Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Childhood-onset neuromuscular diseases are typically inherited genetic diseases characterized by general neurodevelopmental dysfunction, aberrant muscular function and development, and resulting pediatric deformity. This population often requires early orthopedic intervention to address mobility and motor deficits, and in some cases improve the patient’s comfort, hygiene, and general quality of life. This chapter systematically addresses the various childhood-onset neuromuscular diseases that are most frequently seen and treated by orthopedic surgeons through an overview of the disease, a brief review of the orthopedic literature, and a summary of common practices.
Spinal muscular atrophy (SMA) is an autosomal recessive disease affecting the α motor neurons of the anterior horn of the spinal cord. The typical patterns of atrophy are the result of a deletion in one of the survival motor neuron genes ( SMN1 and SMN2 ), which produce a protein necessary for the survival of α motor neurons. A homozygous insult to SMN1 leads to SMA more than 95% of the time because the SMN2 gene produces only 10% to 20% of the functional protein. SMA is one of the leading causes of childhood disability, with an estimated incidence of 1 in 6000 to 10,000 newborns and a carrier frequency of 1 in 40 to 60.
First described by Werdnig and Hoffman, the common presentation of SMA is generalized muscle weakness in early life. Muscle involvement is prominent in muscles supporting the trunk and the lower extremities (proximal muscles are involved to a greater degree than distal muscles). This disease should be considered when children fail to reach developmental milestones, exhibit hypotonia and fasciculations, or demonstrate the typical patterns of muscle weakness with loss of deep tendon reflexes (DTRs). Creatine kinase levels, nerve conduction studies, and EMG may help elucidate the etiology, and if it remains in question, testing for the SMN1 gene deletion can confirm the diagnosis. These patients typically have normal nerve conduction studies, but exhibit abnormal findings on EMG and muscle biopsy. Children with the more severe forms of SMA (I and II) will present early for orthopedic management of early onset scoliosis and hip deformities resulting from muscle imbalance.
The commonly used classification of SMA is based on the age of initial clinical onset. SMA I (also known as Werdnig-Hoffman disease) is the most severe type and has an age of onset between 0 and 6 months. These patients will never sit independently, typically have poor head control, and may also exhibit bulbar dysfunction, tongue atrophy, and an absence of DTRs. Individuals with SMA I have an increased risk of mortality secondary to respiratory failure in early childhood, typically before 2 years of age. Weakness of the intercostal muscles often results in a bell-shaped trunk as the chest wall collapses and the abdomen expands to support breathing. However, recent development of various targeted therapeutic approaches to increase the amount of full-length SMN protein and regenerate or repair damaged motor neurons is reaching preclinical and clinical trial stages.
SMA II has an intermediate age of onset of 7 to 18 months. This group is defined by their ability to maintain an unsupported upright seated position, though they may or may not be able to maintain independent sitting throughout later life. Some may be able to achieve standing with long-leg braces or support frames, though generally these patients are unable to walk. With improved pulmonary toilet and GI care, many patients are now expected to live into adulthood. Joint contractures remain the chief orthopedic issue for this population, and similarly to SMA I patients, the development of kyphoscoliosis secondary to intercostal muscle weakness may lead to life-threatening respiratory complications.
SMA III, also known as Kugelberg-Welander disease or juvenile SMA, is a mild form of the disease with an average age of onset after 18 months old. All patients are ambulatory (with considerable difficulty), although the age at which patients achieve independent walking is variable. The ability to walk may be lost late in the first decade or during early adolescence, and while patients may be able to walk, proximal muscle and hip abductor weakness effectively prevents them from running. Patients are often of above-average intelligence and function well in social, school, and work environments. These patients may develop scoliosis, and musculoskeletal and joint overuse symptoms are common. Patients who maintain ambulation typically have long-term mortality rates similar to that of the general population.
SMA IV has an onset in the second or third decade of life and is characterized by mild motor impairment. These patients typically do not require special orthopedic intervention.
Scoliosis and hip subluxation/dislocation are two major orthopedic concerns in the SMA population ( Figure 52.1 ). SMA I may present with torticollis associated with compensation for scoliosis, generalized muscle weakness, and flexion contracture of the hip, knee, and ankle, necessitating orthotics, bracing, or surgery. The increased risk of lower extremity fracture in SMA II and upper extremity fracture in SMA III has been attributed to osteopenia and osteoporosis, and may be managed nonsurgically with immobilization and fall prevention.
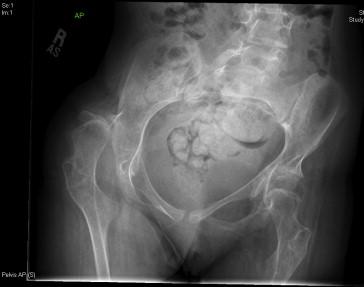
While the current SMA classification system is widely accepted, it is used primarily to predict the spectrum and age of onset of clinical symptoms to be expected. The International SMA Consortium agrees that care should be tailored to address patients’ current functional status rather than their particular classification. Dividing this group into nonsitters, sitters, and walkers may prove more useful with regard to guiding treatment.
In one series of 43 patients, the prevalence of scoliosis was as high as 95%, and at 9 years of age the average curve measured 74, 54, and 23 degrees for SMA I, II, and III, respectively. Granata et al. concluded that nonambulatory patients have larger and more rapidly progressing C-shaped curves than do type III patients, and ambulatory status has been directly correlated to the severity of the curve. Up to 80% of the curves seen in SMA patients are single thoracolumbar curves and are frequently associated with other spinal deformities such as pelvic obliquity and kyphosis.
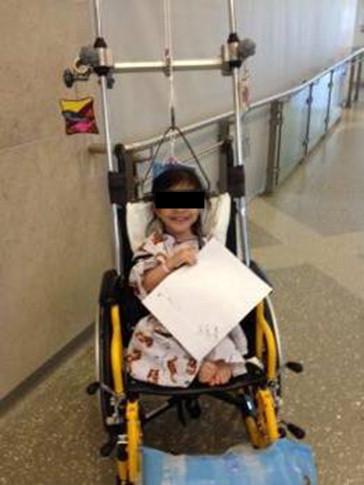
Timing of intervention in scoliosis should take into account the balance between postponement of surgical intervention to preserve growth potential and the deterioration of respiratory function with curve progression. Pulmonary alveolar development happens primarily in early life, and by 8 years of age, children have approximately the same number of alveoli as they will in adulthood. Robinson et al. reported a 4.7% decrease in predicted vital capacity and a 3.3% decrease in peak flow for every 10-degree increase in Cobb angle. As a result of the high likelihood of progression, SMA patients require careful monitoring as there is a limited time window for optimal surgical outcomes. Bracing of scoliosis has been attempted with variable success. In one series of 15 patients, the average curve was 88 degrees, and only three had curves less than 60 degrees; compliance was poor, as only 10 were able to follow the brace-wear prescription, and all were noted to have progression of scoliosis. However, good results from bracing have been described for patients with SMA III. For select patients, the author may opt to use wheelchair traction prior to definitive surgical management ( Figure 52.2 ). For these patients, sitting supports may be cautiously considered as the benefits must be weighed against the potential detrimental effects on breathing and chest wall excursion.
The primary goal of spinal arthrodesis is to improve sitting position and balance during ambulation. Additional indications for surgery include progressive deformity and curve greater than 50 degrees, though in most series the curve has been significantly greater. With the use of Harrington and Luque rods now out of favor, posterior fixation using pedicle screws has shown good results. One study reported an average correction of 62% in a series of 9 patients, all with an improvement in function, although 4 of these had postoperative complications including pulmonary edema and painful instrumentation necessitating removal of hardware. Patients and families have reported satisfaction with surgical outcomes regardless of the change in functional performance, which has been attributed to improved seated balance and cosmesis as well as fair improvement in pulmonary status and self-image. This subjective improvement in pulmonary status has been quantified by Robinson et al. as an increase in vital capacity in 8 of 9 patients undergoing posterior spinal fusion; however, data quantifying long-term changes are not available. Nonetheless, early fusion in SMA and congenital scoliosis in general is not without pitfalls. In a study correlating quality of life, pulmonary function, and radiographic outcomes following early spinal fusion, the authors found that patients with early fusion had shorter spines, worse pulmonary function, and more pain than healthy peers. As a result, growing constructs including chest-wall-based systems (the Vertical Expandable Prosthetic Titanium Rib), rib-to-pelvis, rib-to-spine, and spine-to-spine constructs have been gaining popularity in the SMA population as they allow for curve correction without arthrodesis. However, this modality may increase the risk of potential psychosocial effects given patients’ young age and need for repetitive surgeries. In a series of 15 patients, growing rods achieved a mean correction of 42%, though complications were frequent and were related to implant failure and prominence as well as the need for frequent reoperation to lengthen the implant. Anterior surgery is contraindicated in this population due to the high frequency of progression.
Marked hypotonia and significant pulmonary and nutritional difficulties are the primary contributors to morbidity and mortality. Generalized weakness may limit patients’ abilities to participate in physical, occupational, and speech therapies. Devices to support and improve posture should be considered, and upper arm supports or slings to promote arm and hand function and range of motion may be helpful when used cautiously. In general, however, orthopedic intervention is rarely indicated, and any discussion of surgical treatment should involve the family and multidisciplinary team. Of note, hypotonia may be associated with fractures at birth and suggestive of osteogenesis imperfecta, which may contribute to some diagnostic confusion though these heal quickly with appropriate nonoperative management.
In general, management of SMA is multidisciplinary, involving pediatric neurology, genetics, orthopedics, physical therapy, and pulmonary and GI care. Pulmonary complications are common and result from the combination of weak intercostal musculature and strong diaphragmatic pull forming the bell-shaped torso, which leads to poor airway clearance and nighttime hypoventilation. Poor GI function, including bulbar dysfunction, poor cough, and swallowing difficulty, contributes to the high risk of pulmonary aspiration. Given the high risk of perioperative and postanesthesia pulmonary complications, patients’ respiratory function should be optimized, and consultation with a pulmonologist may be indicated. While the ethical considerations surrounding noninvasive ventilation (NIV) in this population abound, evidence exists to suggest that NIV may actually alter time to onset of chest wall deformity. Postoperative considerations include use of continuous ventilation, direct ICU transfer, and adequate analgesia to minimize hypoventilation and poor airway clearance secondary to pain. Specific recommendations will vary based on a patient’s functional status, and as a general rule, nonsitters are at highest risk for postoperative pulmonary complications while walkers typically require fewer and less invasive interventions.
Hereditary sensory and motor neuropathies (HSMNs) are characterized by the degradation of Schwann cells and the myelin sheath, resulting in progressive peripheral neuropathy. While this group of neuropathies includes a number of disease entities, e.g. congenital hypomyelinating neuropathy and Dejerine-Sottas disease, the most common of these is Charcot-Marie-Tooth disease (CMT). CMT is the most common hereditary neuromuscular disorder with a prevalence of approximately 10 to 82.3 per 100,000 individuals. CMT encompasses a family of diseases with a variable clinical spectrum and patterns of inheritance, and has been linked to at least 40 different genes. Characteristics of presentation and time of onset are dependent on the subtype, though CMT I (HSMN I) is most prevalent with patients typically developing symptoms within the first two decades of life.
Patients commonly present with nonspecific complaints of weakness, clumsiness, and unstable ankles resulting in frequent sprains. The initial signs and symptoms on physical exam include distal weakness and muscle wasting, sensory loss, pes cavus or pes planus, foot drop with steppage gait, and reduced or absent DTRs. Patients may also complain of pain in the lower limbs and lumbar spine. The diagnosis should be suspected based on the typical clinical presentation and a positive family history, and is supported by confirmatory molecular genetic testing. For individuals with a negative family history, it should be noted that spontaneous mutations represent up to half of all cases. The orthopedic concerns associated with this family of diseases most commonly involve the feet, manifesting as varus deformity, progressive pes cavus, and a host of foot deformities, though spinal deformity and hip dysplasia are also frequently seen. Careful examination is important in this population as the differential diagnosis of CMT based on physical exam includes poliomyelitis, spinal cord tumor, syringomyelia, and diastematomyelia.
Historically, CMT had been referred to by the general term “peroneal muscular atrophy,” as the pattern of wasting typically involves the anterior compartment muscles and peroneus brevis while sparing the peroneus longus. The foot deformities in CMT cover a spectrum of patterns including claw toes, forefoot and hindfoot cavus, hindfoot varus and foot drop. Loss of intrinsic foot muscles leads to claw toes early on, and continued unopposed pull from the peroneus longus and extensor hallucis longus (EHL) has been implicated as a possible cause of cavus foot. Similarly, tibialis anterior muscle wasting may result in foot drop, and when the EHL is spared, this may result in a clawed hallux. The presence of bilateral cavus feet is highly suggestive of CMT, and has been reported to be as high as 78%.
A careful physical exam noting the presence or absence of specific muscle weakness and the rigidity or laxity of the deformities will inform surgical management. Clawtoes are an early-manifesting deformity and may be rigid at the time of initial presentation, though fore- and hindfoot deformities are oftentimes flexible and may remain so for years. Once plantar flexion of the first ray becomes rigid, the heel necessarily moves into a varus position during weight bearing. The Coleman block test, in which patients stand on a block and place their weight on the lateral border of the foot, can be used to evaluate the flexibility of this cavovarus deformity. Noting that the talus tilts out of varus and into valgus will indicate that the deformity is still flexible. Conversely, ligamentous laxity is often present in this population and may lead to chronic inversion ankle sprain secondary to talofibular and calcaneofibular insufficiency. Coleman block test results, in addition to ankle films to evaluate joint alignment and bony deformity as well as hindfoot alignment, are crucial to planning conservative or surgical management.
There is no evidence that bracing these children will ultimately prevent progression of foot deformities. However, functional bracing, such as ankle-foot orthoses (AFOs), may be helpful in addressing foot drop and chronic ankle sprain. Higher functioning and athletic patients may compensate on their own by adjusting stride height or increasing circumduction. For these patients, a simple ankle instability brace may be sufficient support to prevent frequent ankle sprain. Stretching the posterior compartment muscles may also help to decrease the rate of progression and allow for longer brace wear versus early surgery, and exercise and physical therapy may also be helpful to address weakness and contractures. Use of botulinum toxin (Botox) to address the imbalance of agonist-antagonist muscles that are the ultimate cause of deformity has been preliminarily investigated and, thus far, found to be ineffective.
When considering surgery in this population, the surgeon must weigh the risks and benefits of early surgery while deformity is relatively flexible versus pursuing conservative management and attempting to stave off surgery until onset of a more rigid deformity. Furthermore, there are no strong data to inform the appropriate timing of surgery. Surgery must be individualized to patients based on the character and location of the deformity while taking into careful consideration the presence of deforming muscular imbalance. The author’s preferred approach is to perform early soft tissue releases and rebalancing to prevent development of bony rigid deformity. Some argue that in patients with sensory deficits, fusion should be avoided.
Often, younger patients will do well with simple soft tissue release procedures. Release of the plantar fascia near its origin can help to correct cavus deformity. Additional soft tissue procedures include transfer of the peroneus longus to the peroneus brevis, which augments foot eversion while decreasing plantar flexion force on the first ray, and the Jones transfer, in which the EHL tendon is transferred to the first metatarsal neck, allowing this muscle to assist in dorsiflexion of the foot while avoiding claw toe deformity. Transfer of the tibialis posterior tendon to the second or third cuneiform or around the second metatarsal may be used to centralize muscular forces and enhance dorsiflexion. When strength of the tibialis anterior is spared, complete or split tendon transfer to the lateral cuneiform or cuboid can help to lateralize forces and augment eversion. This may be used in combination with lengthening of the tibialis posterior to further decrease inversion forces with good long-term results versus triple arthrodesis. There is limited evidence regarding the success of soft tissue procedures when used alone, likely the result of the high diversity in patients’ deformity and deforming forces at play, though in one study a small group of patients undergoing early operative soft tissue intervention avoided triple arthrodesis at long-term follow-up.
Older children and those with rigid deformity often require osteotomies in combination with tendon transfers to achieve adequate correction. Options include dorsiflexion osteotomies for plantar-flexed first ray, midtarsal osteotomy, medial cuneiform osteotomy, and calcaneal osteotomies when hindfoot varus is present. Triple arthrodesis is typically only used as a salvage procedure for fixed deformity as there is a high incidence of developing degenerative joint disease in the foot and ankle and poor patient satisfaction related to appearance, function, pain, and need for reoperation. Good functional results following triple arthrodesis at long-term follow-up have been reported, though in these studies soft-tissue balancing was used when indicated. Despite the high frequency of ankle pain and laxity, ankle arthrodesis is rarely indicated and total ankle replacement is contraindicated due to failure related to uneven medial-sided wear patterns.
Hip dysplasia in CMT was first reported in a case series by Kumar et al. in the early 1980s, and has subsequently been reported at a rate of ~8%. Hip dysplasia also has a greater association with CMT-1 than CMT-2, though the true prevalence remains unclear. It has been suggested that the alteration in gait with resultant change in biomechanics, including increased external rotation and decreased adduction of the hips, may contribute to hip subluxation and dysplasia later in life. While case reports exist documenting the incidence of hip dysplasia as a sentinel sign suggestive of CMT, the association between hip dysplasia and CMT is likely underreported. Occasionally, patients have hip dysplasia present at birth, and in some cases have required proximal femoral or pelvic osteotomy to correct coxa valga and painful subluxation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here