonly used parameter in brain tumor evaluation.
Three main techniques are available to measure perfusion within the brain: T2*-weighted dynamic susceptibility contrast (DSC) imaging, T1-weighted dynamic contrast-enhanced (DCE) perfusion, and arterial spin labeling (ASL).
DSC is the most widely available technique, and, consists of a rapid bolus of intravenous paramagnetic contrast agent administration, followed by a rapid acquisition of a series of echo-planar images during the first pass of contrast material through the capillary bed. The contrast medium in the blood passes through the tissues and results in a signal drop proportional to the blood volume. Challenges to routine use in children include the need to use power injectors and large-bore intravenous catheter placement.
DCE T1-weighted permeability perfusion pharmacokinetic parameters have been useful for discriminating low-grade and high-grade pediatric tumors. In one study, the rate constant from the blood plasma into the extracellular extravascular space (K trans ), rate constant from extracellular extravascular space back into blood plasma (K ep ), and extracellular extravascular volume fraction (V e ) were all significantly correlated with tumor grade; high-grade tumors showed higher K trans , higher K ep , and lower V e .
ASL uses endogenous blood as a tracer agent. Differences in signal intensity changes on ASL are smaller when compared with contrast-enhanced perfusion techniques and depend on other parameters such as the T1 relaxation time of blood and time delay between the tagging pulses and the readout time of the images. ASL has shown promise for hemodynamic evaluation of brain tumors. High-grade pediatric brain tumors display higher CBF compared with low-grade tumors, and CBF is correlated with tumor microvascular density. Lower-grade astrocytomas have relatively lower regional blood volume (rCBV) than higher-grade tumors such as anaplastic astrocytomas and glioblastomas. However, in pediatric patients, low-grade pilocytic astrocytomas can have a high relative blood volume.
Functional MRI (fMRI) is a technique that relies on the fact that oxyhemoglobin is diamagnetic and deoxyhemoglobin is paramagnetic. Due to the relative increased blood flow and consequent increased utilization of oxygen within the activated portions of the brain, the magnetic resonance signal (called the blood oxygen level–dependent [BOLD] signal) is measurably different compared with other parts of the brain. Event-related paradigms (short bursts of stimuli followed by rest) or block design paradigms (alternating blocks of same type of stimuli) are described for use in presurgical planning. fMRI has been successfully used in sedated younger pediatric patients. The primary value of fMRI is in localizing eloquent areas in the brain controlling language, motor functions, and memory. This information helps guide therapeutic approaches to minimize damage to eloquent cortex. The acceptance of fMRI and its capabilities has largely replaced the invasive Wada test in determining hemispheric dominance of memory and language functions. It is important to note that changes in blood flow in and around hypervascular tumors can affect the regional reliability and accuracy of fMRI.
Assessment of regional cerebral blood flow by single positron emission computed tomography (SPECT) has been largely replaced by magnetic resonance perfusion and functional MRI techniques. Positron emission tomography (PET) is an adjunct to MRI and CT brain tumor evaluation, used to determine metabolic activity at diagnosis, to determine response to therapy, and to distinguish between treatment effect and tumor recurrence. Fluorodeoxyglucose ([ 18 F] FDG) is the most commonly used isotope for PET studies in children. Other labeled agents such as amino acid analogues, [ 11 C] methionine and [ 11 C] tyrosine, have shown promise in detecting low-grade tumors in adults, but their role in children is yet to be established. These amino acid analogues are incorporated into tumor proteins, and degree of uptake reflects tumor protein synthesis. Other agents being investigated include cell proliferation agents such as [ 11 C]-labeled fluorothymidine and cell hypoxia imaging agents such as fluoromisonidazole ([ 18 F] MISO) and Cu-diacetyl-bis (N4-methylthiosemicarbazone (Cu-ATSM).
The new 2016 WHO Classification of Tumors of the Central Nervous System uses molecular parameters in addition to histology to define many tumor entities, formulating how CNS tumor diagnoses should be structured in the molecular era. This classification presents major restructuring of the diffuse gliomas, medulloblastomas, and other embryonal tumors, and incorporates new entities that are defined by both histology and molecular features. Some of these tumors are relevant to pediatric practice and will be addressed below. It is hoped that this classification of tumors will further evolve and facilitate epidemiologic studies and improved treatments.
Classification of tumors by location helps limit the differential diagnosis in conjunction with the appearance of the lesion on conventional MRI and advanced imaging techniques ( Box 35.1 ).
TUMORS OF THE CEREBRAL HEMISPHERES
Astrocytomas (WHO grades I-IV)
Supratentorial embryonal tumors
Atypical teratoid rhabdoid tumor
Supratentorial ependymoma
Thalamic astrocytoma
Dysembryoplastic neuroepithelial tumor (DNET)
Meningioangiomatosis
Germinoma
Choroid plexus tumors—papilloma and carcinoma
Neuronal and mixed neuronal-glial tumors (including desmoplastic infantile ganglioglioma [DIG])
SELLAR AND SUPRASELLAR TUMORS
Craniopharyngioma
Chiasmatic/hypothalamic glioma
Hypothalamic hamartomas
Pituitary adenoma
Germ cell tumors
LCH
Rathke cleft cyst
Arachnoid cysts
Dermoid/epidermoid cysts
POSTERIOR FOSSA TUMORS
Intraaxial
Medulloblastoma
Cerebellar astrocytoma
Tectal glioma
Brainstem neoplasms including diffuse midline gliomas
Atypical teratoid rhabdoid tumors (ATRT)
Ependymomas
Teratoma
Hemangioblastoma
Extraaxial
Dermoid
Epidermoid
Teratoma
Schwannoma
Meningioma
Skull base neoplasms
PARAMENINGEAL TUMORS
Osseous, chondroid, or myeloid origin
Other mesenchymal origin tumors
Tumors from notochordal elements
Metastatic disease including leptomeningeal disease from dissemination of primary brain tumors
PINEAL REGION TUMORS
Germ cell tumors
Pineal parenchymal tumors
Tumors of supporting tissues of the pineal gland or adjacent structures
The most recent WHO classification of brain tumors divides astrocytomas into two broad groups. The first group consists of diffuse astrocytic and oligodendroglial tumors, which includes diffuse and anaplastic astrocytoma and glioblastoma. Each of these types is further subdivided into three subtypes based on the presence or absence of isocitrate dehydrogenase (IDH) mutations. For both grade II and III tumors, the great majority falls into the IDH-mutant category. The second group consists of other astrocytic tumors, which includes pilocytic astrocytomas (with pilomyxoid astrocytomas as subtype), subependymal giant cell astrocytomas, and pleomorphic xanthoastrocytoma (with and without anaplastic features).
Pilocytic astrocytomas (PA) are grade I WHO tumors that account for 20% to 30% of all childhood brain tumors. They arise typically in the first two decades of life and there is no definite gender predominance. The most common locations are in the optic pathways, hypothalamus, thalamus, basal ganglia, cerebral hemispheres, cerebellum, and brainstem. Patients with neurofibromatosis type 1 (NF1) have an increased risk of developing pilocytic astrocytomas, including optic pathway tumors, which tend to have a better long-term prognosis than in patients without NF1.
On CT and MRI, a pilocytic astrocytoma is usually a well-defined lesion that demonstrates contrast enhancement of its solid component ( Fig. 35.4 ). Tumoral “cystic” components containing proteinaceous fluid are seen more often in cerebellar lesions. They often contain a contrast-enhancing mural nodule. Increased ADC values due to their low cellularity help to distinguish them from higher-grade astrocytomas. The enhancing components of pilocytic astrocytomas tend to have low perfusion with decreased rCBV, although nodules with increased perfusion may be seen.
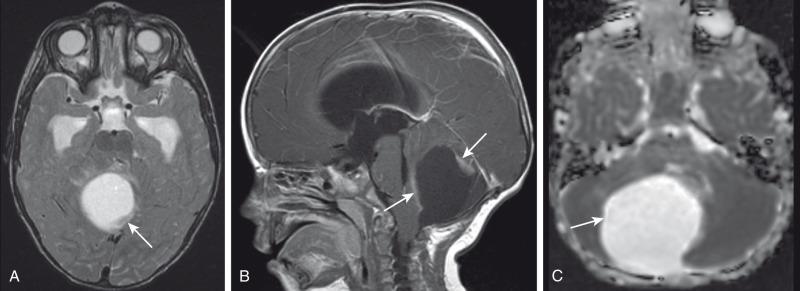
Rarely, pilocytic astrocytomas can present with diffuse leptomeningeal spread, most often seen in association with the diencephalic syndrome (discussed later) or with the pilomyxoid variant. These tumors have an indolent course, but their propensity for slow-growing persistent recurrences makes them difficult to treat. Pilomyxoid astrocytoma (PMA) represents a variant of classical PA with more invasive growth and increased risk of recurrences and dissemination.
Typically, pilocytic astrocytomas exhibit distinct histology with biphasic architecture of loose, microcystic and compact, fibrillary areas. However, some tumors display a heterogeneous histopathologic appearance that can mimic other glial neoplasms. Not infrequently, pilocytic astrocytomas exhibit atypical histologic features suggestive of anaplasia that can suggest a diffuse high-grade glioma. Genetic alterations resulting in aberrant signaling of the mitogen-activated protein kinase (MAPK) pathway have been implicated in the development of pilocytic astrocytomas. The presence of the most commonly identified BRAF-KIAA1549 fusion gene mutation may be associated with improved clinical outcomes.
Gross total resection is often curative, but micrometastases may occur. Recent treatment trials for unresectable recurrent disease have incorporated molecular BRAF status in low-grade glioma treatment with MEK inhibitor drugs. The role of Ras/MAPK alterations seems to be dominant in all pediatric low grade gliomas, but specifically in hemispheric tumors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here