Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In its role as a portal between the ambient environment and the internal milieu, the lung is also the most frequent site of serious infection. A variety of factors predispose to pulmonary infection, including distortions in lung anatomy, decreased mucociliary clearance, and abnormal cellular and humoral immunity. Iatrogenic immunosuppression and that resulting from human immunodeficiency virus type 1 (HIV-1) infection have led to the emergence of opportunistic infections that can present diagnostic challenges for the surgical pathologist.
Because a variety of microbes can potentially infect the lung and the histopathology of noninfectious conditions frequently mimic infection, the differential diagnosis of pulmonary infection is often broad. Although in many cases the clinical history, radiographic findings, and the noninvasive sampling of secretions can establish the cause of infection, lung biopsy is required at times. How to approach the sampling of the lung in infection is an area of some complexity, so that it is incumbent upon both clinicians and pathologists to recognize the advantages and limitations of the currently available methods.
The optimal approach to sampling the lung for infection depends primarily on whether disease is localized or diffuse ( Box 8.1 ). In immunosuppressed patients, diffuse pulmonary infiltrates due to infection can often be diagnosed by sputum induction or bronchoalveolar lavage (BAL). This is particularly the case when the microbial burden is also large. Noninvasive approaches are less sensitive than biopsy in diagnosing localized infections, and they cannot distinguish a colonizing commensal from an invasive pathogen. In addition, the lung biopsy affords an opportunity to evaluate host immunocompetence with accuracy that cannot be achieved by noninvasive or minimally invasive methods. Lung biopsy may also be required to exclude infection definitively and to establish noninfective diagnoses (e.g., acute lung injury due to chemotherapy).
Expectorated sputum
Induced sputum
Bronchoalveolar lavage
Fine-needle aspirate (1 mm)
Bronchial biopsy (1-3 mm)
Transbronchial biopsy (1-3 mm)
Transbronchial needle biopsy (1 mm)
Video-assisted thoracoscopic biopsy (2-3 cm)
Open-lung biopsy (2-3 cm)
Surgical lobectomy
Autopsy
Pathologists prefer to examine generous samplings of lung because diagnoses based on such biopsies are more accurate, yield a greater degree of information with respect to host immunity, and may reveal other potentially treatable disorders.
The lung has approximately the surface area of a tennis court; thus sampling error is an unavoidable pitfall in diagnostic pulmonary pathology. The transbronchial biopsy (TBB) preferentially samples peribronchiolar lung tissue, yielding tissue fragments of 1 to 3 mm in diameter. However, peripherally located lung lesions often cannot be accessed by this approach.
TBB is generally adequate for diagnosing diffuse pulmonary infections and peribronchiolar granulomatous diseases, such as sarcoidosis and lymphangitic spread of tumor or tuberculosis, but at times the findings of a TBB can be nonspecific and misleading. For example, “organizing pneumonia” in a TBB may represent a nonspecific reaction adjacent to focus of infection or malignancy, a nonspecific manifestation of chemotherapy effect, aspiration, or cryptogenic disease. For this reason, the findings gleaned from a TBB must always be thoughtfully correlated with clinical and radiographic findings.
Computed tomography (CT)-guided fine-needle aspiration biopsies have a high yield in the diagnosis of peripheral nodular infiltrates. Biopsies can be semiliquid or include a 1-mm core of tissue. When performed with the assistance of a cytotechnologist, rapid diagnoses can be proffered by examining stained smears directly at the bedside. Fine-needle aspirates are useful in diagnosing localized infections, and cytopathologists can also suggest the pattern of inflammation based on the types of inflammatory cell subsets in the sample and the presence or absence of necrosis.
Transbronchial needle aspiration biopsy of regional lymph node groups is often a low-yield procedure because nonspecific reactive regional lymphadenitis is common in the presence of pulmonary infection. The procedure is prone to artifacts that may present diagnostic difficulties for the surgical pathologist. However, when adopted judiciously, this approach may be adequate for the diagnosis of infection, as in one series in which approximately 50% of cases of tuberculous lymphadenitis were accurately diagnosed.
The technique of endobronchial ultrasound-guided transbronchial and needle biopsies has become standard procedure in many centers. This approach increases the diagnostic yield for localized lesions and allows for mediastinoscopic evaluation. It may be particularly helpful in patients with lung tuberculosis with mediastinal adenopathy.
Video-assisted thoracoscopic surgical (VATS) lung biopsy has replaced open thoracotomy biopsy as the optimal approach for obtaining large samples of lung. The procedure is associated with modest and acceptable morbidity, has the advantage of allowing direct access to widely separated lung segments, and provides generously sized wedge biopsies of 2 to 3 cm. Consequently, VATS should be considered a first line approach when a timely accurate diagnosis is essential.
Appropriate handling of the lung biopsy is essential for obtaining the highest diagnostic yield ( Fig. 8.1 ). Sampling the lung for microbiologic culture should ideally take place under sterile conditions in the operating room, but the pathologist processing the biopsy should try to ascertain that all necessary diagnostic tests have been ordered and be prepared to harvest additional samples for testing that may have been overlooked. When preparing tissue for microbiologic isolation, the lung should be minced rather than crushed because hyphate fungi (e.g., Zygomycoses spp.) may fail to grow in culture following maceration. A pathologist should not place a lung biopsy directly into fixative, without first considering a diagnosis of infection . If questions arise as to which tests to order or how best to transport the specimen to the laboratory, discussions with the hospital microbiology laboratory staff or a hospital infectious disease specialist will be of assistance.
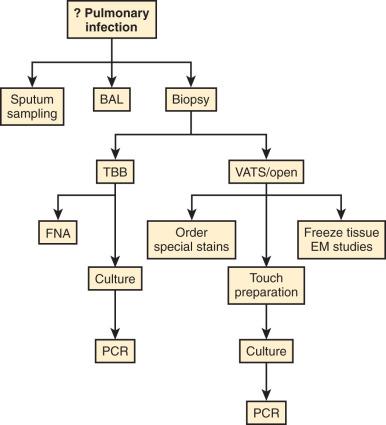
The examination of touch imprints of lung tissue is a simple and rapid way of identifying pathogens. Touch imprints can be prepared from foci of pulmonary consolidation, necrosis, or suppuration and rapidly stained for bacteria, mycobacteria, and fungi in the surgical pathology suite or the microbiology laboratory. Concomitantly biopsies may be harvested for ultrastructural analysis, polymerase chain reaction (PCR) assays, or research purposes. For large biopsies, it may be possible to inflate the lung with 5% formalin via a small (23 to 25) gauge needle to optimize subsequent histologic examination.
The diagnosis of infection requires both an interpretation of the morphologic changes evoked by the pathogen and the identification of a pathogen in situ. The pattern of pulmonary inflammation often suggests the route of entry of an infectious agent and may help to narrow the diagnostic possibilities. It is necessary to be familiar with the multiplicity of response patterns evoked by infection and to recognize that these can vary depending on the route of entry, pathogen load, and the competence of host defenses. For example, whereas herpesvirus 1 can produce a miliary pattern of fibrinoid necrosis in an immunosuppressed patient with viremia, it can also cause ulceration of the tracheobronchial mucosa in a chronically intubated patient.
Microbes are rarely identified randomly or diffusely in infections; rather, they tend to be compartmentalized, so that substantial effort may be wasted in searching for them where they are not likely to be found. Mycobacteria and fungi are usually localized in areas of necrosis; Rickettsia spp. and Bartonella spp. largely target the microvasculature; viruses tend to attack the airways; thus the surgical pathologist must both be acquainted with the pulmonary microanatomy and with the preferential localization of microbes in pulmonary tissues.
The lung is an elastic organ composed of dichotomously branching conducting airways terminating in alveolated surfaces. It has a dual blood supply; the pulmonary circulation arises from the cardiac right ventricle and carries deoxygenated blood at low arterial pressures to the alveolar surfaces; the bronchial circulation arises from branches of the aorta and nourishes both the airways and the connective tissue stroma with oxygenated blood. All new growths within the lung, including regions of infective bronchiectasis, lung abscess, and tuberculous cavities, will evoke neoangiogenesis from the bronchial microcirculation.
Two systems of pulmonary lymphatic channels drain the lung either centrifugally towards the hilum, or centripetally along the convexities of the pleural surfaces before coursing to the hilar lymph nodes. From the lymph nodes, organisms can enter the systemic circulation and spread widely throughout the body. For example, group A streptococci spp. will rapidly invade pulmonary lymphatics and course to the pleura to produce an empyema.
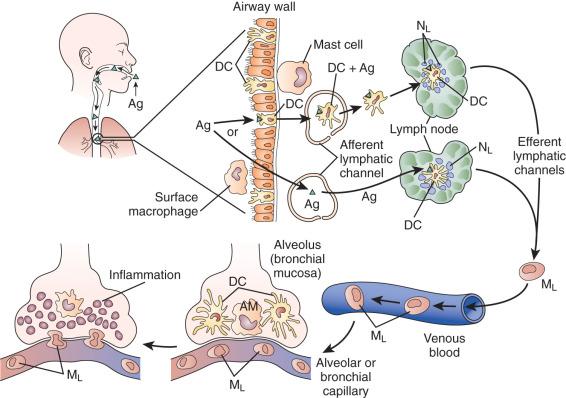
Most microbes are small (<5 µM) and can penetrate to the distal gas-exchanging surfaces of the lung, although the majority are excluded by the defenses of the upper airways or deposit along the conducting airways to be cleared by the mucociliary escalator. Humoral factors, including secretory immunoglobulin A (sIgA) and defensins released by airway cells, limit microbial penetration into tissues. Airway mucosal dendritic cells (DCs) trap microbial antigens and transport them to regional lymph nodes, where they are processed and present to both T and B lymphocytes, evoking adaptive immunity ( Fig. 8.2 ).
Ulceration or thickening of the gas-exchange surface limits diffusion of oxygen and carbon dioxide. For this reason, the alveolus is under normal conditions maintained sterile by resident macrophages that scavenge inhaled particulates and secrete monokines, including interleukin (IL)-10 and transforming growth factor (TGF)-β, that locally suppress inflammation and promote immunotolerance.
When the alveolar lining is injured or when the number of invading organisms exceeds the phagocytotic capacities of resident macrophages, neutrophils and exudate monocytes are recruited to sites of lung infection. Even small numbers of virulent pathogens can greatly amplify inflammation via the release of chemokines, cytokines, and complement by host immune cells. These defenses promote the clearance of infection but can also damage the lung. Lung biopsies afford the pathologist a unique opportunity to assess these dynamic responses directly, in addition to identifying a causative pathogen.
A number of generic patterns of inflammation may be evoked by infection, but how they are distributed is often specific to the involved tissue ( Box 8.2 ). Clinicians and radiologists have developed classification systems with respect to pulmonary infection that are distinct from those of pathologists. For example, a variety of infectious agents yield a picture that clinicians generically term “atypical” interstitial pneumonia, to differentiate them from “typical” bacterial pneumonias. However, the histopathology of an “atypical pneumonia” may be centered on the lung interstitium, small airways, or the alveolar spaces; and because this text is aimed primarily at surgical pathologists, pathologic schemas of classification will be adopted here, with reference to their clinical counterparts when appropriate.
Tracheobronchitis
Bronchiolitis (acute, chronic, necrotizing)
Bronchiectasis
Bronchopneumonia (acute, chronic, necrotizing)
Eosinophilic pneumonia
Pulmonary hemorrhage
Pulmonary edema
Diffuse alveolar damage
Pulmonary nodules and micronodules
Cavitary pneumonia
Vasculitis
Capillary dissemination
Lymphatic dissemination
Pulmonary hypertension
Pleuritis
Many pathogens target the conducting airways to produce tracheobronchitis and bronchiolitis. Pathologic changes range from superficial erosion of the lining respiratory epithelium, to ulceration and repair. The type of inflammation will vary from intralumenal neutrophilic exudates ( Fig. 8.3A ) to airway cuffing by lymphocytes and histiocytes (see Fig. 8.3B ), depending on the offending pathogen.
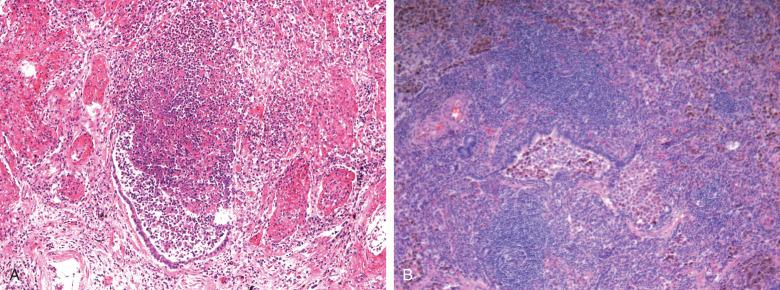
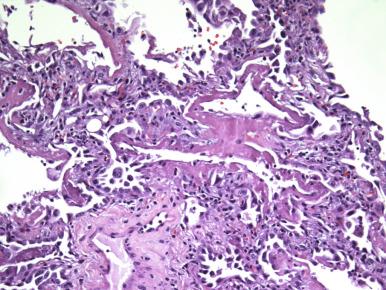
Disease of the gas-exchange alveolar surfaces can show a spectrum of changes, including acute ulceration and septal infiltration by chronic inflammatory cells. Diffuse alveolar damage (DAD) represents a global injury to the gas-exchange surfaces due to disruption of the blood–air barrier leading to exudative edema and fibrosis and resulting in severely impaired blood and tissue oxygenation ( Fig. 8.4 ). The sine qua non of DAD is the hyaline membrane that is composed of necrotic alveolar lining cell debris and an extravascular fibrin coagulum apposed to an ulcerated alveolar wall, which yields a gel that entraps lung water ( Fig. 8.5 ). Although DAD is the most frequent pathologic cause of the clinical entity, the adult respiratory distress syndrome (ARDS), other diseases, including extensive bronchopneumonia and acute pulmonary hemorrhage, can also lead to ARDS ( Box 8.3 ). The pathology of the exudative phase of DAD can focally mimic acute bacterial infection, and one must maintain a high threshold for making the diagnosis of acute infection in this setting ( Fig. 8.6 ).
Pulmonary infection
Systemic infection with sepsis
Vasodilatory shock
Aspiration
Drugs
Radiation
Trauma
Accelerated phase of chronic interstitial pneumonia
Idiopathic
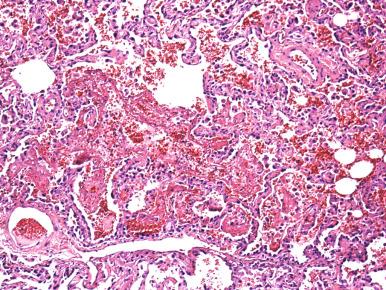
Viruses are the most common infectious cause of DAD, although bacteria, fungi, and parasites also produce diffuse lung injury. DAD can result from sepsis that complicates either pulmonary or extrapulmonary infection. Whenever DAD is present, the surgical pathologist must examine the lung for evidence of viral-induced cytopathic changes. These vary with the type of viral infection, and some viruses do not produce cytopathic changes, so that viral infection is always in the differential diagnosis of DAD ( Table 8.1 ). A common pitfall in diagnosis is to mistake the hyperplastic reparative alveolar type II cells of DAD that can exhibit prominent nucleoli with viral infected cells, particularly when examining rapidly frozen sections, in which these changes may be especially prominent ( Fig. 8.7 ).
| Organism | Cytopathic Change |
|---|---|
| Influenza | No cytopathic change |
| SARS (Coronavirus) | No cytopathic change |
| Respiratory syncytial virus | Polykaryons, inconspicuous cytoplasmic inclusions |
| Parainfluenza | Polykaryons, intracytoplasmic inclusions |
| Measles | Polykaryons, intranuclear inclusions |
| Adenovirus | Intranuclear inclusions (smudge cells) |
| Herpesvirus | Intranuclear inclusions, polykaryons |
| Cytomegalovirus | Intranuclear and cytoplasmic inclusions |
| Varicella-Zoster | Intranuclear inclusions |
| EBV | No cytopathic change |
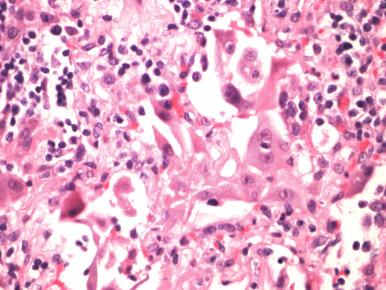
Due to the introduction of noninvasive and rapid PCR diagnoses of respiratory viruses from nasal swabs and respiratory secretions, it has become increasingly unusual to encounter these infections in the routine practice of surgical pathology, except at autopsy in lethal cases.
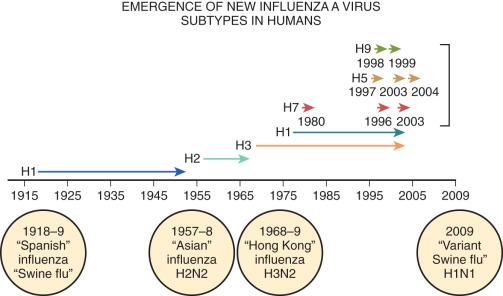
Influenza is a rod-shaped RNA virus that can cause either bronchiolitis or DAD without apparent cytopathic changes. Influenza infection recurs each year due to a high incidence of mutation of its hemagglutinin (H) and neuraminidase (N) antigens, and these determine its virulence. When mutations occur concomitantly in both the H and N antigens, pandemics with potentially high degrees of morbidity due to the lack of immunity may ensue ( Fig. 8.8 ). H1N1 influenza was responsible for an epidemic in this country, and sporadic cases have persisted. Patients dying with this infection were often relatively young and their lungs showed DAD with prominent areas of hemorrhage. Currently, epidemiologists are carefully monitoring the evolution of an avian influenza in Southeast Asia for evidence of spread to humans.
Influenza is the most common cause of viral pneumonia, although most cases are subclinical. The virus most commonly causes a diffuse tracheobronchitis/bronchiolitis in which the normal ciliated respiratory epithelium is sloughed. However, when DAD develops, it can carry a high mortality even in the absence of acute bacterial superinfection ( Fig. 8.9 ). The lungs in DAD due to influenza in patients with prolonged survival often develop prominent squamous metaplasia of bronchoalveolar lining cells ( Fig. 8.10A ). Although these findings are characteristic, they are also nonspecific, so that immunostains, in situ hybridization, electron microscopy, or viral antigen detection may be required to establish the diagnosis (see Fig. 8.10B ). Superinfection by pyogenic bacteria, including Haemophilus influenza , group A Streptococcus , and Staphylococcus are a well-recognized complication and may mask evidence of a healing influenza infection.
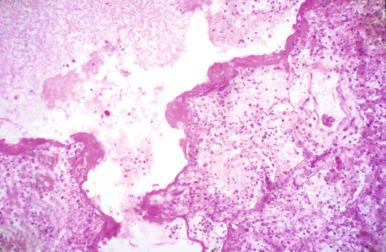
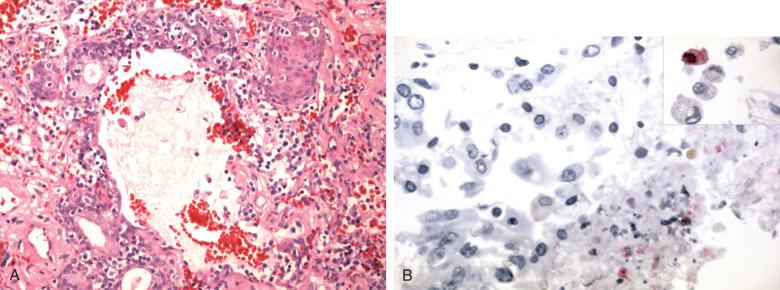
The epidemic of the zoonotic coronavirus infection termed severe acute respiratory syndrome (SARS) fortunately has not recurred, as the virus led to acute respiratory distress with high mortality. The lungs at autopsy showed DAD with scattered multinucleated giant cells of uncertain diagnostic significance. The virus otherwise produced no cytopathic changes and was essentially histologically indistinguishable from DAD due to influenza.
Like SARS, Middle East respiratory syndrome (MERS) is also caused by a coronavirus. The first cases were reported in Saudi Arabia in the fall of 2012, but cases had occurred in Jordan earlier that year. All cases of MERS to date have been linked through travel to or residence in countries in and near the Arabian Peninsula. The largest known outbreak of MERS outside the Arabian Peninsula occurred in the Republic of Korea in 2015 and was associated with a traveler returning from the Arabian Peninsula. MERS-Corona virus (Co-V) spreads from ill people to others via close contact, such as caring for or living with an infected person, and patients have ranged in age from infants to nonagenarians. Unfortunately, the detailed histopathology of patients with MERS has not been reported but is likely to resemble that due to other coronaviruses.
Respiratory syncytial virus (RSV) causes a benign respiratory infection in older children and has been recognized as a cause of adult community-acquired pneumonia, acute bronchiolitis, and DAD in the immunosuppressed host. The infection targets the respiratory lining epithelium producing syncytial giant cells with nonprominent eosinophilic inclusions ( Fig. 8.11 ). Human metapneumovirus produces changes comparable to RSV and must be included in its differential diagnosis.
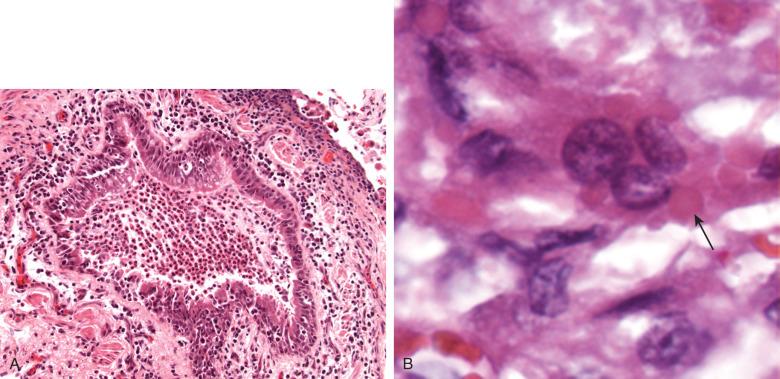
Parainfluenza causes a benign upper respiratory infection in children that rarely progresses to DAD, although severe disease may develop in the immunosuppressed host. Like RSV, parainfluenza produces bronchiolitis and DAD with syncytial giant cells and epithelial cell intracytoplasmic inclusions. However, the latter are both more frequent and larger than those seen in RSV ( Fig. 8.12 ).
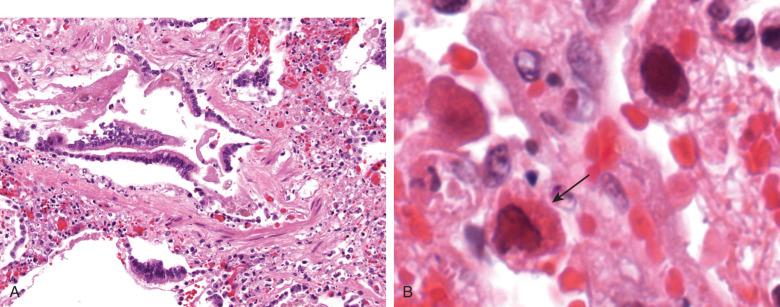
Measles pneumonia is a rare and serious complication of the childhood viral exanthem. The pathology of pulmonary measles infection ranges from bronchiolitis ( Fig. 8.13A ) to DAD. The virus produces multikaryons with prominent glassy eosinophilic nuclear Cowdry type A inclusions (see Fig. 8.13B ). The differential diagnosis of giant cell pneumonia includes RSV and hard-metal pneumoconiosis; however, the giant cells in the latter disorders lack intranuclear inclusions, and hard-metal pneumoconiosis specifically lacks the exudative features of an acute infection.
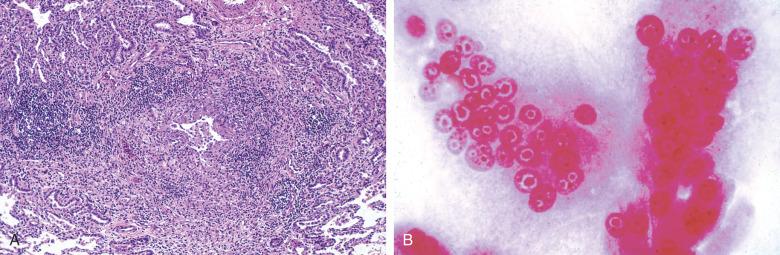
Adenovirus primarily affects the immunosuppressed host but can produce outbreaks in healthy subjects living at close quarters (e.g., military recruits). Adenovirus typically produces (1) ulcerative bronchiolitis with karyorrhexis ( Fig. 8.14A ), (2) neutrophilic pneumonia (see Fig. 8.14B ), (3) acute intrapulmonary necrosis with hemorrhage (see Fig. 8.14C ), or (4) DAD. Infected cells can exhibit amphophilic intranuclear inclusions with perinuclear clearing that mimic herpesvirus infection but more characteristically produce “smudge cells” (see Fig. 8.14C ), showing hyperchromatic nuclei extruding beyond the confines of their nuclear membranes.
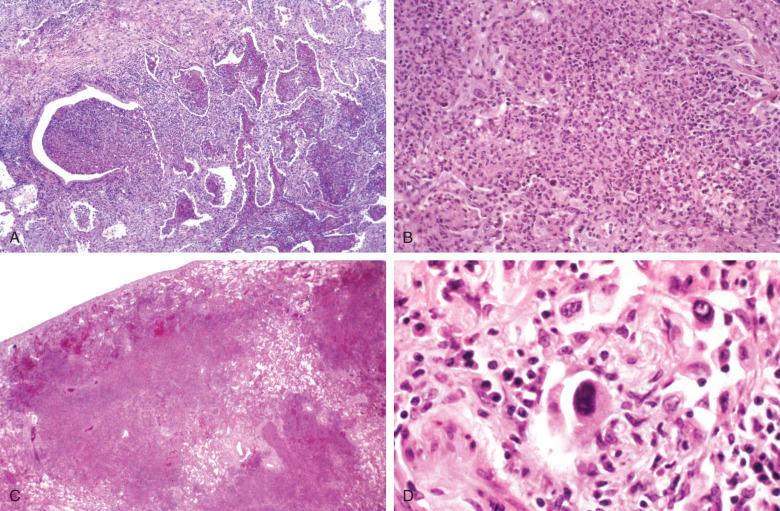
The appearance of “smudge cells” can be mimicked by pulmonary cytotoxic drug injury or by epithelial repair in the early proliferative phase of DAD. For this reason and because of the potential overlap with herpesvirus-induced cytopathic changes, the diagnosis of adenovirus infection should always be confirmed by immunohistochemical staining, ultrastructural examination, or viral isolation.
Cytomegalovirus (CMV) occurs at the extremes of age or as a result of immunosuppression, and it is a common infection in HIV/acquired immune deficiency syndrome (AIDS). The number of cells showing cytopathic changes can vary considerably and parallels the severity of infection. CMV primarily targets pulmonary macrophages and endothelial cells, but virtually any cell can show cytopathic features. The most common distribution is blood-borne miliary disease ( Fig. 8.15 ), but bronchiolitis and DAD also occur. The diagnostic features of infection are (1) cytomegaly, (2) intranuclear inclusions with characteristic Cowdry type B inclusions ( Fig. 8.16A ), and (3) ill-defined amphophilic intracytoplasmic inclusions that are seen with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and Gomori methenamine silver (GMS) stains (see Fig. 8.16B ). In patients receiving prophylactic treatment with antivirals, CMV infection may fail to exhibit cytopathic changes. However, immunostains and in situ hybridization will continue to identify intracellular CMV antigens (see Fig. 8.16C ).
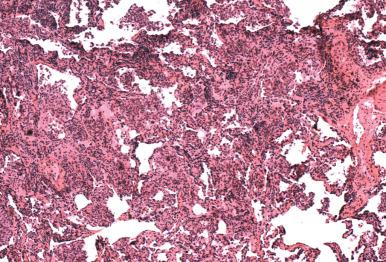
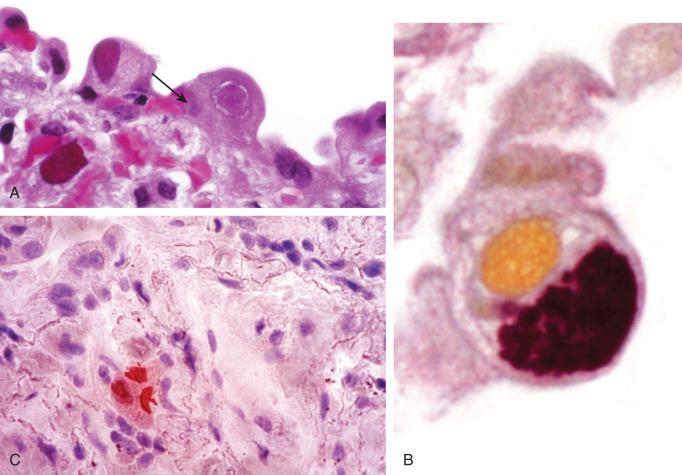
CMV is a frequent copathogen in the immunosuppressed patient and a cause of immunosuppression in its own right. Patients with active pulmonary infections will generally have circulating CMV DNA as judged by PCR assay of peripheral blood. CMV infection can also be seen together with other viral infections, Pneumocystis jiroveci , or opportunistic fungal infections ( Fig. 8.17 ).
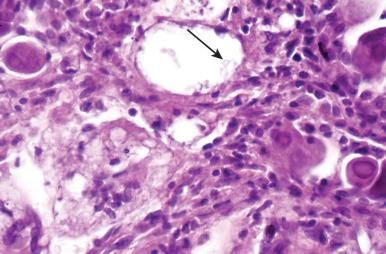
Herpesvirus types 1 and 2 both infect the lung. The incidence of herpesvirus infection increases with immunosuppression and when mucosal barrier defenses have been breached. Herpesvirus characteristically elicits a prominent neutrophilic response that mimics pyogenic bacterial infection, but foci of necrosis, cell karyorrhexis, and piled up virus-infected cells with amphophilic nuclei confirm the diagnosis ( Fig. 8.18 ). Diagnostic cytopathic changes include type A or type B Cowdry nuclear inclusions showing molding of adjacent cells with multikaryon formation ( Fig. 8.19 ). Immunosuppressed patients with herpesvirus viremia develop miliary foci of hemorrhagic necrosis with prominent fibrinous exudates ( Fig. 8.20A and B ). In a recently reported case, herpesvirus type 2 was demonstrated to occur as a superinfection in a patient recovering from a bacterial superinfection of influenza pneumonia (see Fig. 8.20C ).
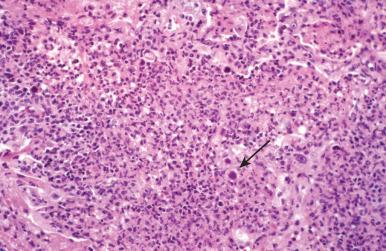
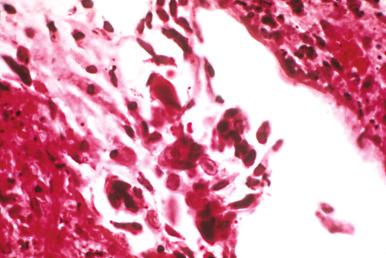
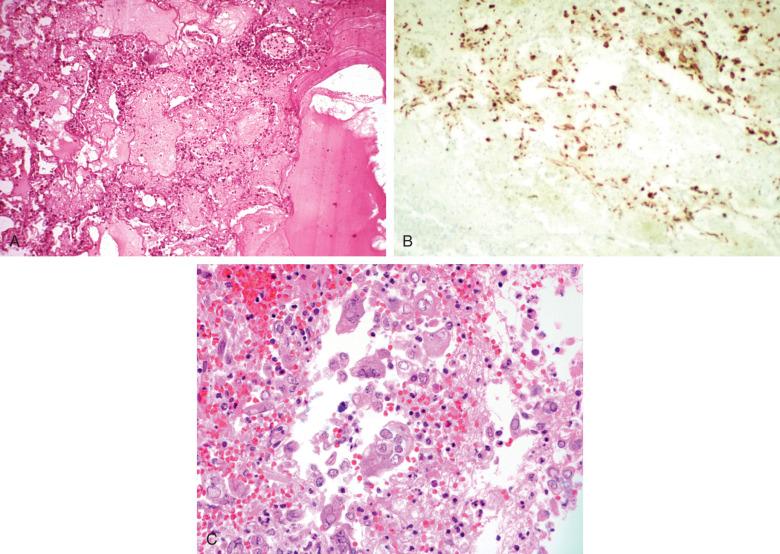
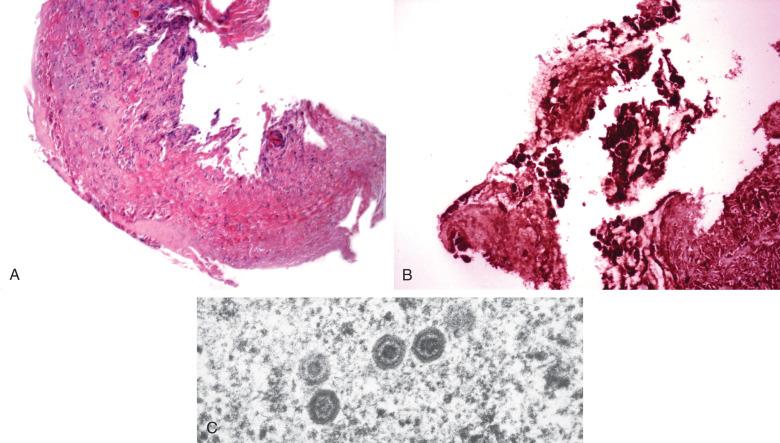
Herpesvirus pulmonary infections also develop in patients with structural abnormalities of the airways or as complications of primary infections of the oropharynx and esophagus. Intubated patients receiving chronic ventilatory support are at increased risk due to local barotrauma from inflated endotracheal tubes. The respiratory mucosa is the primary target ( Fig. 8.21 ). At times, extensive necrosis of an ulcerated airway suggests the diagnosis, but immunostaining for herpes viral antigen can demonstrate high background staining, obscuring the diagnosis. When this is the case, examining paraffin-embedded tissues by electron microscopy can reveal diagnostic virions ( Fig. 8.22A to C ).
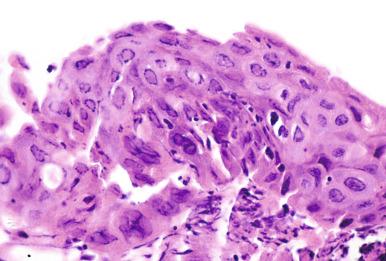
Varicella zoster pneumonia is a rare complication of the childhood chickenpox, and it is more commonly encountered as the consequence of reactivated virus in the immunocompromised host. Following nonlethal pulmonary infections in childhood, the lung shows multiple calcified miliary lesions. Cases coming to biopsy or autopsy show miliary nodules of hemorrhagic necrosis in lung and pleura or DAD ( Fig. 8.23 ). Infected cells with primarily Cowdry type A inclusions may be seen at the edges of the lesion, but they are harder to identify than in herpesvirus pneumonia.
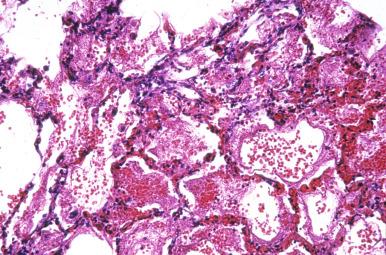
This virus produced an epidemic in the four corners region of the southwestern United States in 1993. The infection is a zoonosis transmitted by infected rodent feces. The most common radiographic presentation is diffuse pulmonary edema with pleural effusions mimicking congestive heart failure. Histologically the lung shows pulmonary edema with scant poorly formed hyaline membranes ( Fig. 8.24A ), with atypical lymphocytes circulating within the pulmonary vasculature (see Fig. 8.24B ). Confirmation of the diagnosis requires specific immunohistochemistry, serologic evidence of hantavirus-specific IgM, PCR, or ultrastructural identification of the causative virions.
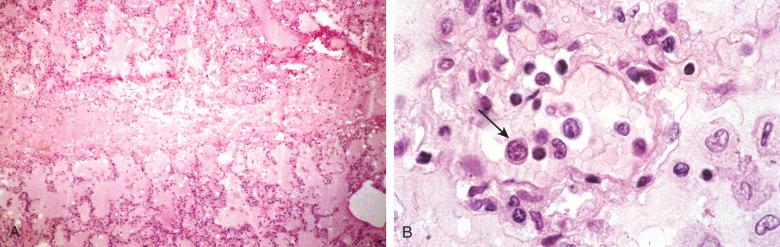
Mycoplasma are the smallest (0.2 to 0.8 µM) free-living bacteria, but they lack a true cell wall. They are facultative anaerobes, except for Mycoplasma pneumoniae , the most common pulmonary pathogen, which is a strict aerobe. Mycoplasma pneumonia occurs worldwide with no increased seasonal activity, but epidemics predictably occur every 4 to 8 years. Although primarily an infection of young adults, it can attack older adults. The most common clinical syndrome is tracheobronchitis, with one-third of patients developing a mild but persistent pneumonia.
Mycoplasma pneumonia is rarely biopsied, because positive cold agglutinin and specific complement fixation antigen assays will establish the diagnosis. Biopsied cases show lymphocytic or neutrophilic bronchiolitis with alveolar wall inflammation and fibrinous exudates ( Fig. 8.25 ). Similar changes are seen in both Chlamydia ( Fig. 8.26 ) and Coxiella pneumonia.
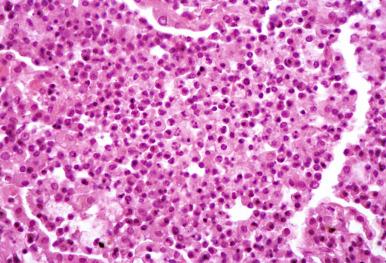
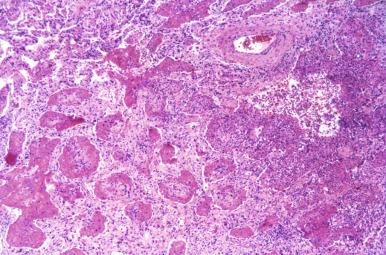
Epstein-Barr virus (EBV) has been implicated in disorders ranging from the mononucleosis syndrome to malignant lymphoid neoplasia. The mononucleosis syndrome includes pharyngitis, lymphadenitis, and hepatosplenomegaly. Pulmonary involvement can occur as part of the syndrome, but it is unusual and rarely biopsied. EBV pneumonia shows patchy peribronchiolar and interstitial polyclonal lymphoid infiltrates with scant interstitial and intra-alveolar fibrin exudates ( Fig. 8.27 ). The diagnosis is generally established serologically by EBV antigen titers but can be confirmed by in situ hybridization.
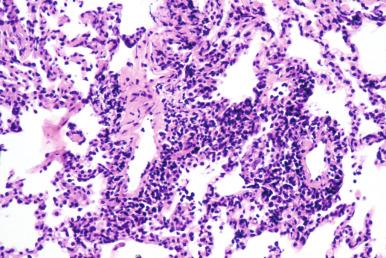
For years, the organism formerly known as Pneumocystis carinii was thought to be a protozoon; however, it is now confidently classified as a fungus. Originally described as a plasma-cell interstitial pneumonia in malnourished children, it was subsequently seen in patients with hematologic malignancies and in those receiving chemotherapy or chronic corticosteroids. In the 1980s pneumocystis pneumonia became a signal infection in establishing the diagnosis of AIDS. It was encountered in epidemic proportions until prophylactic use of trimethoprim-sulfa (Bactrim) became a routine aspect of HIV/AIDS management. P. jiroveci is currently most often diagnosed by sputum induction, and lung biopsy is reserved for diagnostic challenges.
Although the disease presents radiographically as an atypical “interstitial” pneumonia, interstitial inflammation is not its most striking pathologic feature. In pneumocystis pneumonia the alveoli are filled with a frothy eosinophilic exudate that mimics exudative pulmonary edema or alveolar lipoproteinosis ( Fig. 8.28A ). The pulmonary interstitium shows a mild plasma cell–rich pneumonitis and prominent alveolar type II cell hyperplasia.
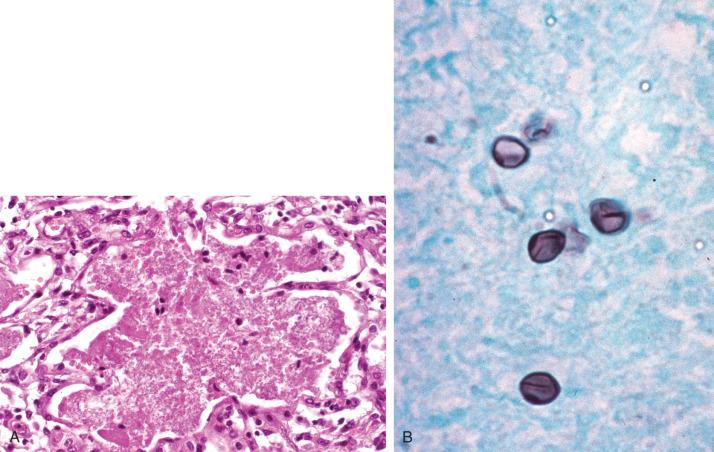
The diagnosis is confirmed by the presence of oval-, helmet-, or crescentic-shaped GMS-positive “cysts,” 4 to 6 µM in greatest dimension, within the alveolar froth (see Fig. 8.28B ). Pneumocystis is distinguished from GMS-positive fungal yeast forms by the absence of budding, pericapsular accentuation, and a so-called intracytoplasmic dot, in the former. However, when these features are absent and when the organism load in the biopsy is low, it may be difficult to confidently exclude Histoplasma or Cryptococcus. In these cases, specific immunohistochemistry can confirm the diagnosis.
Multiple patterns of unusual host reactions to P. jiroveci have been recognized. These include DAD ( Fig. 8.29A ), solitary necrotizing granulomas (see Fig. 8.29B ), miliary infection, lymphoid interstitial pneumonia, and regional lymphadenitis. Microcalcifications may be seen in areas of infection, and thin-walled cyst formation may develop.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here