Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As improved therapies prolong the lives of cancer patients, the prevalence of oncologic emergencies continues to increase. However, nonspecific clinical features misattributed to the underlying cancer complicate their diagnosis. In this chapter, we review febrile neutropenia, metastatic spinal cord compression (MSCC), malignant pericardial disease, hypercalcemia of malignancy, tumor lysis syndrome (TLS), leukostasis, superior vena cava (SVC) syndrome, and complications of cancer immunotherapies including monoclonal antibodies, T-lymphocyte checkpoint inhibition, and adoptive cell transfer therapy.
Patients whose absolute neutrophil count is or is expected to soon be 500 cells/mm 3 or lower are considered severely neutropenic. A single temperature of 38.3°C or sustained temperature of 38.0°C for 1 to 2 hours or longer is considered fever.
Any neutropenic patient with fever or with infectious signs or symptoms (even in the absence of fever) should be evaluated for an infectious source, including drawing of blood cultures, and started on empirical antibiotics. Those with high-risk features (e.g., prolonged or profound neutropenia, pneumonia, hypotension, abdominal pain, neurologic changes, Multinational Association for Supportive Care in Cancer (MASCC) score < 21) should be started on an antipseudomonal beta-lactam (e.g., cefepime, piperacillin-tazobactam, antipseudomonal carbapenem). Those with low-risk features may be appropriate for oral antibiotics. Empirical gram-positive bacterial, antifungal, and antiviral coverage is unnecessary unless the clinical situation dictates otherwise.
Neutropenic patients with fever should generally be hospitalized, including all high-risk patients. Select low-risk patients may be managed as outpatients.
Whether due to underlying malignancy or as a cytotoxic effect of chemotherapeutics, cancer patients regularly experience depleted levels of circulating neutrophils. Risk of infection rises when a patient’s absolute neutrophil count (ANC) drops below 1000 cells/mm . However, the increase in risk is most marked in patients with ANC less than 500 cells/mm . Historically, ANC of 1000 to 1500 cells/mm 3 has been considered mild, 500 to 1000 cells/mm 3 moderate, and less than 500 cells/mm 3 severe. Current guidelines do not emphasize these gradations, and neutropenia is defined as an ANC less than 500 cells/mm 3 or an ANC expected to drop below this threshold within 48 hours. , The ANC is calculated from a complete blood count (CBC) with differential count by the following formula:
Neutropenic patients are particularly susceptible to infection, even from their own existing microbial flora, and such infection carries significant mortality risk. Twenty to thirty percent of patients with neutropenic fever require hospitalization, and of those patients, about 10% will not survive to discharge. Most commonly, neutropenic fever is caused by pneumonia, anorectal lesion, skin infection, pharyngitis, or urinary tract infection. However, because local inflammatory responses are dampened by the absence of granulocytes, the only sign of infection may be fever, defined as a single temperature 38.3°C or greater or a sustained temperature of 38.0°C or greater for 1 hour or more. However, fever is not a requisite for infection; any neutropenic patient with signs or symptoms of infection should be treated as having neutropenic fever, whether actually febrile or not. In fact, suppressed temperature on presentation (<36.5°C) may portend higher mortality than fever or normothermia.
Due to underlying immunosuppression, neutropenic patients often do not manifest infectious signs or symptoms beyond fever, making a thorough history and physical examination crucial. The interview should include a standard infectious review of systems, questioning for presence of diarrhea, nausea or vomiting, headache, neck stiffness, rashes, dysuria, cough, dyspnea, and pain at any location, including the abdomen, chest, joints, throat, sinuses, and ears. Specific note should be made of indwelling venous catheters, because these increase the risk of bacteremia and skin infection. To evaluate for perianal infection, the perineal region should be examined and inquiry made about pain with defecation. Although there is no hard evidence, expert opinion suggests against digital rectal examination to avoid compromise of the barrier between blood and rectal flora. Due to the chemosensitivity of the rapidly dividing epithelial cells of the mouth, mucositis is a common adverse effect of chemotherapy and provides a portal for oral flora into the bloodstream. This can be evaluated by examination and inquiry about oral pain.
Causes of fever in cancer patients are diverse and include infection, venous thrombus or embolus, adverse effect of chemotherapy or other medication, and direct effect of tumor burden. A clear source of infection is identified in only about one-third of neutropenic fever cases. Nonetheless, because of the potentially life-threatening effects of an infection in this population, all febrile neutropenic patients should receive empirical antibiotics and a full evaluation for an infectious source.
Neutropenic patients with fever should have at least two sets of blood cultures drawn before administration of antibiotics. Both may be drawn peripherally in patients without preexisting central access. In patients with a preexisting central line, one of the two blood cultures should be obtained peripherally, whereas other cultures should be simultaneously drawn off each lumen of the central catheter. Bacterial growth in the catheter-drawn samples greater than 2 hours prior to the peripheral samples may suggest a catheter-associated infection. Patients with neutropenic fever should also have a CBC with differential count performed to assess severity of neutropenia, as well as urinalysis, urine culture, chemistries, and renal and hepatic function tests. Serum lactate should be measured if sepsis is suspected. Additional cultures may be sent according to the clinical presentation (e.g., sputum culture if productive cough, stool culture, and testing for Clostridium difficile if diarrhea or abdominal pain). ,
Initial imaging usually consists of chest x-ray, although this is often low-yield in the absence of specific respiratory symptoms. If fever persists for 72 hours without identified source, empirical computed tomography (CT) scan of the chest and sinuses, and bronchoalveolar lavage may be considered to evaluate for occult fungal infection. Patients with a history of invasive aspergillosis, and those with profound or prolonged neutropenia are at higher risk of fungal pneumonia, and consideration should be given to pulmonary imaging if there is little clinical response within the first day of therapy. Similarly, directed CT scans may be performed sooner in the setting of appropriate clinical signs (e.g., chest CT for a patient with coughing and bronchial breath sounds, but a clear chest x-ray, abdominal CT for a patient with unexplained abdominal tenderness).
As nucleotide sequencing becomes more reliable, assays employing this technique for pathogen identification are being aggressively developed. Although these assays provide a useful adjunct, and may direct antibiotic therapy prior to culture growth, they are not yet sufficiently developed to stand in lieu of conventional diagnostics, and a full set of cultures should always be obtained.
Febrile neutropenic patients should receive antibiotics prior to confirmation of an infectious source. Recommendations for specific regimens are based on risk of clinical decompensation. According to the Infectious Disease Society of America (IDSA), high-risk features include an expected duration of neutropenia greater than 7 days, expected nadir ANC less than 100 cells/mm , hypotension, pneumonia, new-onset abdominal pain, neurologic changes, or existence of other significant medical comorbidities. A current or prior infection with a resistant organism and treatment at a center with a high prevalence of resistant organisms should also be viewed as high risk. Alternatively, several scoring systems exist to assess risk of deterioration in neutropenic fever. The Multinational Association for Supportive Care in Cancer (MASCC) risk index identifies low-risk patients based on clinical features ( Table 112.1 ). Patients scoring at least 21 points are considered low-risk, because nearly 90% of cases have uncomplicated resolution of their fever within 5 days. Similarly, the Clinical Index of Stable Febrile Neutropenia (CISNE) index ( Table 112.2 ) has been validated to indicate patients with low (score 0), intermediate (score 1–2), and high (score ≥ 3) risk of clinical deterioration.
| Clinical Feature | Point Value |
|---|---|
| Age < 60 years | 2 |
| Onset of fever while outpatient | 3 |
| Overall moderate symptom burden | 3 |
| Absence of dehydration | 3 |
| No prior fungal infections or solid tumor type | 4 |
| No history of chronic obstructive pulmonary disease | 4 |
| Absence of hypotension | 5 |
| Asymptomatic or overall mild symptom burden | 5 |
a A score of ≥ 21 suggests low risk of complication and likely resolution of fever within 5 days.
| Clinical Feature | Point Value |
|---|---|
| Eastern Cooperative Oncology Group Performance status ≥ 2 | 2 |
| Stress-induced hyperglycemia | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Chronic cardiovascular disease | 1 |
| Mucositis of grade ≥ 2 | 1 |
| Monocyte count < 200 per μL | 1 |
a A score of zero indicates low risk of clinical deterioration prior to resolution of the episode of neutropenic fever.
High-risk patients should receive a parenteral broad-spectrum antibiotic regimen. Local antibiograms should be considered when choosing specific agents, but guidelines recommend monotherapy using a broad-spectrum beta-lactam with antipseudomonal coverage, such as ceftazidime, cefepime, piperacillin-tazobactam, or antipseudomonal carbapenem. , Among head-to-head studies of these agents in neutropenic patients, all have shown good effectiveness and none has consistently out-performed the others. For patients with signs of sepsis or septic shock, double coverage for gram-negative bacteria with a fluoroquinolone or aminoglycoside in addition to beta-lactam therapy should be considered; however, a meta-analysis of studies comparing beta-lactam monotherapy with combination beta-lactam/aminoglycoside therapy showed that patients receiving aminoglycoside had no survival benefit and were more likely to suffer adverse events, including nephrotoxicity and fungal superinfection. We therefore recommend antipseudomonal beta-lactam monotherapy for patients with neutropenia and fever but without signs of sepsis and septic shock. Regimens meeting these criteria for patients with normal renal function include piperacillin-tazobactam 4.5 g IV q6 hours or cefepime 2 g IV q8 hours; dosing adjustments must be made for patients with renal impairment. Further empirical antibiotics administered will depend on clinical presentation and consultation with oncology or infectious disease consultants.
Despite increasing rates of bacteremia with gram-positive organisms in cancer patients, , randomized controlled studies and meta-analyses have failed to demonstrate a survival benefit to immediate empirical gram-positive–specific coverage and have previously suggested increased rates of adverse effects. Empirical gram-positive–specific therapy, such as with intravenous vancomycin or other glycopeptides, is not recommended except in cases of suspected cellulitis, catheter-associated infection, or pneumonia, or in the case of clinical instability. Similarly, randomized controlled trials have not shown a benefit in immediate, empirical antifungal therapy, and guidelines suggest against empirical antifungal agents, unless there is specific concern for a fungal source. Recent guidelines do suggest, however, that evaluation for fungal pneumonia with high-resolution chest CT scan, serum beta-galactomannan assay, and consideration of bronchoscopy with lavage is beneficial in patients with prolonged or profound neutropenia. In cases requiring empirical antifungal coverage, echinocandins (e.g., micafungin 100 mg IV q24 hrs) compare well with other agents both in terms of effectiveness and safety profile. ,
Neutropenic patients with community-acquired pneumonia should be covered for atypical pathogens and possibly pneumocystis pneumonia (PCP). For atypical pathogens we recommend either levofloxacin 750 mg IV q24 hrs or azithromycin 500 mg IV q24 hrs and doxycycline 100 mg q12 hrs (levofloxacin dose must be adjusted for impaired renal function). For PCP coverage, the preferred regimen is trimethoprim-sulfamethoxazole (TMP-SMX) at 15 to 20 mg of TMP per kilogram of patient’s total body weight per day IV, divided into 3 or 4 doses per day (e.g., 5 mg/kg IV q6 hrs will result in a 20 mg/kg/day dose). TMP-SMX dosing must be adjusted for impaired renal function. For patients with gastrointestinal symptoms, one randomized controlled trial suggests improved 28-day survival with cefepime/metronidazole combination therapy when compared to piperacillin-tazobactam monotherapy. We recommend cefepime 2 g IV q8 hrs and metronidazole 500 mg PO q8 hrs (cefepime dose must be adjusted to renal function). Patients with a vesicular rash or other evidence of herpes infections should receive empirical acyclovir; an initial regimen of 5-10 mg/kg IV every 8 hrs is appropriate for severe cases. In the case of renal impairment, acyclovir dosing must be adjusted.
Although no specific timing of antibiotic administration is recommended in the current IDSA guidelines, evidence is mounting that delays in initiation of antibiotic therapy are correlated with worsened outcomes. , We therefore recommend that high-risk patients receive antibiotic therapy as soon as possible after obtaining blood cultures. Many process improvement efforts have been shown to reduce time to antibiotics in the ED setting, including implementation of neutropenic fever order sets and a dedicated neutropenic fever response team, elevation of patients with neutropenic fever in the triage queue, and establishment of a protocol for initiation of antibiotics by the bedside nurse in appropriate patients.
Observational studies and a meta-analysis have demonstrated that low-risk patients may be treated with an enteral regimen, usually amoxicillin/clavulanate (875 mg PO q12 hrs) and a fluoroquinolone (e.g., ciprofloxacin 500 mg PO q12 hrs). Both amoxicillin/clavulanate and levofloxacin doses must be adjusted in the case of impaired renal clearance. Alternatively, low-risk patients may be started empirically on parenteral broad-spectrum therapy as outlined previously with transition to an enteral regimen after 24 to 48 hours if no complications arise. ,
Regardless of risk category, the majority of febrile neutropenic patients will be hospitalized for observation and initial treatment, , ideally to a unit specialized for oncology patients. Hemodynamically unstable patients and those with a deteriorating course should be admitted to an intensive care unit (ICU). A small fraction of patients may be safely treated with enteral antibiotics in the outpatient setting. , These patients should (1) meet the low-risk criteria of a MASCC score of 21 or less; (2) have no evidence of pneumonia, line infection, cellulitis, or organ failure; (3) have reliable daily follow-up with their oncologist; (4) demonstrate clinical stability during observation in the ED for 4 hours or longer; (5) carry low suspicion of infection with a drug-resistant organism. , Prior to discharge, an initial dose of parenteral antibiotics should be given in the ED, reliable follow-up and access to the outpatient antibiotic regimen must be ensured, and discharge should be coordinated with the patient’s oncologist. ,
Vertebral metastasis and spinal cord compression should be considered in any cancer patient, particularly those who have back pain, peripheral strength or sensory loss, or bowel or bladder dysfunction.
MRI of the spine is the preferred diagnostic test when evaluating spinal cord compression. CT of the spine with myelography may be performed if MRI is contraindicated or unavailable. Plain films are not sufficiently sensitive to rule out spinal cord compression.
Intravenous corticosteroids (dexamethasone 10 mg bolus) should be given to any patient with neurologic deficits from known or suspected MSCC. Consideration should be given to emergent surgical and radiotherapeutic intervention if compatible with goals of care.
Malignancy-related compromise of the spinal cord most commonly occurs from an extradural neoplasm that has metastasized to the vertebral column. The lesion then typically expands locally from the marrow space through a vertebral vein foramen to invade the spinal canal. Although the resulting cord injury is termed metastatic spinal cord compression (MSCC), direct nerve compression by tumor is uncommon. Cord injury is more commonly caused by occlusion of the epidural venous plexus, leading to breakdown of the blood-cord barrier and vasogenic edema. If untreated, tumor expansion eventually leads to arterial obstruction, causing cord ischemia and infarct. Less commonly, direct compression of the cord over time may lead to demyelination and axonal injury.
The most common tumors causing MSCC are prostate, breast, and lung cancer, each accounting for about 15% to 20% of total cases. Renal cell cancer, non-Hodgkin lymphoma, and multiple myeloma each account for an additional 5% to 10% of all cases. Most cases of MSCC affect the thoracic spine (60%), with the lumbosacral and cervical spine each making up 25% and 15% of cases, respectively. Twenty to forty percent of patients with MSCC have multiple loci of spinal metastasis.
Back pain, weakness, sensory loss, and autonomic function loss are the most frequent presenting symptoms of MSCC. Back pain occurs in more than 95% of patients with MSCC and is the most common initial symptom. Extremity weakness occurs in up to 75% and generally (but not always) precedes sensory loss. Patients with MSCC may also present with autonomic nerve dysfunction, including loss of bowel or bladder function, but it is a late finding and rarely presents in isolation.
In addition to MSCC, patients with back pain with or without neurologic symptoms should be considered for nonmalignant musculoskeletal etiologies (e.g., muscle strain, ligamentous sprain, pathologic fracture, disc displacement, radicular stenosis, vertebral osteoarthritis) and paraspinal or vertebral infections (e.g., paraspinal abscess, vertebral osteomyelitis, discitis). In patients with known malignancy, new back pain and neurologic deficits (motor, sensory, or autonomic) carry high specificity for MSCC, and this diagnosis should be presumed and investigated. In patients without known cancer, however, new back pain is the heralding symptom of cancer in 20% of cases of MSCC, which commonly takes up to two months from original presentation to diagnose.
A thorough physical examination should be performed, including palpation of the entire spine, as well as testing of strength, sensation, deep tendon reflexes, and rectal tone. The diagnosis is confirmed by imaging, for which magnetic resonance imaging (MRI) has become the gold standard with sensitivity of 93% and specificity of 97% ( Fig. 112.1 ). Even in patients in whom MSCC has been established by another modality, MRI should still be performed when possible, because its added resolution changes treatment strategy in approximately 50% of cases. Because multiple separate lesions can occur simultaneously, both the thoracic and lumbar spine should be imaged in any patient with MSCC. Although the incidence of a second lesion in the cervical spine is much lower, this segment should also be included when possible.
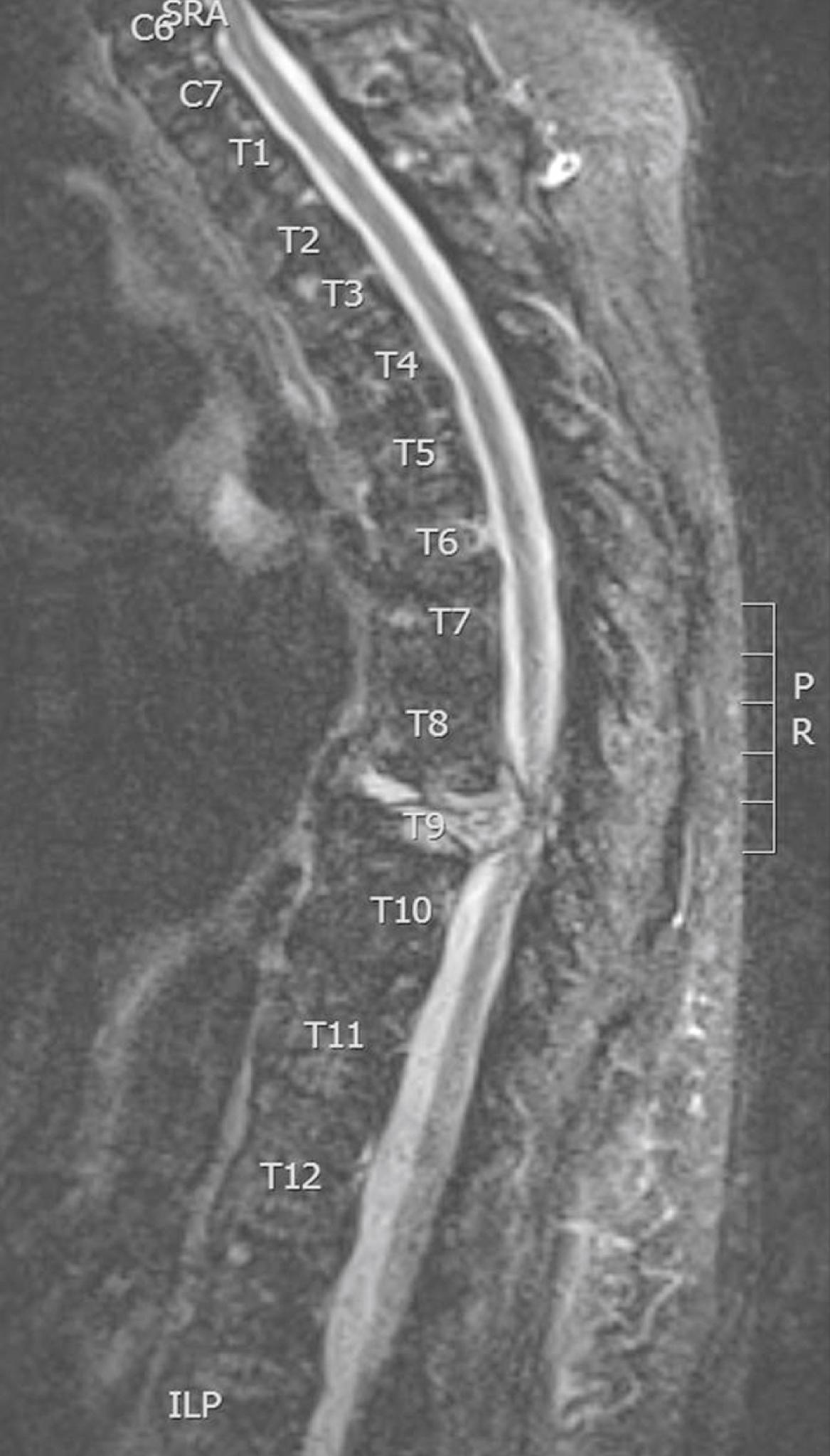
If MRI is unavailable or contraindicated (e.g., patients with incompatible pacemakers), CT scan of the spine is the next most informative study. If vertebral metastasis is seen on initial scans, presence of cord compression can be assessed by CT myelography, in which contrast is introduced into the subarachnoid space. Sensitivity of this technique rivals that of MRI, but MRI offers similar information noninvasively. Thus, CT myelography is reserved for those rare patients in whom MRI is contraindicated yet radiographic confirmation of cord compression as the cause of symptoms is required prior to intervention. Plain radiographs, positron emission tomography (PET) scans, and radionuclide scans may demonstrate vertebral metastasis, but sensitivity is limited. Furthermore, these techniques provide no information about the state of the spinal cord itself or the precise location of suspected compression. These studies are therefore insufficient to rule out MSCC.
Treatment of MSCC in the ED entails administration of corticosteroids and initiation of definitive treatment with surgery, radiation therapy, or both. , Corticosteroids provide the most immediately available therapy for cord compression; unlike surgery or radiation, their administration does not require significant logistical planning or knowledge of the exact anatomic location of tumor. Early steroid administration has been shown to improve ambulation rates at 3- and 6-month intervals, and patients receiving corticosteroids have improved long-term pain scores. Current guidelines suggest a 10 mg intravenous bolus of dexamethasone followed by 16 mg orally per day in divided doses for any patient with neurologic deficits believed secondary to MSCC. Patients with severe deficiencies, such as paraplegia, may receive a higher dose of 100 mg intravenous dexamethasone, followed by 96 mg orally per day in divided doses. Steroids are generally unnecessary in patients with vertebral metastases on imaging but without neurologic deficits.
Corticosteroids temporize vasogenic cord edema, but cord damage will ensue without definitive correction with radiation therapy, surgery, or both. For patients who can tolerate surgery and have goals of care in line with surgery, a combination of surgical decompression followed by radiation therapy provides better long-term rates of continence, ambulation, and survival than radiation therapy alone, and combined management is recommended in the most recent guidelines. , Surgical intervention is especially important in patients with spinal instability or cord compression by bony fragments. Although surgery carries a high complication rate, development of mini-open approaches , and use of postoperative stereotactic body radiation therapy to limit the degree of necessary resection show promise to reduce complication rates and shorten recovery times. For patients unable to tolerate surgery or with incompatible goals of care, radiation alone may be pursued. Conventional fractionated radiotherapy has previously been the standard of care and continues to be an important modality for radiation therapy. Stereotactic body radiation therapy (SBRT), which employs advanced tumor mapping and beam-focusing techniques to allow safe delivery of significantly higher bolus doses of radiation to tumor cells without collateral injury to nearby tissues, is rapidly developing a central role in the management of MSCC. This is particularly true for patients with tumor histology known to be insensitive to fractionated radiotherapy, and for those with tumor in an area that has already received a maximum allowable radiation dose. Neuro-interventional radiology technologies such as injection of cement into spinal column fractures, or intra-arterial tumor embolization and other ablative techniques also show promise for patients whose goals of care are incompatible with major surgery.
Although some recovery of lost neurologic function is possible after decompression, often the greater impact of treatment is prevention of further damage. In fact, neurologic status at initiation of treatment is the strongest indicator of functional outcome, and every effort should be made to expedite appropriate therapy to prevent further neurologic decline. No direct evidence exists to guide the exact timing of treatment, but most experts recommend definitive treatment within 24 hours whenever possible.
Following corticosteroid administration, patients with neurologic deficits (i.e., motor, sensory, or autonomic) should be hospitalized for definitive therapy. Asymptomatic patients with incidentally noted vertebral metastasis may be managed as outpatients, provided they have reliable follow-up. Given the complexity intrinsic to management of MSCC, a multidisciplinary approach involving oncology, radiation oncology, and neurosurgery should be employed regardless of disposition.
No clinical sign or symptom is entirely sensitive for cardiac tamponade, but echocardiographic findings of a large pericardial effusion (anechoic circumferential stripe around the heart) and right atrial or ventricular collapse during diastole, combined with clinical findings of shock are highly suggestive.
If compatible with goals of care, pericardial effusion causing tamponade should be emergently drained. Intravenous fluid or inotrope administration may be trialed as a temporizing measure, but these therapies are unreliable and should not delay definitive management.
Pericardial manifestations of malignant disease, including pericarditis, pericardial neoplasm (usually metastatic), and pericardial effusion affect greater than 10% of cancer patients and cause about 25% of all effusive pericardial disease in the developed world. Approximately two-thirds of malignancy-associated pericardial disease is clinically insignificant. However, in the remaining one-third of cases, hemodynamic compromise, organ failure, or death occurs.
Neoplastic disease is believed to cause pericardial effusion when lymphatic flow, which normally drains fluid from the pericardium, becomes obstructed or reversed by congestion in proximal malignant lymph nodes. An effusion develops both by obstruction of fluid outflow, and by metastatic spread to the pericardial lining, leading to a nonphysiologic increase in pericardial fluid production. The most common culprits in this process are lung, breast, and hematologic tumors, as well as melanoma. Effusions not directly caused by malignancy may also develop in cancer patients secondary to hypoalbuminemia or as an adverse effect of radiation or chemotherapy.
Although the pericardial sac has the elastic potential for gradual expansion to greater than 1 L, it is poorly distensible in short (i.e., hours to days) time frames, and rapid accumulation of even a few hundred milliliters of fluid may precipitate cardiac tamponade. This life-threatening condition occurs when intrapericardial pressures rise to match or surpass those of the atria and then ventricles, reducing or eliminating cardiac output and leading to shock.
The classic presenting symptoms of pericardial disease are dyspnea and chest pain. Weakness and fatigue are often associated, and large, slowly accumulating effusions may result in mass effect on nearby structures, causing nausea, early satiety, cough, hiccups, hoarseness, or dysphagia. Cardiac tamponade presents with shock, but other classic stigmata of the disease are unreliable. Pulsus paradoxus, defined as a 10 mm Hg systolic blood pressure gradient between inspiration and expiration in the respiratory cycle, is the most sensitive sign, present in about 80% of cases. Kussmaul sign (paradoxically increased jugular venous pressure [JVP] with inspiration) and Beck triad (hypotension, elevated JVP, and muffled heart sounds) are seen in less than half of cases. Hypotension may not even be a presenting symptom, particularly in patients with underlying hypertension.
Because of its nonspecific presentation, the differential diagnosis for malignant pericardial effusion is broad. Considerations should include acute coronary syndrome, acute heart failure or valve failure, pulmonary embolism, pleural effusion, pneumonia, and pneumothorax.
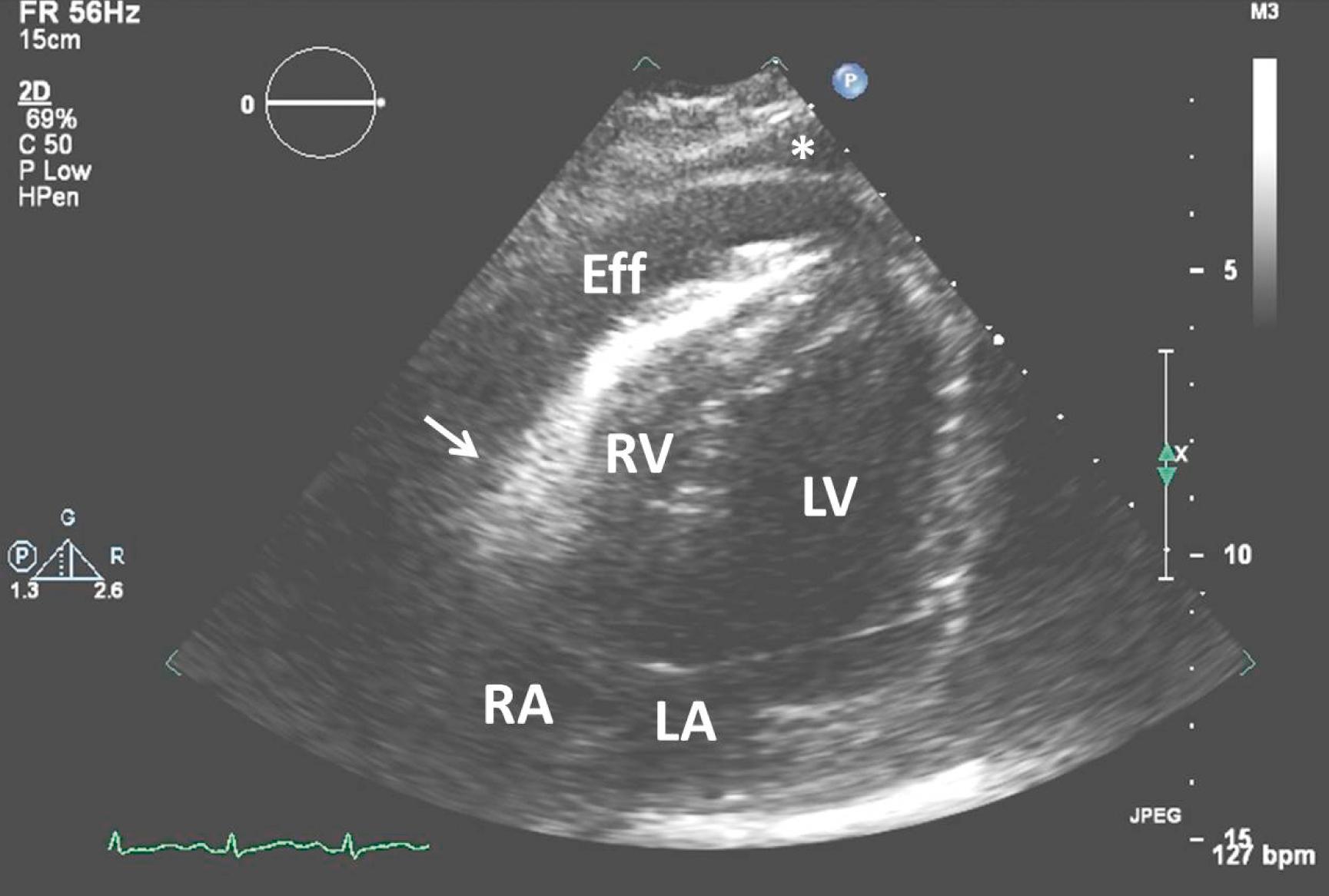
Evaluation for a patient with suspected malignant pericardial effusion should include chest radiography, electrocardiogram (ECG), and transthoracic echo (TTE). Electrocardiographic manifestations may include nonspecific ST or T changes, or low amplitude QRS voltage. Electrical alternans (alternating high and low QRS amplitudes) is only seen in 10% of cases. An enlarged cardiac silhouette on chest x-ray may suggest a large effusion. Echocardiography, which approaches 100% sensitivity and specificity for pericardial effusion, can also identify characteristics of tamponade physiology, such as cardiac chamber collapse and abnormal tissue imaging markers (e.g., tricuspid valve annular plane systolic excursion). Emergency clinicians, who are trained in basic bedside cardiac ultrasound, can reliably identify pericardial effusion and should perform the initial examination to hasten diagnosis ( Fig. 112.2 ).
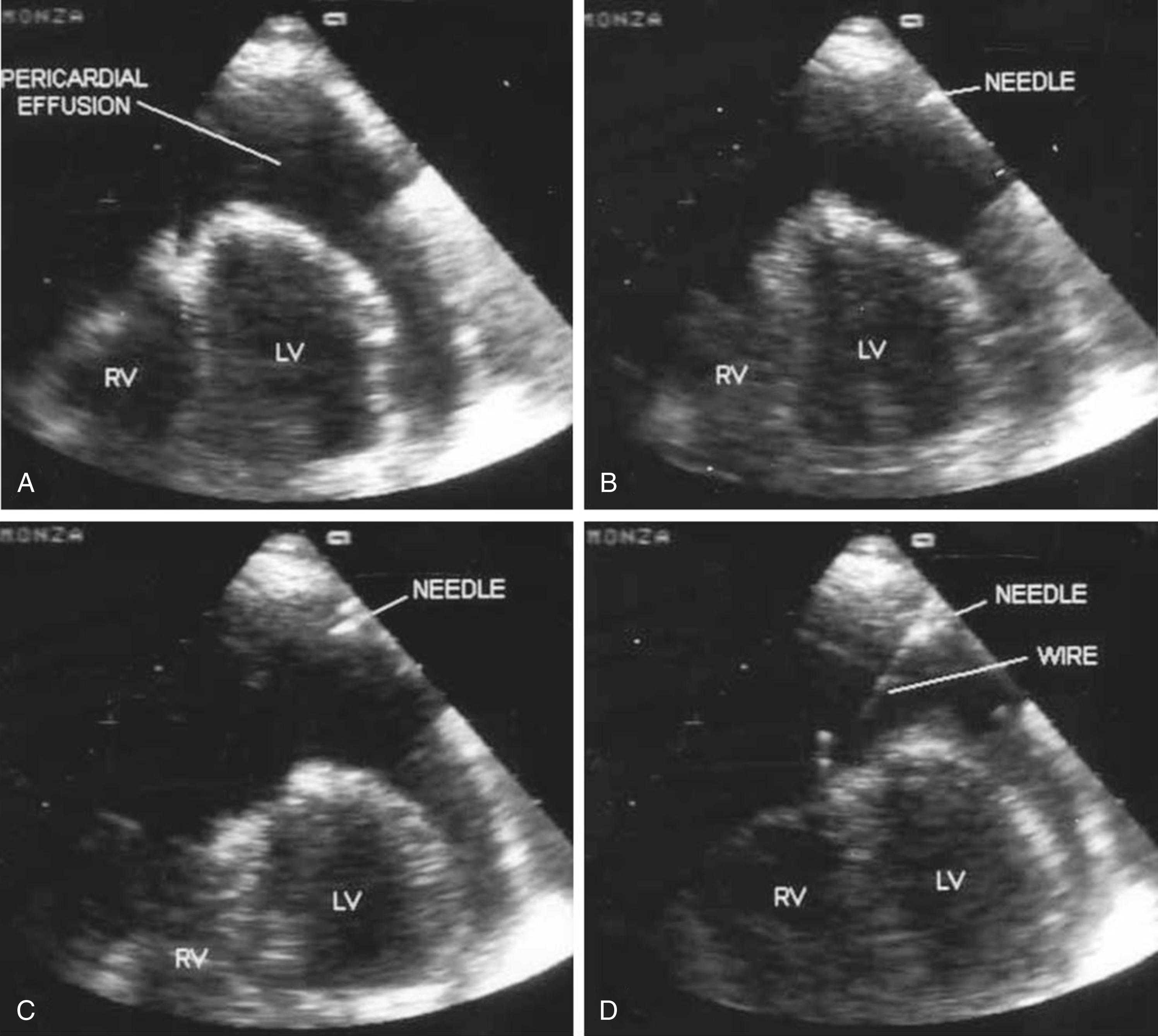
Malignant pericardial effusion generally serves as evidence of advanced cancer, and therapies should be tailored to match each patient’s goals of care. If aggressive therapy is desired, cardiac tamponade is a medical emergency and warrants immediate drainage, ideally under real-time ultrasonographic guidance ( Fig. 112.3 ). Ultrasound-guided drainage by the intercostal approach results in fewer complications and higher success rates than blind drainage by the subxiphoid approach. If ultrasound is unavailable, a subxiphoid approach should be employed by inserting the needle at a 15-degree angle to horizontal between the xyphoid process and left costal margin. After clearing the rib cage, the needle should be leveled and advanced toward the left shoulder until return of fluid is achieved. Temporizing measures such as inotropes (e.g., epinephrine in hypotensive patients, dobutamine in normotensive patients) or intravenous fluids may be attempted, but, despite early successes in trials on anesthetized animal models, these measures have not demonstrated reproducible benefit in humans and should not be viewed as a substitute for timely drainage.
Effusion without tamponade can be managed nonemergently. Fluid sampling for cytology and tumor marker analysis can help confirm etiology of effusion. This also allows intrapericardial chemotherapy or injection of sclerosing agents in a more controlled fashion. Malignant effusions tend to recur, so a long-term evacuation strategy by way of percutaneous drain, surgical window, or percutaneous balloon pericardiotomy should be considered.
Disposition depends on the hemodynamic effect of the pericardial effusion. Patients without tamponade or with a low likelihood of tamponade in the near future (i.e., slow evolution of symptoms) can be managed nonemergently as an outpatient. Those with tamponade or rapid development of effusion should undergo pericardiocentesis and be hospitalized to monitor for fluid reaccumulation.
Calcium levels in hypercalcemic patients should be assessed by measuring ionized calcium concentration, rather than total calcium concentration.
First-line management of hypercalcemia includes intravenous fluids (1–2 L crystalloid bolus followed by 200–250 mL/hr), and loop diuretics only for volume management, as well as bisphosphonate therapy (pamidronate 90 mg or zoledronate 4 mg, intravenously). Calcitonin is faster acting than bisphosphonates, but tachyphylaxis may develop; consider calcitonin in hypercalcemic patients with active cardiac or neurologic symptoms (e.g., dysrhythmias, seizures).
Serum calcium regulation is achieved by parathyroid hormone (PTH) and calcitriol (the activated form of vitamin D), which both increase serum calcium level, and to a lesser extent by calcitonin, which decreases it ( Fig. 112.4 ). Approximately a third of cancer patients will experience dysregulation of calcium homeostasis, usually caused by one or more of the following: (1) synthesis of the PTH analog PTH-related protein (PTHrP), (2) overproduction of calcitriol, (3) bone osteolysis due to direct spread of tumor, or, (4) less commonly, ectopic production of PTH. In most cases, malignancy-associated hypercalcemia signifies advanced disease, with median survival of less than two months.
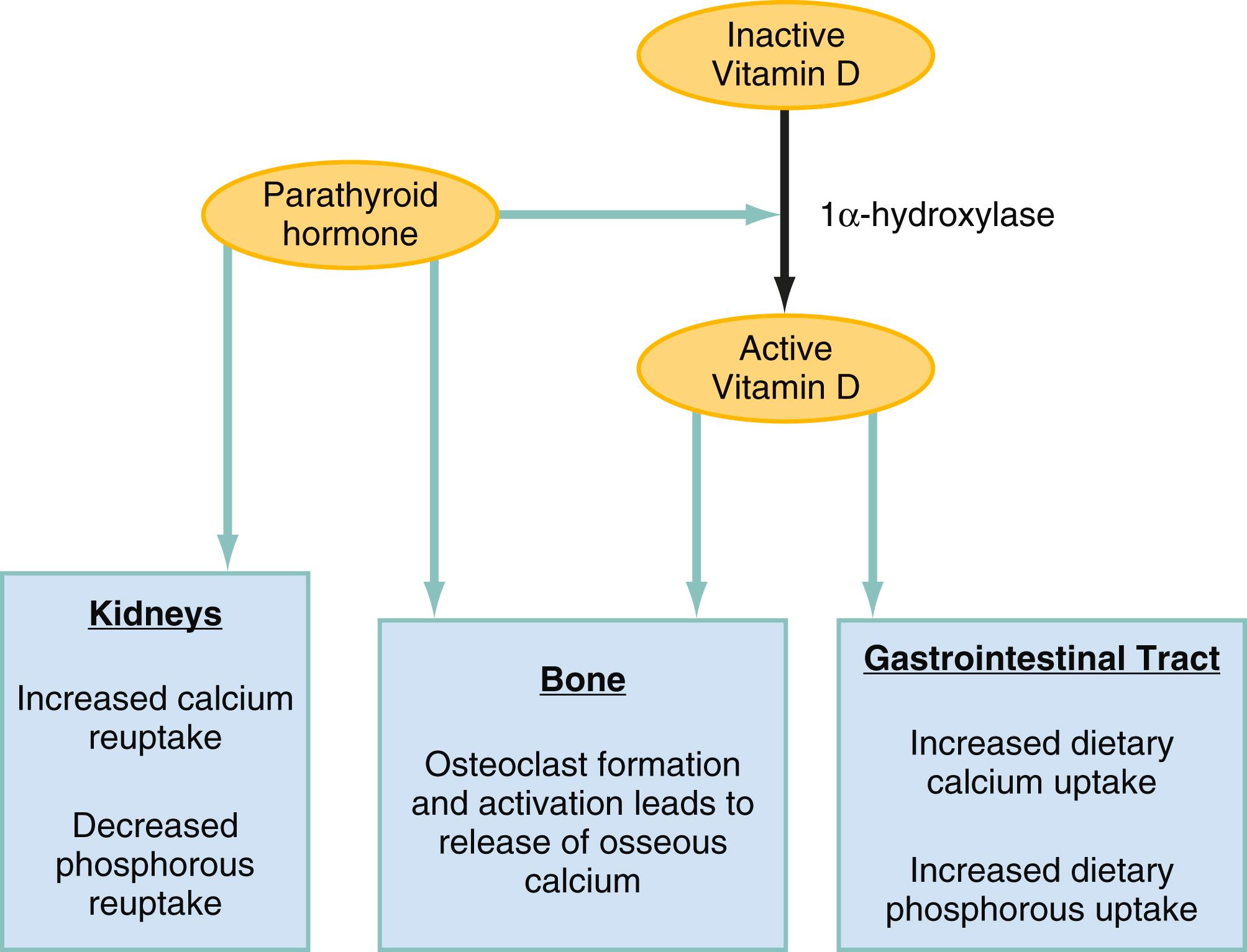
Synthesis of PTHrP, classically called humoral hypercalcemia , causes about 80% of cases of malignancy-associated hypercalcemia, and is usually associated with squamous cancers, such as head and neck, lung, esophageal, cervical, ovarian, and endometrial carcinomas. Calcitriol overproduction is usually seen in Hodgkin and non-Hodgkin lymphomas, in which secreted cytokines inappropriately activate the vitamin D–activating enzyme 1α-hydroxylase in macrophages. Bony metastasis can cause local cytokine-induced osteolysis and, if extensive, can lead to hypercalcemia. Ectopic PTH production is a rare feature of malignancies, mainly limited to case reports. Primary hyperparathyroidism occurring coincidentally with cancer is much more common.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here