Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients with Marfan syndrome are a clinically diverse group, but they classically exhibit tall stature, long thin limbs, long thin spider-like digits (arachnodactyly), dislocation of the ocular lens, and cardiac anomalies. In 1896, Marfan, a French pediatrician, described to his colleagues the clinical features of Gabrielle, a 5-year-old girl with long thin limbs (leading him to describe the girl’s condition by the term dolichostemelia ), spider-like digits, and joint contractures that prevented her from walking. Marfan’s drawings of Gabrielle’s hands and feet have characteristics of Beals syndrome as well as Marfan ( Fig. 37.1 ). In 1902, Achard named the syndrome arachnodactyly. It was only after the identification of associated cardiac anomalies by Baer and colleagues in 1943 that the description of the major features of Marfan syndrome was complete. Excellent reviews of Marfan syndrome have been published by several other authors. , , , ,
Marfan syndrome is transmitted in an autosomal dominant manner. There is great variability in the nature and severity of clinical manifestations, and an unwary physician may not recognize the syndrome in mildly affected patients. Although a family history of Marfan syndrome is an important diagnostic criterion, the incidence of spontaneous mutations is thought to be between 15% and 30%. The lack of a specific laboratory test to confirm the diagnosis of Marfan syndrome and the variable clinical expression of the disorder, including mild manifestations, make it difficult to estimate its incidence with certainty. The prevalence is thought to be approximately 1/5000 population in the United States.
Marfan syndrome is caused by a defective gene, FBN1, located on the long arm of chromosome 15. , , , This gene encodes for fibrillin-1, a large glycoprotein closely associated with elastin. The biochemistry of fibrillin correlates with the syndromic features of Marfan and Beal syndromes. Fibrillin monomers normally link head to tail in forming microfibrils, which form two- and three-dimensional structures that add elasticity to tissues. Abnormal or absent fibrillin alters tissue elasticity. Fibrillin also alters the bioavailability of transforming growth factor-β (TGF-β). In addition to their presence in the aortic media and suspensory ligaments of the lens, fibrillin microfibrils are found in skin, tendon, cartilage, and periosteum. a
a References , , , , , , , , , , , , .
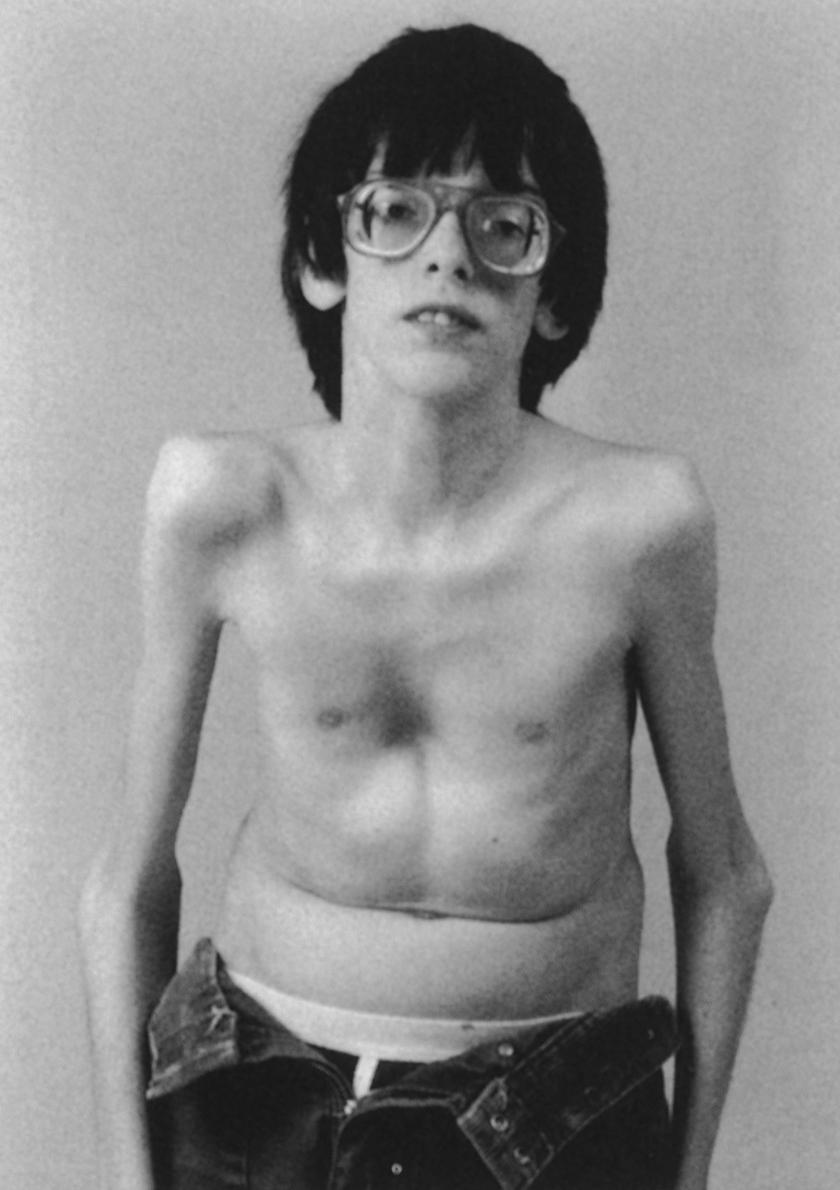
A person with classic florid Marfan syndrome is easily recognized as an unusually tall, lanky individual with arachnodactyly, disproportionately long arms, chest wall deformity, extreme myopia, and a loud cardiac murmur ( Fig. 37.2 ). b
b References , , , , , , , A, , , .
Many patients have much less florid manifestations, however, and the clinician should rely on an awareness of the condition and strict fulfillment of specific criteria to make the diagnosis. Some individuals may have subtle deformities suggestive of the condition, including a taller-than-average appearance, ligamentous laxity, myopia, and minimal cardiac abnormalities, such as mild mitral valve prolapse. In the past, such patients were characterized as having forme fruste Marfan syndrome. , , 60a , , 74a ,
A number of historical figures are thought to have had Marfan syndrome, the most famous being Abraham Lincoln. Although Lincoln was tall and lanky and had large hands, lending credence to this suggestion, he had no known family history of the disorder and no cardiac anomalies, and he wore glasses for reading only after 50 years of age. More likely to have been affected with Marfan syndrome were violinist Nicolo Paganini , and composer Sergei Rachmaninoff. Certain to have had Marfan syndrome was US volleyball player Flo Hyman, whose 1986 death on the volleyball court emphasizes the importance of identifying the presence of the condition and screening for its sequelae.
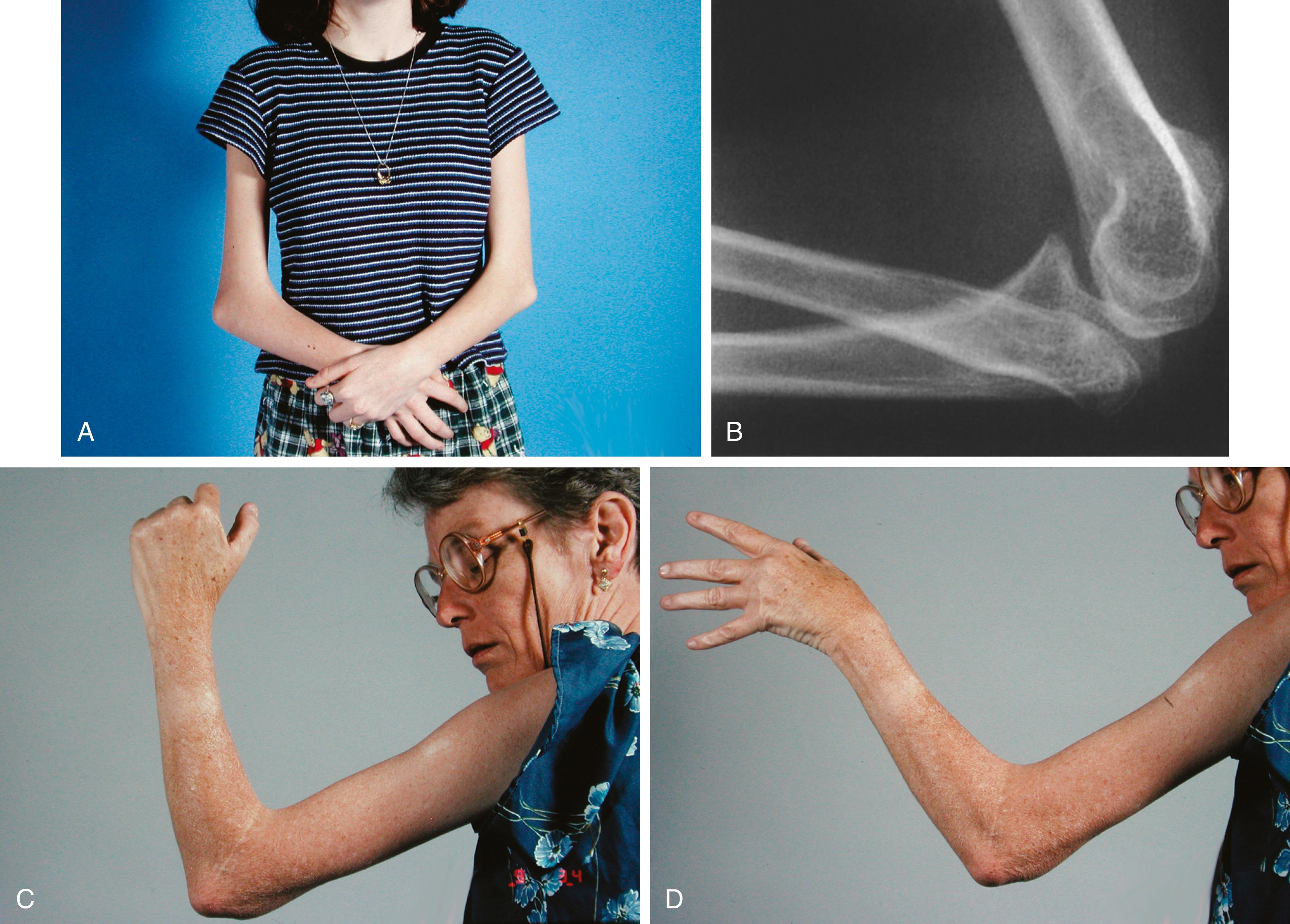
Patients with Marfan syndrome are typically tall—usually attaining a height greater than 6 feet in adult life—and have disproportionately long, thin limbs. The distal bones of the limbs exhibit the excess length most strikingly, resulting in long slender hands and feet, with spider-like digits (arachnodactyly). The ratio of the upper body segment (measured from the top of the symphysis pubis to the top of the head, or by subtracting the measurement of the lower body segment from the total height) to the lower body segment (measured from the sole to the top of the symphysis pubis) is abnormally low (the upper body–to–lower body ratio is 0.93 in the normal adult population). In addition, the arm span usually exceeds the patient’s total height.
Patients with Marfan syndrome often have a high-arched palate, a long narrow face, and prognathism. The increased height of the skull is associated with enlargement of the frontal sinus.
The hallmark of ocular involvement is ectopia lentis (dislocated lens) caused by lax or broken suspensory ligaments (zonules). The lens typically dislocates upward and laterally and may manifest as iridodonesis (fluttering of the iris). The dislocation is best seen on slit lamp examination after dilation of the pupil. There is usually extreme myopia as a consequence of changes in the shape of the globe. Strabismus, cataract, glaucoma, and detached retina also may occur.
Dilation of the ascending aorta and mitral valve insufficiency are the most common associated cardiovascular anomalies. c Ascending aortic dilation may result in aortic valvular incompetence and frequently leads to the formation of a dissecting aneurysm. Aneurysms and dissections may also occur in the descending or thoracoabdominal aorta. In a review of 732 cases of Marfan syndrome followed for 6 years, there were five deaths and two aortic dissections. These events were four times more likely to occur if the aortic diameter exceeded 50 mm. These cardiovascular anomalies are the most frequent cause of death in patients with Marfan syndrome, and their presence must be carefully sought. d
c References , , , , , , , , , , , A, , , , a, , , .
d References , , , , , , , .
Pectus excavatum is common and is caused by excessive longitudinal growth of the ribs. The anteroposterior (AP) diameter of the thoracic cage is reduced. Recurrence after surgical correction is more common than in the general population. , Pectus carinatum is also frequently seen in patients with Marfan syndrome.
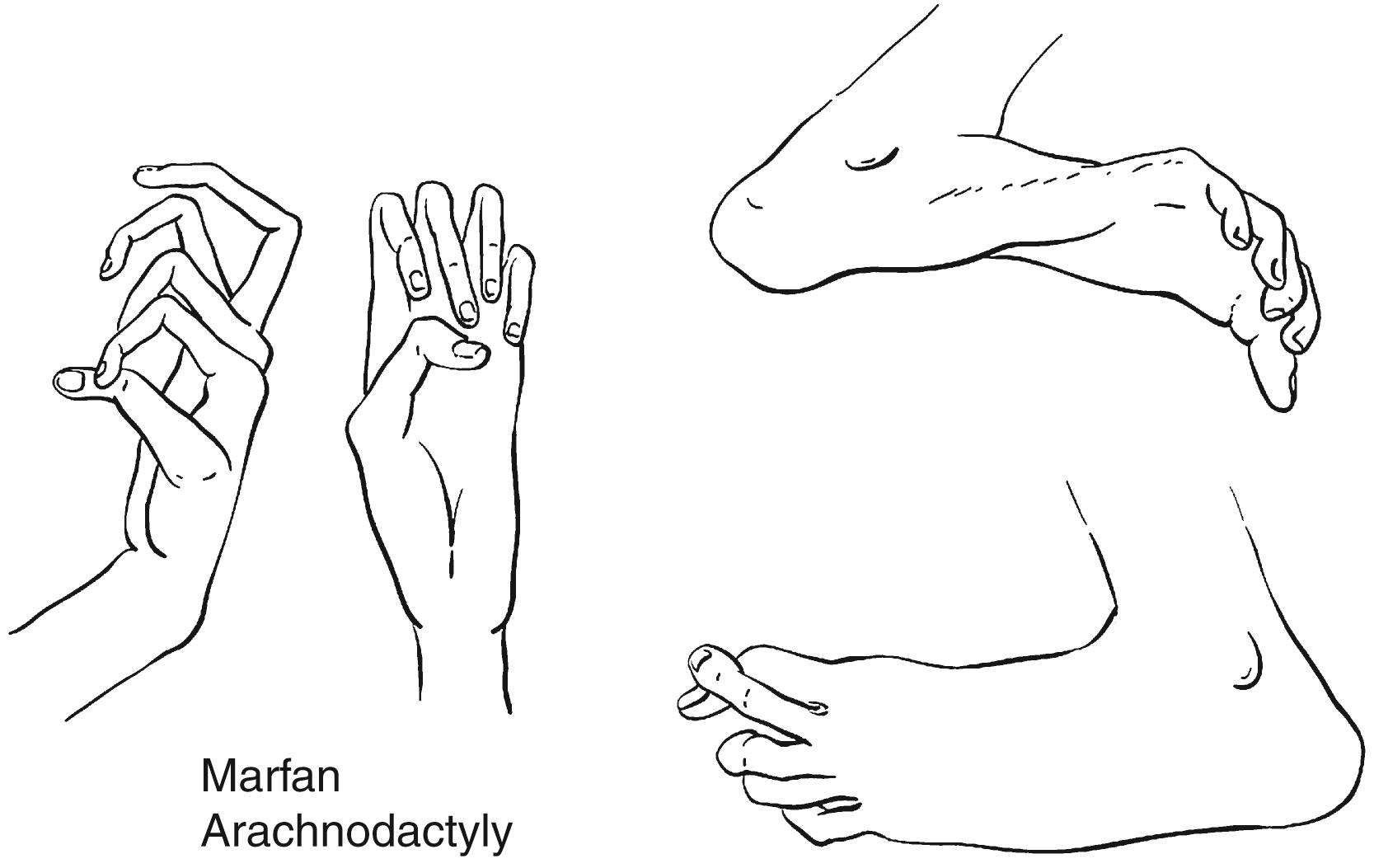
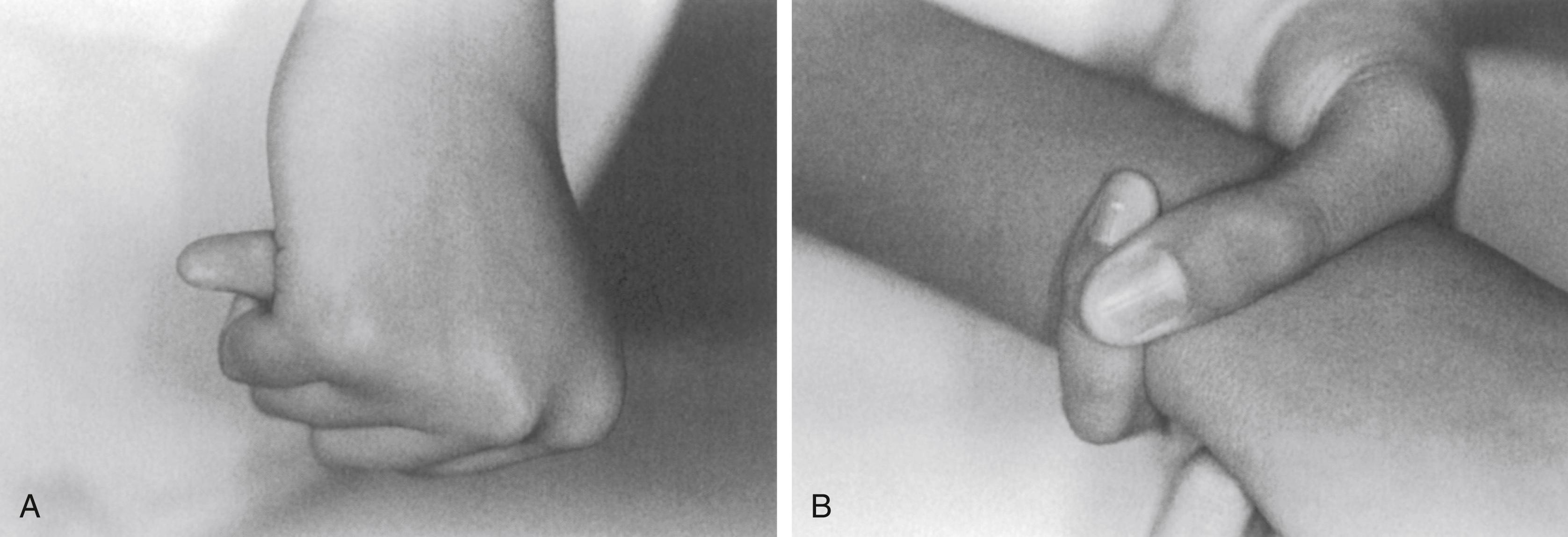
Joint laxity is another hallmark of the disease. Marked pes planovalgus and genu recurvatum are typical. Dislocations of the hip, either developmental or presenting later, and of other joints can occur. Perilunate dislocations have been reported and are caused by excessive carpal ligamentous laxity. Extreme planovalgus deformity of the feet is a common feature. The combination of joint laxity and long digits results in several clinical signs indicative of, but not pathognomonic for, Marfan syndrome. One of these is the thumb sign ( Fig. 37.3A ), in which the nail of the flexed thumb extends beyond the ulnar border of the clenched fist, often referred to as the Steinberg sign. The wrist sign refers to the patient’s ability to encircle the opposite wrist with the thumb and small finger, with the thumb overlapping the terminal phalanx of the small finger (see Fig. 37.3B ). ,
The vertebral column is significantly affected in patients with Marfan syndrome. e
e References , , , , , .
Radiographs typically show tall vertebral bodies with elongated transverse processes. The position of the sacrum is low in relation to the iliac crests. The spinal canal may appear widened in the lumbar region, with concavity of the posterior borders of the vertebral bodies. Increased localized kyphosis and evidence of ligamentous instability have been noted in the cervical spine. Scoliosis is common, reported in 30% to 100% of patients. f
f References , , , , , , , , , , , , , .
The curve pattern in Marfan syndrome is often double or multiple. Pain is frequently associated with these curves. , , Spondylolisthesis of L5–S1 is also relatively common in Marfan syndrome. , , Several cases of atlantoaxial rotatory instability have been reported in patients with Marfan syndrome, including one case of sudden death. ,
Other anomalies associated with Marfan syndrome include polysyndactyly, myopathy, talipes equinovarus, muscular underdevelopment, and hypotonia. Protrusio acetabuli can be seen radiographically and may manifest as hip pain and stiffness in patients with Marfan syndrome. g
g References , , , , , , , , .
Inguinal, femoral, diaphragmatic, and incisional hernias may be present. Subcutaneous fat is usually sparse. The skin is relatively elastic, and striae may be present.
Although the defective gene for Marfan syndrome has been identified, the diagnosis remains a clinical one, based on the fulfillment of diagnostic criteria. These criteria were updated by De Paepe and colleagues and are summarized in Box 37.1 . In the past, an increased metacarpal length-to-width ratio (metacarpal index) was thought to aid in the diagnosis of Marfan syndrome. However, there is disagreement regarding the usefulness and specificity of this radiographic assessment, and it should probably not be used as a criterion for the diagnosis of Marfan syndrome. , , ,
For the skeletal system to be considered involved, at least two major criteria or one major criterion plus two minor criteria must be present.
Pectus carinatum
Pectus excavatum requiring surgery
Reduced upper segment–to–lower segment ratio, or arm span–to-height ratio >1.05
Wrist and thumb signs
Scoliosis >20 degrees or spondylolisthesis
Reduced extension at the elbows (<170 degrees)
Medial displacement of the medial malleolus, causing pes planus
Protrusio acetabuli of any degree (ascertained on radiographs)
Pectus excavatum of moderate severity
Joint hypermobility
High-arched palate with crowding of teeth
Facial appearance (dolichocephaly, malar hypoplasia, enophthalmos, retrognathia, down-slanting palpebral fissures)
In addition to the major criterion, at least two minor criteria must be present for the ocular system to be considered involved.
Ectopia lentis
Abnormally flat cornea (as measured by keratometry)
Increased axial length of globe (as measured by ultrasonography)
Hypoplastic iris or hypoplastic ciliary muscle causing decreased miosis
For the cardiovascular system to be considered involved, one major criterion or one minor criterion must be present.
Dilation of the ascending aorta with or without aortic regurgitation and involving at least the sinuses of Valsalva
Dissection of the ascending aorta
Mitral valve prolapse, with or without mitral valve regurgitation
Dilation of the main pulmonary artery, in the absence of valvular or peripheral pulmonic stenosis or any other obvious cause, in a patient <40 years
Calcification of the mitral annulus in a patient <40 years
Dilation or dissection of the descending thoracic or abdominal aorta in a patient <50 years
For the pulmonary system to be considered involved, at least one minor criterion must be present.
None
Spontaneous pneumothorax
Apical blebs (ascertained by chest radiography)
For the skin and integument to be considered involved, at least one minor criterion must be present.
None
Striae atrophicae (stretch marks)
Recurrent or incisional hernia
For the dura to be considered involved, the major criterion must be present.
Lumbosacral dural ectasia
None
For the family or genetic history to be considered contributory, one major criterion must be present.
Having a parent, child, or sibling who meets these diagnostic criteria independently
Presence of a mutation of FBN1 known to cause Marfan syndrome
Presence of a haplotype around FBN1, inherited by descent, known to be associated with unequivocally diagnosed Marfan syndrome in the family
None
If the family or genetic history is not contributory, major criteria in at least two different organ systems and involvement of a third organ system must be present.
If a mutation known to cause Marfan syndrome in others is detected, one major criterion in one organ system and involvement of a second organ system must be present.
Presence of a major criterion in the family history, and one major criterion in one organ system and involvement of a second organ system
Clinicians, particularly primary care physicians and those working in scoliosis clinics, must be alert to the possibility of this condition because the presenting anomaly is usually scoliosis or a heart murmur. The physician should seek a family history of the disorder or its manifestations, especially tall stature, ligament laxity, poor vision, cardiac anomalies, and sudden or premature death. The examiner should check for the typical manifestations, including tall stature, reduced upper body–to–lower body ratio, high-arched palate, spinal deformity, thumb and wrist signs, and cardiac murmur. A survey of patients presenting to a musculoskeletal practice found that craniofacial features, thumb and wrist signs, pectus excavatum, and hindfoot valgus were the physical features with the highest diagnostic yield. Consultation should be sought with an ophthalmologist, who should perform a slit lamp examination to identify the presence of a dislocated lens, and a cardiologist, who should perform echocardiography to assess the diameter of the aortic root and look for evidence of mitral valve prolapse.
As detailed in Box 37.1 , for an individual with a family history of Marfan syndrome (i.e., definite family history of the disease or genetic confirmation in a first-order relative), one major criterion in one organ system and involvement of a second organ system are required to make the diagnosis. For an individual with a noncontributory family history, the presence of major criteria in at least two different organ systems and involvement of a third organ system are required to make the diagnosis of Marfan syndrome.
A number of conditions have clinical features suggestive of Marfan syndrome and should be considered in the differential diagnosis. The principal considerations for orthopaedic surgeons are homocystinuria, congenital contractural arachnodactyly (Beals syndrome), Loeys-Dietz syndrome, and juvenile ophthalmoarthropathy (Stickler syndrome).
Homocystinuria can closely resemble Marfan syndrome clinically. Patients whose appearance suggests Marfan syndrome and who have mental retardation or mental deficiency should be suspected of having homocystinuria. This diagnosis can be confirmed by testing the urine for the presence of excessive homocystine or its byproducts (see later, “Homocystinuria”).
Congenital contractural arachnodactyly (Beals syndrome) also shares some phenotypic similarities with Marfan syndrome, in that patients have long extremities and usually marked arachnodactyly. However, the condition appears to occur sporadically and is essentially obvious from birth, and affected patients have joint contractures rather than ligamentous laxity and more problematic kyphoscoliosis rather than the scoliosis seen in Marfan syndrome. As noted earlier, Beals syndrome is caused by an abnormality of fibrillin-2 due to a defect in the FBN2 gene located on chromosome 5 (see later, “Congenital Contractural Arachnodactyly”).
Several findings in the Loeys-Dietz syndrome are shared with Marfan syndrome. These patients have arachnodactyly, pectus excavatum, pectus carinatum, aortic aneurysms and dissection, and joint laxity. In addition, they have widely spaced eyes, bifid uvula and or cleft palate, and craniosynostosis. Vascular complications are often quite severe, often leading to early death from aneurysm rupture. Diagnosis is made by clinical features and genetic variants of TGFBR1 and TGFBR2.
Patients with Stickler syndrome have some phenotypic features similar to those of Marfan patients in that they tend to have long thin limbs (dolichostemelia); this condition is also autosomal dominant, so a family history is often found. However, Stickler syndrome has features of a skeletal dysplasia radiographically. Furthermore, the primary ocular manifestation is a tendency toward retinal detachment, which also occurs in patients with Marfan syndrome, but much less frequently than lens dislocation (see later, “Hereditary Progressive Arthro-Ophthalmopathy”).
Other conditions with features of Marfan syndrome primarily affecting organ systems other than the skeletal system must be considered by geneticists, ophthalmologists, and cardiologists. They include familial thoracic aortic aneurysm, familial aortic dissection, familial ectopia lentis, familial Marfan-like habitus, MASS (myopia, mitral valve prolapse, mild aortic regurgitation, and skin [striae] and skeletal [minor Marfan’s criteria] involvement) phenotype, and Shprintzen-Goldberg syndrome (Marfan-like skeletal changes, craniosynostosis, neurodevelopmental abnormalities, and occasionally aortic dilation). ,
Although there is no specific treatment for patients with Marfan syndrome, physicians should be alert to the various manifestations that may be initial evidence of the disorder. Orthopaedic surgeons should assure themselves that patients presenting with flatfeet, joint instability, or scoliosis do not have other manifestations of Marfan syndrome. If the skeletal features are consistent with those of Marfan syndrome, a cardiologist and ophthalmologist should be consulted.
The most common cardiovascular manifestations of Marfan syndrome are mitral valve prolapse, with or without regurgitation, and aortic aneurysm. Aortic aneurysm most commonly involves the ascending aorta and can lead to aortic valve incompetence or dissection of the aortic wall. All patients suspected of having Marfan syndrome should be evaluated by a cardiologist, and echocardiography should be performed to evaluate for the presence of these two conditions. Patients with confirmed Marfan syndrome should undergo serial echocardiography to monitor for progressive dilation of the aortic root, which normally measures approximately 3 cm in adults, depending on body size. Prophylactic aortic root resection is recommended when the dilation reaches 5 to 6 cm; it is important to monitor this condition in confirmed cases of Marfan syndrome because aortic aneurysm rupture is the most common cause of death in patients with the syndrome, and the perioperative mortality is much higher in urgent or emergent repairs than in elective repairs. h
h References , , , , , , , , , , , , , .
Another treatment to be considered is the use of β-blockers, which slow the rate of aortic root dilation in adults. , , , , Some authors also recommend their use in children. Finally, cardiologists often exclude children with Marfan syndrome from contact sports or strenuous activities because of the increased incidence of sudden death related to the cardiac manifestations of the syndrome.
Scoliosis is one of the most common and important manifestations of Marfan syndrome from an orthopaedic perspective and is often the presenting complaint. The incidence of scoliosis is 30% to 100% in different series, i
i References , , , , , , , , , , , , .
with the variation likely related to the heterogenicity of study populations. The treatment of scoliosis in patients with Marfan syndrome parallels that in patients with idiopathic scoliosis. In general, observation is sufficient for curves less than 25 degrees, bracing should be considered for progressive curves greater than 25 to 30 degrees, and spinal fusion and instrumentation should be considered for curves greater than 45 to 50 degrees. However, the results of scoliosis treatment in patients with Marfan syndrome differ from the results in those with idiopathic scoliosis. j
j References , , , , , , , , , , , , , .
Specifically, most reports indicate poor patient tolerance of bracing and a relatively high frequency of deformity progression, despite bracing. Vertebral stapling has been reported to have a high failure rate. In younger patients, growing rods may be considered, but again with a fairly high rate of complications. One case is reported of heart failure after rod lengthening, with resolution of the failure with relief of the distraction.
Surgical management is recommended for larger curves, and it is not uncommon for neglected cases to present with extremely severe kyphoscoliosis, in comparison with idiopathic scoliosis. Some authors report success with posterior fusion alone, whereas others recommend combined anterior-posterior fusion. , Current reports note comparable clinical outcomes with posterior fusion alone, allowing for shorter operative times and less blood loss. Anterior instrumentation and fusion alone have not been successful. A recent study comparing 34 Marfan patients to a group of idiopathic adolescents found that the Marfan patients had greater kyphosis correction, more fusion levels, more fusions to the pelvis, and greater correction of sagittal imbalance. They also had more CSF leaks, more instrumentation complications, more reoperations, and lower SRS-22 scores. Preoperative halo traction combined with vertebral column resection has been shown to improve outcome in very severe deformities. Marfan patients undergoing spinal fusion and instrumentation may have a higher likelihood of pseudarthrosis, mandating secure instrumentation and careful observation for its development.
In addition to scoliosis, kyphotic deformities and spondylolisthesis occur regularly in patients with Marfan syndrome. These deformities may require treatment, using the same guidelines that apply to patients without Marfan syndrome. Finally, patients with Marfan syndrome have a higher incidence of back pain, with or without spinal deformity, which may require symptomatic care. , , , ,
Another skeletal manifestation of Marfan syndrome is protrusio acetabuli. , , , ,
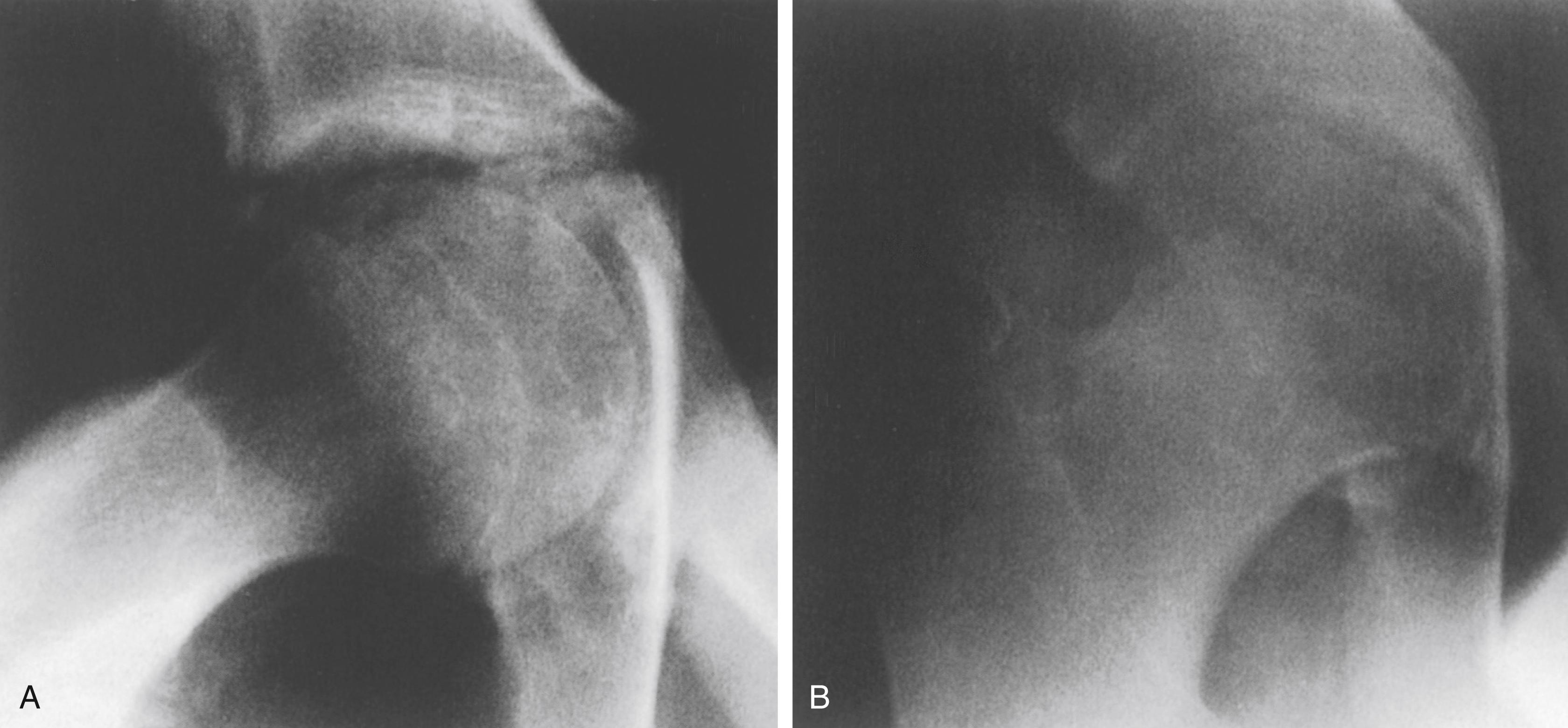
This condition , , is characterized clinically by hip joint stiffness and pain and radiographically by collapse of the acetabular teardrop ( Fig. 37.4 ). Steel described surgical closure of the triradiate cartilage in skeletally immature patients who were symptomatic or in whom increasing acetabular deepening was demonstrated radiographically. In his report on 19 hips followed to skeletal maturity, radiographic indices normalized in 12, improved in 4, and were unchanged in 3 (these 3 patients were operated on between 13 and 14 years of age); all hips were asymptomatic. He recommended surgical closure of the triradiate cartilage for patients between 8 and 10 years of age; older patients had symptomatic relief but little improvement in the radiographic appearance of their hips. Others have reported that the incidence of protrusio acetabuli in patients with Marfan syndrome is as high as 31%; however, not all patients are symptomatic, and prophylactic closure of the triradiate cartilage in asymptomatic patients is not recommended. , , ,
Patients with Marfan syndrome may present with developmental dysplasia of the hip as newborns or infants. In one report, Pavlik harness treatment was unsuccessful in obtaining reduction of the hip, and closed reduction and spica cast immobilization under anesthesia were required. However, the affected hips stabilized in the usual amount of time, and further treatment was not required.
The generalized joint laxity that characterizes patients with Marfan syndrome may manifest as severe pes planovalgus or joint dislocations, , especially of the patellofemoral joint. In general, treatment of these conditions should be as conservative as possible, because obtaining and maintaining joint stability can be difficult in these patients.
A rare variant of Marfan syndrome is a relatively severe form presenting in infancy, known as infantile or congenital Marfan syndrome. k
k References , , , , , .
The clinical characteristics, as described by Morse and colleagues, include serious cardiac pathology (often present at birth), congenital contractures (64% of their cases; 47% of cases reported in the literature), arachnodactyly, dolichocephaly, high-arched palate, micrognathia, hyperextensible joints, pes planus, anterior chest deformity, iridodonesis, megalocornea, and dislocated lenses. Cardiac anomalies include mitral valve prolapse, valvular regurgitation, and aortic root dilation. Three of the 22 patients identified by Morse and colleagues died during the first year of life; one-third of the survivors developed severe scoliosis, joint dislocations, or both. Sponseller and associates reported that of 14 patients with infantile scoliosis (onset before age 3 years) and Marfan syndrome, 4 died during treatment. Most cases of infantile Marfan syndrome are likely caused by a sporadic mutation in a single germ cell of one parent, because there was a positive family history in only one patient in each series. ,
In 1972, the predicted average life span of patients with true Marfan syndrome was 45 years, with premature deaths (almost all a result of cardiac complications) occurring at an average age of 32 years. Beginning with the introduction of aortic root surgery by Bentall and De Bono, the life span of Marfan patients has substantially increased. , , , , With periodic echocardiographic screening, treatment with β-blockers, and early elective aortic root surgery, the predicted average life span has increased to 72 years, with premature deaths occurring at an average age of 41 years. These improvements and an appreciation of the potentially dire consequences of not identifying the condition should remind orthopaedists to be alert for this syndrome.
Hereditary progressive arthro-ophthalmopathy is an autosomal dominant disorder that was first described in 1965 by Stickler and associates. , It is more commonly referred to as Stickler syndrome. Genetic linkage analyses in some families suggest that the disorder is due to a mutation of the COL2A1 gene, which encodes for type II collagen. , , Other studies have identified homozygous loss-of-function mutations in COL9A1, COL9A2, and COL9A3 genes, which code for collagen IX.
Clinically, the condition is characterized by mild hereditary spondyloepiphyseal dysplasia, premature degenerative arthritis, hearing loss, and congenital myopia that is compounded over time by chorioretinal degeneration and retinal detachment. l
l References , , , , , .
In addition, patients often have micrognathia or cleft palate, somewhat reminiscent of Pierre Robin syndrome. , Mandibular lengthening is feasible and may eliminate the need for tracheostomy. Herrmann and associates called attention to Stickler syndrome in the 1970s, believing that it was underdiagnosed and might be confused with Pierre Robin syndrome. , , Establishing a correct diagnosis is important because of the genetic implications and the need for periodic ophthalmologic assessment because retinal detachment occurs and is preventable. ,
The orthopaedic manifestations consist of a mild spondyloepiphyseal dysplasia. Coxa valga with or without acetabular protrusion, acetabular dysplasia, valgus slipped capital femoral epiphysis, and hip subluxation have been described. , , Typically, early degenerative changes occur in the hip because of the dysplasia. The digits may have fusiform enlargement. Vertebral body changes are similar to those of Scheuermann kyphosis. Endplate irregularities are seen, and scoliotic or kyphotic deformities can occur. Reduced bone mass and reduced bone turnover may account for some of the skeletal manifestations.
Treatment includes genetic counseling concerning the autosomal dominant trait and referral to an ophthalmologist for the management of eye complications. Progressive scoliosis or kyphosis may require orthotic treatment or spinal fusion. Total joint arthroplasty may be necessary in midlife because of premature degenerative joint disease.
Congenital contractural arachnodactyly (CCA), or Beals syndrome, is a rare condition that resembles and has been confused with Marfan syndrome, including the case Marfan described in 1896. m
m References , , , , , , , , , .
Epstein and associates described a case in 1968. In 1971, Beals and Hecht delineated this syndrome in a report of two kindreds. CCA is usually differentiated from Marfan syndrome by the absence of lens dislocation and aortic root dilation (although eye and heart problems can occur) and by the presence of joint contractures (maximal at the knee) that spontaneously improve with growth and walking.
CCA is transmitted as an autosomal dominant trait. Genetic linkage studies have identified the defect as a mutation of the FBN2 gene located on chromosome 5, which encodes for the protein fibrillin-2. , , , (As noted earlier, Marfan syndrome is caused by a mutation of FBN1 on chromosome 15, which encodes for fibrillin-1.) Fibrillin is a large glycoprotein and one of the structural components of the elastin-associated microfibrils. The similarity of the two FBN genes accounts for the phenotypic similarities between CCA and Marfan patients, although definitive amino acid sequence differences have been identified.
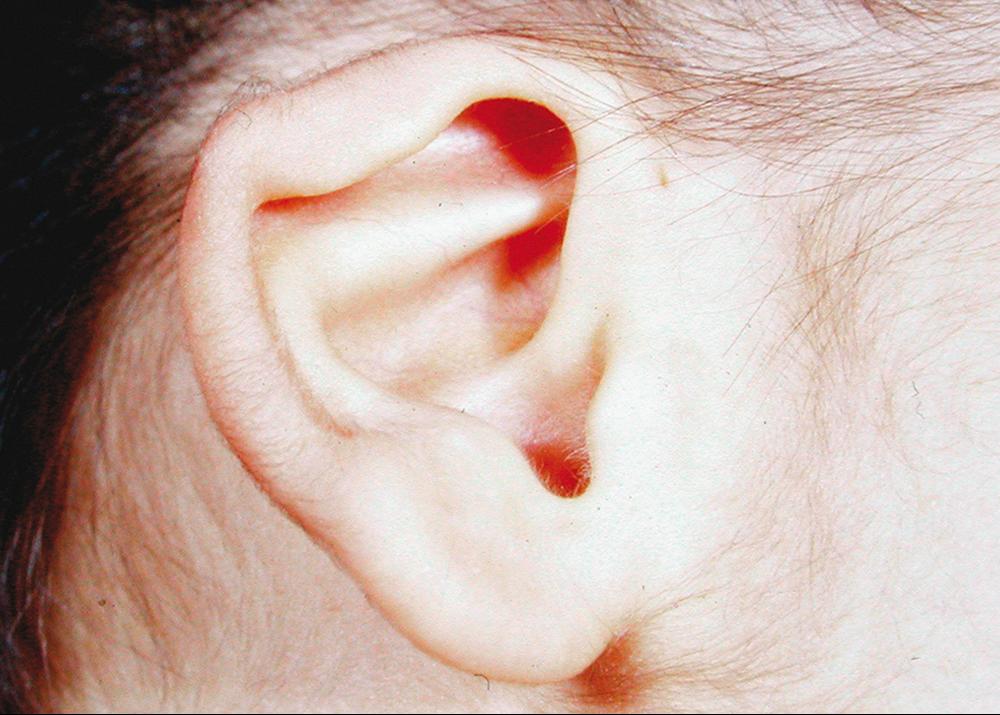
In patients with CCA, there is a tendency toward retrognathia and a small mouth. The crumpled appearance of the external ear is a distinctive clinical feature. It is caused by extra and prominent crura in the antihelix, partial obliteration of the concha, and flattening of the helix ( Fig. 37.5 ). There may be mild restriction of range of motion of the temporomandibular joints. Intelligence is generally normal, although some cases of neurodevelopmental delay have been reported. ,
The initial descriptions of CCA suggested that patients with this condition did not exhibit eye or heart abnormalities. As more cases were described, however, it was observed that these patients can have myopia and cardiac abnormalities that are milder than those seen in Marfan syndrome (typically, mitral valve prolapse with regurgitation). , , ,
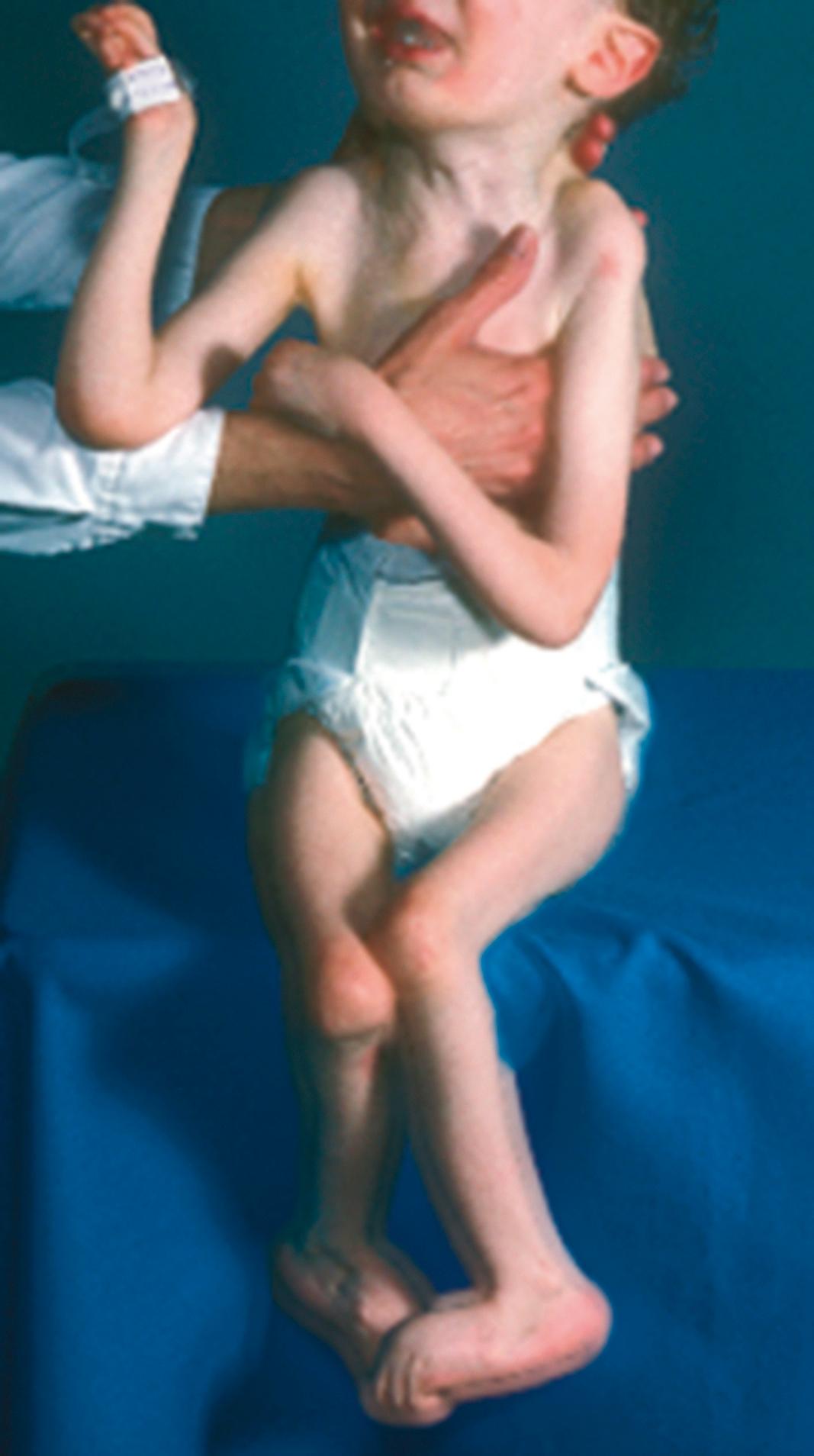
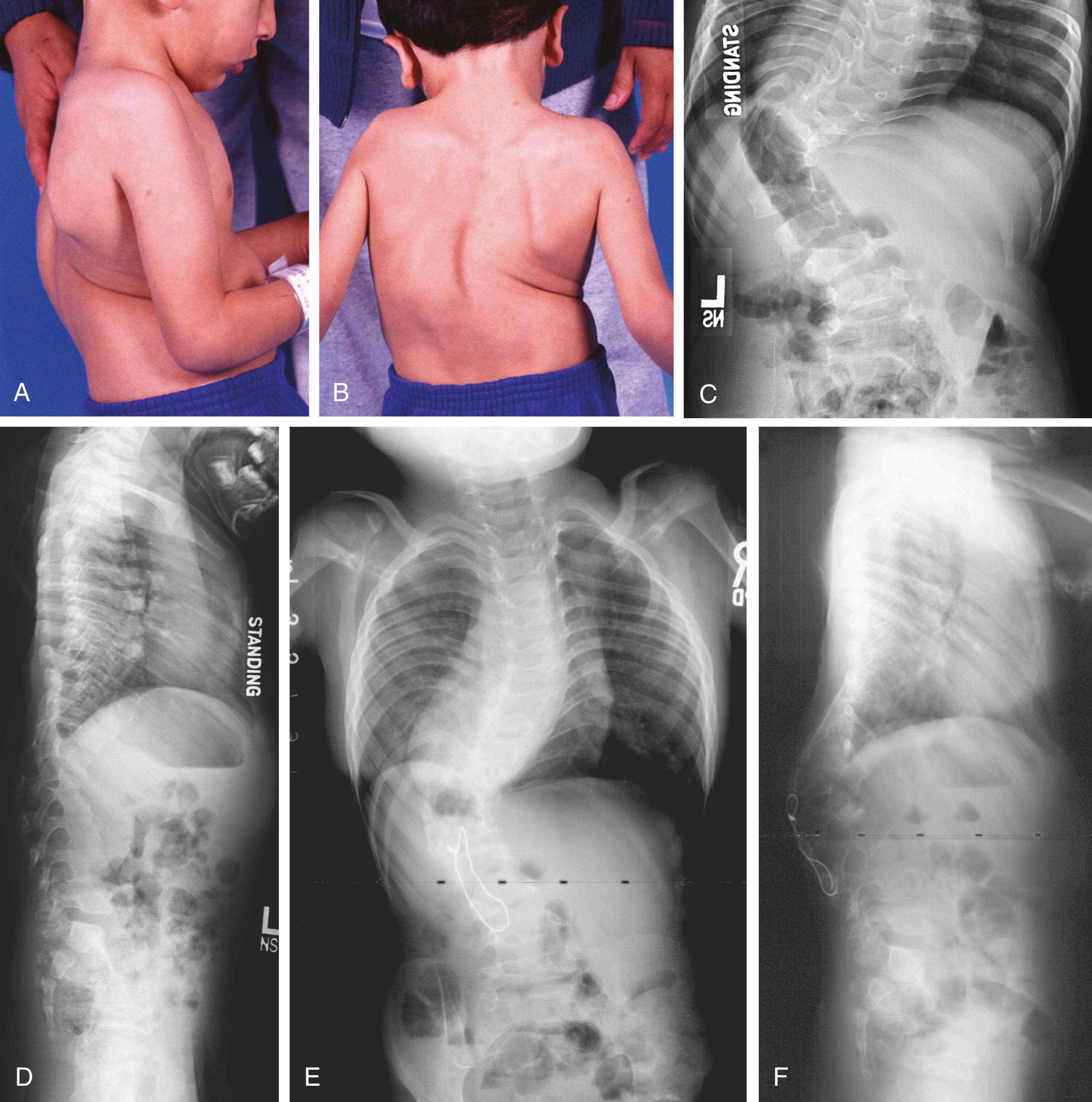
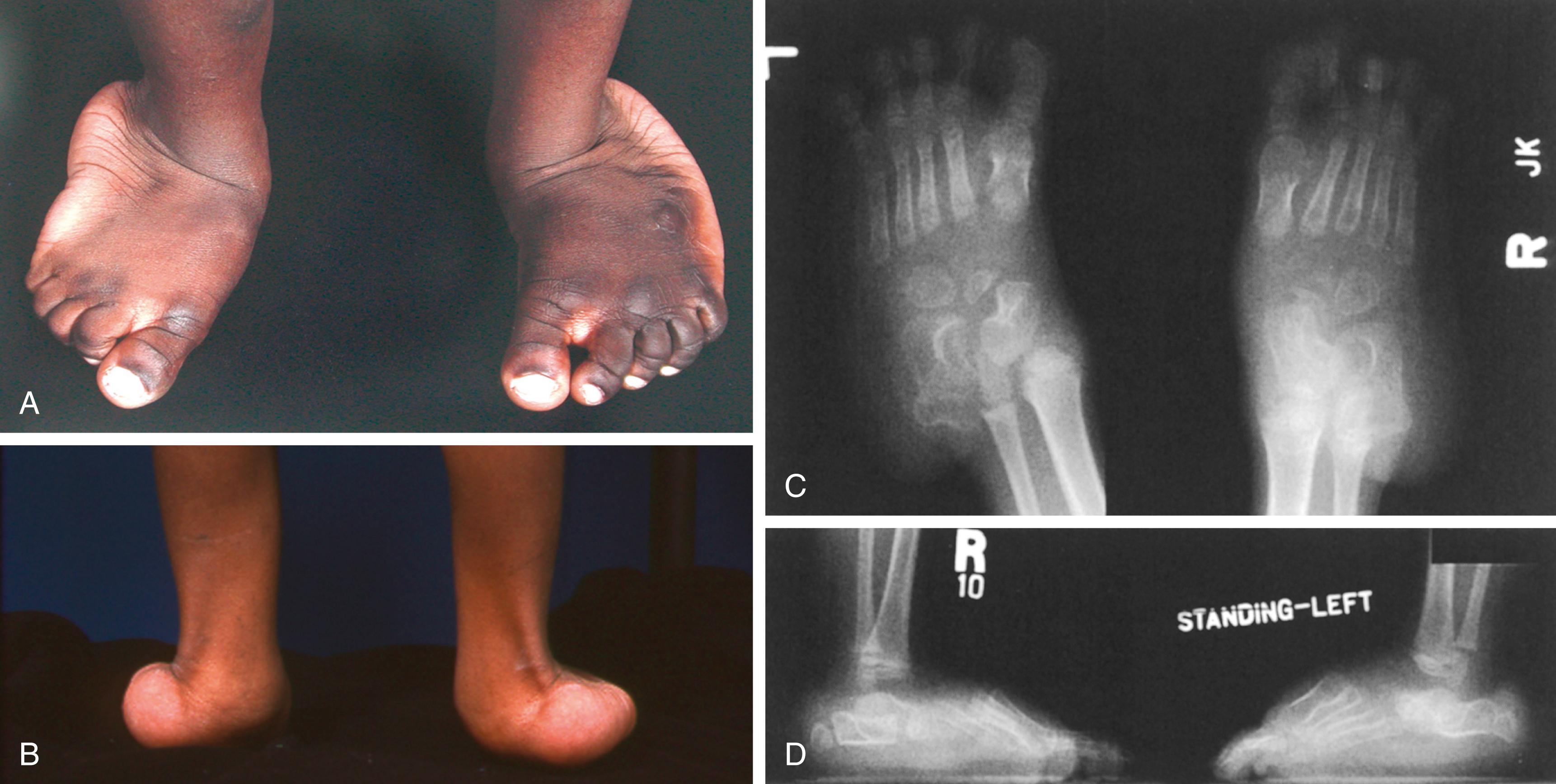
Aortic root enlargement has also been reported in three cases. The extremities often exhibit muscle hypoplasia, exacerbating patients’ asthenic appearance. The joint contractures in CCA are present at birth. Knee flexion contracture is the most severe; it may be as great as 90 degrees ( Fig. 37.6 ) and may delay walking. The hips are normal. The ankles may be in the calcaneus position, with limited plantar flexion and excessive dorsiflexion; alternatively, they may demonstrate vertical talus. In the hands and feet, there are flexion contractures of the metacarpal phalangeal and proximal interphalangeal joints ( Fig. 37.7 ). The elbows do not extend completely, and the forearms are restricted in supination, pronation, or both. The joint contractures tend to improve spontaneously with growth and development. This restoration of joint motion may be almost complete, making the diagnosis of CCA somewhat difficult in adolescents.
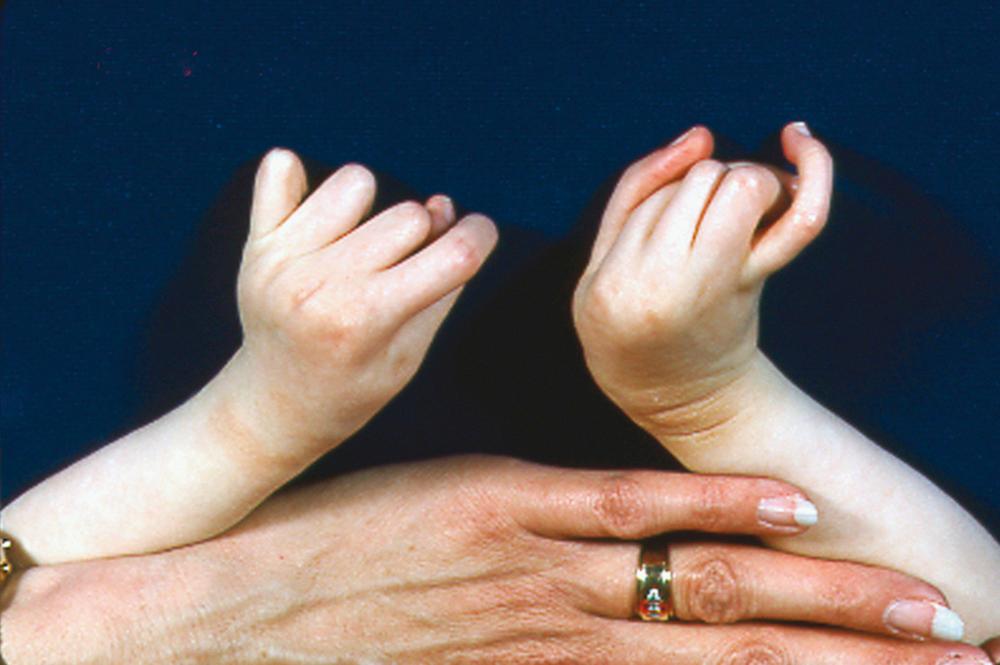
The limbs, fingers, and toes are long and gracile (see Figs. 37.6 and 37.7 ). The long narrow foot may show metatarsus varus. Vertical talus and equinovalgus are also common. Progressive scoliosis develops in the spine and may be severe ( Fig. 37.8 ).
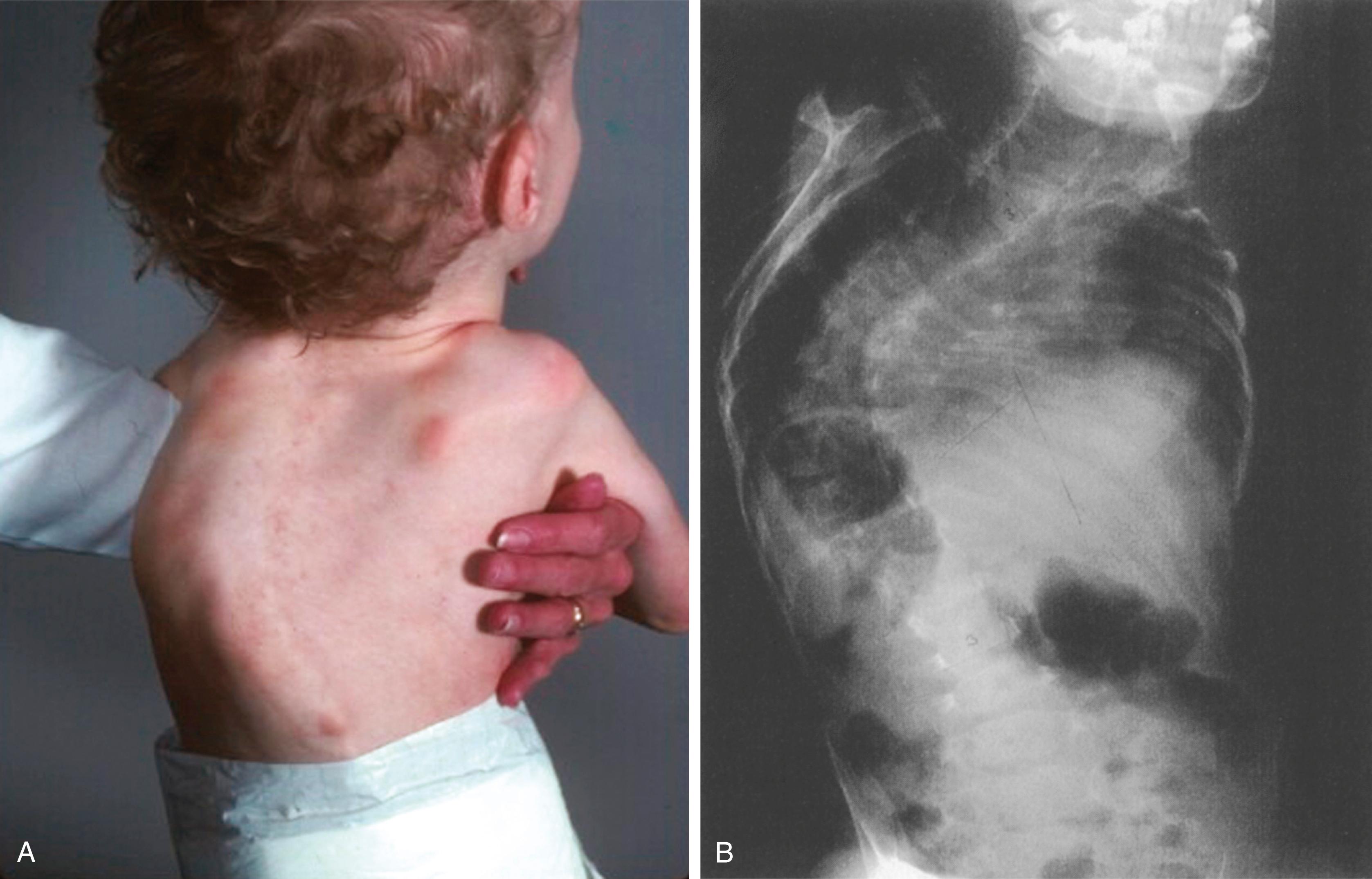
Radiographs show the elongated appearance of the diaphysis of the long bones, especially of the digits. Scoliosis is evident, and osteopenia may also be present (see Fig. 37.8 ). Long bone films in infancy may suggest osteogenesis imperfecta because of bowing and the appearance of diaphyseal fragility ( Fig. 37.9 ). Interestingly, birth fractures are rare. Scoliosis is often evident early, with a characteristic wedging or dysplasia of apical vertebrae, reminiscent of neurofibromatosis ( Fig. 37.10 ; see also Fig. 37.8 ).
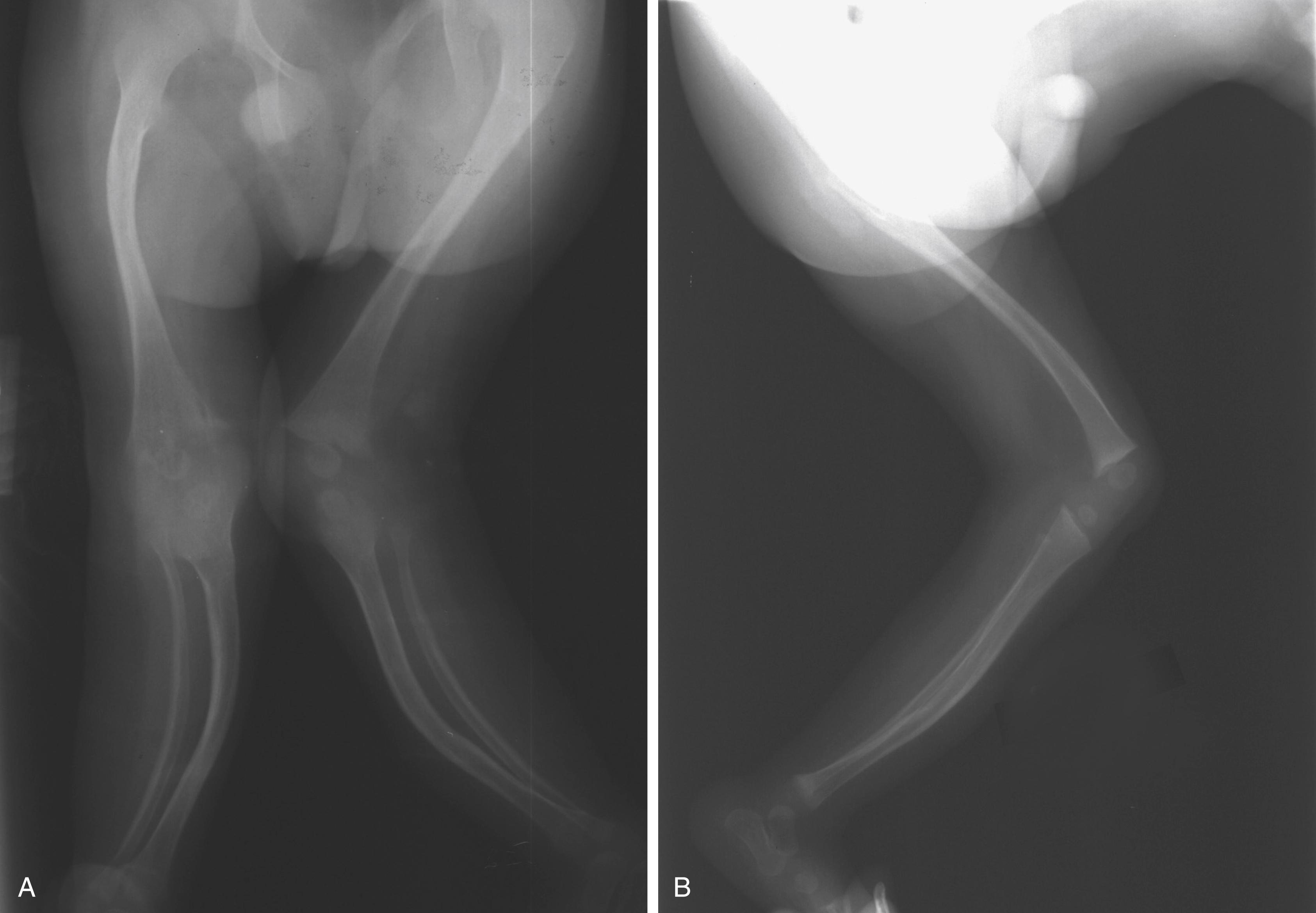
CCA should be differentiated from Marfan syndrome, homocystinuria, arthrogryposis multiplex congenita, and Achard syndrome. Homocystinuria is distinguished by the presence of lens dislocation, cardiovascular disease (especially thromboembolism), mental retardation, and characteristic biochemical abnormalities in the urine and tissues. These findings are usually not observed in CCA.
Arthrogryposis is characterized by congenital joint contractures that are usually more rigid than those seen in CCA, have little tendency to resolve, and are often associated with other structural anomalies such as talipes equinovarus, vertical talus, dislocated hips, and dislocated or hyperextended knees.
In Achard syndrome, arachnodactyly is present, but it lacks the dolichostenomelia and contractures that are present in CCA. Achard syndrome is differentiated from Marfan syndrome by the absence of lens dislocation and cardiac abnormalities.
A cardiac examination, including echocardiography, should be performed, with periodic reassessment as indicated. Contracted joints are treated by gentle passive stretching exercises and splinting to maintain and increase range of motion. Surgical soft-tissue release, particularly of the knee (where the flexion contracture is most severe), should be delayed until the deformity is clearly impeding normal ambulation. Such releases may not be required at all, however, because the contractures tend to resolve spontaneously.
Joint contractures and finger deformities can resemble those of arthrogryposis and are often treated similarly. The scoliosis, however, may be progressive and severe, and although orthotic management can be attempted, surgery is often required. Significant deformity may result, similar to infantile or neuromuscular scoliosis, requiring aggressive treatment such as growing instrumentation and early anterior and posterior fusion. Like other conditions in which osteopenia coincides with dysplastic bone, fixation is often tenuous in CCA, and there is a tendency toward pseudarthrosis (see Fig. 37.10 ). Because CCA patients are generally thin and asthenic, skin coverage for spinal implants may be problematic, especially in young children (see Fig. 37.10 ).
Homocystinuria is an inborn error of methionine metabolism inherited as an autosomal recessive trait. In its classic form, it is caused by deficiency of the enzyme cystathionine synthase, sometimes called cystathionine β-synthase or cystathionine synthetase. n
n References , , , , , , , , , , , , .
Clinically, the disorder is characterized by mental retardation, a tendency for thromboembolic complications, lens dislocation (ectopia lentis), and various skeletal abnormalities that strongly resemble those of Marfan syndrome. The condition was first described by Field and associates in 1962 and was later reported independently by Gerritsen and Waisman. Before these reports, cases of homocystinuria were likely misdiagnosed as Marfan syndrome, and this probably still occurs.
The worldwide prevalence of homocystinuria is reported to be approximately 1 in 335,000. This prevalence varies regionally, from 1 in 65,000 in Ireland to 1 in 900,000 in Japan. A bacterial inhibition assay or amino acid chromatography can be used to screen children at birth for homocystinuria; however, approximately 20% of documented cases are missed by these methods. Because of the relatively low prevalence of the disorder and inaccuracy of screening methods, routine screening for homocystinuria has been discontinued in many countries.
A deficiency in the enzyme cystathionine synthase (or synthetase) blocks the conversion of homocysteine to cystathionine ( Fig. 37.11 ). The abnormally accumulated homocysteine is converted to homocystine, causing an elevated level of homocystine in the tissues and plasma and the excretion of large quantities of homocystine in the urine. The plasma concentration of methionine is also increased. Cystathionine is normally present in brain tissue and is a precursor to cysteine. In a homocystinuric patient, cystathionine cannot be found in the brain, and cystine becomes an essential amino acid for the patient.
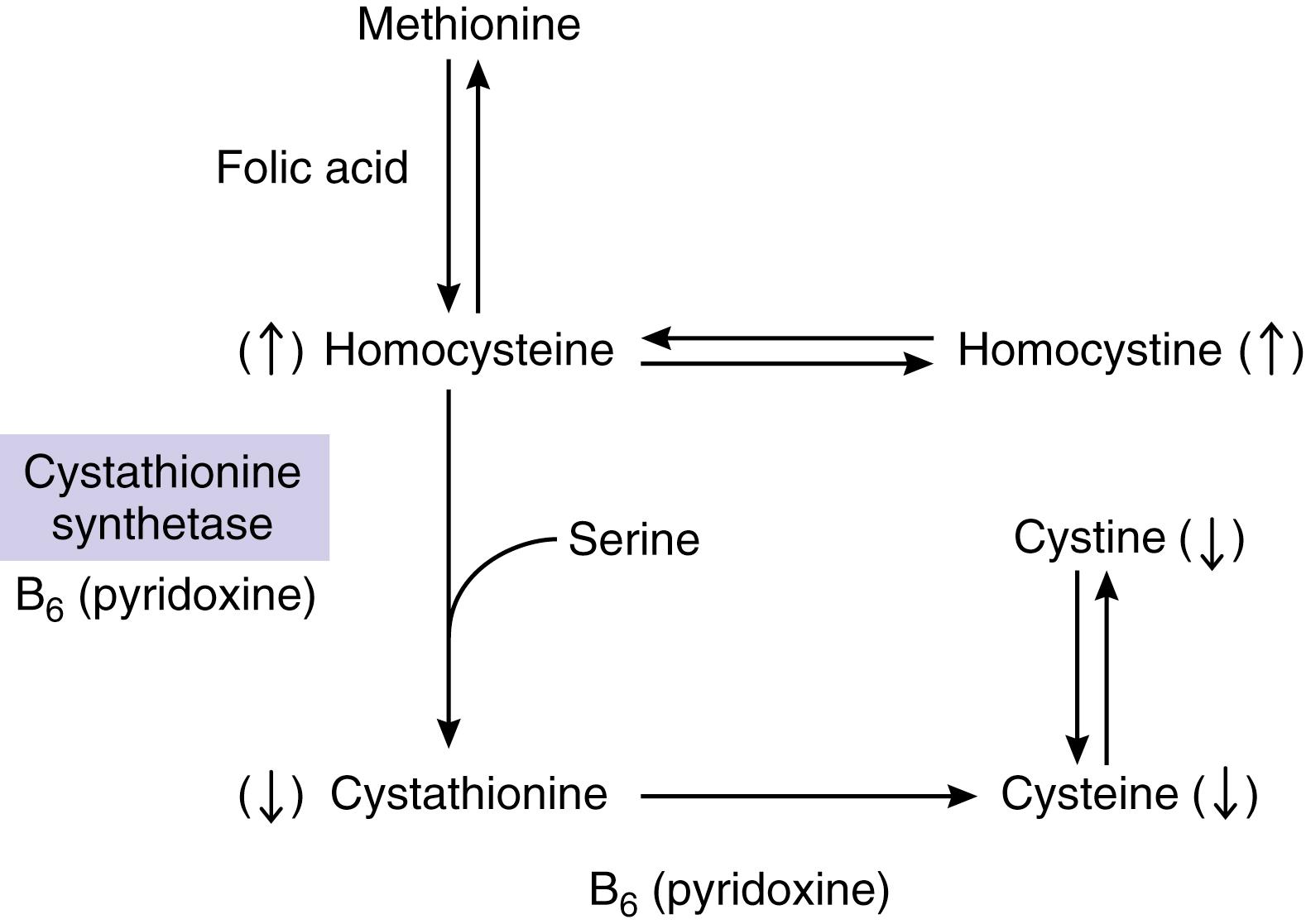
There are several other extremely rare causes of homocystinuria, such as vitamin B 12 malabsorption syndrome (Imerslund-Graesbeck syndrome), vitamin B 6 depletion, 5-methyltetrahydrofolate-homocysteine methyltransferase deficiency caused by defective production of cofactor methylcobalamin, and 5,10-methylene tetrahydrofolate reductase deficiency. The latter two causes of homocystinuria are inheritable metabolic defects. In this chapter, only classic homocystinuria is discussed.
The clinical features consist of a tendency toward venous and arterial thrombosis, , mental retardation, , , dislocation of the lens, and skeletal changes resembling those of Marfan syndrome, along with the additional findings of osteoporosis and metaphyseal enlargement (particularly around the knee). o
o References , , , , , .
The lesions in the blood vessels consist of intimal fibrosis, abnormality of the elastic laminae, atherosclerosis, and thrombosis. Homocysteine itself is a very toxic substance, causing endothelial lesions. Another factor in the pathogenesis of thrombosis is platelet stickiness. Venous and arterial thrombosis can become life-threatening. Death can occur from myocardial infarction, pulmonary embolism, mesenteric thrombosis, or cerebrovascular accident. Approximately 20% of patients with homocystinuria develop serious thrombosis. Because the incidence of venous thrombosis is very high following surgery, the need for any surgery must be considered carefully and if surgery is required, antithrombotic measures must be taken to minimize the danger.
Mental retardation is common but is not uniformly present; some patients with homocystinuria have normal intelligence. In those who are retarded, the IQ is usually approximately 50, and it can deteriorate with age. Whether the mental retardation is caused by biochemical abnormalities or by intermittent cerebral thrombosis is not certain. Hypertensive hydrocephalus has been reported. In general, homocystinuric patients who respond to pyridoxine (vitamin B 6 ) have less severe mental retardation. Adolescents with homocystinuria may exhibit schizophrenia-like states. Patients may develop seizures and have abnormal electroencephalograms.
Dislocation of the lens is not observed in infancy but develops later in childhood. It appears to be caused by a defective suspensory ligament. Lens dislocation can produce glaucoma.
The limbs are excessively long and thin (dolichostenomelia), with the arm span exceeding the patient’s height. The measurement from the head to the symphysis pubis is less than that from the symphysis pubis to the heel. Arachnodactyly may be present but is not as severe or as frequent as in Marfan syndrome. There may be fixed flexion deformity of the digits and flexion deformity of the elbow, with limited supination. The feet and toes are long. Severe pes planovalgus is common, and some patients have cavus feet. Most patients have scoliosis—usually a left lumbar, right thoracic curve, with the lumbar curve greater than the thoracic one. Osteoporosis, especially in the spine, is often a striking feature. The vertebral bodies frequently exhibit biconcave endplates in addition to the generalized osteopenia.
Widening of the metaphyses and enlargement of the epiphyses of the long bones, particularly at the knees, are important features of homocystinuria. These are usually not present in Marfan syndrome. Associated anomalies are pectus carinatum or excavatum, high-arched palate, facial appearance characterized by malar flush, and light-colored hair.
Several studies indicate that the diagnosis of homocystinuria is frequently delayed, despite the presence of typical clinical features. , The authors emphasize that unusual myopia (high, abnormally progressive, or occurring at a very young age), especially when combined with skeletal manifestations, should alert the attending physician to the possibility of homocystinuria.
Homocystinuria is apparently caused by a number of potential genetic or biochemical abnormalities, as suggested by the spectrum of clinical manifestations and the variable response to pyridoxine. Thus confirmation of the diagnosis with laboratory tests is not always simple. , The measurement of sulfur amino acid concentrations, especially total homocysteine, in plasma or urine is a good screening tool. In patients whose clinical features and laboratory results suggest the diagnosis of homocystinuria, cystathionine synthase assays can be done using fibroblasts cultured from skin biopsy.
Features distinguishing homocystinuria from Marfan syndrome are presented in Table 37.1 .
| Parameter | Homocystinuria | Marfan Syndrome |
|---|---|---|
| Inheritance | Autosomal recessive | Autosomal dominant |
| Cause | Deficiency in enzyme cystathionine synthase | Defect in fibrillin-1 |
| Neurologic features | Mental retardation present in most but not all Convulsions; schizophrenia-like state |
Absent |
| Vascular changes | Tendency to thrombosis of veins and arteries High incidence of thromboembolism |
Dissecting aneurysm Rupture of aorta Prolapse of mitral valve |
| Lens dislocation | Present, usually downward; not present at birth | Present, usually upward; can be present at birth |
| Skeletal changes | Osteoporosis with platyspondyly and biconcave vertebrae Dolichostenomelia Flaring of metaphysis with enlargement of epiphysis at knee Joint laxity; dislocation rare Moderate arachnodactyly Pectus excavatum, pectus carinatum Scoliosis |
Osteoporosis absent or minimal Dolichostenomelia Epiphysis and metaphysis normal Joint laxity typical Severe arachnodactyly Pectus excavatum, pectus carinatum Scoliosis |
Patients diagnosed with homocystinuria should have a trial of pyridoxine; approximately 50% of patients respond to such treatment, with reversal of the biochemical abnormalities. , , This biochemical reversal may reduce the risk of thromboembolic complications and prevent the progression of osteopenia or mental retardation, but the extent is uncertain. Preservation of good vision is enhanced if the diagnosis is made and treatment initiated within the first 6 weeks of life. In addition, Wilcken and Wilcken demonstrated a significant reduction in thromboembolic events (myocardial infarction and pulmonary edema) in a group of patients treated with a combination of pyridoxine, folic acid, and hydrocobalamin. Approximately 50% of patients do not respond to pyridoxine therapy, presumably because of individual variations in the exact nature of the metabolic disorder. ,
Progressive scoliosis in skeletally immature patients is initially treated with a thoracolumbar orthosis. However, this treatment often fails to prevent progression of the deformity, and spinal fusion and instrumentation must be considered. Vertebral body osteopenia and the risk of thromboembolic complications must be taken into consideration when advising and proceeding with surgery.
In 1820, Chatelain described a patient with congenital anomalies of the nails, elbows, and patellae, the earliest report of nail dystrophy associated with skeletal dysplasia. In 1897, Little quoted a description by Sedgwick of a family in which 18 members from four generations had no thumbnails and no patellae, suggesting the hereditary nature of this disorder. Involvement of the elbows was reported by Wrede in 1909. A detailed study of this triad of anomalies was made by Osterreicher in 1931.
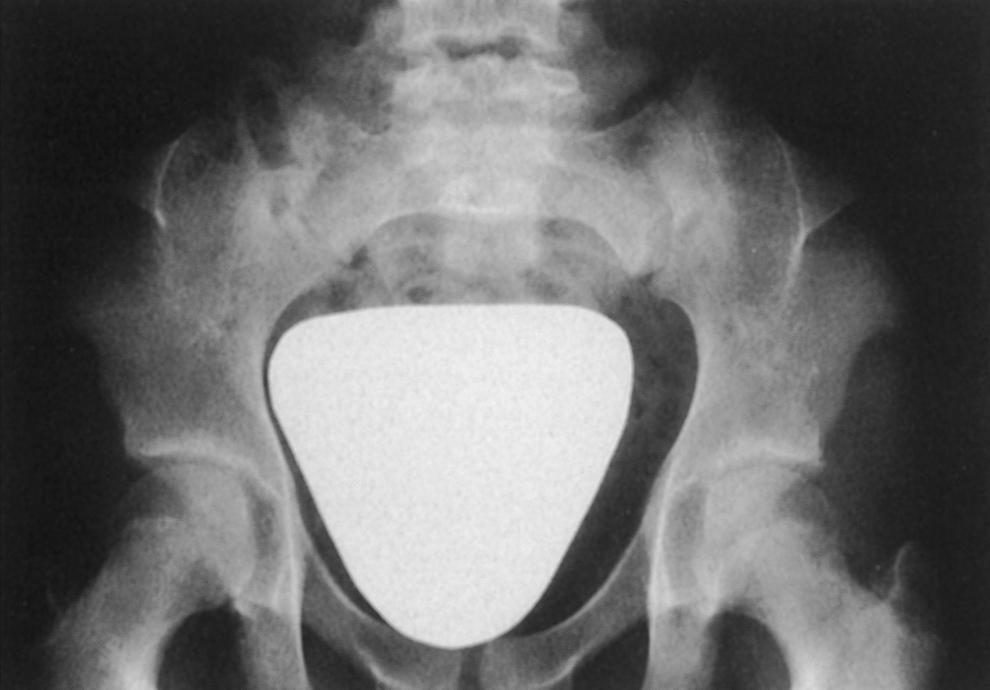
In 1933, Turner observed flaring of the iliac crests and prominence of the anterior superior iliac spines in some affected patients. Fong, in 1946, during routine pyelography, noted conical bony projections on the posterior ilia, which he termed iliac horns; he did not, however, associate them with any syndrome. A few years later, these iliac horns were observed in association with knee, elbow, and nail anomalies and reported by other authors. , Thus iliac horns were established as an important constituent of this syndrome ( Fig. 37.12 ).
Nail-patella syndrome is the popular name applied to this triad of anomalies, but in 1957 Love and Beiler proposed the term hereditary osteo-onychodysplasia. It is also referred to as Fong syndrome and hereditary onycho-osteodysplasia.
The incidence of nail-patella syndrome in the United States is 4.5/1 million population. Wynne-Davies and associates reported a probable prevalence of approximately 1/1 million population.
Onycho-osteodysplasia is transmitted as an autosomal dominant trait. Despite this type of inheritance, there is a marked degree of intrafamilial and interfamilial variation in the clinical severity of the disease. , The gene for nail-patella syndrome is LMX1B, located on chromosome 9. , Many different mutations in the gene have been described, which lead to a loss of function of the protein. LMX1B is a transcription factor involved in normal patterning of the dorsoventral axis of the limbs and early morphogenesis of the glomerular basement membrane.
Dystrophy is greatest in the thumbnails and becomes less severe in the more ulnar digits ( Fig. 37.13 ). The little finger is rarely affected. Abnormalities of the toenails have been noted in some patients. The thumbnail may be absent, bifid, or hemiatrophic; the ulnar side of the nail is usually the part that is absent. The nails may be reduced in length and show numerous longitudinal cracks. Nail deformity is present in 98% of cases.
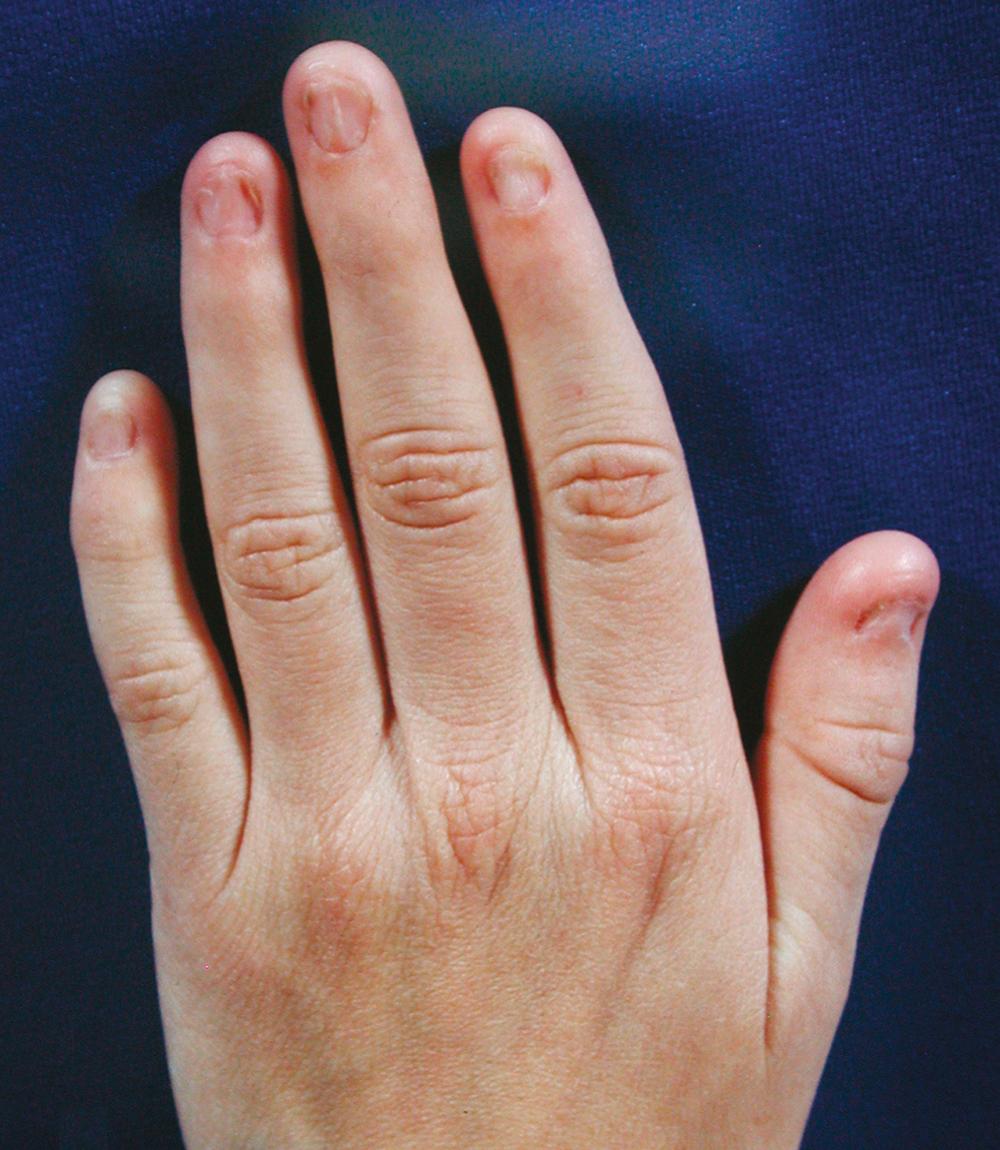
Bony abnormalities of the digits have not been demonstrated. The mesodermal tissues of the fingers appear to be involved to some extent. The terminal pulp may extend from the volar aspect onto the dorsal surface. The dorsal skin creases over the distal interphalangeal joints may be absent or poorly developed. There may also be laxity of the ligaments of the metacarpophalangeal and interphalangeal joints.
The patella is absent or hypoplastic ( Fig. 37.14 ). The hypoplastic patella may be ovoid, triangular, or irregular in shape and may arise from several ossification centers ( Fig. 37.15 ). It may be located more distally than in the normal knee.
![FIG. 37.14, Anteroposterior (A) and lateral (B) radiographs of the knee of a boy aged 4 years 7 months with nail-patella syndrome. Note the absence of the patella. Magnetic resonance imaging (lateral [C] and transverse [D] views) shows a lack of normal patellar development. FIG. 37.14, Anteroposterior (A) and lateral (B) radiographs of the knee of a boy aged 4 years 7 months with nail-patella syndrome. Note the absence of the patella. Magnetic resonance imaging (lateral [C] and transverse [D] views) shows a lack of normal patellar development.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/OrthopaedicRelatedSyndromes/13_3s20B9780323567695000375.jpg)
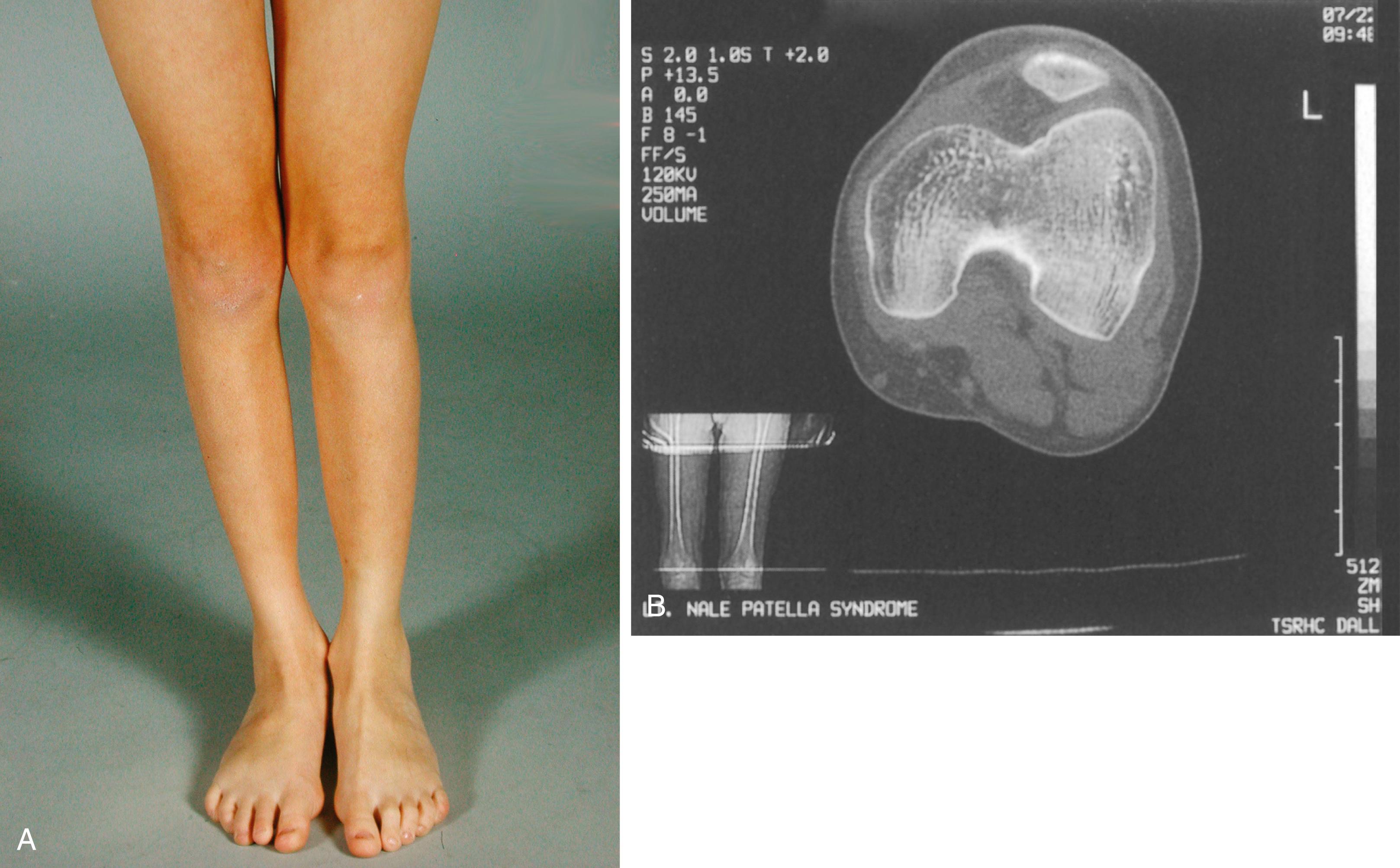
The presenting complaint may be knee pain or recurrent lateral dislocation of the patella caused by hypoplasia of the lateral femoral condyle. Some degree of genu valgum is usually present. The medial femoral condyle is frequently large and prominent, whereas the lateral femoral condyle is underdeveloped. The medial tibial plateau may slip downward and medially or may even be grooved. The medial margin of the proximal tibial metaphysis tends to sweep upward and medially in a characteristic arc. Valgus of the foot and ball-and-socket ankle joint have been reported.
The carrying angle of the elbow joint is increased, with varying degrees of cubitus valgus. There is hypoplasia of the lateral side of the elbow joint involving not only the capitellum and lateral condyle but also the radial head. The radial head may articulate normally with the capitellum, or there may be subluxation or dislocation posteriorly ( Fig. 37.16 ). There may be a pointed exostosis of the lateral aspect of the coronoid process. Range of motion of the elbow joints is usually limited.
Iliac horns and flaring of the iliac crests, with prominence of the anterior superior iliac spines, are the two types of pelvic abnormalities encountered. Iliac horns, one of the most common features of onycho-osteodysplasia, are bilateral, present in 80% of cases, and may be visible, palpable, or impalpable, according to their size. The horns are located at the origin of the gluteus medius and project posterolaterally. Secondary centers of ossification may occur at their tips. They are present early in life. When outflaring of the iliac crests and prominent anterior superior iliac spines is combined with iliac horns, the appearance of the pelvis has been likened to that of an elephant’s ear.
Radiographs of the knee reveal a number of patellar and femoral abnormalities. Patellar aplasia is present in 4% while patellar hypoplasia is found in 86% of patients. Shortening of the lateral femoral condyle is present in 55% and a flat anterior surface of the medial femoral condyle is seen in 92% of patients.
Other anomalies that may be found in association with the foregoing main lesions include clubfoot and other congenital foot abnormalities, , developmental dysplasia of the hip, scoliosis, glenoid and acromial dysplasia, and congenital contracture of the little finger. Abnormal pigmentation of the iris occurs in approximately 50% of cases. Plummer-Vinson syndrome (dysphagia, hypochromic anemia, and koilonychia) has also been associated with nail-patella syndrome.
Approximately 40% of individuals with nail-patella syndrome have kidney disease. Most have accelerated age-related loss of filtration function, which is usually asymptomatic. A minority, approximately 5% to 10% of people with nail-patella syndrome, have more severe disease, with nephritic syndrome, in their youth, which progresses to end-stage kidney failure. Later in life, usually during the third or fourth decade, nephropathy and proteinuria develop, with subsequent renal failure. p
p References , , , , , , , .
Nephropathy occurs in approximately 40% of affected individuals, is more frequent in female patients, and may be linked to the specific mutation location in the LMX1B gene.
Glaucoma is also seen with increased frequency in patients with nail-patella syndrome. , Because of the association with both renal failure and glaucoma, patients with nail-patella syndrome should be screened for these serious associated conditions.
There is no specific treatment for the disorder. The iliac horns do not affect gait and should not be resected. Recurrent dislocation of the patella may occur; if this is disabling, it is treated by proximal or distal realignment. , In a series reported by Guidera and associates, approximately 50% of children with nail-patella syndrome underwent knee surgery to treat instability. A rare finding of a synovial band blocking the patella from engaging the trochlear groove has been treated arthroscopically. Foot deformities often require posteromedial clubfoot releases.
The magnitude of the task of managing patients with Larsen syndrome has been recognized since the first description of the disorder by Larsen and associates in 1950. The syndrome is associated with so many orthopaedic deformities requiring treatment that its management has been referred to as a herculean task. Dislocated joints, foot deformities, and spinal deformities, including a potentially lethal cervical kyphosis, may all need to be addressed in the patient. Once an infant has been diagnosed, a thorough investigation of the entire musculoskeletal system is necessary to prioritize the management of these multiple deformities. Even with optimal care of each deformity, the functional outcome at maturity is guarded at best because of the additive morbidity and possible complications of managing bilateral dislocations of the hips and knees, independent of any spinal deformities and foot surgeries that might also be required.
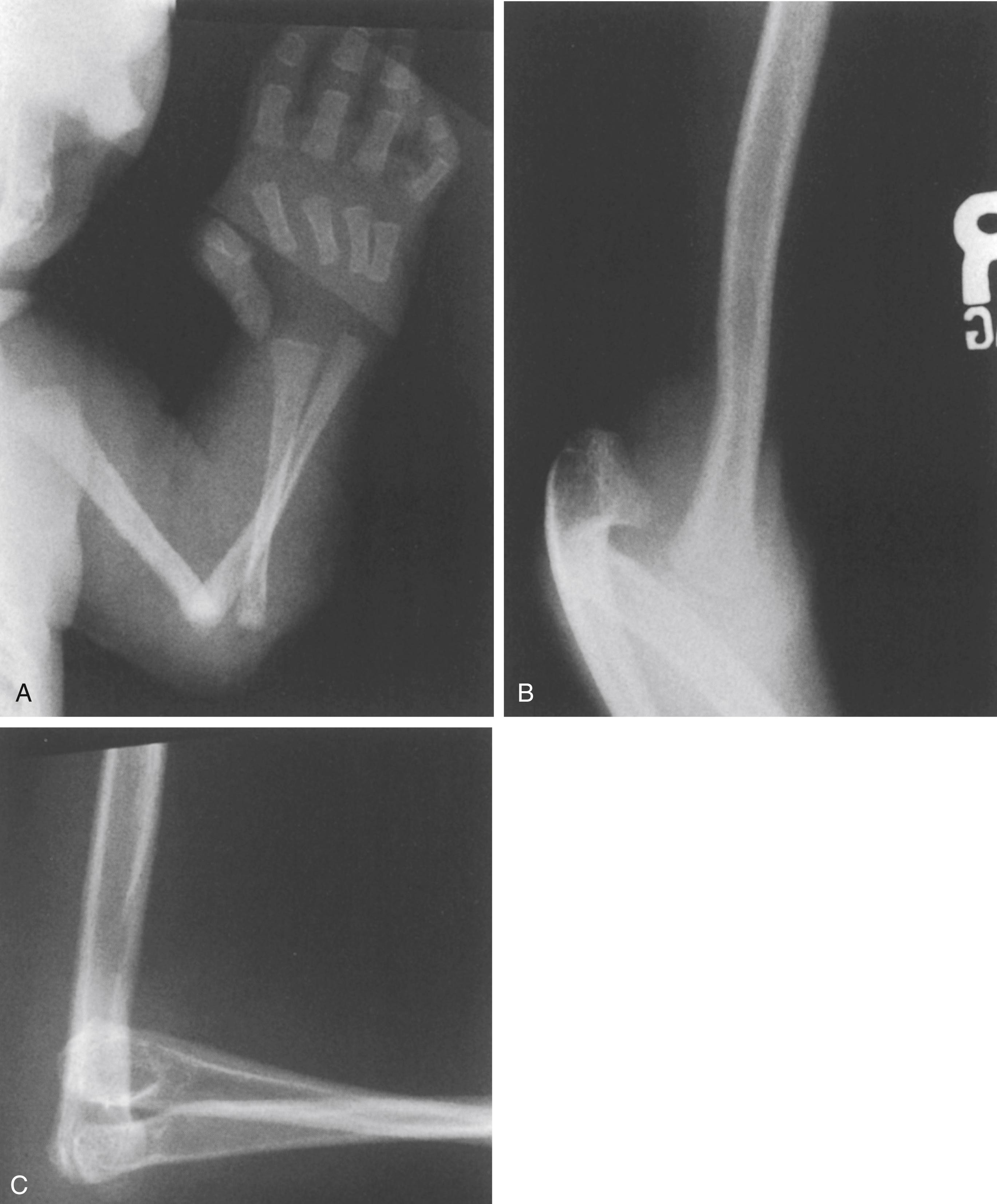
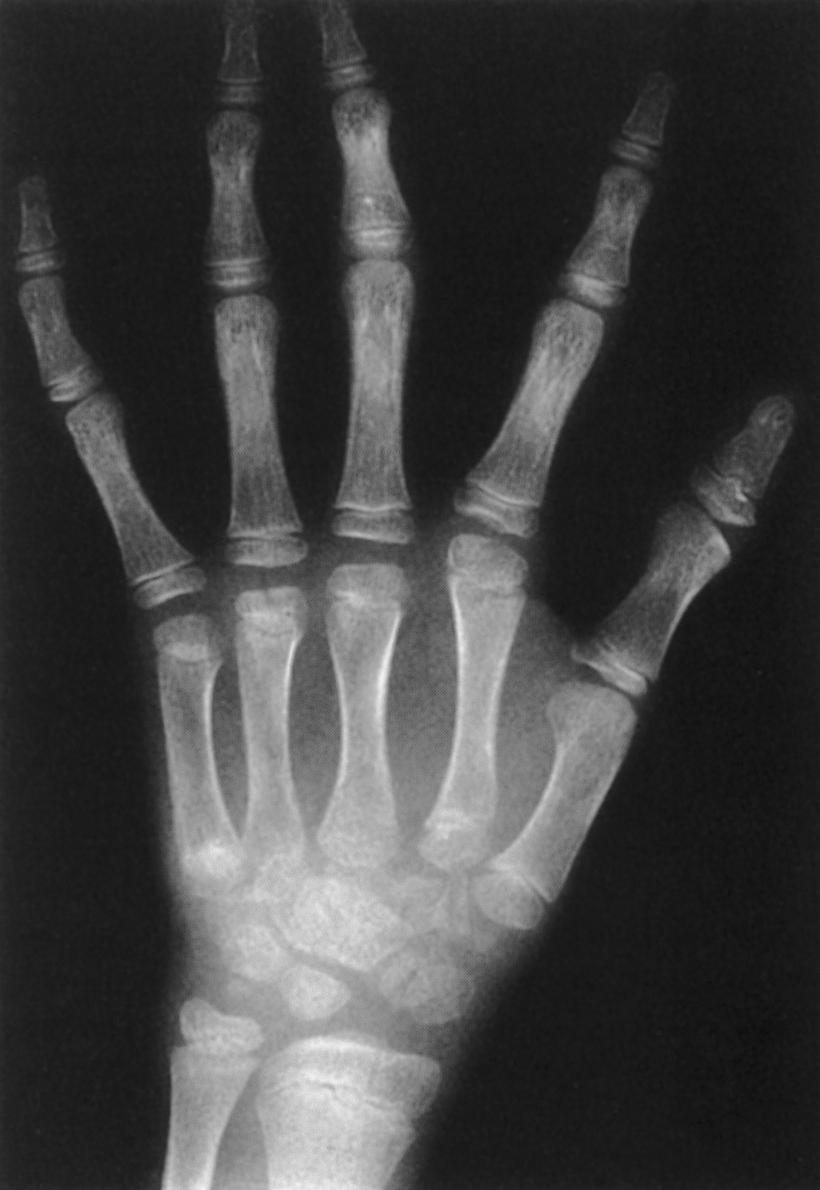
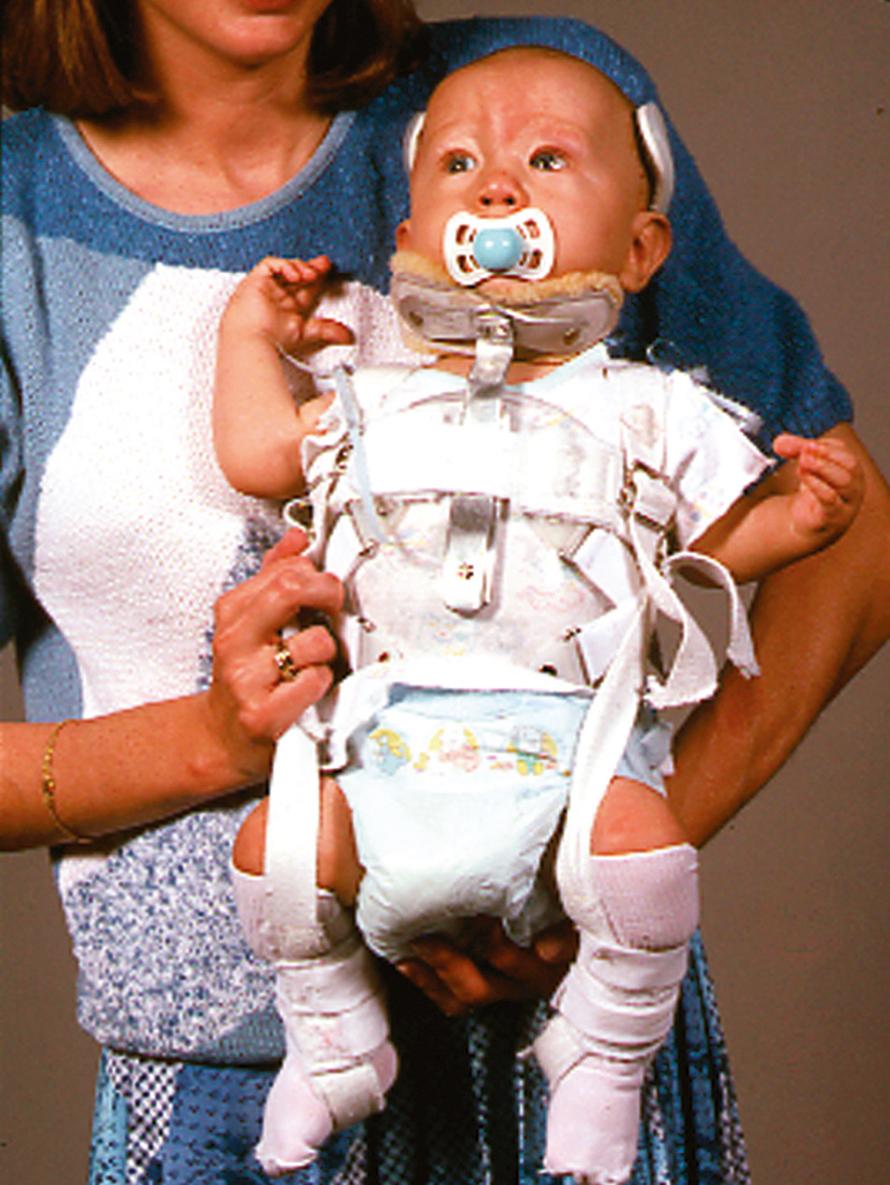
An initial evaluation is often requested soon after birth because the orthopaedic manifestations are so dramatic. The lower extremities most frequently exhibit bilateral hyperextension deformities of the knees and fairly rigid clubfeet. The knee deformity spans a spectrum from simple congenital hyperextension deformity to complete (grade 3) anterior dislocation of the tibia on the femur ( Fig. 37.17 ), with the more severe dislocation being common. The hips are often teratologically dislocated, with obvious foreshortening of the thighs, but there is often remarkable mobility, reflecting the characteristic generalized ligamentous laxity. Other obvious skeletal manifestations include deformities of the hands and elbows. The elbows frequently demonstrate radiohumeral dislocation, with lateral prominence of the radial heads, even in infants; there may be more extensive dislocation, with total disruption of the radiocapitellar and humeroulnar joints ( Fig. 37.18 ). In the latter case, the elbow is fixed by webbing in the antecubital space, with a flexion contracture varying from 60 to 90 degrees. The fingers are usually long and cylindric, with a series of shortened metacarpals and a spatulate thumb, where the terminal phalanx is short and wide. Dislocation of the metacarpal joint of the thumb can occur (see Fig. 37.18 ). The appearance of the fingers is distinctly different from classic arthrogryposis, one of the other possible diagnoses to be considered in this setting.
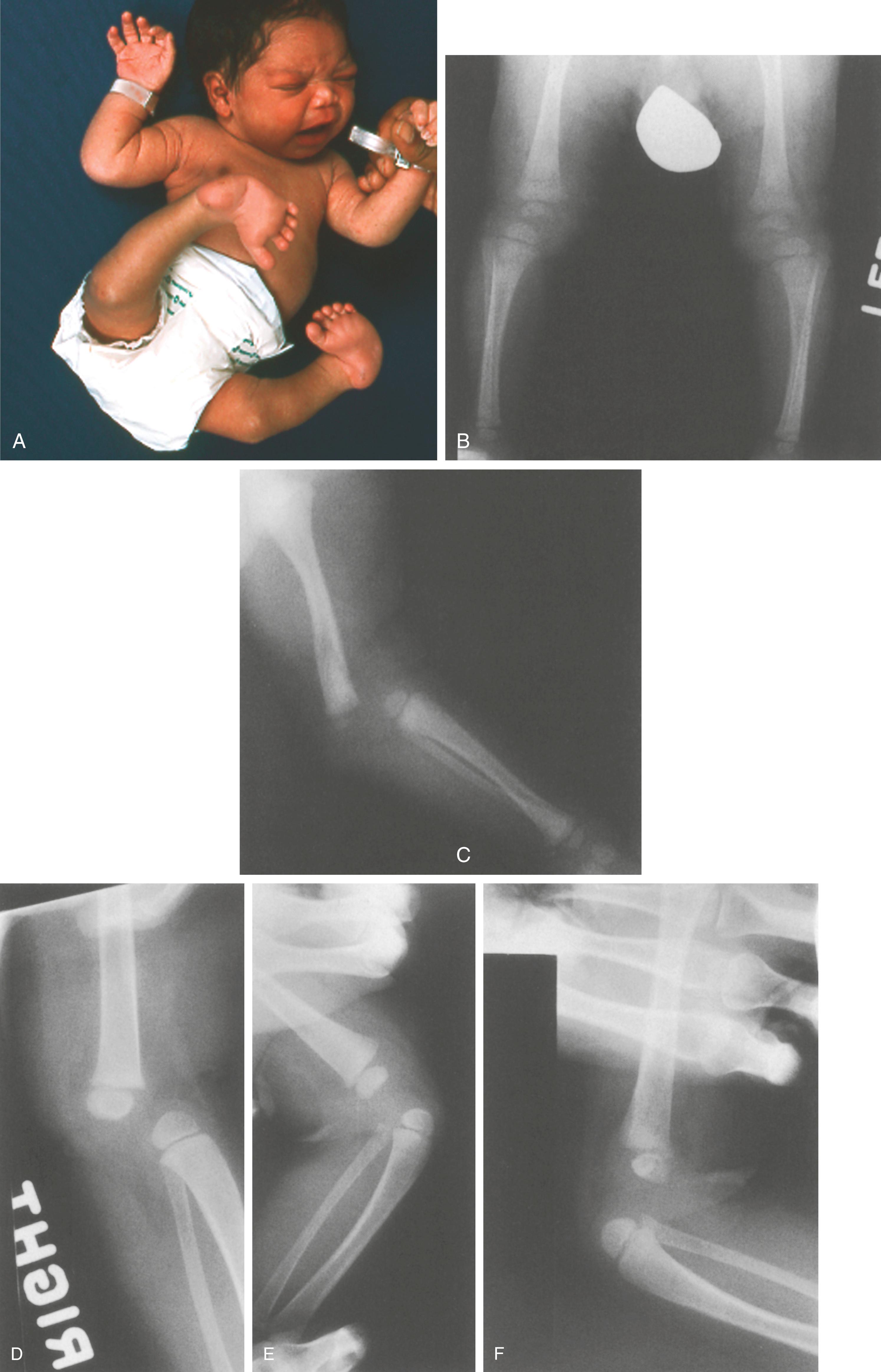
Facial dysmorphia aids in the immediate identification of patients with Larsen syndrome, who characteristically have a flattened nasal bridge, relatively widely spaced eyes, and a prominent forehead (hypertelorism). Depression of the nasal bridge is a universal characteristic that makes the facial findings, in conjunction with the orthopaedic deformities, pathognomonic. Other nonorthopaedic involvement includes elasticity of the thoracic cage, tracheomalacia, and laryngomalacia. Respiratory failure and early respiratory death have been reported as part of the syndrome. , , , Acquired lesions of the mitral valve and aorta, similar to those found in Marfan syndrome and other hyperelasticity conditions, can further complicate the surgical management of these children. , ,
The neurologic evaluation of children with suspected Larsen syndrome is critical. These patients are often described as being hypotonic, a condition associated with hyperelasticity syndromes and often implicated in the delayed achievement of certain motor skills, such as the ability to walk. In infants who are floppy or who are late in reaching milestones, it may be a dangerous oversimplification to attribute such delay to the syndrome per se or to the presence of multiple joint dislocations or foot deformities; the possibility of cervical cord compression must always be entertained in any infant with hypotonicity. In patients with a known associated cervical kyphosis, the presence of cord compression must be determined as early as possible to avoid chronic myelopathy and neurologic morbidity. Should cervical cord compression occur before complete myelinization, the classic signs of spasticity or hyperactive deep tendon reflexes indicating spinal cord compression are likely to be absent, and the patient may exhibit a flaccid-type paresis (hypotonicity). Although the tracheomalacia or bronchomalacia observed in patients with Larsen syndrome , , can be responsible for pulmonary compromise or death in infancy, patients with cord compression and flaccid paresis also suffer respiratory weakness and failure. , Thus the importance of early neurologic evaluation and radiographic identification of cervical kyphosis cannot be overemphasized in this patient population. Furthermore, a review of the literature strongly suggests that cervical kyphosis and cord impingement are underdiagnosed. The cervical spine deformities were not emphasized in the original description of the syndrome and, with the exception of sporadic reports, , , , , there has been little emphasis on this critical and potentially catastrophic lesion in patients with Larsen syndrome.
Other entities that share features with Larsen syndrome must be ruled out. These include other hyperelasticity syndromes, such as Marfan and Ehlers-Danlos syndromes, and, because of the contractures and extremity deformities, arthrogryposis multiplex congenita and Beals syndrome (congenital contractural arachnodactyly). The latter two are usually differentiated by the fact that patients with Larsen syndrome have a relatively normal muscle mass. Patients with Beals syndrome or classic arthrogryposis may suffer birth fractures as a result of rigid joints. Arthrogrypotic patients lack normal flexion creases, and with the exception of chest cage elasticity in patients with Larsen syndrome, which may manifest in newborns as paradoxical motion on inspiration and stridor, infants with Larsen syndrome are generally more robust and actively moving. Patients with hyperelasticity syndromes do not exhibit the multiple joint dislocations typical of Larsen syndrome. A milder form of Larsen syndrome lacking full-blown joint dislocations may be confused with other hyperelasticity syndromes. In this case, the dysmorphic facies of Larsen syndrome is the physical finding that settles the issue.
Larsen syndrome is classically inherited by an autosomal dominant pattern, , so a family history of the disorder is an important factor in a patient with an otherwise uncertain diagnosis. Evidence of mutations clustered in the FLNB gene indicate that a molecular diagnostic test may be available to assist in diagnosis and genetic counseling.
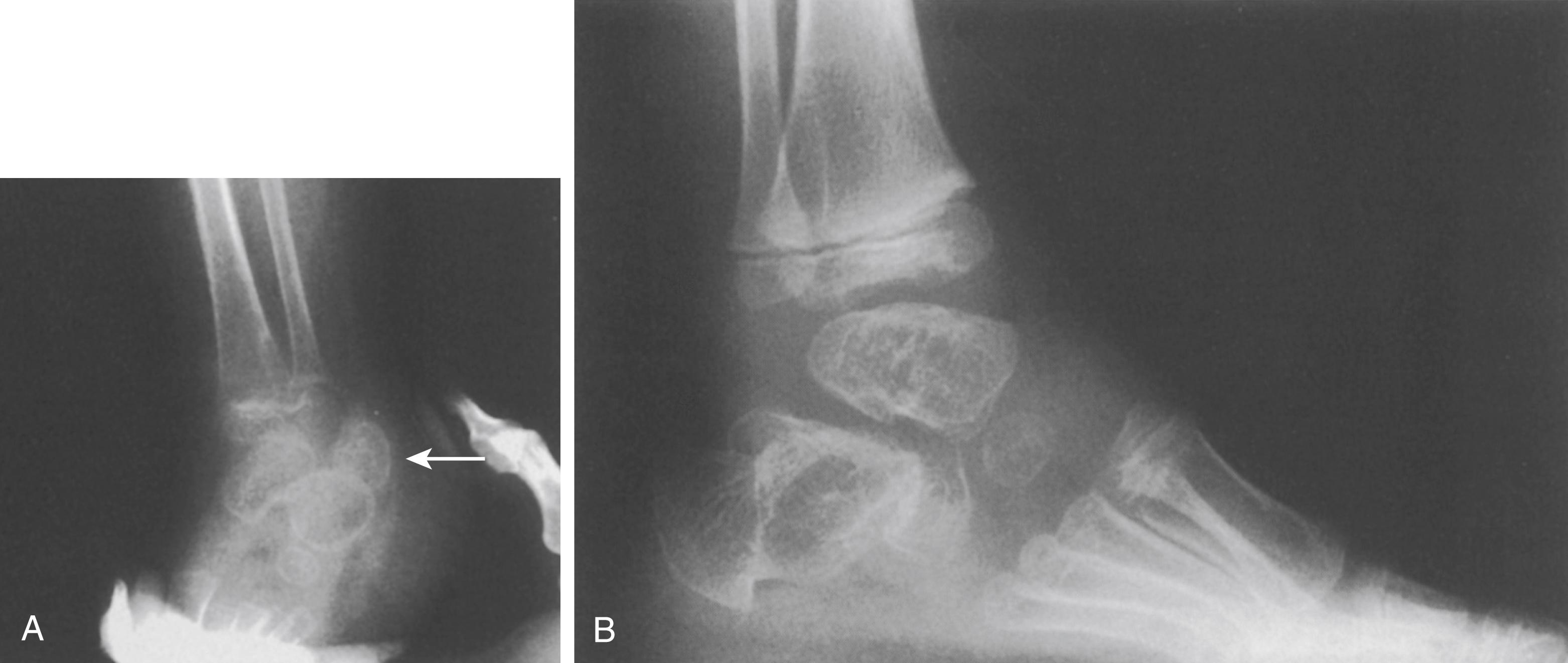
An unusual radiographic finding—a separate, additional ossification center for the calcaneus ( Fig. 37.19 )—may aid in the diagnosis. , The additional calcaneal apophysis is a fairly consistent finding, and its absence casts doubt on a diagnosis of classic Larsen syndrome. Extra carpal ossification centers have also been observed ( Fig. 37.20 ). , , The presence of multiple joint dislocations (hip, knee, elbow), with or without cervical kyphosis and spondylolysis, is an additional confirmatory finding. In the spine, particularly at cervical levels, spondylolysis at multiple levels is common, which may represent unossified fibrocartilage or may be associated with AP dissociation. ,
Because of the multiple deformities presenting simultaneously, the order of treatment should be determined as early as possible. In infants with the full-blown syndrome, we recommend that treatment be prioritized as follows:
Cervical kyphosis (or other threatened instability)
Congenital dislocation of the knee
Congenital dislocation of the hip
Foot deformities requiring correction to reach plantigrade position
Congenital dislocation of the elbow or radial head rarely requires treatment; scoliosis should be treated as described in Chapter 9 .
To minimize morbidity from unsuspected or undiagnosed cervical kyphosis, definitive management of this deformity is the first priority. Although surgical stabilization of a cervical kyphosis need not precede the treatment of other deformities, the presence of cervical kyphosis must be acknowledged, even if spinal cord impingement is not present, and appropriate anesthetic precautions must be taken during surgical procedures for other deformities to avoid possibly catastrophic complications. , ,
Complex deformities such as C3–4 spondyloptosis with atlantoaxial dislocation have been reported. Reports of AP dissociation in the cervical spine have highlighted a new and previously unreported complication of Larsen cervical dysplasia. , In the presence of such dissociation between the anterior vertebral column and posterior elements, simple posterior fusion will fail to stabilize a kyphosis because of the lack of bony continuity between the dysplastic anterior vertebrae and posterior tether. Computed tomography (CT) scanning is the best modality to identify this dissociation, which should be suspected when multiple wide cervical pars defects are noted on plain radiographs. The absence of pedicles clearly identifies the AP dissociation. Continued or worsening myelopathy is the hallmark of such dissociation, even after circumferential fusion. Unexpectedly long anterior or posterior fusion is required to stabilize these cases, , essentially bypassing the levels of dissociation and fusing to anatomically intact levels. In an extreme case, this may require fusion extension from the occiput to the upper thoracic levels.
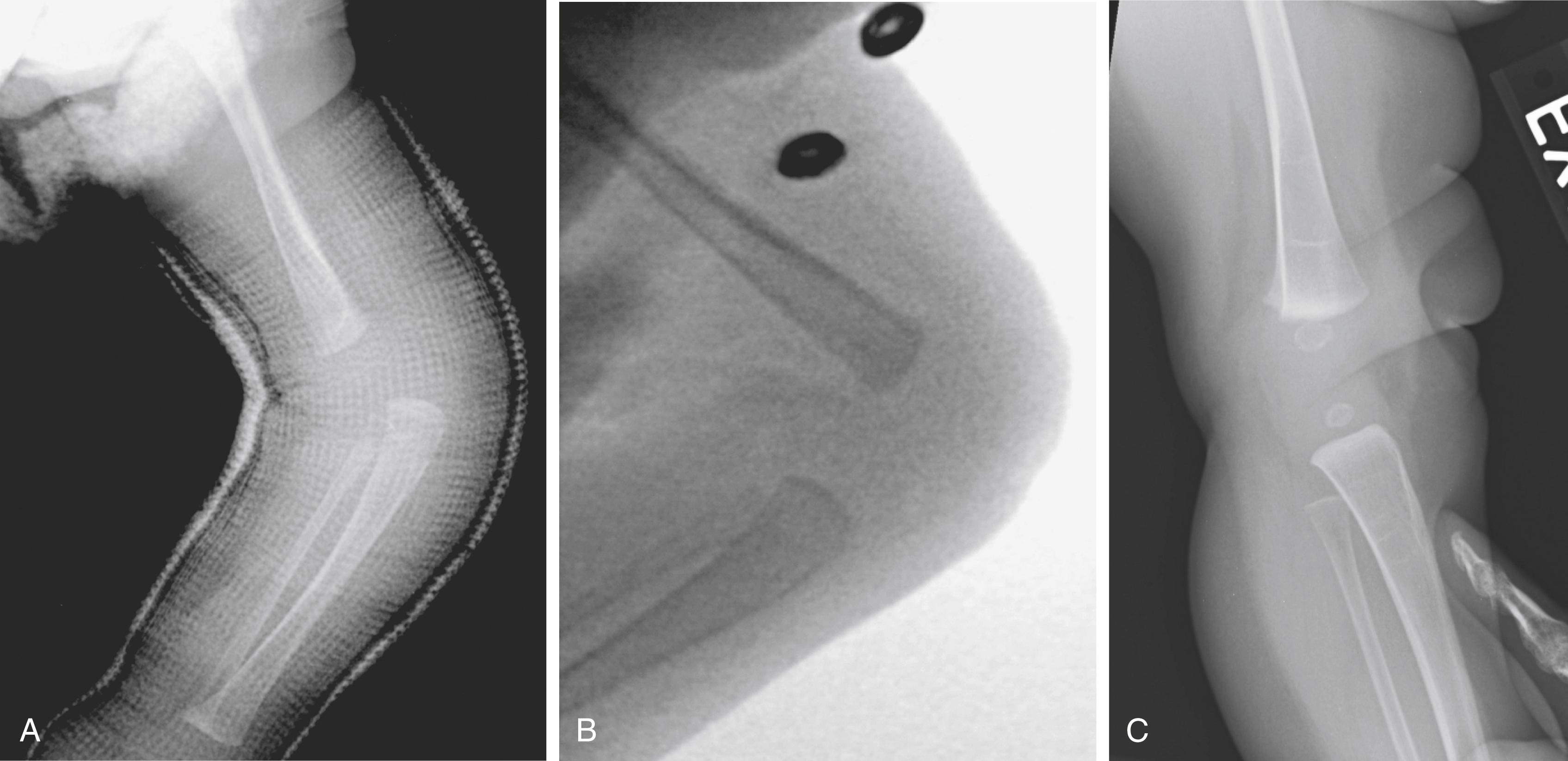
The full spectrum of knee dislocation has been observed in Larsen syndrome. It is occasionally amenable to nonoperative treatment in infancy. If the knee is merely hyperextended (grade 1), it may be possible to use serial casting and quadriceps stretching to achieve reduction by flexion. More often than not, however, the quadriceps contracture is more severe, and obliteration of the suprapatellar pouch is complete. In this case, open reduction is necessary to gain concentric femorotibial motion. We have also been successful in obtaining closed reduction by inducing neuromuscular blockade of the quadriceps with botulinum toxin (Botox; Fig. 37.21 ). An early attempt at closed reduction by flexion and serial casting should be made in newborns, and if the reduction is successful, it can be maintained by splinting, which may include the use of a Pavlik harness to maintain the knees in a flexed position ( Fig. 37.22 ). In a relatively mild case, knee hyperextension and hip dislocation can be treated simultaneously by a Pavlik harness in the neonatal period, provided that knee flexion greater than 40 to 45 degrees can be achieved.
Open reduction of congenital dislocation of the knee is probably the second most important operative procedure, after cervical spine stabilization, if the patient is to have a good functional outcome and ambulatory ability as an adult. The best results are obtained when the knees are reduced by age 2 years. q
q References , , , , , .
Traditional treatment involves extensive quadriceps mechanism lengthening to achieve flexion, as well as an anterior arthrotomy to release intraarticular and extraarticular adhesions, preventing congruous knee flexion, and to mobilize the patellofemoral joint. However, the common end result of such lengthening is an incompetent quadriceps mechanism, producing extensor weakness and poor ambulatory function. If the knee is also unstable because of ligamentous insufficiency (particularly cruciate), or if extensive intraarticular release is required to achieve reduction, the quadriceps weakness further reduces function, and severe valgus or frank subluxation may result, making the patient brace-dependent. Marked instability at the time of reduction, requiring temporary transarticular fixation, portends the later development of valgus, subluxation, and fixed flexion contracture caused by resubluxation of the tibia. In such a clinical scenario, impaired knee function becomes the most significant disability ( Fig. 37.23 ).
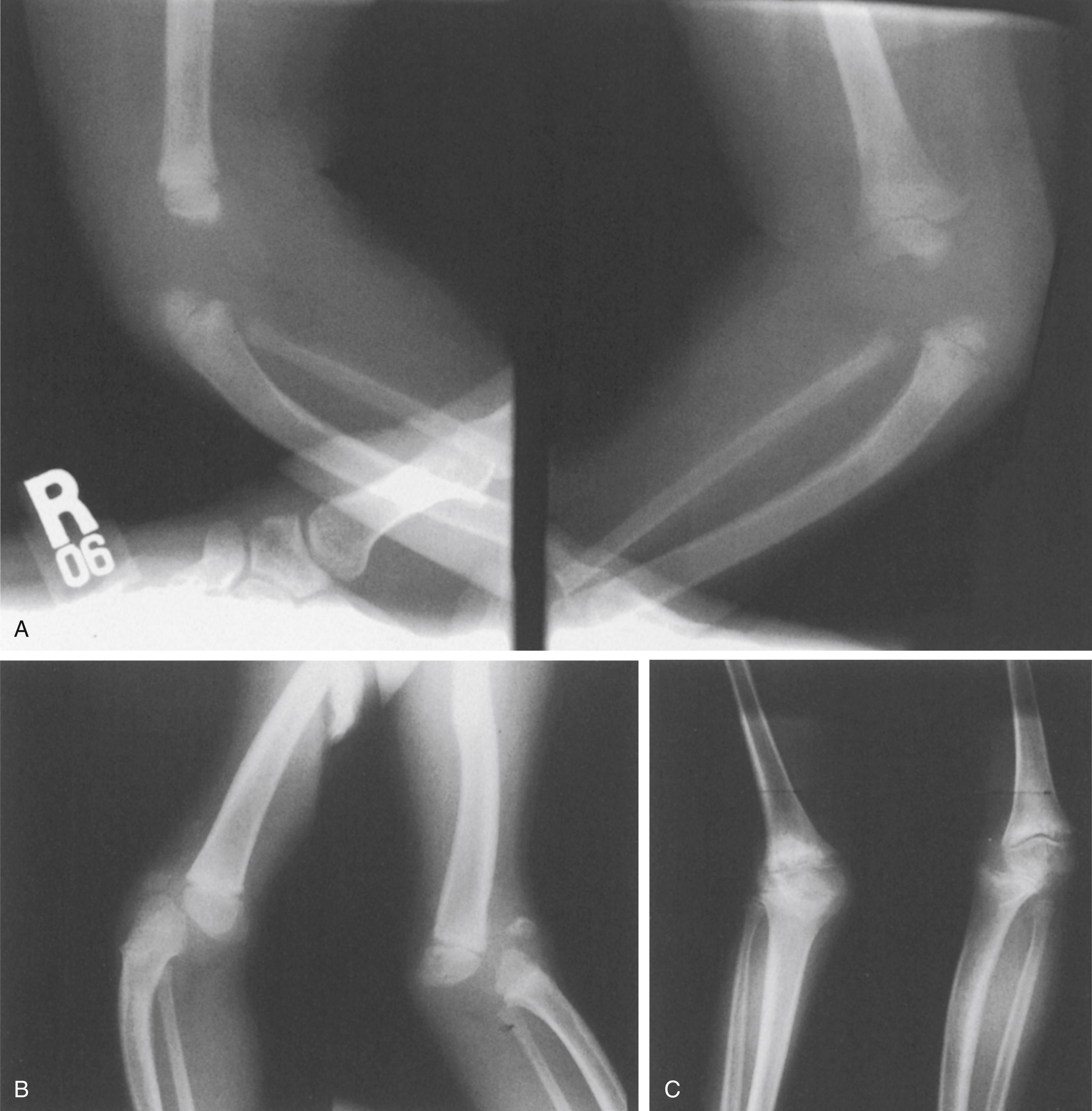
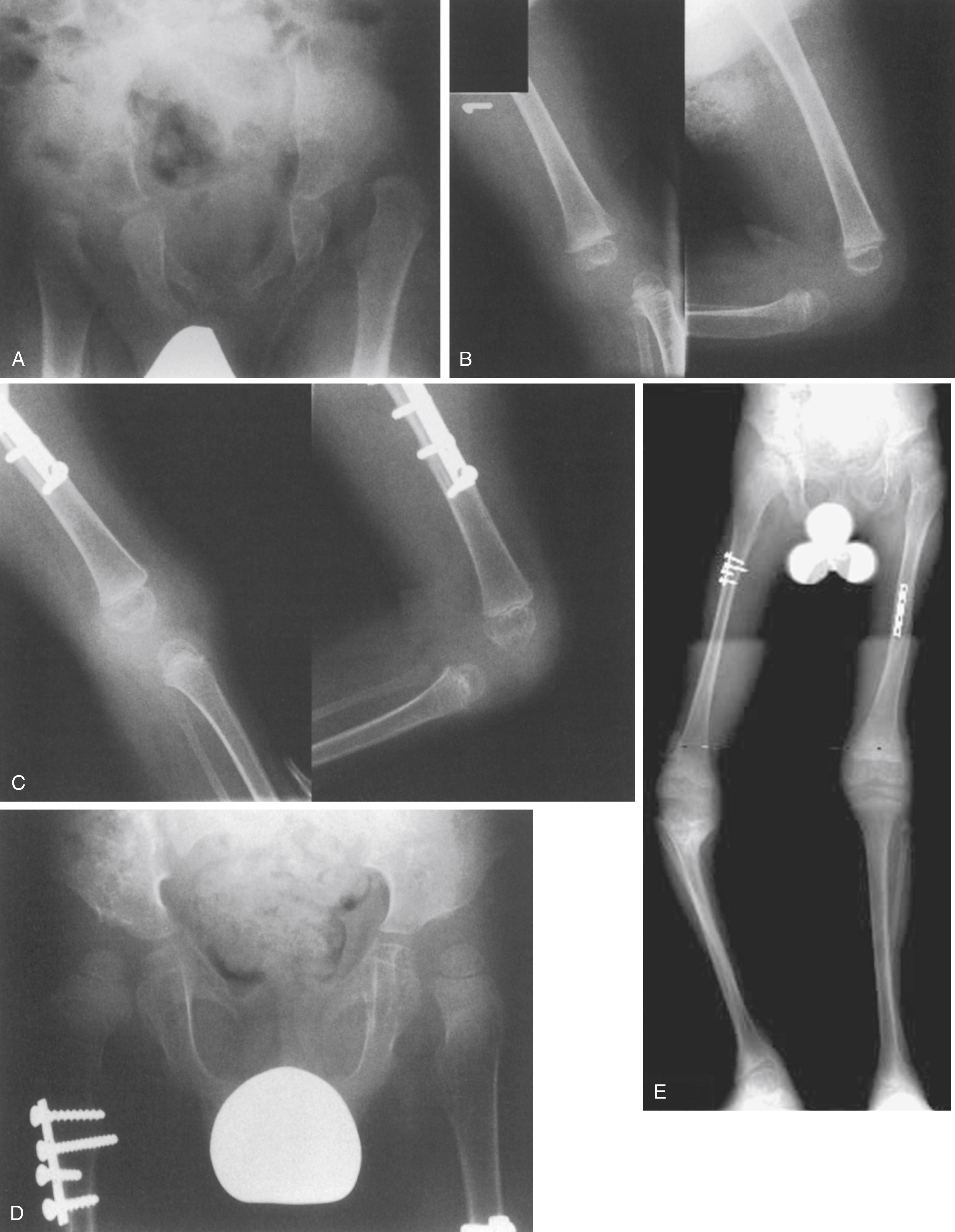
Experience with arthrotomy and primary femoral shortening to gain reduction and flexion of the knee has been more encouraging ( Fig. 37.24 ). The purpose of femoral shortening is to lengthen the quadriceps mechanism without extensive dissection and lengthening of the muscle-tendon unit itself. With shortening of the femur, the extension contracture is decompressed, and with a more limited arthrotomy, intraarticular and extraarticular obstructions to reduction of the knee can be released or excised without damage to the suprapatellar quadriceps mechanism itself. The patellofemoral joint can be realigned by extending the arthrotomy proximally on the lateral side of the knee, freeing the patella from its laterally dislocated position, and realigning it in its appropriate intercondylar groove, again aided by femoral shortening. The bony shortening is stabilized by an appropriately sized small or mini-DCP plate (dynamic compression plate). After bony healing, the knees are splinted in a flexed position and gradually brought to full extension as the child grows and ambulatory status develops. Additional details of this technique are available elsewhere.
Late angular deformity, primarily valgus, is common following knee reduction surgery. If the valgus is associated with marked anterolateral instability, ligamentous reconstruction can be attempted. We have used the iliotibial band transfer to substitute for the anterior cruciate ligament, combined with imbrication of the posteromedial corner to stabilize such knees, with definite short-term improvement. Over the longer term, subluxation may recur because of gradual stretching of the ligamentous replacement. As always, any transphyseal cruciate ligament transfer in an immature knee risks a growth disturbance of the proximal tibial physis (see Fig. 37.24E ). However, the stability obtained by the ligament and capsular reconstruction is invaluable to the ultimate function of the knee and is well worth the risk of physeal growth disturbance, which can be addressed later by appropriate lengthening and angular correction. Bony realignment by varus osteotomy may also be appropriate for valgus alignment. Although it does not directly address the pathologic ligamentous and articular structures, realignment may improve symptomatic valgus by preventing further stretching of the medial and posterior capsules.
Congenital dislocation of the hip may be amenable to closed treatment in newborns. Although this deformity is often considered teratologic in patients with Larsen syndrome, closed reduction and stabilization have been performed successfully. As noted, knee hyperextension and congenital dislocation of the hip can be treated simultaneously in neonates by means of a Pavlik harness. If hip stability is not achieved with the harness, maintaining the knees in flexion is still beneficial for subsequent formal closed or open reduction of the hips, which are then immobilized in a spica cast. Knee flexion is obviously helpful when applying an appropriate spica cast.
If teratologic dislocation persists after closed treatment in infancy, which should not be unexpected, an attempt at anterior open reduction and capsulorrhaphy should be planned. Hyperlaxity usually contributes to the hip instability, necessitating capsulorrhaphy; this is best done through a traditional anterior ilioinguinal approach. I have no experience with medial open reduction without capsulorrhaphy in patients with Larsen syndrome.
Anterior open reduction and capsulorrhaphy of the hip are best performed around 1 year of age. Simultaneous reduction of both the hip and knee, aided by a single diaphyseal femoral shortening, has proven to be the most efficacious method of achieving joint reduction with a minimum of surgical procedures. Because femoral shortening may be required to achieve a stable hip reduction without risking avascular necrosis, we perform a mid-diaphyseal femoral shortening to aid in the simultaneous reduction of both the hip and knee (see Fig. 37.24 ). With the knee flexed, the quadriceps realigned, and the incision closed, the acetabulum is cleared, capsulorrhaphy is performed, and the hip wound is closed. The involved extremity is then immobilized in a spica cast for an appropriate period. The contralateral extremity with one or both joints dislocated can be approached in 2 to 3 months.
If a patient presents late with dislocated hips, or if open reduction has failed, leaving the hips unreduced is a viable option. Larsen and colleagues did not treat the dislocated hips in some of their patients because of the excellent motion and absence of hyperlordosis.
Foot deformities in Larsen syndrome usually include equinovarus or equinovalgus. Foot deformities are usually addressed after the knee and hip have been stabilized. As with other congenital foot problems involving significant equinus, waiting until the patient is ambulatory and fully weight bearing is beneficial in terms of maintaining correction. Although operative treatment is frequently required for equinovarus deformities, it is not unreasonable to attempt closed correction with casting or other stretching techniques while the hip and knee joints are undergoing treatment. The equinus of Larsen syndrome tends to be resistant, however, and Achilles tendon lengthening and posterior release will likely be required to achieve a plantigrade foot. Because of the overall ligamentous laxity, caution is recommended when releasing other components of the clubfoot, because hyperpronated valgus overcorrection is common. An apparently rigid clubfoot in an infant may later become pathologically flexible. Minimal release of the equinus and hindfoot varus in a 1-year-old who has completed treatment for other joint dislocations may be all that is required.
Equinovalgus deformities may not require treatment at all or may require only Achilles tendon lengthening. Patients with Larsen syndrome also tend to develop serpentine or Z-foot deformities, in which hindfoot valgus is combined with forefoot adductus ( Fig. 37.25 ). Because of the ligamentous laxity, the feet of patients with Larsen syndrome frequently appear to have significant deformity in the weight-bearing position but remain asymptomatic. Surgical intervention is rarely required, and supportive shoes or orthoses are usually sufficient. On occasion, however, it may be necessary to correct the hyperpronated portion of the deformity by hindfoot stabilization.
Congenital dislocation of the elbow or radial head is a fairly frequent finding in patients with Larsen syndrome but rarely requires treatment. , Patients with radial head dislocation are treated like any other patients with this congenital deformity; excision of the radial head is performed at maturity if the patient is symptomatic. Because the arms remain functional, with an adequate range of motion of the elbow, the skeletal anomaly is generally ignored. In the more severe situation of humeroulnar dislocation, the deformity appears to require treatment in that the elbows are frequently in a flexed position, with webbing and significant contracture (see Fig. 37.18 ). However, because of the bizarre absence of a distal humerus in these patients, treatment is generally declined because of the inability to restore a normal articulation by open reduction. Proximal radioulnar synostosis, although reported in two-thirds of the patients in Laville and associates’ series, is an uncommon elbow abnormality with few therapeutic implications because of the lack of symptoms or functional impairment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here