Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Michael Jansen, Arie Perry, Reid R. Heffner, Jr., and David N. Louis were authors of this chapter in the previous edition.
Tumors of the nervous system, like other human neoplasms, are dysregulated clonal proliferations that arise secondary to changes in cellular genetics and physiology. In many cases, alterations involving growth-promoting oncogenes, growth-checking tumor suppressors, and cell death genes are involved in brain tumorigenesis. However, recent work in brain tumors as well as other cancer types has expanded conventional notions of cancer-related genes, implicating chromatin-binding proteins and other regulators of cellular epigenetics in the oncogenic process ( ). Some of the specific genes affected in human nervous system tumors are discussed with their respective tumor types later in this chapter. Because oncogenic transformation requires cell division, postmitotic cells, such as neurons, should not be susceptible to tumorigenic events. However, it remains possible that nervous system tumors arise when oncogenic changes occur in precursor cells rather than mature nervous system components. Such precursor cells reside in the brain into adult life ( ).
While much progress has been made in recent years in characterizing the underlying genetics and epigenetics of brain tumors, precise etiological mechanisms remain unclear. To date, radiation and hereditary predisposition are the only clearly implicated factors in the genesis of nervous system tumors. See Chapter 71 for a discussion of ionizing radiation as a risk factor for specific types of brain tumors. Hereditary predisposition to brain tumors is rare and confined to specific syndromes (e.g., neurofibromatosis type 1 [NF1], neurofibromatosis type 2 [NF2], tuberous sclerosis, von Hippel–Lindau disease, Li-Fraumeni syndrome, Turcot syndrome, and Gorlin syndrome). These conditions highlight the critical roles played by specific genes in brain tumorigenesis. Tumors arising with these syndromes are discussed at multiple points within this chapter.
The foundation of the first comprehensive classification of nervous system tumors, formulated by Percival Bailey and Harvey Cushing in 1926, presumed parallels between embryological and neoplastic cells. In large part, this histogenetic model based on the presumed cell of origin still forms the basis for current nomenclature, although molecular factors are becoming increasingly important. In 1949, as a means of enhancing the clinical utility of tumor classification, Kernohan contributed a tumor grading system whose purpose was assessing patient prognosis. Russell and Rubinstein modified and updated the Bailey and Cushing system during the 1960s, 1970s, and 1980s. Further updates were incorporated into the World Health Organization (WHO) classification, first completed in 1979 and then revised in 1993, 2007, and most recently in 2016 ( ). The WHO classification has become the system most widely used by neuropathologists today. The latest 2016 effort is the first to incorporate defining molecular features into specific diagnostic entities.
The WHO classification currently lists more than 100 types of nervous system tumors and their variants. This level of complexity may seem daunting at first, but consideration of key clinical and imaging characteristics typically narrows the differential diagnosis to only a few common possibilities ( Table 72.1 ). Specific differential diagnoses vary substantially for supratentorial versus infratentorial, pediatric versus adult, and enhancing versus nonenhancing tumors.
| Location | Child/Young Adult | Older Adult |
|---|---|---|
| Cerebral/supratentorial | Ganglioglioma (temporal lobe, cyst-MEN) DNT (temporal lobe, intracortical nodules) AT/RT (infant) |
Grade II–III glioma (NE) Glioblastoma (ring E, butterfly) Metastases (gray/white junctions, E) Lymphoma (periventricular, E) |
| Cerebellar/infratentorial | Pilocytic astrocytoma (cyst-MEN) Medulloblastoma (vermis, E) Ependymoma (fourth ventricle, E) Choroid plexus papilloma (fourth ventricle) AT/RT (infant) |
Metastases (multiple, E) Hemangioblastoma (cyst-MEN) Choroid plexus papilloma (fourth ventricle) |
| Brainstem | Diffuse midline glioma Pilocytic astrocytoma (dorsal brainstem) |
Gliomatosis cerebri (multifocal) |
| Spinal cord (intra-axial) | Ependymoma Pilocytic astrocytoma (cystic) |
Ependymoma Diffuse astrocytoma (ill-defined) Paraganglioma (filum terminale) |
| Extra-axial/dural | Secondary lymphoma/leukemia | Meningioma Metastases Secondary lymphoma/leukemia |
| Intrasellar | Pituitary adenoma Craniopharyngioma Rathke cleft cyst |
Pituitary adenoma Rathke cleft cyst |
| Suprasellar/hypothalamic/optic pathway/third ventricle | Germinoma/germ cell tumor Craniopharyngioma Pilocytic astrocytoma |
Colloid cyst (third ventricle) |
| Pineal | Germinoma/germ cell tumor Pineocytoma Pineoblastoma Pineal cyst |
Pineocytoma Pineal cyst |
| Thalamus | Pilocytic astrocytoma Anaplastic astrocytoma/glioblastoma |
Anaplastic astrocytoma/glioblastoma Lymphoma |
| Cerebellopontine angle | Vestibular schwannoma (NF2) | Vestibular schwannoma Meningioma |
| Lateral ventricle | Central neurocytoma SEGA (tuberous sclerosis) Choroid plexus papilloma Choroid plexus carcinoma (infant) |
Central neurocytoma SEGA (tuberous sclerosis) Choroid plexus papilloma Subependymoma |
| Nerve root/paraspinal | Neurofibroma (NF1) MPNST (NF1) |
Schwannoma Meningioma Secondary lymphoma Neurofibroma (NF1) MPNST |
Light microscopic examination of hematoxylin and eosin–stained sections is the basis of most classification and grading systems for nervous system tumors. The approach to nervous system neoplasia recapitulates that used to classify human tumors from all parts of the body; thus, many neuropathological terms derive from the discipline of general pathology. The fundamental concept of grading utilizes distinct histopathological features to stratify nervous system tumors on the basis of anticipated biological behavior. In the WHO scheme, grade I is equivalent to benign; II is low-grade malignant; III is intermediate-grade malignant, often associated with the term anaplastic ; and IV is high-grade malignant. Grading , which depends on histological assessment, should not be confused with tumor staging , which depends on gross/radiographic characteristics and extent of tumor spread.
Intraoperative frozen sections are more difficult to interpret than fixed tissue sections, but their use has the advantage of speed, requiring only a few minutes to prepare. This technique is requested for several reasons, including simple curiosity and a desire to provide rapid feedback to patients and their families. However, the most important reasons for performing this technique are to ensure that the pathologist obtains a representative sample for permanent sections and to provide information that may alter the surgical procedure. For example, a neurosurgeon may opt to stop at a limited biopsy for a suspected lymphoma, aggressively resect for an ependymoma, or send additional material to the microbiology laboratory for an abscess. Because the process of tissue freezing produces significant cytological artifacts, another popular technique has been the preparation of touch imprints, or smears, from fresh tissue. Although underlying tissue architecture is lost, sample requirements are minimal, and cytological preservation is typically excellent. This may be critical for diagnoses based primarily on nuclear cytology, such as reactive gliosis versus low-grade diffuse glioma. It is also important to realize that the frozen section technique results in substantial tissue loss and morphological artifacts in the thawed residual specimen. Therefore, it is best to omit its use in limited biopsies for which the surgical procedure will not be altered. In other words, it is imperative to save at least some optimally fixed nonfrozen tissue for final diagnosis on sections from paraffin-embedded material.
Immunohistochemistry is an ancillary diagnostic technique for detecting protein expression within tumor cell nuclei, cytoplasm, and cell membranes. It is used most commonly to determine lines of cellular differentiation, proliferation indices, or the status of disease-defining molecular markers. Monoclonal antibody technology and recently improved antigen-retrieval methods have greatly expanded the versatility of this technique in routine formalin-fixed paraffin-embedded tissue, although a number of pitfalls remain, and considerable experience is necessary to make accurate determinations. Commercial antibodies vary greatly in terms of sensitivities, specificities, and clinical utilities. Most laboratories use the immunoperoxidase technique because staining is permanent and the reaction product is visible by conventional light microscopy. The application of several antibodies is usual for studying the more common CNS tumors:
Lineage markers : Glial fibrillary acidic protein (GFAP), an intermediate filament, remains the primary molecular marker used to designate glial cell lineage. Although it is commonly considered an astrocytic marker, it does not reliably distinguish astrocytic from oligodendroglial or ependymal ontogeny. Likewise, some degree of glial differentiation or GFAP expression may occur in choroid plexus tumors, medulloblastomas, gangliogliomas, and even some peripheral nerve sheath and cartilaginous tumors.
Neurofilaments are heteropolymers composed of three subunits with molecular weights of 68, 150, and 200 kD that are unique to neurons and their axonal processes. Normal neurons and mature neuronal tumors (e.g., gangliogliomas) stain for neurofilament protein, although primitive neuronal tumors, such as medulloblastoma, are often negative. Nevertheless, the staining of axons has great utility for highlighting a tumor’s growth pattern. For example, discrete tumors such as metastases and ependymomas will push axon-bearing parenchyma to the side, whereas diffuse gliomas will contain entrapped neurofilament protein-positive axons within their substance. Another commonly utilized neuronal cell marker is synaptophysin , a 38-kD glycosylated polypeptide that is a component of presynaptic vesicle membranes. It is a relatively reliable marker of neuronal differentiation and is typically found even in the most primitive neuronal tumors, such as medulloblastoma. This marker, along with chromogranin , is also useful for highlighting normal and neoplastic ganglion cells, as well as neuroendocrine tumors such as pituitary adenomas, carcinoids, and paragangliomas. Lastly, NeuN (neuronal nuclear antigen), a protein marker of relatively mature neuronal differentiation, has the advantage of clearly marking tumor nuclei rather than surrounding neuropil, but it also clearly labels normal neurons.
S100 protein expression is common to neuroectodermal cells, including melanocytes, glia, Schwann cells, chondrocytes, and the sustentacular cells in tumors such as paraganglioma, pheochromocytoma, and olfactory neuroblastoma. To a lesser extent, it also stains neuronal tumors and fibrous meningiomas. S100 protein is particularly useful for demonstrating Schwann cell differentiation in benign and malignant peripheral nerve sheath tumors. Cytokeratins are a class of intermediate filaments primarily expressed in epithelial cells. Antibodies against cytokeratin are commonly useful in the diagnosis of metastatic carcinomas but can also identify craniopharyngiomas, chordomas, and choroid plexus tumors. Epithelial membrane antigen (EMA) is a glycoprotein constituent of normal and neoplastic epithelial cells. In the central nervous system (CNS), EMA is a particularly useful marker for meningiomas, which unlike true epithelial tumors, display minimal to no cytokeratin expression in most cases.
Cell proliferation markers : The simplest and least expensive method for estimating cellular proliferation is the mitotic index , usually expressed as either the average or the maximal attainable count per 10 high-power fields (HPF). Difficulties in this type of proliferative assessment stem from pyknotic nuclei mimicking mitoses in poorly preserved specimens, variable field sizes among microscopes, and lack of uniform methods for counting. Underestimates of the proliferation index are common as well, given that the cells undergoing mitosis represent only a small fraction of cycling cells within a tumor.
Most proliferation antigens are nuclear constituents manifested during one or more proliferative phases in the cell cycle, allowing the recognition of cycling cells by routine immunohistochemistry. These markers are already valued in the diagnosis and management of brain tumors. The monoclonal antibody Ki-67 (also known as MIB-1 ) binds to a human nuclear protein expressed during the cellular growth fraction (G1, S, G2, and M phases of the cell cycle). In most tumor types, the Ki-67 or MIB-1 labeling indices increase with degree of malignancy. Phospho-histone H3 (pHH3) is a protein expressed exclusively during mitosis, and accordingly labels mitotically active cells, localizing to the spindle formation itself. Automated, computer-assisted algorithms to precisely quantify the extent of Ki-67 and/or pHH3 labeling further enhance the utility of both methodologies to accurately ascertain proliferative index.
Disease-defining and disease-associated markers : Several proteins directly involved in the pathogenesis of brain tumors are assessable by immunostaining. These include markers of diagnostic significance, such as IDH1 R132H, p53, ATRX, and BRAF V600E as well as markers reflecting the activation state of important oncogenic signaling networks, such as PTEN. Widespread use of markers like these has greatly facilitated the incorporation of molecular criteria into brain tumor diagnostics. Their precise application is discussed more extensively with associated tumor entities below.
As stated above, tumors arise when alterations occur in key molecular mechanisms governing cell growth, proliferation, differentiation, and cell death. The recent identification of many such alterations has greatly clarified the pathogenesis of brain tumors and led to significant revisions of the WHO classification scheme ( Table 72.2 ). Relevant molecular abnormalities are detectable at the level of genomic DNA, messenger ribonucleic acid (mRNA), and protein. Some, such as large chromosomal translocations and/or deletions, can even be visualized cytogenetically as “signature” events. However, most abnormalities are undetectable by routine karyotyping and must be ascertained with molecular approaches. Focused assays based on loss of heterozygosity (LOH), fluorescence in situ hybridization (FISH), and quantitative polymerase chain reaction (PCR) enable the detection of specific biomarkers of interest (e.g., 1p/19q codeletion) at relatively low cost, as do immunohistochemical surrogates, which are available for IDH1 R132H, ATRX, BRAF V600E, and INI1, among others. However, the increasing requirement for more sophisticated disease stratification based on multiple biomarkers of interest has necessitated the development of multiplexed assays, which have now been adopted as clinical platforms in most large medical centers. These most notably include next-generation sequencing approaches that assess mutational profiles in hundreds of genes simultaneously ( ), RNA sequencing techniques to detect fusion transcripts ( ), and array-based technology to determine global DNA copy number ( ). Finally, recent work has demonstrated that global DNA methylation profiling effectively segregates primary neuroepi-thelial tumors by lineage and molecular features, designating previously unappreciated diagnostic categories ( ). The importance of methylation profiling to routine brain tumor classification is likely to become evident in the next several years.
| Pilocytic astrocytoma | KIAA1549-BRAF fusion , BRAF p.V600E |
| Rosette-forming glioneuronal tumor | PIK3CA and FGFR1 |
| Ganglioglioma | BRAF p.V600E, KRAS , FGFR1/FGFR2 |
| Pleomorphic xanothoastrocytoma | BRAF p.V600E |
| Low-grade diffuse astrocytoma | IDH1/IDH2, ATRX, TP53 |
| Ependymoma | C11orf95-RELA fusion (supratentorial location) |
| Oligodendroglioma | IDH1/IDH2, TERT promoter, 1p deletion, 19q deletion |
| Glioblastoma (adults) | EGFR, PTEN, CDKN2A, TERT promoter, NF1 |
| Glioblastoma (pediatric) | H3F3A (H3.3 p.K27M—diffuse midline glioma) (H3.3 p.G34R/V—hemispheric location) |
| ETMR | C19MC amplification |
| Craniopharyngioma | Adamantinomatous type— CTNNB1 Papillary type—BRAF p.V600E |
| Primary CNS lymphoma | MYD88 p.L265P |
Primary neuroepithelial tumors are nervous system neoplasms that arise from resident cells of origin within the CNS (i.e., glia, neurons, or their precursors).
Gliomas constitute the largest group of neuroepithelial tumors from a clinical standpoint, and the term diffuse glioma encompasses all widely infiltrative glioma subtypes: astrocytomas and oligodendrogliomas (grade II to III), and glioblastoma (grade IV). While the histopathology and molecular foundations of these tumors vary, they are united by a shared tendency to widely infiltrate surrounding brain, a property that renders them incurable at present. Recent large-scale molecular profiling efforts have established striking biological distinctions between adult and pediatric gliomas, as well as between WHO grade II–III and WHO grade IV gliomas (see discussion in the sections that follow).
Diffuse astrocytoma is a slow-growing tumor with a median age at the time of diagnosis of 35 years. Radiographically, these tumors are ill-defined nonenhancing cerebral masses with a small proportion of diffuse astrocytomas occurring in the spinal cord, cerebellum, and brainstem. Microscopically, these tumors are characterized by hypercellularity, mild cellular pleomorphism, and a low mitotic count. Genetically, most diffuse astrocytomas exhibit a combination of mutations in the IDH1, ATRX , TP53 genes (see below). This molecular signature is a strong prognostic indicator in diffuse gliomas. Astrocytomas with an IDH1 or IDH2 mutation have a better prognosis than IDH1/IDH2 -wild-type astrocytomas. Moreover, the prognostic significance of IDH1/IDH2 mutations in diffuse astrocytomas is superior to that based on histological features alone. As a result, the presence or absence of IDH1/IDH2 mutations has been incorporated in the diagnosis of astrocytoma within the larger classification scheme for diffuse gliomas.
Grossly, diffuse astrocytomas are poorly circumscribed tumors in which the CNS parenchyma variably expands, but the overall disruption of anatomy is minimal, although there can be significant edema and mass effect. Given its diffusely infiltrative nature and general lack of solid mass formation, large portions of the tumor may appear invisible to the naked (or radiographic) eye (i.e., microscopic disease). Also, this infiltrative growth pattern makes therapy a challenge because it has not been possible to address microscopic foci of disease adequately with focal treatments such as surgery and radiation. Particularly widespread infiltration involving multiple lobes and even the brainstem (gliomatosis cerebri) is rare and tends to have a poor prognosis regardless of histological grade.
While the morphology of diffuse astrocytoma varies, most are at least partially composed of irregular elongated hyperchromatic nuclei, either appearing “naked” in an otherwise fibrillary background or displaying discernible cytoplasmic processes ( Fig. 72.1 ). GFAP immunostains highlight the latter, although this is neither absolutely sensitive nor specific, because (1) some astrocytomas harbor minimal quantities of GFAP-positive cytoplasm, (2) interpretation may be difficult owing to staining of nonneoplastic astrocytic elements or high background in general, and (3) other gliomas display GFAP immunoreactivity. Gemistocytic or “stuffed cell” architecture, characterized by strongly GFAP-positive cells with eccentric bellies of eosinophilic cytoplasm, may also be present to varying degrees. By definition, grade II astrocytomas lack significant mitotic activity, microvascular proliferation, and necrosis (see Glioblastoma [WHO Grade IV]). Ancillary staining for MIB-1 (Ki-67) generally reveals a low proliferative index as well.
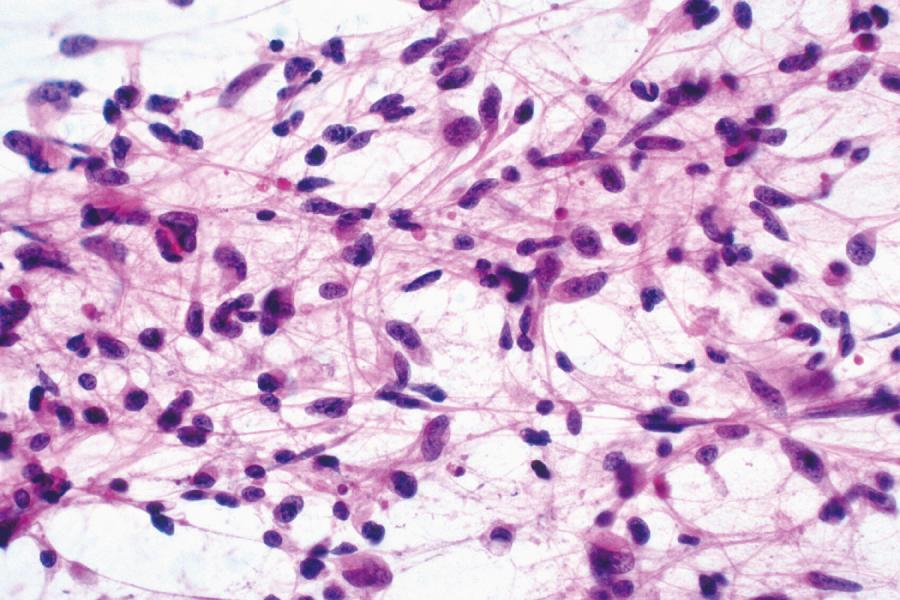
The clinical presentation and radiographic features of anaplastic astrocytoma (WHO grade III) are similar to those of grade II astrocytoma, except that some cases show contrast enhancement on magnetic resonance imaging (MRI). Histologically, anaplastic astrocytomas are more cellular than grade II counterparts and display a greater degree of proliferation. The presence of mitotic figures primarily defines these tumors, as does a generally higher Ki-67 proliferative index on immunostains. Necrosis and microvascular proliferation are absent by definition.
Oligodendrogliomas most commonly present as grade II tumors, occurring in young to middle-aged adults. Most are hemispheric masses, with the frontal lobe representing a favored location. On imaging studies, oligodendrogliomas are virtually indistinguishable from astrocytomas. However, gyriform calcifications are an uncommon but characteristic imaging feature. The prognosis for grade II oligodendrogliomas is significantly better than for astrocytomas, with average survival times of 10 years or more and improved chemosensitivity profiles. Oligodendrogliomas are defined by a specific molecular signature that includes a combination of IDH1 or IDH2 mutations and the codeletion of the 1p and 19q chromosomal regions (1p/19q codeletion). Accordingly, these genomic features are now included in the formal diagnosis of both WHO grade II and WHO grade III oligodendroglioma.
The classic microscopic features of oligodendroglioma include uniformly round nuclei, bland chromatin, clear perinuclear haloes imparting a “fried egg” appearance, and a rich branching capillary network reminiscent of “chicken wire” ( Fig. 72.2 ). Less specific findings include cortical involvement, microcalcifications, mucin-rich microcystic spaces, and perineuronal satellitosis. Although helpful, the so-called fried-egg appearance is a formalin fixation artifact that is neither necessary for diagnosis nor encountered in frozen sections or rapidly fixed specimens. The morphological spectrum includes two strongly GFAP-positive cells: minigemistocytes (or microgemistocytes) and gliofibrillary oligodendrocytes. The former are gemistocyte-like cells with small bellies of eosinophilic cytoplasm, round bland nuclei resembling those of classic oligodendroglioma nuclei, and no cytoplasmic processes. The latter are histologically identical to classic oligodendroglioma cells but exhibit a thin perinuclear rim of GFAP immunoreactivity. Hypercellularity, numerous mitoses, and microvascular proliferation define anaplastic oligodendroglioma (grade III). Some oligodendrogliomas have regions that are histologically similar to diffuse astrocytoma, which caused significant diagnostic difficulty in the past. However, IDH1 or IDH2 mutations combined with 1p/19q codeletion represent defining molecular alterations for oligodendrogliomas, and the application of these biomarkers to routine clinical diagnosis has eliminated the diagnostic uncertainty associated with ambiguous and overlapping microscopic features.
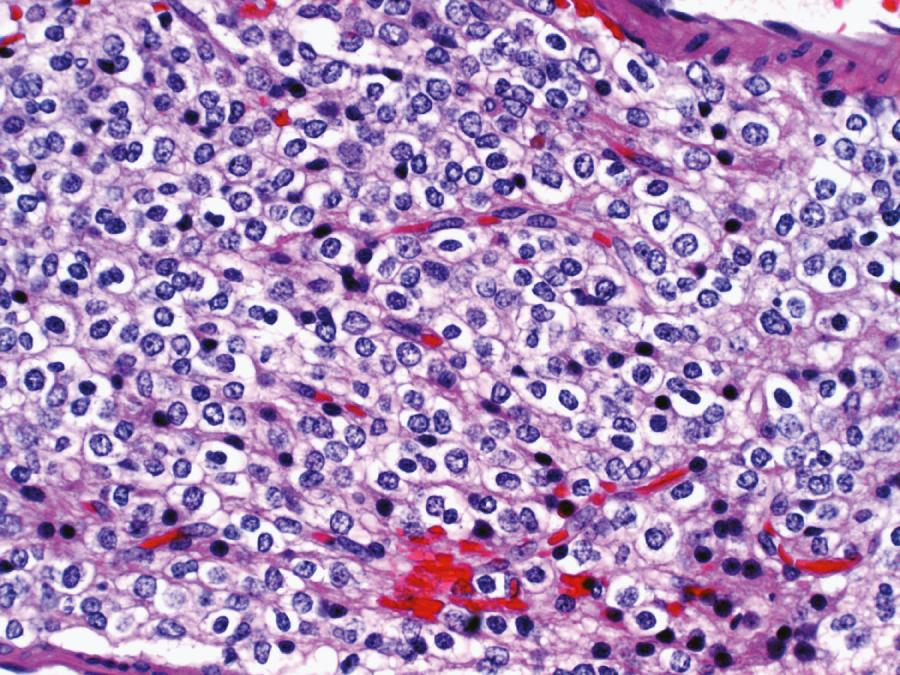
Previous editions of this book included a description of oligoastrocytomas. These tumors were characterized by the presence of microscopic features classically associated with both astrocytomas and oligodendrogliomas in the same lesion. However, the diagnosis of oligoastrocytoma demonstrated little agreement between pathologists and the incorporation of genetic alterations as defining features for brain tumor classification has led to the understanding that the vast majority of oligoastrocytomas can be classified as either astrocytomas or oligodendrogliomas based on highly recurrent molecular alterations.
Glioblastoma (previously known as glioblastoma multiforme ) is the most common malignant primary brain tumor in adults, accounting for approximately 80% of all infiltrating gliomas. The peak age at onset is 50–60 years. Unfortunately, several decades of basic and clinical research have had little impact on the clinical outcomes of glioblastoma, and average survival remains at approximately 1–1.5 years following radiation therapy and chemotherapy. Most glioblastomas arise de novo—primary glioblastomas—in a fully malignant state, while a minority emerges from lower-grade (WHO grade II or III) astrocytomas (secondary glioblastomas).
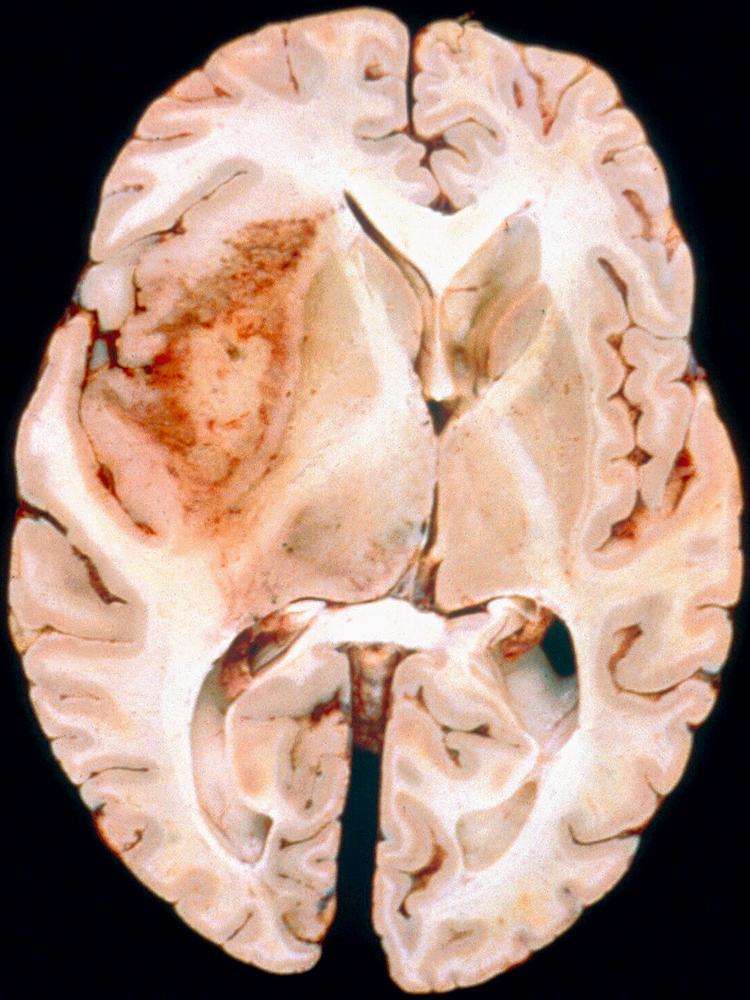
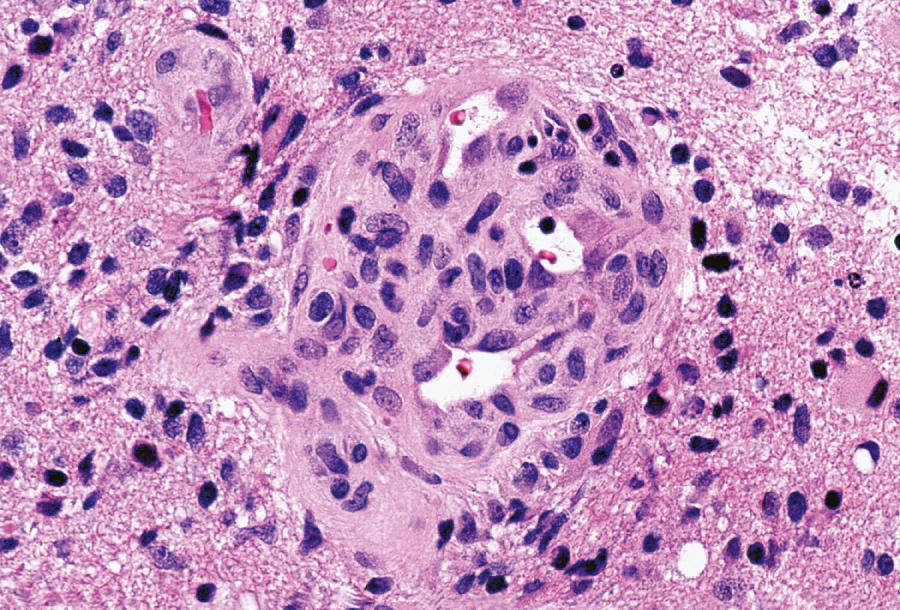
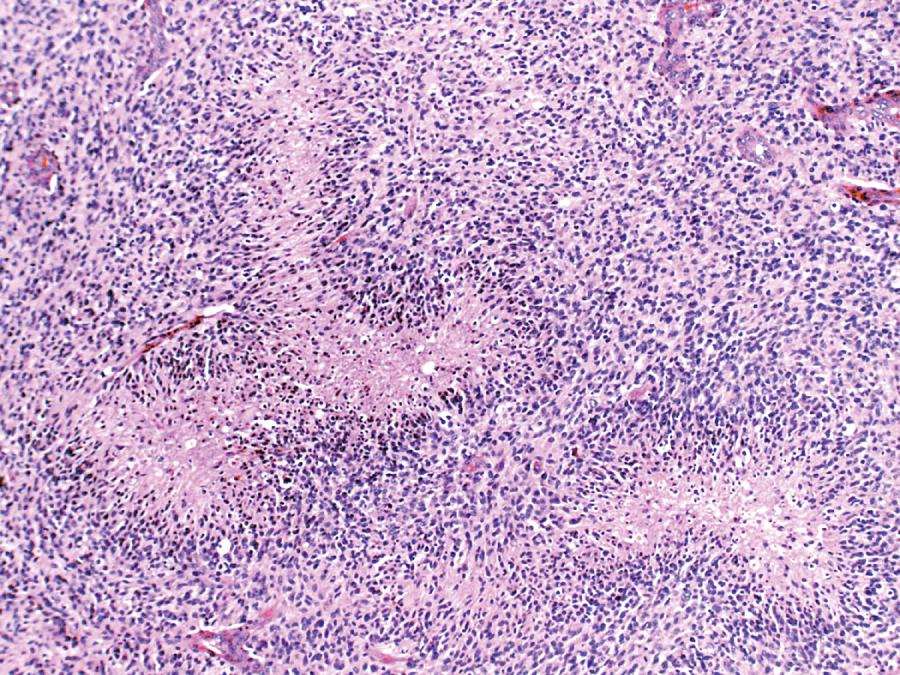
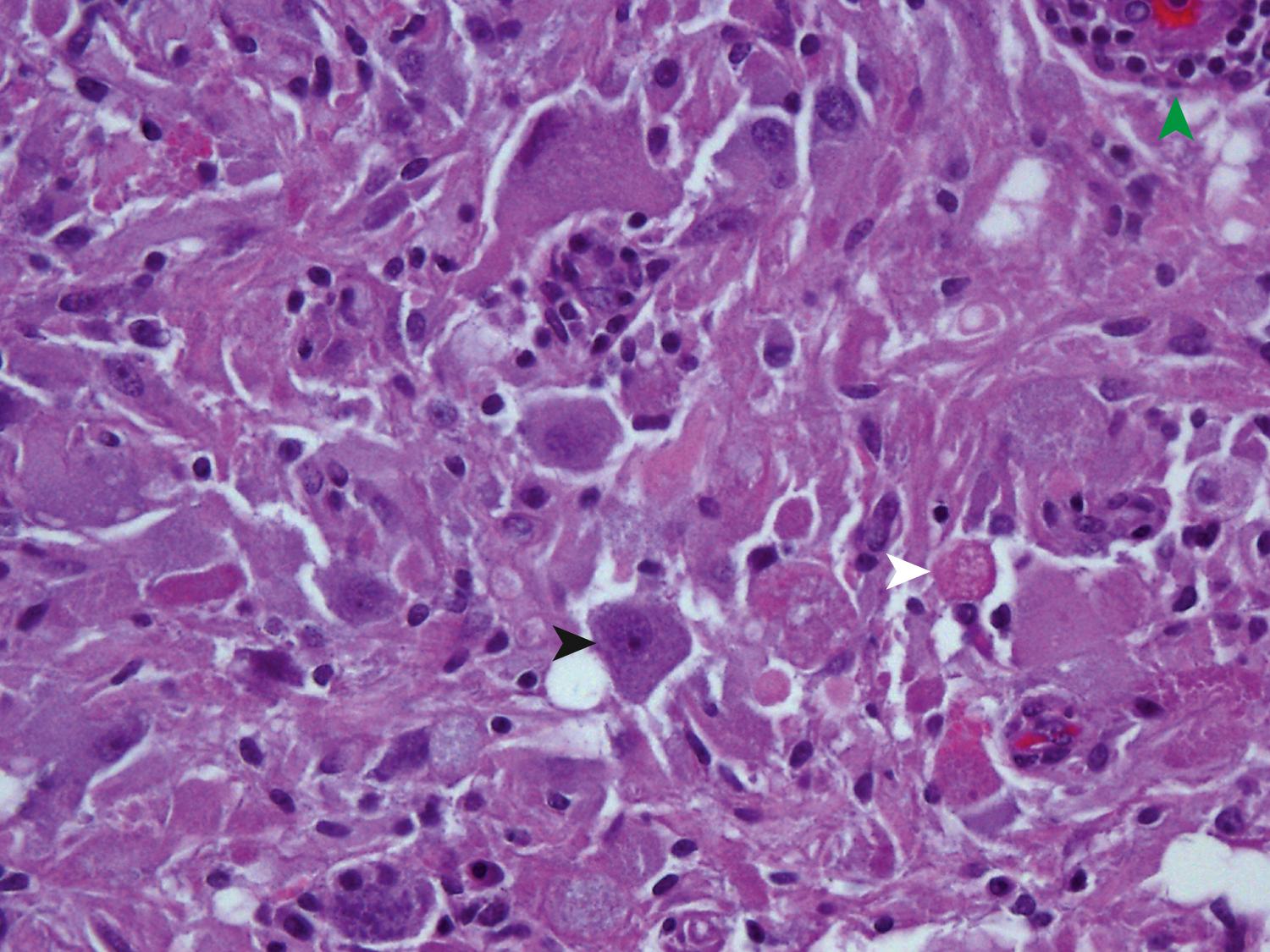
Glioblastoma most commonly occurs in the deep white matter, basal ganglia, or thalamus and is rarely found in the cerebellum or spinal cord. On imaging, these tumors typically present as an irregular ring of contrast enhancement, with a necrotic center, and significant edema. Gross and microscopic features are heterogeneous. Replacing the affected portion of the brain is a single mass that may grossly appear deceptively well circumscribed but microscopically infiltrates widely, often spreading to the opposite hemisphere via the corpus callosum (butterfly glioblastoma). Multifocal tumors may occur and, in some cases, they likely represent separate regions of malignant transformation within a widely disseminated astrocytoma. In advanced stages, the tumor may extend into the meninges or the ventricles. Seeding of the neuraxis as multiple implants on the brain or ventricular surfaces is an atypical growth pattern, and extracranial metastases are extremely rare. Glioblastomas exhibit a variegated appearance ( Fig. 72.3 ) characterized by central yellow or white zones of necrosis and hemorrhage surrounded by a hyperemic ring (endothelial hyperplasia) and “edematous” brain with variable mixtures of vasogenic edema, gliosis, and tumor infiltrates. Microscopically characterizing glioblastomas are all the features of anaplastic astrocytoma plus endothelial hyperplasia or necrosis. Endothelial hyperplasia is defined as thickened or glomeruloid vessels with multilayering ( Fig. 72.4 ), representing a form of tumor-induced angiogenesis. The necrosis is often associated with a characteristic serpiginous distribution and associated nuclear pseudopalisading ( Fig. 72.5 ). Several histological variants exist, including giant cell glioblastoma, small-cell glioblastoma, and gliosarcoma. Characteristic of the latter is a sarcomatous element, currently felt to arise most likely from mesenchymal metaplasia within a glioblastoma. No significant clinical differences are identifiable in these variants when compared with conventional glioblastoma. However, some histological variants are associated with specific molecular alterations, which may have diagnostic or therapeutic relevance.
This recently defined disease entity includes midline infiltrating astrocytomas (thalamus, brainstem, and spinal cord) that occur in children and younger adults, including the majority of tumors previously designated “diffuse intrinsic pontine glioma” (DIPG). Histologically, these tumors frequently present with features of high-grade infiltrating astrocytomas or glioblastomas. However, traditional histological grading has been eliminated in the diagnosis of these tumors, given its poor correlation with tumor behavior. Diffuse midline gliomas are defined by the pathogenic K27M mutation involving genes encoding histone 3 monomers (H3-K27M; see below) and correspond to WHO grade IV, regardless of the presence/absence of histological features classically associated with grade IV tumors (i.e., increased mitotic activity, vascular proliferation, and necrosis). This represents the first example, in the WHO classification system for CNS tumors, of a molecular alteration replacing histological features as criteria for predicting tumor behavior. However, the diagnosis itself is predicated on the tumor in question being a diffusely infiltrating glioma arising in the midline, as very recent literature has identified H3-K27M mutations in other tumor types (e.g., pilocytic astrocytoma) characterized by less aggressive clinical course ( ).
As alluded to earlier, extensive molecular profiling has greatly clarified the molecular foundations of infiltrating glioma. In particular, a defined set of highly recurrent molecular alterations appear to transcend histopathological distinctions and have been incorporated into the diagnosis of more biologically uniform disease entities. The striking biological distinctions between primary glioblastoma and its lower-grade counterparts are most notably delineated by the presence or absence of point mutations in IDH1/IDH2 , which occur in 70%–90% of astrocytomas and in 100% of oligodendrogliomas as currently defined ( ). In this way, IDH1/IDH2 mutations represent a defining molecular alteration segregating rapidly progressive infiltrating glioma from more indolent variants. Indeed, glioma patients with IDH -mutant tumors are younger and exhibit longer overall survival than those with IDH -wild-type tumors, with the latter distinction appearing to transcend conventional WHO grading. IDH proteins are metabolic enzymes that normally convert isocitrate to α-ketoglutarate as core components of the citric acid cycle. Glioma-associated IDH mutations alter enzymatic activity such that large amounts of the metabolite D-2-hydroxyglutarate (D-2-HG) are generated ( ). While many of the downstream effects of D-2-HG accumulation remain unclear, it has been shown to dramatically alter cellular epigenomes and DNA methylation profiles. In particular, IDH mutation is sufficient to generate the so-called CpG island hypermethylator phenotype (G-CIMP) seen in IDH -mutant gliomas ( ).
Additional highly recurrent molecular alterations almost invariably co-occur with IDH1/IDH2 mutations. By definition, oligodendrogliomas exhibit 1p/19q codeletion, which has been associated with both prolonged survival and a favorable response to cytotoxic therapy in both WHO grade III (Cairncross et al., 1998; Kaloshi et al., 2007) and WHO grade II variants (Kaloshi et al., 2007). Two of the likely tumor suppressors targeted by 1p/19q codeletion— CIC on chromosome 19q and FUBP1 on chromosome 1p—were identified by high-throughput sequencing approaches ( , ). Oligodendrogliomas also harbor activating mutations in the promoter region of the TERT gene, which encodes the catalytic core of the telomerase enzyme, in virtually all cases ( ). In contrast to oligodendrogliomas, astrocytomas have long been associated with mutations in TP53 and/or chromosome 17p losses ( ). More recently, loss-of-function mutations in ATRX have also been identified in >70% of IDH -mutant tumors without 1p/19q codeletion ( ). Therefore, the vast majority of IDH -mutant gliomas appear to harbor either 1p/19q codeletion and TERT mutation or TP53 and ATRX mutations, with almost complete mutual exclusivity.
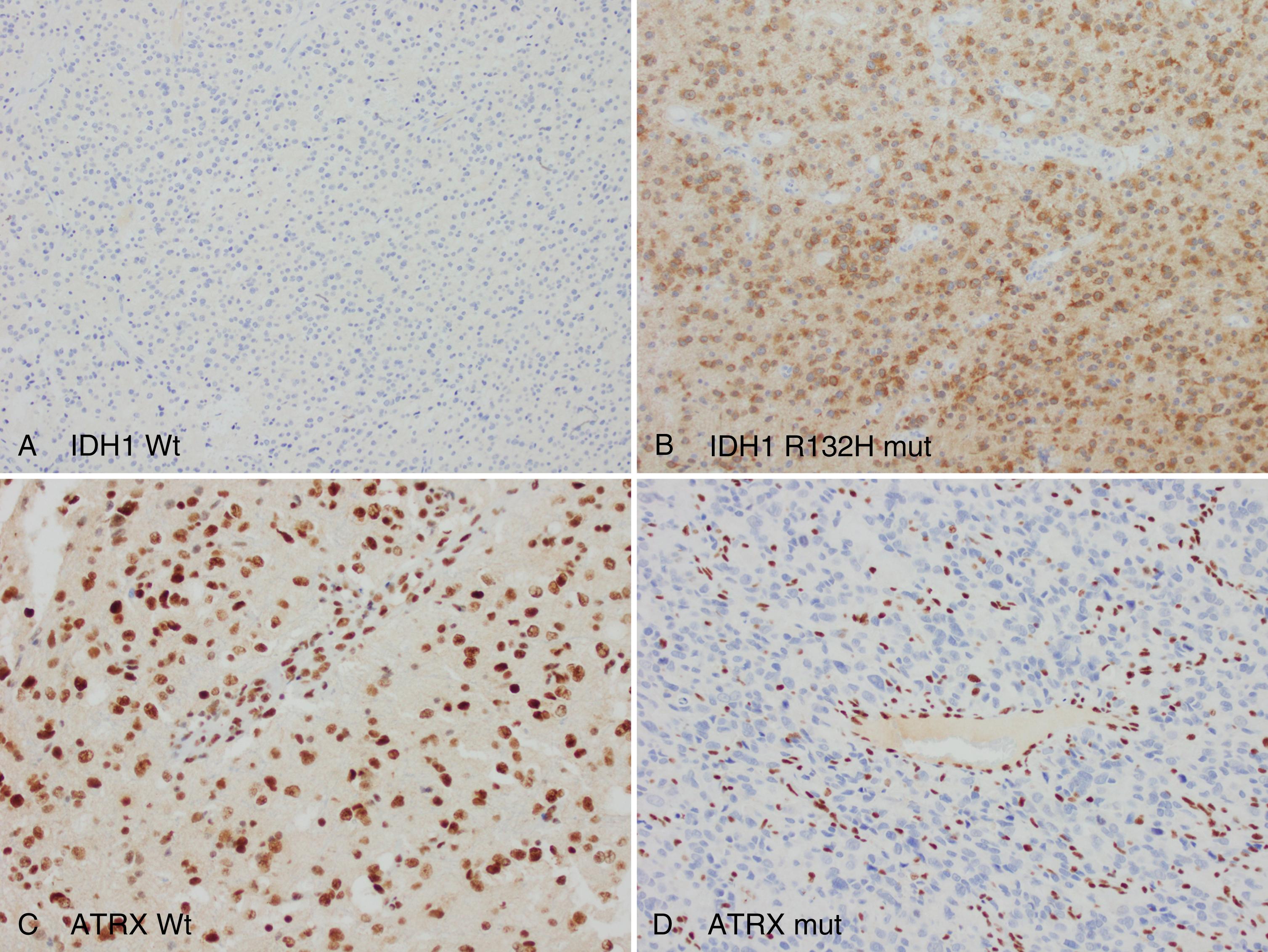
Early data indicate that 1p/19q-codeleted tumors are associated with longer overall survival than their TP53/ATRX -mutant counterparts, with both IDH -mutant variants exhibiting considerably better outcomes than IDH -wild-type gliomas ( ). Moreover, these distinctions appear to be more marked than those stratified by conventional histopathology alone. Thus, assessments of IDH1/IDH2 , TP53 , and ATRX mutations, along with 1p/19q codeletion, carry clinical relevance, and should be obtained whenever possible. Fortunately, rapid immunohistochemical assays exist for the detection of the most common IDH mutation (IDH1 p. R132H; Fig. 72.6 , A ), nuclear p53 accumulation (which frequently co-occurs with IDH1/IDH2 mutation), and ATRX deficiency (see Fig. 72.6 , B ), and well-validated methodologies, most commonly FISH-based, are widely used for 1p/19q analysis.
Primary glioblastomas often harbor numerous cytogenetic and molecular genetic aberrations with frequent involvement of core components in the PI3K/MAPK, p16/CDK4/RB, and p53 signaling networks ( ). Promoter mutations in TERT, identical to those found in oligodendrogliomas, are also common ( ). Mutations in IDH1 , IDH2 , and ATRX are rarely seen. Instead, 30%–40% of primary glioblastomas harbor EGFR amplifications, often with an associated constitutively activating mutation ( ). EGFR amplifications are also particularly common (≈70%) in the small-cell histopathological variant of glioblastoma. Alterations of the p16/CDK4/RB pathway are nearly universal in glioblastoma, and many cases harbor chromosome 10 loss. Moreover, the PTEN gene on chromosome 10q23 is mutated in a subset of these. The list of additional glioblastoma-associated alterations is growing rapidly. Recent large-scale sequencing studies have shown that glioblastomas harbor somatic mutations of multiple genes ( ). These studies have confirmed the incidence of mutated and/or amplified genes such as EGFR , PTEN , RB1 , and PIK3CA and showed higher prevalence for mutations in TP53 , NF1 , and PIK3R1 , as well as new candidate genes involved in tumorigenesis.
Methylation of the MGMT gene promoter has emerged as a prognostic and predictive biomarker in glioblastoma. MGMT is a DNA repair enzyme, and promoter methylation leads to downregulation of expression, presumably reducing the capacity of tumor cells to correct DNA damage caused by cytotoxic chemotherapy. Patients whose tumors harbor MGMT promoter methylation demonstrate improved survival following treatment with the alkylating agent temozolomide ( ). Furthermore, resistance to alkylating chemotherapy in glioblastoma is associated with therapy-related inactivation of MSH6, a DNA mismatch repair enzyme, allowing increased mutagenesis and the emergence of a hypermutated phenotype (Cahill et al., 2007; ). Similar observations have been made recently for WHO grade II astrocytoma ( ).
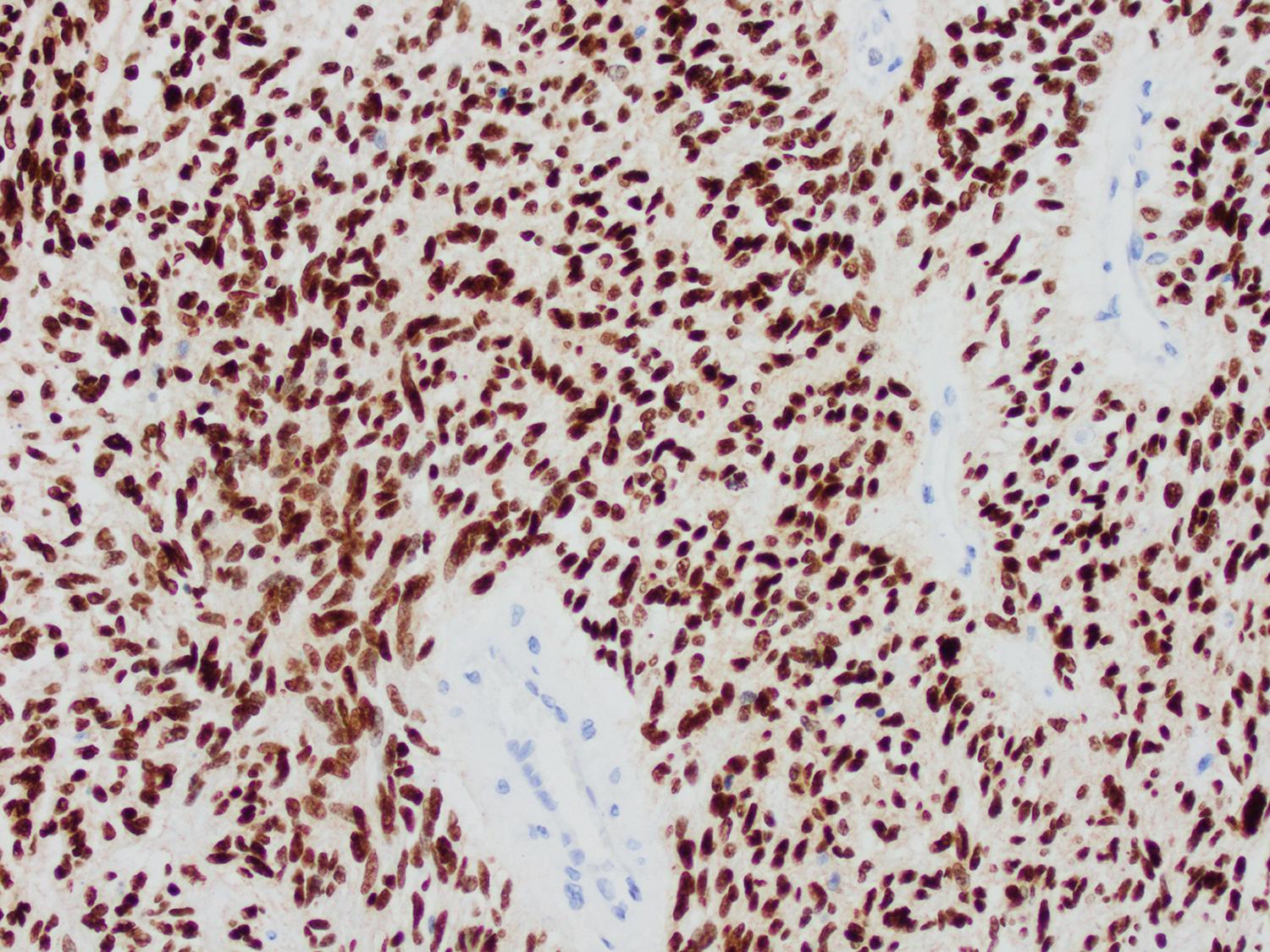
Finally, recent sequencing studies have identified mutations in genes encoding for H3.3 core histone proteins— H3F3A and HIST1H3B —as highly recurrent in aggressive gliomas arising most often in the pediatric population ( ). Intriguingly, these mutations invariably involve amino acid residues either at or close to sites of post-translational modification in the histone tail known to impact associated gene expression, suggesting the potential for epigenomic consequences. Subsequent data have confirmed this conjecture, with H3.3-mutant tumors demonstrating unique gene methylation profiles and globally altered patterns of histone modification ( ). Additionally, H3.3 mutations frequently co-occur with ATRX and TP53 mutations, suggesting pathogenic parallels with adult gliomas. TERT mutations are uncommon. Striking correlations between tumor location and precise H3.3 mutation exist in these tumors. As described above, diffuse midline gliomas, are defined by the H3-K27M mutation; by contrast, hemispheric pediatric glioblastomas frequently (∼30%) harbor the H3-G34R or H3-G34V mutations. A specific antibody targeting the H3-K27M mutant epitope has greatly facilitated the diagnosis of diffuse midline glioma ( Fig. 72.7 ).
A broad spectrum of glial and glioneuronal tumors impact pediatric and adult populations at lower rates than infiltrating gliomas. Many of these tumor variants are characterized by relatively indolent growth and discrete demarcation, although some can widely permeate surrounding brain. In general, clinical outcome associated with these tumors compares favorably to that seen in infiltrating gliomas. Molecular profiles are also distinct from those described above for astrocytomas, oligodendrogliomas, and glioblastomas, exhibiting high rates of abnormalities in FGFR1/2 , BRAF , and other MAPK signaling pathway constituents. Finally, several of these morphologically diverse tumor types are strongly associated with seizure activity, and the broad designation “LEAT” (long-term epilepsy associated tumor) has been used to describe their functional intersection ( ). While the precise classification of tumors within this group remains a work in progress, several entities are well established (see below).
Usually, pilocytic astrocytomas are well-circumscribed tumors, both grossly and radiographically, although limited degrees of microscopic infiltration are not unusual. They arise in both children and adults, and are more common in the cerebellum, hypothalamus, third ventricle, optic nerve, spinal cord, and dorsal brainstem but may also involve the cerebrum. The adjective juvenile often added to pilocytic astrocytoma is misleading because most adult cases are histologically and clinically indistinguishable from their pediatric counterparts. Outcome depends on the surgical accessibility of the tumor but is usually excellent (80% 20-year survival), and many are curable with resection alone. Gross appearance varies somewhat with anatomical location. Cerebellar tumors, which are often hemispheric, are typically composed of a large fluid-filled cyst with an enhancing mural nodule. Hypothalamic and optic nerve tumors are usually solid. Optic nerve gliomas appear as a focal segmental nerve swelling. Both unilateral and bilateral optic nerve gliomas are particularly common in NF1 patients, a setting in which most tumors are indolent and do not progress to a point requiring surgical intervention.
The distinctive histological feature of pilocytic astrocytoma is a biphasic pattern with compact pilocytic areas interspersed with microcystic, spongy, or loose areas ( Fig. 72.8 ). Dense portions contain piloid (hairlike) or bipolar astrocytes with long spindle-shaped processes. Rosenthal fibers are common. These are aggregates of intracellular astrocytic filaments, fusiform or corkscrew shaped, with a hyaline appearance (see Fig. 72.8 , A ). In addition, mulberry-shaped eosinophilic granular bodies (EGBs) also occur in most cases (see Fig. 72.8 , B ). Although not entirely specific, both Rosenthal fibers and EGBs are generally signs of an indolent process and represent important diagnostic clues that distinguish pilocytic astrocytoma from diffuse astrocytoma or glioblastoma. NF1 gene inactivation occurs in NF1-associated pilocytic astrocytomas but is much less common in sporadic examples. Instead, most (∼70%) pilocytic astrocytomas are characterized by a unique KIAA1549:BRAF fusion protein that serves as a constitutively active BRAF isoform ( ). Additional BRAF fusion events and the known oncogenic BRAF p. V600E mutation are also found in lower percentages of tumors. Finally, mutations in FGFR1 and PTPN11 , along with NTRK2 fusion genes, have been identified in small subsets of pilocytic astrocytoma ( ).
Pilomyxoid astrocytoma is a variant of pilocytic astrocytoma that was historically associated with more aggressive behavior corresponding to WHO grade II. This tumor mainly affects children younger than 3 years of age and occurs predominantly in the hypothalamic region. The most recent WHO classification of CNS tumors in 2016 recommends not grading pilomyxoid astrocytomas. It is now believed that the more unfavorable course associated with pilomyxoid histology might reflect the limitations of obtaining complete surgical resection in the hypothalamic region rather than more inherently aggressive tumor biology. Pilomyxoid astrocytoma lacks a biphasic appearance and Rosenthal fibers but shows piloid cells, often with an angiocentric arrangement in a myxoid matrix. EGBs are often rare or absent.
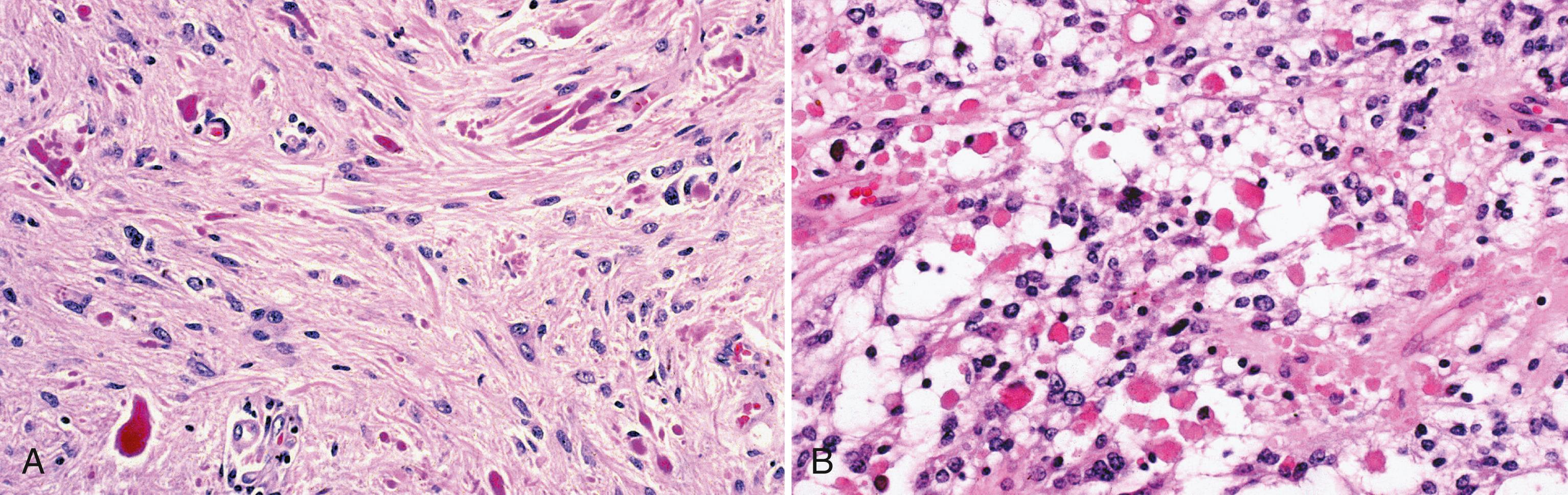
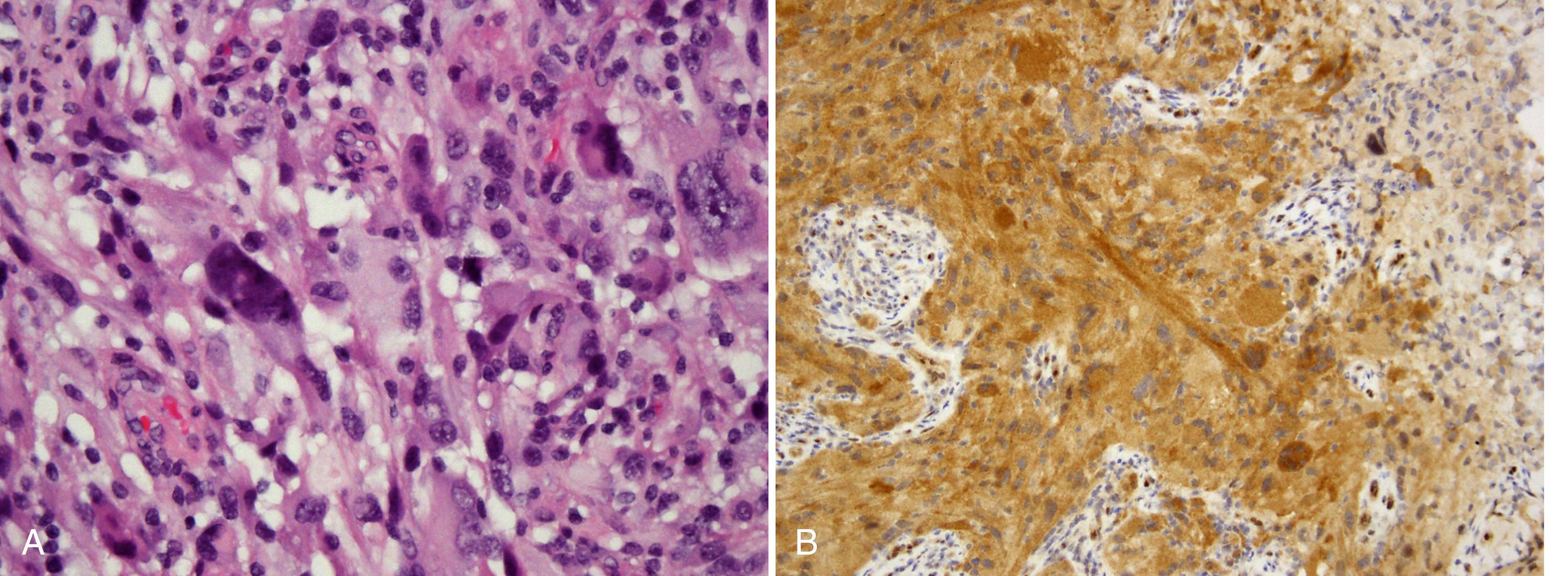
PXA is a rare and distinctive astrocytic neoplasm associated with a favorable prognosis and has often been misdiagnosed in the past as glioblastoma due to its marked cellular pleomorphism. The average age at diagnosis is 26 years, and a history of seizures often precedes diagnosis (Giannini et al., 1999). PXA usually involves the cerebral cortex and overlying meninges, and the preferred site is the temporal lobe. Histological features include hypercellularity and many atypical and pleomorphic tumor astrocytes ( Fig. 72.9 , A ). Bizarre giant cells are present, but mitoses are unusual. Probably the most helpful pathological finding is that of EGBs, since these do not occur in glioblastomas. Despite its name, xanthomatous cells (lipidized astrocytes) with foamy lipid-filled cytoplasm only occur in approximately one-fourth of cases. PXA has a relatively favorable prognosis (WHO grade II), with postoperative survival averaging 81% at 5 years and 70% at 10 years. However, it is estimated that 15%–20% of these tumors undergo malignant transformation (WHO grade III) and are associated with an aggressive clinical course. When fully malignant, PXAs often acquire a discohesive cellular architecture, reminiscent of malignant epithelioid neoplasms like melanoma, and are often classified as “epithelioid glioblastoma.” Recent studies have shown that BRAF p.V600E mutations occur in the majority of PXAs (see Fig. 72.9 , B ), whether WHO grade II or III, emphasizing the importance of the MAPK signaling pathway in the pathogenesis of these tumors ( ).
Most subependymal giant cell astrocytomas (SEGAs) are associated with tuberous sclerosis, and the suggestion is that SEGA patients lacking other features of tuberous sclerosis have a forme fruste of this disorder. An elongated, sausage-like, or lobulated gross appearance is typical. Histologically identical smaller masses resembling candle gutterings (the drippings of tallow from a burning candle) on the wall of the lateral ventricle are common in tuberous sclerosis–associated cases. Hydrocephalus may result secondary to obstruction of the foramen of Monro.
The rich vascularity of the tumor gives the cut surfaces a red and beefy appearance. Calcification is almost invariable and is at times so extensive that the mass has the consistency of stone. SEGAs are moderately cellular, consisting of closely packed astrocytes with abundant cytoplasm. Tumor cells often sweep in fascicles or around blood vessels, analogous to the pseudorosettes in ependymomas. Some SEGAs may have a gemistocyte-like or spindled morphology.
Some tumor cells are clearly of astrocytic origin, and GFAP fills the cytoplasm. Other tumor cells resemble neurons having prominent nucleoli, and many have intermediate features with astrocytoma-like cytoplasm and neuronal-like nuclei. Positive immunohistochemical staining for neuronal markers further suggests neuronal differentiation. Tumor cells may stain with both neuronal and glial markers or with neither, explaining the preference of some neuropathologists for the term subependymal giant cell tumor rather than SEGA. These tumors are related to alterations in the TSC1 (hamartin) and TSC2 (tuberin) genes and thus involve aberrant signaling through downstream growth-regulatory pathways.
Most gangliogliomas occur before age 21 and comprise 4%–8% of all pediatric brain tumors. Gangliogliomas grow slowly and tend to show benign biological and clinical behavior. The most common site of involvement is the temporal lobe, and seizures are a typical presenting symptom. Other lobes of the cerebral hemispheres, cerebellum, and spinal cord are less often affected. Tumors are often cystic and well circumscribed, extending to the surface of the brain. Solid portions are firm, gray, and gritty due to calcium deposits that are evident on computed tomography (CT) scans.
Portions of the tumor resemble a low-grade astrocytoma, either pilocytic or fibrillary in nature. Unlike native entrapped neurons within an infiltrative glioma, some of the tumor ganglion cells have a dysmorphic appearance, as evidenced by their lack of polarity, clustering, cytoplasmic vacuolation, increased nuclear pleomorphism, or multinucleation ( Fig. 72.10 ). Binucleate or multinucleate neurons are particularly helpful for diagnostic purposes when present. Otherwise, the most useful features to distinguish this tumor from diffuse gliomas include relative circumscription and eosinophilic granular bodies. Perivascular lymphocytic cuffing, microcystic spaces, and fibrosis with collagen deposition are other common findings. Rosenthal fibers are common, particularly at the edges of the lesion. Those without an obvious astrocytic component are sometimes referred to as gangliocytoma , although it is not yet clear that this distinction has any clinical relevance. The term ganglion cell tumor is less specific and incorporates both entities. Rare cases demonstrate signs of anaplasia (grade III), almost invariably in the glial component, but grading criteria and their predictive value have yet to be firmly established. GFAP expression is abundant in the astrocytic component, whereas the ganglion cell component expresses most markers of mature neurons such as synaptophysin, chromogranin, neurofilament, and NeuN. While the molecular basis of these tumors remains unclear, a significant minority has been shown to harbor BRAF V600E mutations ( ). Mutations in other genes involved in the MAPK pathway such as KRAS, NF1 , and FGFR1/FGFR2 , have also been associated with gangliogliomas. The recently described multinodular and vacuolating neuronal tumor (VNT) may represent a unique morphological variant of gangliocytoma, and is also characterized by mutations in genes encoding MAP kinase pathway effectors (e.g., MAP2K1 , BRAF ) ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here