Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hemostasis preserves vascular integrity by balancing the physiologic processes that maintain blood in a fluid state under normal circumstances and prevent excessive bleeding after vascular injury. Preservation of blood fluidity depends on an intact vascular endothelium, and a complex series of regulatory pathways that maintain platelets in a quiescent state and keep the coagulation system in check. In contrast, arrest of bleeding requires rapid formation of hemostatic plugs at sites of vascular injury to prevent severe hemorrhage. Perturbation of hemostasis can lead to bleeding or thrombosis. Bleeding will occur if there is failure to seal vascular leaks either because of defective hemostatic plug formation, or because of premature breakdown of the plugs. In contrast, thrombosis may occur if prothrombotic stimuli are unregulated, or if thrombus resolution is attenuated.
Thrombosis can occur in arteries or veins and is a major cause of morbidity and mortality. Arterial thrombosis is the most common cause of acute coronary syndrome, ischemic stroke, and limb gangrene, whereas thrombosis in the deep veins of the leg leads to post-thrombotic syndrome and pulmonary embolism, which can be fatal.
Most arterial thrombi form on top of disrupted atherosclerotic plaques, because plaque rupture exposes thrombogenic material in the plaque core to the blood. This material then triggers platelet aggregation and fibrin formation, which results in the generation of a platelet-rich thrombus that temporarily or permanently occludes blood flow. Temporary occlusion of blood flow in coronary arteries may trigger unstable angina, whereas persistent obstruction causes myocardial infarction. The same processes can occur in the cerebral circulation, where temporary arterial occlusion may manifest as a transient ischemic attack, and persistent occlusion can lead to a stroke. Likewise, critical limb ischemia can occur if there is superimposed thrombosis on ruptured atherosclerotic plaques in the major arteries supplying blood to the lower extremities.
In contrast to arterial thrombi, venous thrombi rarely form at sites of obvious vascular disruption. Although they can develop after surgical trauma to veins, or secondary to indwelling venous catheters, they usually originate in the valve cusps of the deep veins of the calf or in the muscular sinuses, where there is stasis. Sluggish blood flow in these veins reduces the oxygen supply to the avascular valve cusps. Hypoxemia induces endothelial cells lining the valve cusps to become activated and express adhesion molecules onto their surfaces. Tissue factor–bearing leukocytes and microparticles adhere to these activated cells and induce coagulation. Impaired blood flow exacerbates local thrombus formation by reducing clearance of activated clotting factors and consumption of regulatory molecules. These responses constitute the three axes of Virchow’s triad associated with development of thrombosis: stasis, hypercoagulability of the blood, and activation or disruption of the endothelium. A further complication of thrombosis is embolism, where venous or arterial thrombi can dislodge and travel to the lungs, brain, or other organs to produce pulmonary embolism, ischemic stroke, or systemic embolism, respectively.
Arterial and venous thrombi contain platelets and fibrin, but the proportions differ. Arterial thrombi are rich in platelets because of the high shear on this side of the circulatory system. In contrast, venous thrombi, which form under low shear conditions, contain relatively few platelets, and consist mostly of fibrin and trapped red blood cells. Because of the predominance of platelets, arterial thrombi appear white, whereas venous thrombi appear red.
The antithrombotic drugs used for prevention and treatment of thrombosis target components of thrombi and include antiplatelet drugs, which inhibit platelets; anticoagulants, which attenuate coagulation; and fibrinolytic agents that induce fibrin degradation (see Chapter 143 ). With the predominance of platelets in arterial thrombi, strategies to inhibit or treat arterial thrombosis focus mainly on antiplatelet agents, although in the acute setting, strategies often include anticoagulants and fibrinolytic agents. When arterial thrombi are occlusive and rapid restoration of blood flow is imperative, mechanical and pharmacologic methods enable thrombus extraction, compression, or degradation. Parenteral anticoagulants are often given in conjunction with reperfusion therapy to prevent re-occlusion and clotting on guidewires and catheters. Anticoagulants may also be given for secondary prevention in patients with arterial thrombosis. Thus, when compared with placebos in stabilized patients with acute myocardial infarction, low-dose rivaroxaban administered on top of antiplatelet therapy reduced recurrent ischemic events and stent thrombosis. Likewise, dual pathway inhibition with aspirin plus low-dose rivaroxaban is superior to either agent alone for prevention of major adverse cardiac and limb events in patients with coronary artery or peripheral artery disease.
Anticoagulants are the mainstay for prevention and treatment of venous thromboembolism because fibrin is the predominant component of venous thrombi (see Chapter 143 ). Antiplatelet drugs are less effective than anticoagulants because of the limited platelet content of venous thrombi. Nonetheless, aspirin is as effective as rivaroxaban for extended thromboprophylaxis after hip or knee arthroplasty. Selected patients with venous thromboembolism benefit from fibrinolytic therapy—for example, patients with massive pulmonary embolism achieve more rapid restoration of pulmonary blood flow with systemic, or catheter-directed, fibrinolytic therapy than with anticoagulant therapy alone. Certain patients with extensive deep vein thrombosis in the iliac and/or femoral veins may also have a better outcome with catheter-directed fibrinolytic therapy, and/or mechanical thrombus extraction in addition to anticoagulants.
This chapter provides an overview of hemostasis and thrombosis by highlighting the processes involved in platelet activation and aggregation, blood coagulation, and fibrinolysis.
The major components of the hemostatic system are the vascular endothelium, platelets, and the coagulation and fibrinolytic systems. The complex interplay between cellular and soluble factors is termed immunohemostasis. Although these highly integrated processes maintain normal hemostasis under physiological conditions, their dysregulation can lead to pathological responses (see box on Immunohemostasis ).
A monolayer of endothelial cells lines the intimal surface of the circulatory tree and separates the blood from the prothrombotic subendothelial components of the vessel wall (see Chapter 122 ). As such, the vascular endothelium encompasses about 10 13 cells and covers a vast surface area. Rather than serving as a static barrier, the healthy vascular endothelium is a dynamic organ ( Fig. 121.1 ) that actively regulates hemostasis by inhibiting platelets, suppressing coagulation, promoting fibrinolysis, and modulating vascular tone and permeability. Defective vascular function can lead to bleeding if the endothelium becomes more permeable to blood cells, if vasoconstriction does not occur, or if premature degradation of hemostatic plugs reopens repaired vasculature.
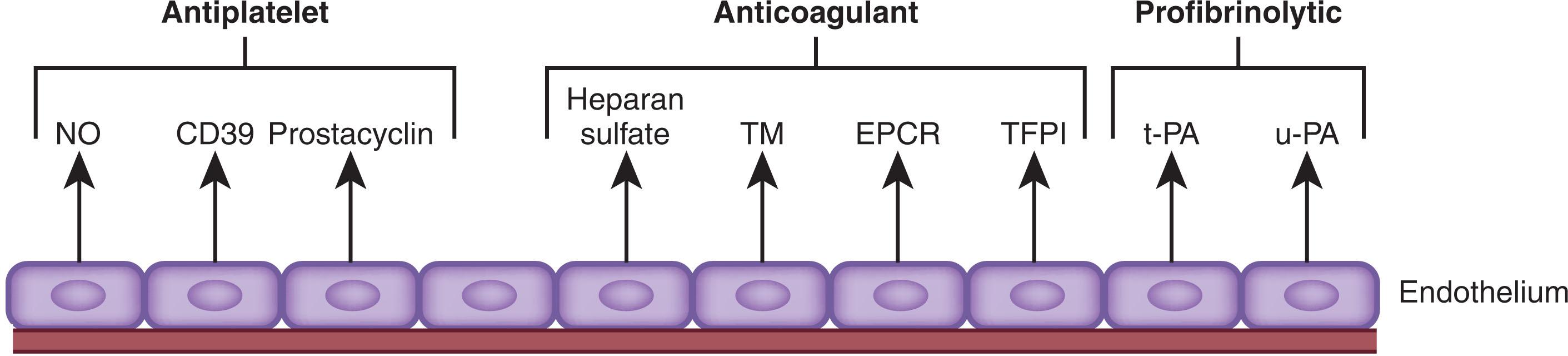
Endothelial cells synthesize prostacyclin and nitric oxide and release them into the blood. These agents not only serve as potent vasodilators, but also inhibit platelet activation and subsequent aggregation by stimulating adenylate cyclase and increasing intracellular levels of cyclic adenosine monophosphate (cAMP). In addition, endothelial cells express CD39 on their surfaces, a membrane-associated ecto-adenosine diphosphatase (ADPase). By degrading adenosine diphosphate (ADP), which is a platelet agonist, CD39 attenuates platelet activation.
Intact endothelial cells play an essential part in the regulation of thrombin generation through a variety of mechanisms. Endothelial cells produce heparan sulfate proteoglycans, which bind circulating antithrombin and accelerate the rate at which it inhibits thrombin and other coagulation enzymes. Tissue factor pathway inhibitor (TFPI), a naturally occurring inhibitor of coagulation, binds heparan sulfate on the endothelial cell surface. Administration of heparin or low-molecular-weight heparin (LMWH) displaces glycosaminoglycan-bound TFPI from the vascular endothelium, and released TFPI may contribute to the antithrombotic activity of these drugs.
Endothelial cells regulate thrombin generation by expressing thrombomodulin and endothelial cell protein C receptor (EPCR) on their surfaces. Thrombomodulin binds thrombin and alters its substrate specificity such that thrombin no longer acts as a procoagulant but becomes a potent activator of protein C (see Chapter 125 ). Activated protein C (APC) serves as an anticoagulant by degrading activated factor V and factor VIII (factor Va and VIIIa, respectively), key cofactors involved in thrombin generation. Protein S acts as a cofactor in this reaction, and EPCR enhances this pathway by binding protein C and presenting it to the thrombin–thrombomodulin complex for activation. In addition to its role as an anticoagulant, APC also regulates inflammation and preserves the barrier function of the endothelium.
The vascular endothelium promotes fibrinolysis by synthesizing and releasing tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA), which initiate fibrinolysis by converting plasminogen to plasmin (see Chapter 125 ). Endothelial cells in most vascular beds synthesize t-PA constitutively and release it in response to stimuli such as thrombin or bradykinin. In contrast, perturbed endothelial cells produce u-PA in the settings of inflammation and wound repair.
The hemostatic system is not the sole mechanism through which the vasculature is protected from disruption. There is overwhelming evidence that inflammation plays an equally important part and that the two processes are tightly integrated and mutually dependent. The terms immunohemostasis and thromboinflammation have been coined to capture this interplay. The inflammatory axis can be integrated into the cell-based model of hemostasis because leukocytes, platelets, and the endothelium are critical components of both processes. For example, tissue factor expression by monocytes is upregulated in response to inflammatory cytokines. The reductionist view of hemostasis and inflammation considers these pathways in isolation. However, it is now apparent that inflammation plays an integral part in stroke, deep-vein thrombosis, and myocardial infarction, processes previously attributed to dysregulation of hemostasis. In agreement with their similar protective and reparative roles, both thrombosis and inflammation are initiated in response to tissue damage or pathogen infection.
Platelets provide an obvious link between the hemostatic and immune systems because they respond to bacterial or viral infection, as well as to ischemia and cancer. Because of their proximity, platelets are among the first cells to arrive at sites of injury where they interact with leukocytes and endothelial cells. Upon activation, platelets expose ligands that mediate their interaction with immune cells. Such ligands include P-selectin, intercellular adhesion molecule (ICAM)-2, macrophage-1 antigen (MAC-1), and CD40L. Furthermore, platelets express toll-like and Fc receptors and release soluble effectors such as cytokines, chemokines and platelet factor 4. Platelets also link the complement system to the coagulation and immune systems because platelets bind C3b via P-selectin and C3 via GPIb-alpha, and because they release bactericidal mediators such as thrombocidin. Consequently, platelets link hemostasis with innate and adaptive immunity.
Leukocytes provide another link in the thromboinflammation axis. Activated platelets and endothelial cells express P-selectin on their surface. Leukocytes bind P-selectin via P-selectin glycoprotein ligand-1 (PSGL-1), a counter receptor that is constitutively expressed on their surface. This interaction results in the formation of platelet-leukocyte aggregates and the tethering of leukocytes to the endothelium, interactions that promote the release of pro-inflammatory cytokines, reactive oxygen species, and proteolytic enzymes and facilitate neutrophil transmigration to sites of inflammation. Therefore, leukocytes contribute to innate immunity.
Leukocytes also contribute to coagulation. Activated monocytes express tissue factor, which triggers coagulation via the extrinsic pathway. Activated neutrophils undergo NETosis, which involves programmed release of neutrophil extracellular traps (NETs) that originate from decondensed chromatin and consist of DNA, histones, and metalloproteases such as myeloperoxidase (MPO) and elastase. The mesh-like structure of NETs provides a scaffold for platelet adhesion where histones can activate them and the DNA in NETs triggers coagulation via the contact system. Confirmation of the role of neutrophils and NETs in coagulation comes from the observations that neutrophil depletion or DNase administration attenuates thrombosis in murine models.
Enhanced understanding of thromboinflammation has identified new disease pathways and has revealed novel targets for directed therapy. The hypercoagulability in patients with novel coronavirus disease (COVID-19) illustrates the reciprocal and tight integration of thrombosis and inflammation (see Chapter 153 ). Such integration is also evident in chronic inflammatory diseases and explains why patients with inflammatory bowel disease, systemic lupus erythematosus and rheumatoid arthritis are at higher risk for thrombosis than those without these conditions. These revelations have prompted routine thromboprophylaxis for hospitalized patients with COVID-19, the use of global Janus kinase (JAK) inhibitors to attenuate inflammation in patients with rheumatic diseases, and the evaluation of novel agents such as soluble thrombomodulin for treatment of DIC.
Endothelial cells also produce type 1 plasminogen activator inhibitor (PAI-1), the major inhibitor of both t-PA and u-PA. Therefore, net fibrinolytic activity depends on the dynamic balance between the release of plasminogen activators and PAI-1. Fibrinolysis is localized to the endothelial cell surface because these cells express annexin II, a coreceptor for plasminogen and t-PA that promotes their interaction. Therefore, healthy vessels actively resist thrombus formation and help maintain platelets in a quiescent state.
In addition to synthesizing potent vasodilators, such as prostacyclin and nitric oxide, endothelial cells also produce a group of counter-regulatory peptides known as endothelins that induce vasoconstriction. Endothelial cell permeability is influenced by the connections that join endothelial cells to their neighbors. Macromolecules traverse the endothelium via patent intercellular junctions, by endocytosis, or through transendothelial pores. Vasodilatation, severe thrombocytopenia, and high doses of heparin can increase endothelial permeability, which may contribute to bleeding. Bradykinin, generated through degradation of high molecular weight kininogen by kallikrein, also induces vascular leakage and inflammation. APC combats these effects by attenuating inflammation and vascular permeability, thereby enhancing the barrier function of the endothelium.
Platelets are anucleate cellular particles released into the circulation after programmed fragmentation of bone marrow megakaryocytes (see Chapter 123 ). Because they are anucleate, platelets have limited capacity to synthesize proteins. Consequently, platelet protein composition is determined by the parent cell as well as by those factors endocytosed from the circulation. Thrombopoietin, a glycoprotein synthesized in the liver and kidneys, regulates megakaryocytic proliferation and maturation as well as platelet production. Once they enter the circulation, platelets have a life span of 7 to 10 days.
Damage to the intimal lining of the vessel exposes the underlying subendothelial matrix. Platelets home to sites of vascular disruption and adhere to the exposed matrix proteins (see Chapter 124 ). Adherent platelets undergo activation and not only release substances that recruit additional platelets to the site of injury, but also promote thrombin generation and subsequent fibrin formation ( Fig. 121.2 ). A potent platelet agonist, thrombin amplifies platelet recruitment and activation. Activated platelets then aggregate to form a plug that seals the leak in the vasculature. An understanding of the steps in these highly integrated processes helps pinpoint the sites of action of antiplatelet drugs, and rationalizes the utility of anticoagulants for the treatment of arterial thrombosis and venous thrombosis.
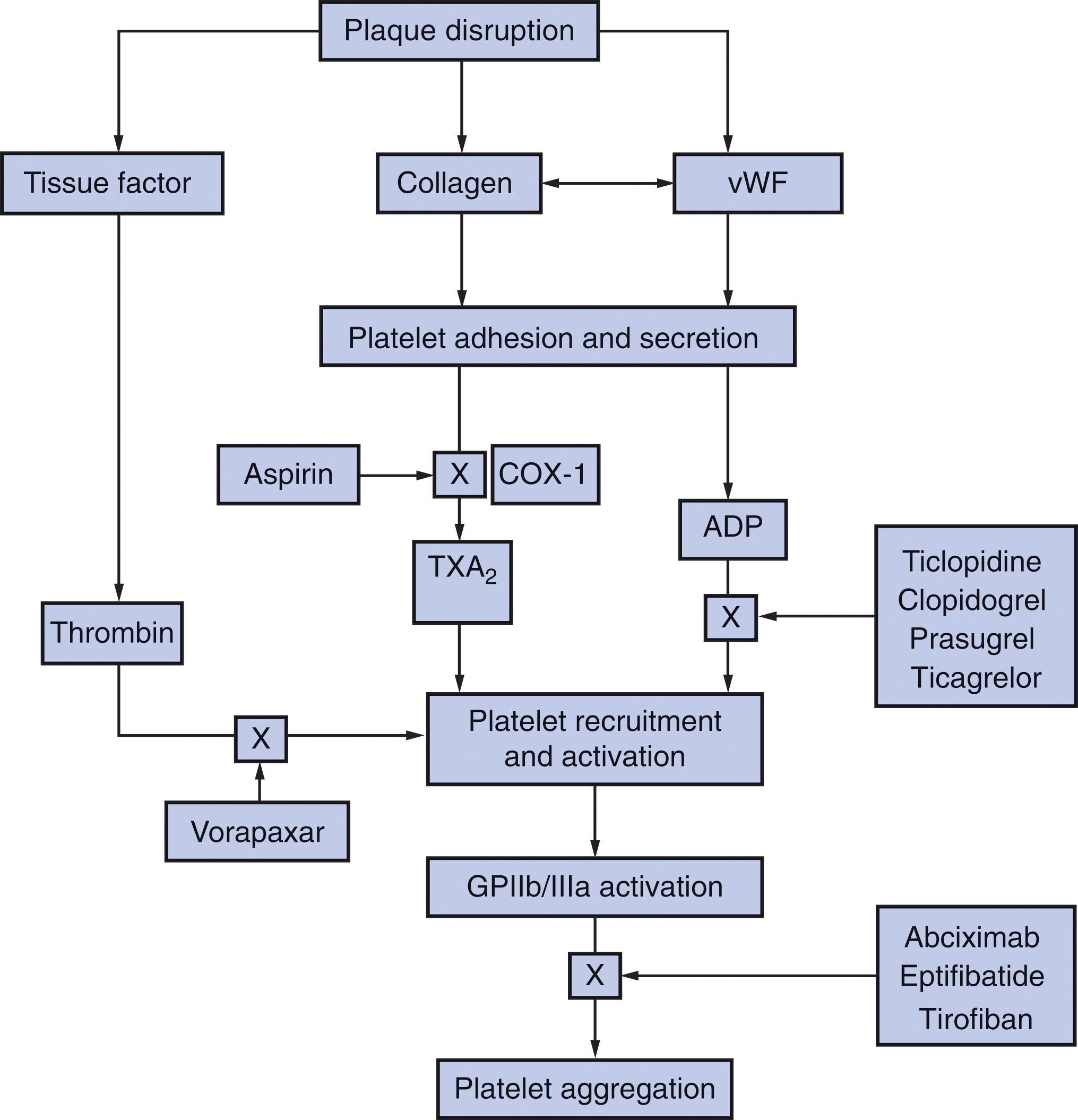
Platelets adhere to von Willebrand factor (vWF) and collagen that originate from endothelial cells and the subendothelium, respectively. The platelet monolayer promotes thrombin generation and subsequent fibrin formation. These events depend on constitutively expressed receptors on the platelet surface, α2β1 and glycoprotein (GP) VI, which bind collagen, and GPIbα and GPIIb/IIIa (αIIbβ3), which bind vWF. The platelet surface is crowded with receptors, but those involved in adhesion are the most abundant: every platelet has about 40,000 to 80,000 copies of GPIIb/IIIa and 25,000 copies of GPIbα. Receptors cluster in cholesterol-enriched subdomains, which render them more mobile, thereby increasing the efficiency of platelet adhesion and subsequent activation (see Chapter 124 ).
Under low shear conditions, such as in the venous circulation, collagen can capture and activate platelets on its own. Captured platelets undergo cytoskeletal reorganization that causes them to flatten and adhere more closely to the damaged vessel wall. Under high shear conditions in the arterial system, however, collagen and vWF must act in concert to support optimal platelet adhesion and activation. vWF synthesized by endothelial cells and megakaryocytes assembles into multimers that range from 550 to over 10,000 kDa. When released from storage in the Weibel-Palade bodies of endothelial cells or the α-granules of platelets, most of the vWF enters the circulation, but the vWF released from the abluminal surface of endothelial cells accumulates in the subendothelial matrix, where it binds collagen via its A3 domain. This surface-immobilized vWF can simultaneously bind platelets via its A1 domain. In contrast, circulating vWF does not react with unstimulated platelets. This difference in reactivity likely reflects vWF conformation; circulating vWF is in a coiled conformation that prevents access of its platelet-binding domain to vWF receptors on the platelet surface, whereas immobilized vWF assumes an elongated shape that exposes its A1 domain. Shear forces at sites of vascular injury also unfold vWF, thus contributing to the abundance of platelets in arterial thrombi. In this extended conformation, large vWF multimers serve as the molecular glue that tethers platelets to the damaged vessel wall with sufficient strength to withstand higher shear forces. Large vWF multimers provide additional binding sites for collagen and heighten platelet adhesion because platelets have more vWF receptors than collagen receptors.
The size of the vWF multimers is regulated by ADAMTS13, a circulating metalloprotease and member of A D isintegrin A nd M etalloprotease with T hrombo S pondin type 1 repeats (ADAMTS) family. Synthesized in hepatic stellate cells and found in other cells including endothelial cells, megakaryocytes and platelets, ADAMTS13 is secreted into plasma as a constitutively active enzyme. ADAMTS13 cleaves vWF, thereby preventing accumulation of ultra large multimers. Such cleavage is essential for normal hemostasis. Thus, deficiency of ADAMTS13 because of autoantibody formation or inherited mutations leads to thrombotic thrombocytopenic purpura (see Chapter 132 ), which is characterized by microvascular occlusion by platelet aggregates induced by ultra large vWF multimers.
Platelet adhesion to collagen or vWF results in platelet activation and secretion, the next steps in platelet plug formation.
Adhesion to collagen and vWF initiates signaling pathways that result in platelet activation. These pathways induce cyclooxygenase-1 (COX-1)–dependent synthesis and release of thromboxane A2 and trigger the release of ADP from storage granules. Thromboxane A2 is a potent vasoconstrictor, and like ADP, locally activates ambient platelets and recruits them to the site of injury. This process results in expansion of the platelet plug. To activate platelets, thromboxane A2 and ADP must bind to their respective receptors on the platelet membrane. The thromboxane receptor (TP) is a G protein–coupled receptor that is found on platelets and on the endothelium, which explains why thromboxane A2 induces vasoconstriction as well as platelet activation. ADP interacts with a family of G protein-coupled receptors on the platelet membrane. Most important of these is P2Y12, which is the target of the thienopyridines, but P2Y1 also contributes to ADP-induced platelet activation, and maximal ADP-induced platelet activation requires activation of both receptors. A third ADP receptor, P2X1, is an adenosine triphosphate (ATP)–gated calcium channel. Platelet storage granules contain ATP as well as ADP; ATP released during the platelet activation process may contribute to the platelet recruitment process in a P2X1-dependent fashion.
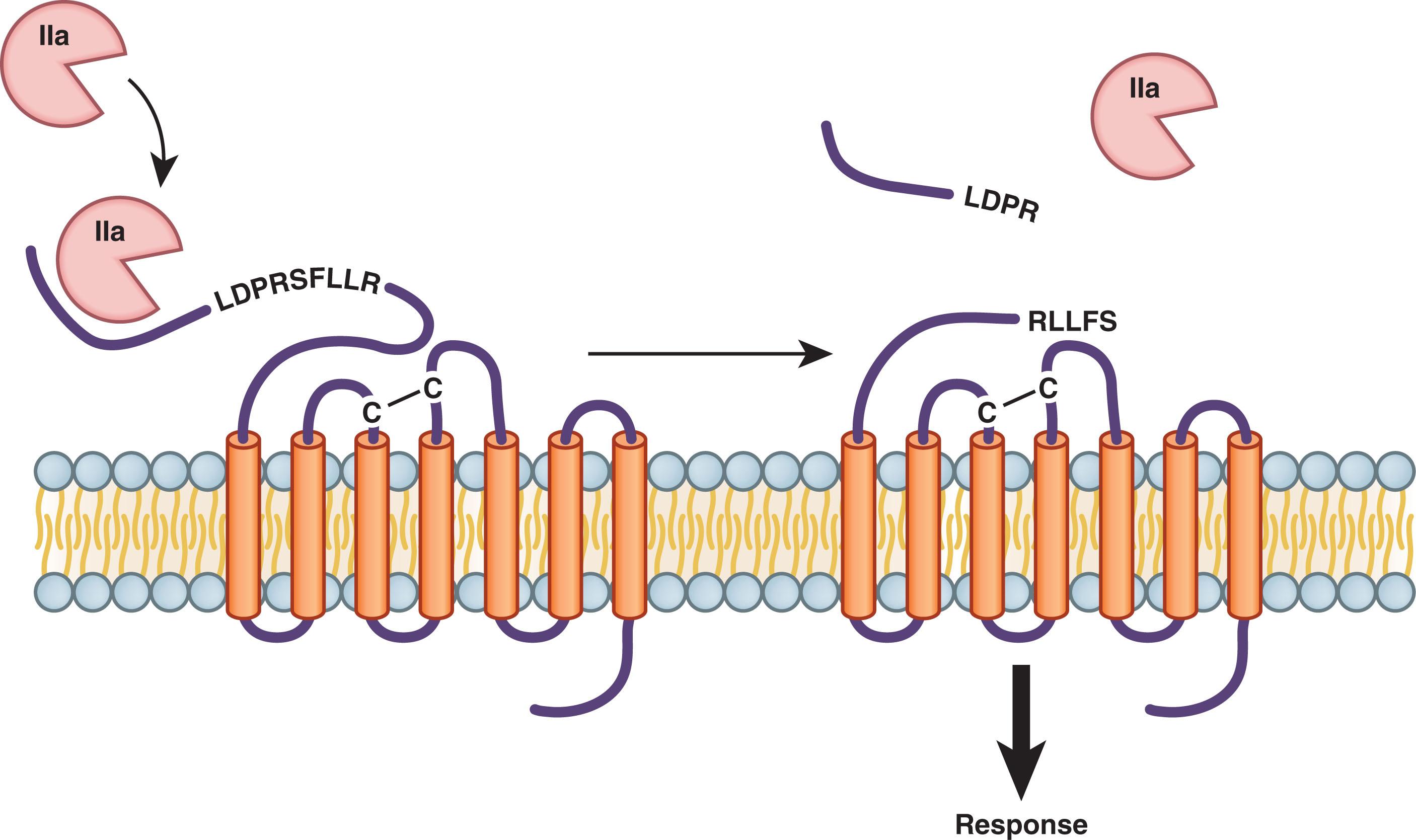
Although TP and the various ADP receptors signal through different pathways, they all trigger an increase in the intracellular calcium concentration in platelets. This, in turn, induces shape change via cytoskeletal rearrangement, granule mobilization and release, and subsequent platelet aggregation. Activated platelets promote coagulation by cycling phosphatidylserine from the inner membrane bilayer to the outer layer. Surface exposure of this anionic phospholipid is essential for assembly of coagulation factor complexes (see Chapter 125 ). Once assembled, these clotting factor complexes trigger a burst of thrombin generation and subsequent fibrin formation. In addition to converting fibrinogen to fibrin, thrombin amplifies platelet recruitment and activation, thus promoting expansion of the platelet plug. Thrombin binds to protease-activated receptor (PAR) types 1 and 4 (PAR1 and PAR4, respectively) on the platelet surface and cleaves their extended amino-termini, thereby generating new amino-termini that serve as tethered ligands that bind internally and activate the receptors ( Fig. 121.3 ). Whereas low concentrations of thrombin cleave PAR1, PAR4 cleavage requires higher thrombin concentrations. Cleavage of either receptor triggers platelet activation.
In addition to providing a surface on which clotting factors assemble, activated platelets also promote fibrin formation and subsequent stabilization by releasing factor V, factor XI, fibrinogen, and factor XIII (see Chapters 124 and 125 ). Thus, there is coordinated activation of platelets and coagulation, and the fibrin network that results from thrombin action helps anchor the platelet aggregates at the site of injury. Activated platelets also release adhesive proteins, such as vWF, thrombospondin, and fibronectin, which augment platelet adhesion at sites of injury, as well as growth factors, such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β), which promote wound healing. Platelet aggregation is the final step in the formation of the platelet plug.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here