Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter is dedicated to Professor Bruno Taccardi—a scientist, a gentleman, and a dear friend. He will be greatly missed. I am indebted to my outstanding graduate students, research and clinical fellows, and many collaborators who helped make ECGI a reality of great research and clinical promise. Studies presented in this chapter were supported by grants R01-HL33343 and R01-HL49054 from the National Institutes of Health–National Heart, Lung, and Blood Institute.
Electrocardiographic imaging (ECGI; also called electrocardiographic mapping) is a noninvasive method for electroanatomic mapping that provides continuous panoramic maps of activation and repolarization on the epicardial surface of the heart. , With ECGI, recordings can be obtained under real-world conditions without the need for sedation, surgery, or other invasive procedures. Over time, this allows for the evaluation of rapidly changing arrhythmias, potentially allowing for the evaluation of polymorphic ventricular tachycardia (VT)/ventricular fibrillation (VF) and atrial fibrillation (AF). –
The ECGI methodology has been detailed elsewhere. , In brief, body surface electrocardiographic (ECG) potentials are acquired at 1-ms intervals using 250 electrodes mounted in a vest or in strips. The electrode positions and the patient-specific epicardial geometry are simultaneously imaged using an ECG-gated computed tomography (CT) or magnetic resonance imaging (MRI) scan. ECGI algorithms combine the body surface ECG data and CT anatomic data to noninvasively construct potential maps, electrograms (EGMs), activation sequences (isochrone maps), and repolarization patterns on the epicardial surface of the heart.
To image the electrophysiologic (EP) substrate associated with scar, EGM magnitude maps, EGM deflection maps, and EP scar maps (ESMs) are constructed during sinus rhythm (SR). Low-voltage regions and very-low-voltage regions associated with scar are defined by EGMs with amplitude less than 30% and 15% of the maximum in a given patient’s heart, respectively. The so-called electrical scar (abnormal EP substrate associated with a scar) is defined as a region with EGMs characterized by low-voltage and multiple deflections (fractionation); it is presented visually as an ESM. For studies of repolarization, local recovery time (RT) and activation-recovery interval (ARI; a surrogate for local action potential duration) are determined from each EGM, and epicardial maps of RT and ARI are constructed.
ECGI was validated extensively for reconstruction of EGMs, activation sequences, and repolarization. Validation studies were conducted in animal experiments, in a human-shaped torso-tank containing a beating dog heart placed in the correct anatomic position for humans, and in patients undergoing open-heart surgery and invasive catheter mapping. A summary of the validation studies is provided in the appendix.
The substrate of post–myocardial infarction scars , is characterized by altered electrical properties because of cellular EP remodeling and structural remodeling that involves gap junction changes and regional fibrosis (see Chapters 46 and 85 Chapter 46Chapter 85 ). As a consequence, conduction of excitation through a heterogeneous scar is slow and discontinuous, a property that is reflected in low voltages and fractionation of local EGMs and delayed local activation (i.e., late potentials). – These properties have provided the basis for substrate-based ablation strategies in the treatment of VT. ECGI can reconstruct the EGM characteristics associated with a scar, as demonstrated in canine experiments. ,
Fig. 68.1 presents a representative example of an ECGI-reconstructed EP scar substrate in a patient with an anterior myocardial infarction (MI) scar (data from 24 patients). The electrical scar reconstructed by ECGI is compared with the anatomic scar imaged with delayed enhanced MRI. – Fig. 68.1A depicts the electrical scar in red . The top image is based on the low-voltage criterion alone; the bottom image combines the low-voltage and EGM fractionation criteria to define the electrical scar. Note that incorporation of the EGM fractionation criterion eliminates the basal part of the scar, which is a low-voltage region in the ECGI potential map and a bright region (suggesting an anatomic scar) in the MRI. These characteristics of the ECGI and MRI likely reflect fat tissue in this region rather than a true scar; the fractionation criterion can remove this artifact. Fig. 68.1B–C shows representative EGMs from the electrical scar region (red) and from regions outside the scar (blue). The scar EGMs are of low amplitude and long duration, and they exhibit multiple deflections (i.e., fractionation). Fig. 68.1D compares the ECGI-reconstructed electrical scar (red) to the anatomic scar imaged with delayed enhanced MRI.
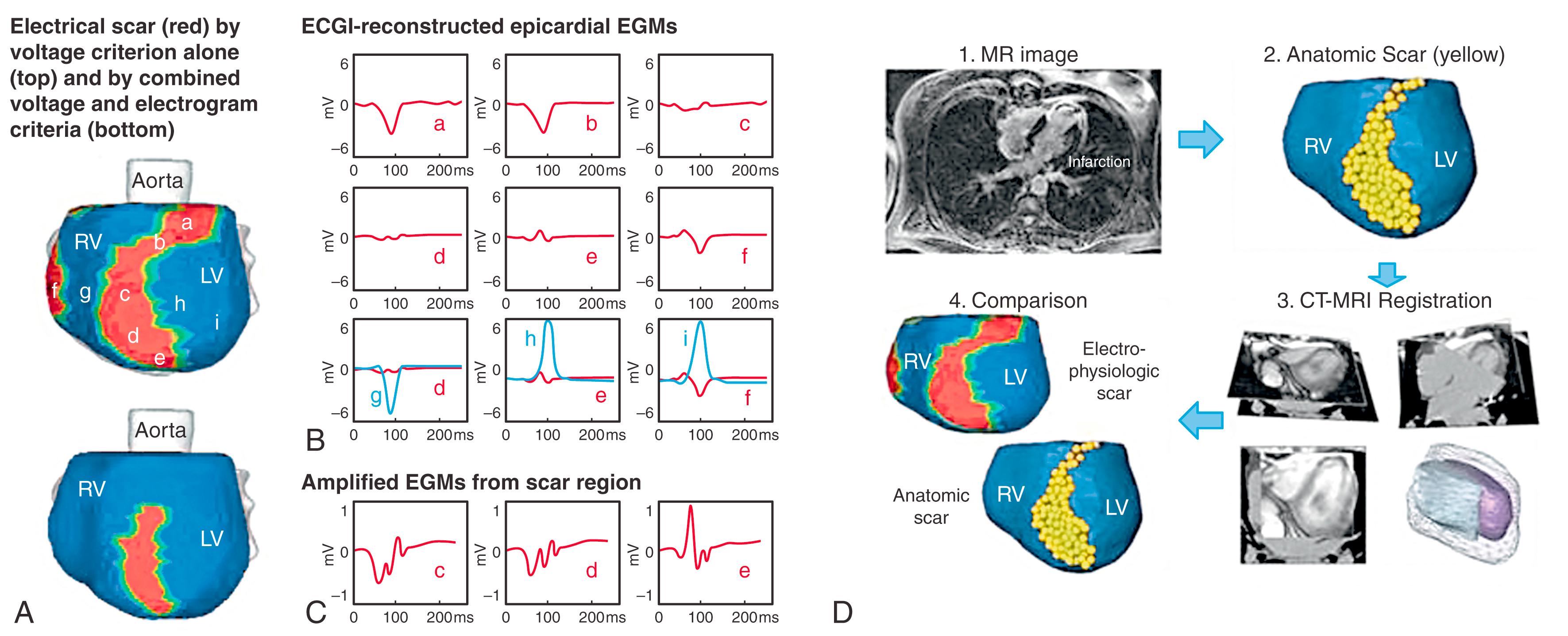
Fig. 68.2 provides a second example of an ECGI scar reconstruction. The ESM is apical, consistent with the MRI anatomic scar. The presence of the electrical scar influences the pattern of epicardial activation (AI map). The earliest epicardial breakthrough location (asterisk) is normal, but the activation wavefront encounters a line of block along the inferior and apical aspect of the scar. Consequently, left ventricular (LV) activation is from base to apex (arrows), with the region near the apical scar activating last. ECGI also reconstructed late potentials that in almost all cases (94%) were within the electrical scar.
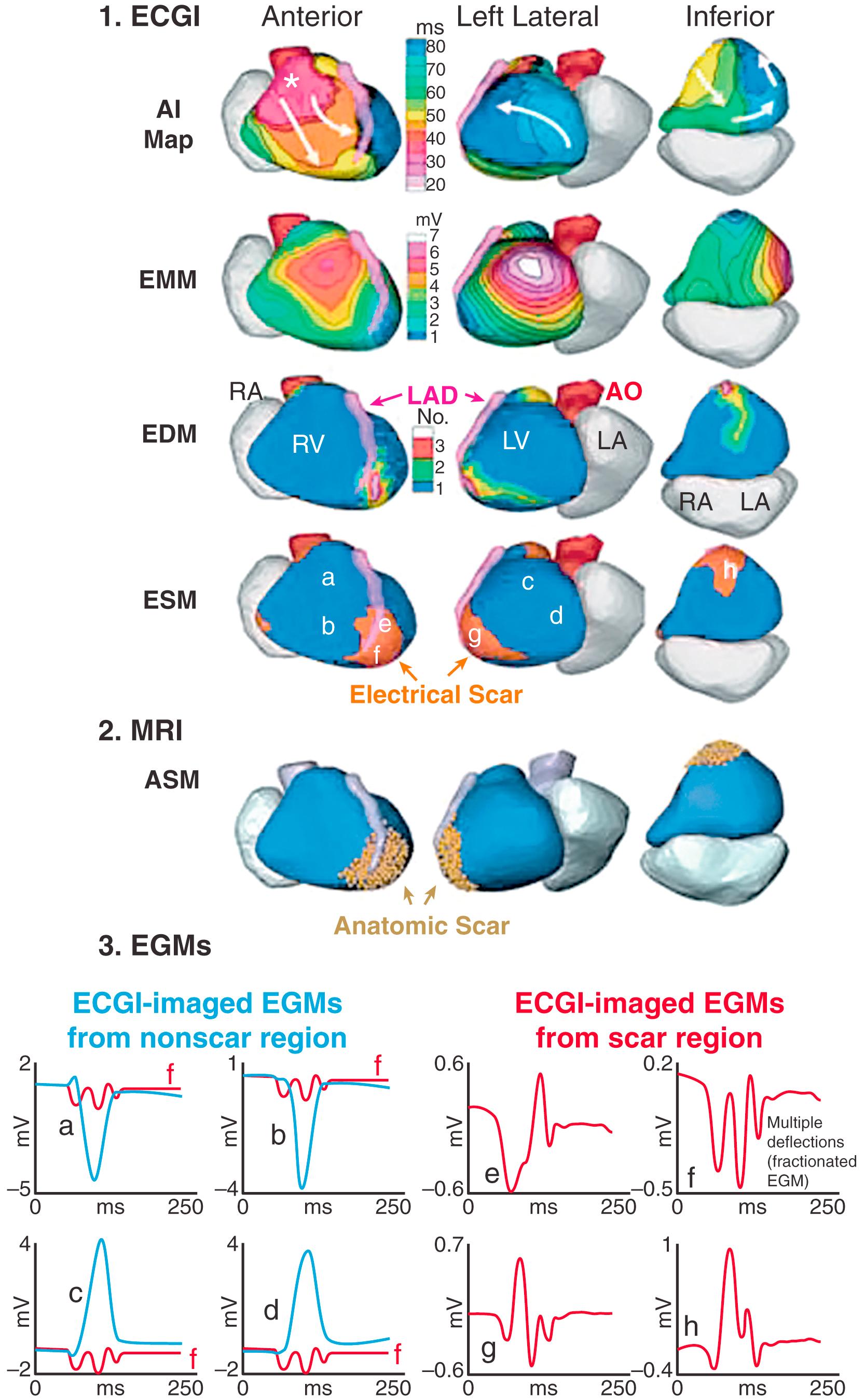
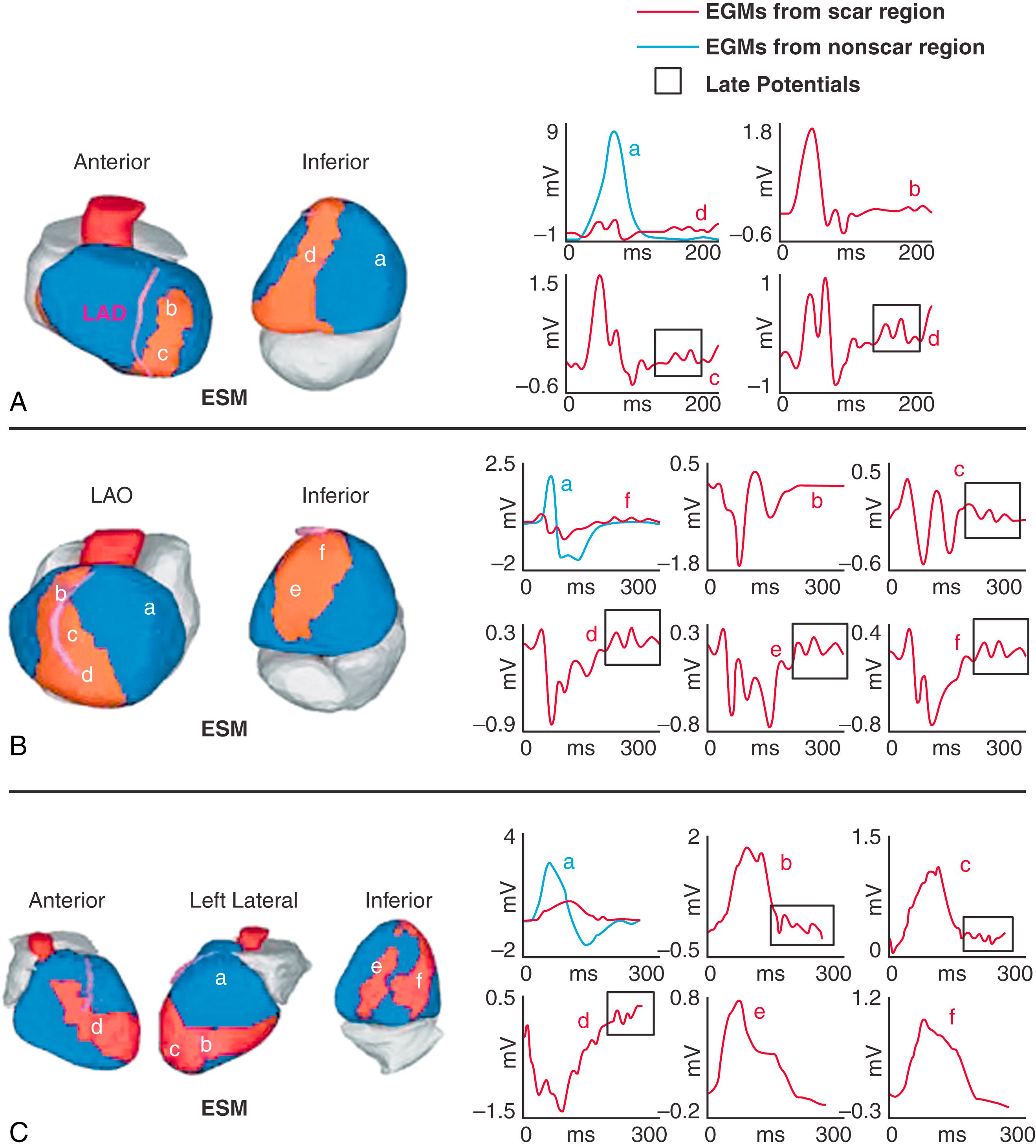
Fig. 68.3 shows three examples of scar EGMs with late potentials, reflecting delayed activation in the scar region during SR.
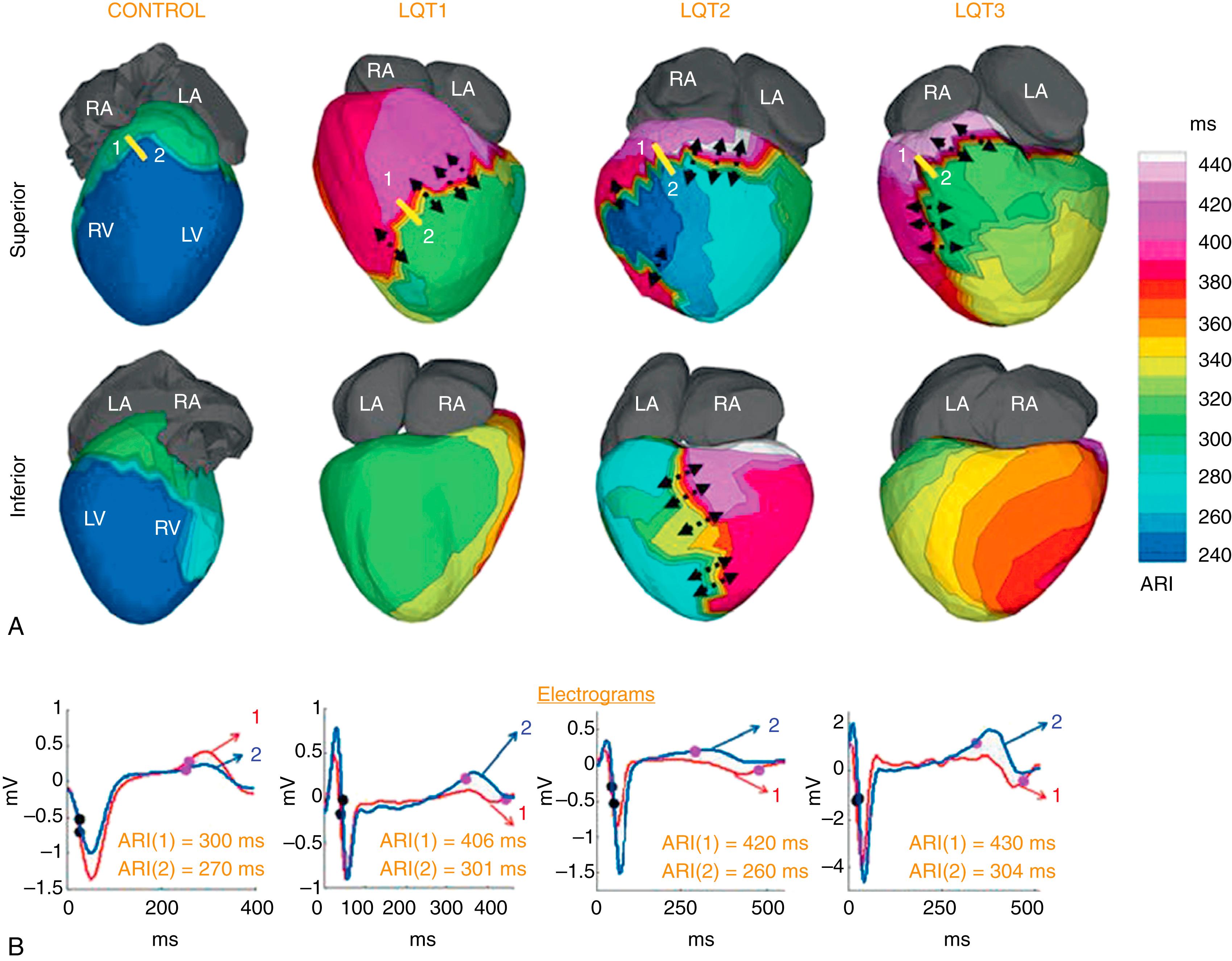
Congenital long QT syndrome (LQTS; see Chapter 96 ) is an inherited cardiac channelopathy that causes syncope and sudden death in young adults with structurally normal hearts. , Arrhythmias in these patients are classified as polymorphic VT (torsades de pointes). Genetic and molecular studies in humans have provided insight into the molecular basis of the disease. , Nevertheless, the arrhythmogenic substrate in the heart of LQTS patients has not been fully defined. ECGI was used to map the EP substrate in 25 patients with genotype-positive, phenotype-positive LQTS. Based on identified mutations, there were nine LQT1 patients (loss-of-function mutations in KCNQ1), nine LQT2 (loss-of-function mutations in KCNH2), five LQT3 (gain-of-function mutations in SCN5A), and two LQT5 (loss-of-function mutations in KCNE1). Epicardial activation during SR (not shown here; see Vijayakumar et al. ) was normal in all LQTS patients, with normal right ventricular (RV) breakthrough, uniform spread of the excitation wavefront, and latest activation in the LV base. The total ventricular activation time was around 50 ms, comparable to that in the normal control group (47 ± 9 ms). In contrast to activation, repolarization in LQTS patients of all types was very abnormal, with significant prolongation of the action potential on the ventricular epicardium compared with normal control. This prolongation was spatially heterogeneous, introducing regions with steep dispersion of repolarization. Fig. 68.4 shows representative ARI maps for control, LQT1, LQT2, and LQT3 in superior and inferior views. The ARI values were corrected for heart rate. For example, the maximum ARI in LQT3 was 430 ms compared with only 320 ms in control. Importantly, the regional, heterogeneous prolongation of ARI introduced steep gradients of repolarization in LQTS (shown by black arrows in Fig. 68.4 ). The mean ARI gradient was 92 ms.cm (± 18 ms/cm) in LQT1, 117 ms/cm (± 29 ms/cm) in LQT2, and 129 ms/cm (± 4 ms/cm) in LQT3, compared with only 2.0 ms/cm (± 2.0 ms/cm) in control ( P < .05). The presence of regions with steep dispersion of repolarization creates a substrate for asymmetrical conduction (unidirectional block) and reentrant arrhythmias, not detectable by the surface ECG. Interestingly, the location and magnitude of the steep gradient varied between patients, even among patients with the same genetic mutation. Nevertheless, repolarization gradients were steeper in symptomatic patients (12 patients, gradient = 130 ± 27 ms/cm) than in asymptomatic patients (13 patients, gradient = 98 ± 19 ms/cm), suggesting a possible role for ECGI in arrhythmic risk stratification in LQTS.
Brugada syndrome (BrS; see Chapter 95 ) is a highly arrhythmogenic inherited cardiac disorder associated with an increased incidence of sudden death; it predominantly affects men in their 40s. , , It presents on the ECG as an atypical right bundle branch block (RBBB) pattern and ST segment elevation (STE) in leads V 1 through V 3 . BrS is considered a primary electrical cardiac disease because no structural abnormalities are detected by conventional imaging. About 30% of BrS patients test positive for mutations in SCN5A and loss of sodium channel function. As with LQTS, the arrhythmogenic substrate of BrS in the intact human heart has not been well defined, with two leading hypotheses about mechanisms that underlie the phenotype: (1) the abnormal repolarization hypothesis (based on a canine wedge preparation) and (2) the abnormal conduction hypothesis. – Understanding the cardiac EP substrate that determines the phenotype requires high-resolution panoramic EP mapping of the ventricles, which cannot be achieved with invasive catheter mapping. To this end, ECGI was applied in 25 BrS patients to reconstruct epicardial EGMs and to map ventricular activation and repolarization during SR.
In BrS patients, the exclusive localization of the abnormal substrate to the RV outflow tract (RVOT) was a striking difference from LQTS patients, where location of the abnormal substrate (steep repolarization gradients) was highly variable. EGMs in the RVOT (but nowhere else) were characterized by ST segment elevation and inverted T wave morphology and by low amplitude and presence of multiple deflections (fractionation); examples can be found in Zhang et al. Fig. 68.5A shows activation isochrone maps from three BrS patients, with a normal control map provided for reference (left panel). The BrS patients had normal RV epicardial breakthrough (asterisk) on the RV free wall (indicative of a normally functioning conduction system) and normal activation of most of the RV but delayed activation of the RVOT (blue), which was last to activate (in normal hearts, latest activation is in the lateral LV base). The mean time needed for activation of the RVOT among all patients was 36 ms (± 16 ms), whereas the RV free wall took 16 ms (± 3 ms) to activate, and the entire RV (including the RVOT) took 40 ms (± 14 ms), indicating that most of the RV activation duration was taken by slow spread of excitation in the RVOT.
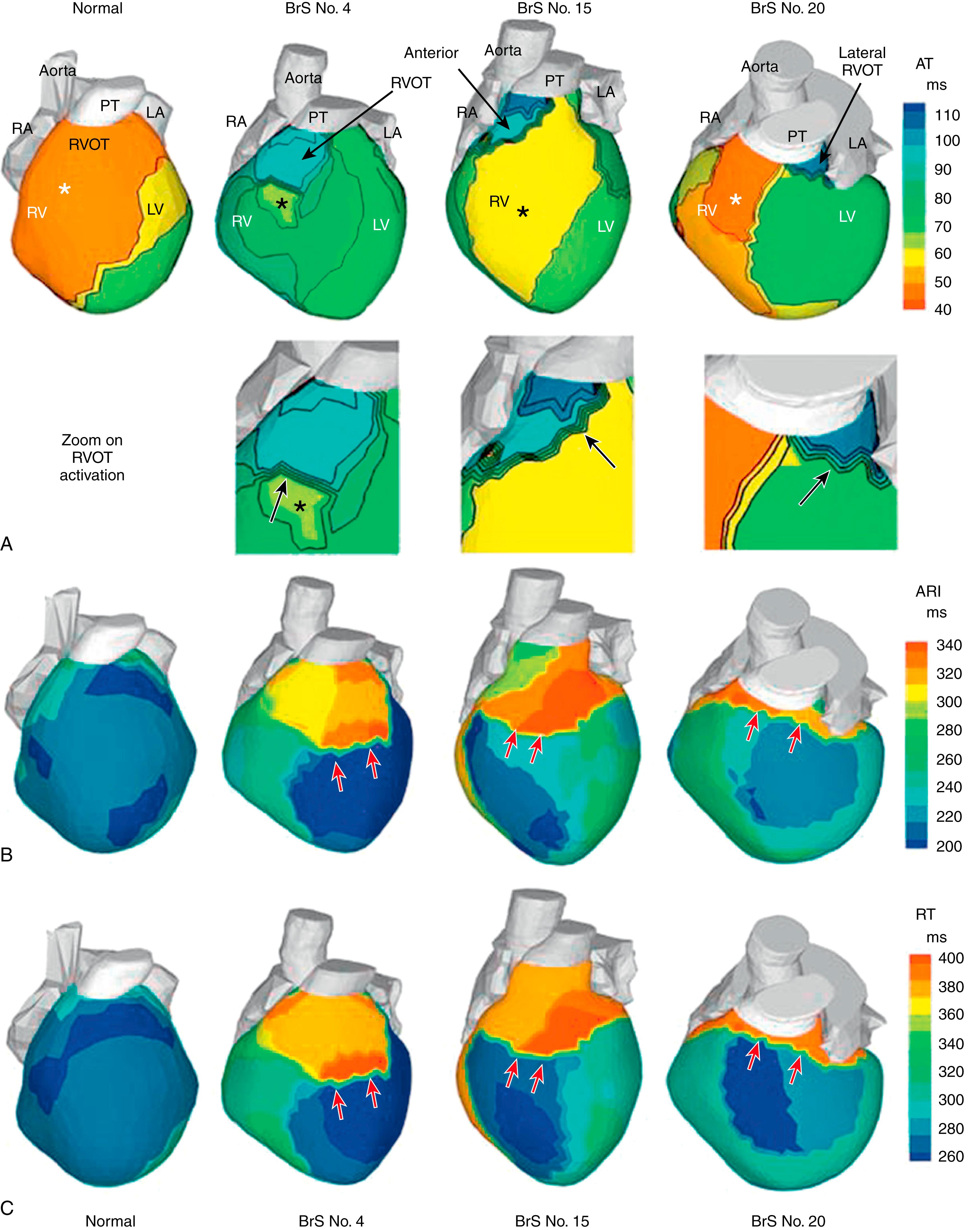
Fig. 68.5B–C shows representative maps of repolarization from the same three patients, together with normal repolarization maps for reference (left column). Fig. 68.5B displays ARI maps that reflect local repolarization (local action potential duration), independent of the activation sequence; Fig. 68.5C displays RT maps that are determined by both local repolarization and the activation sequence. That the patterns of the ARI maps and RT maps are similar indicates that local repolarization is the major determinant of the repolarization pattern. ARI prolongation (318 ± 32 vs. 241 ± 27 ms in control) and RT prolongation (381 ± 30 vs. 311 ± 34 ms in control) were observed primarily in the RVOT but extended somewhat into adjacent regions. This localized prolongation caused steep gradients of ARI and RT at the RVOT-RV free wall or RVOT-LV free wall borders (see red arrows in Fig. 68.5B–C ).
The results shown in Fig. 68.5 demonstrate the presence of both abnormal repolarization and abnormal conduction in the RVOT of BrS patients. Abnormal repolarization during phase 1 of the action potential can generate potential gradients that give rise to the STE in the EGMs and ECG. Slow discontinuous conduction in the RVOT can give rise to the fractionation and low voltage of RVOT EGMs. Increasing the heart rate should have an effect on both. It diminishes the phase 1 notch of the action potential (by not providing sufficient time for cardiac transient outward potassium current [I to ] recovery from inactivation between beats) and thereby reduces potential gradients and STE. It also compromises conduction further by reducing sodium current (I Na ) availability. To examine these hypotheses, we performed ECGI in six BrS patients during increased heart rate through exercise or isoprenaline administration (mean increase from 71 to 133 beats/min). As expected, STE was reduced and fractionation was increased with an increase in heart rate (see Zhang et al. ), providing additional supporting evidence for the coexistence of conduction and repolarization abnormalities in the RVOT substrate of BrS patients.
As previously stated, the ECG in BrS is of atypical RBBB pattern. It can be challenging to differentiate BrS from non-BrS right bundle branch (RBB). Nevertheless, accurate diagnosis is of utmost clinical importance because BrS and RBBB differ greatly in their arrhythmogenicity, with BrS being highly arrhythmogenic. We examined whether ECGI can distinguish between these abnormalities by mapping six non-BrS patients with RBBB, without structural heart disease, during SR. The results (see Zhang et al. ) established the ability of ECGI to do so, based on the following: (1) EGMs with the BrS morphology and with low voltage and fractionation were not found in RBBB; (2) normal RV epicardial breakthrough was observed in BrS, reflecting a functioning RBB, but not in RBBB where RBB is defective; (3) delayed activation in BrS was confined to the RVOT, whereas, in RBBB, the delay occurred across the septum; and (4) repolarization abnormalities were not present in RBBB. We concluded that noninvasive substrate mapping with ECGI can assist with a BrS-RBBB differential diagnosis in the clinical setting and with the clinical diagnosis of BrS when it is masked by an RBBB pattern on the body surface ECG.
ECGI was performed in 25 patients with symptomatic VT or premature ventricular contractions who also underwent invasive, catheter-based EP study (EPS). , In patients with spontaneous abnormal ventricular rhythms (18 patients), ECGI was performed before EPS. In other patients, ECGI was performed during EPS, where the rhythm was induced.
Based on the invasive EPS, VT in 18 patients was judged to be focal. In all 18 patients, ECGI isochrone maps showed a radial spread of excitation from the site of origin. An example from a patient with nonischemic cardiomyopathy is provided in Fig. 68.6 . ECGI was applied in the EP laboratory during induction of VT by programmed electrical stimulation. Fig. 68.6A shows isochrones during baseline pacing (cycle length = 600 ms) from a catheter in the RV apex (plus sign). There is a line of block (thick black line) in the lateral LV that is circumvented by the excitation wavefront. Premature pacing (S1–S2 interval = 280 ms) functionally extends the line of block (see Fig. 68.6B ), forcing the wavefront to make a longer arc around this line and activate a more inferior region of the LV last (minus sign). The first VT beat is shown in Fig. 68.6C ; it originates from the location (asterisk) of latest activation by the S2 premature paced beat in Fig. 68.6B , suggesting that triggered activity is the mechanism of initiation of this focal VT. Note that the local EGM at the site of VT initiation has a pure Q wave morphology, indicating an epicardial origin. shows the dynamic progression in this case.
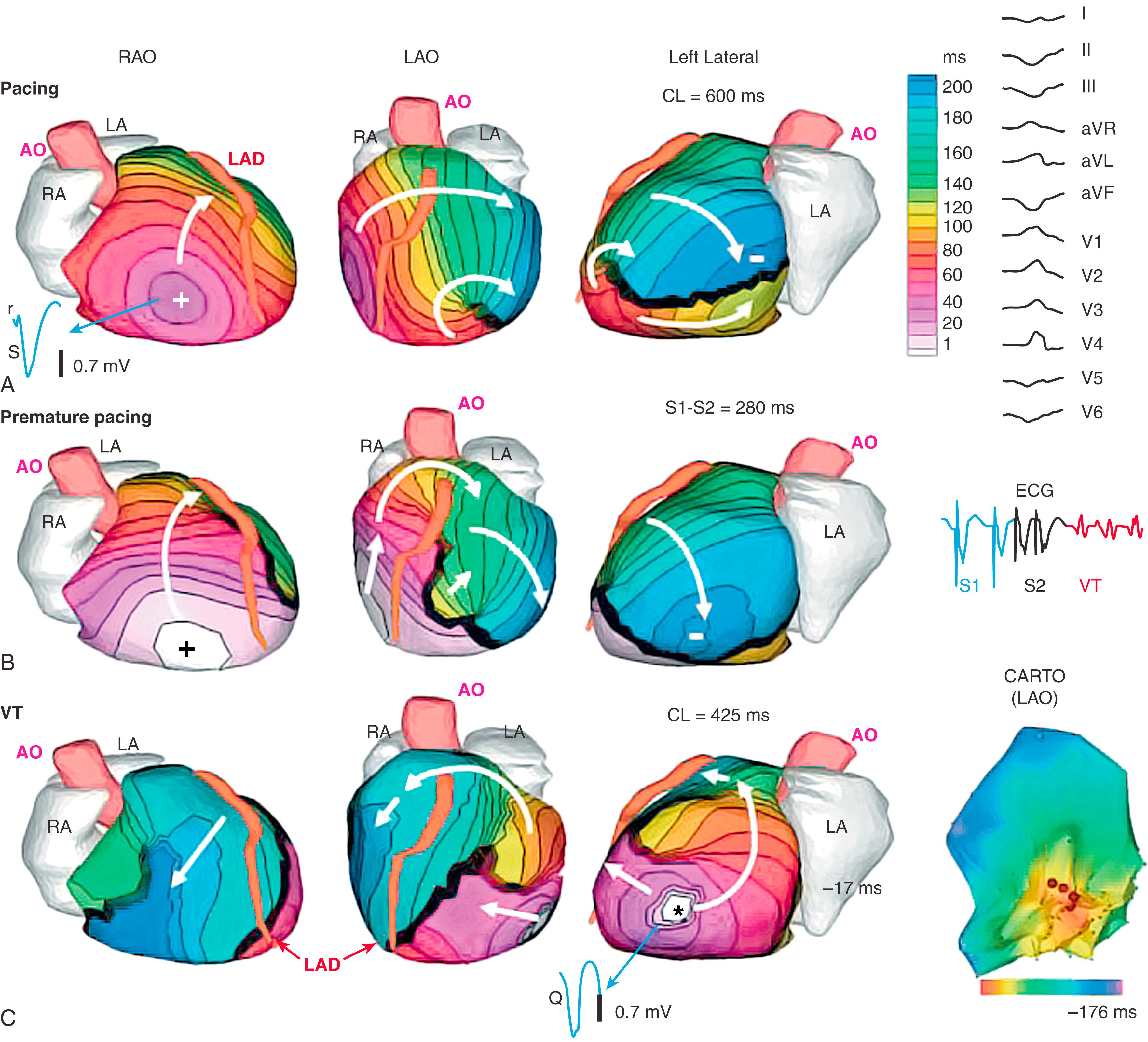
Five patients had reentrant VT as determined during invasive EPS. Noninvasive ECGI maps showed high-curvature rotational wavefronts, with the excitation wave returning to its site of initiation. This pattern was consistent in all five patients and related to a ventricular scar in all cases. An example is provided in Fig. 68.7 and . This patient had an extensive inferoseptal scar from a prior inferior wall MI (see single-photon emission computed tomography image in Fig. 68.7C ). He had a slow, hemodynamically tolerated VT, occasionally interrupted by sinus capture (SC) beats (see ECG lead V 2 in inset to Fig. 68.7B ; red is a VT beat and blue is an SC beat). Earliest activation of the VT beat (see Fig. 68.7B ) is near the scar border in the inferior basal septum (red in the left anterior oblique and left lateral views). The main reentrant wavefront propagates clockwise with a high curvature (white arrows) and completes the beat by reentering the area of earliest activation. Immediately before the VT beat, presystolic activation is detected near the inferior scar border where the VT beat begins (the “exit site”; see ). A second wavefront (pink arrows) propagates toward the base, where it turns slowly counterclockwise into the RV. It then propagates to the inferior base, back toward the scar. In the inferobasal septal region, ECGI-reconstructed EGMs were of low amplitude and highly fractionated, which is consistent with a scar substrate. Invasive catheter mapping confirmed this ECGI finding. EPS during the VT confirmed its inferior septal origin and, using entrainment criteria, its reentrant mechanism. Ablation in this region terminated the VT.
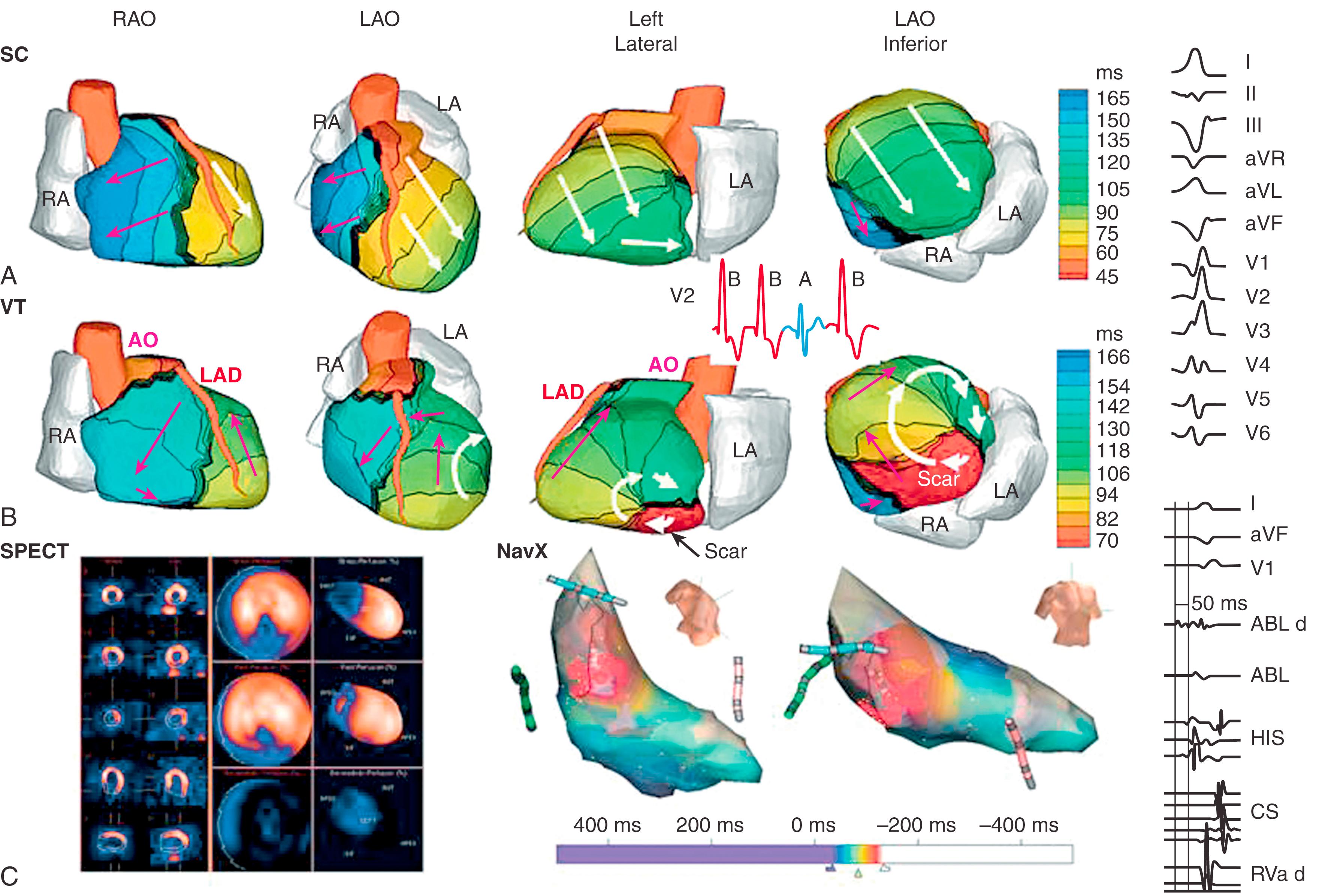
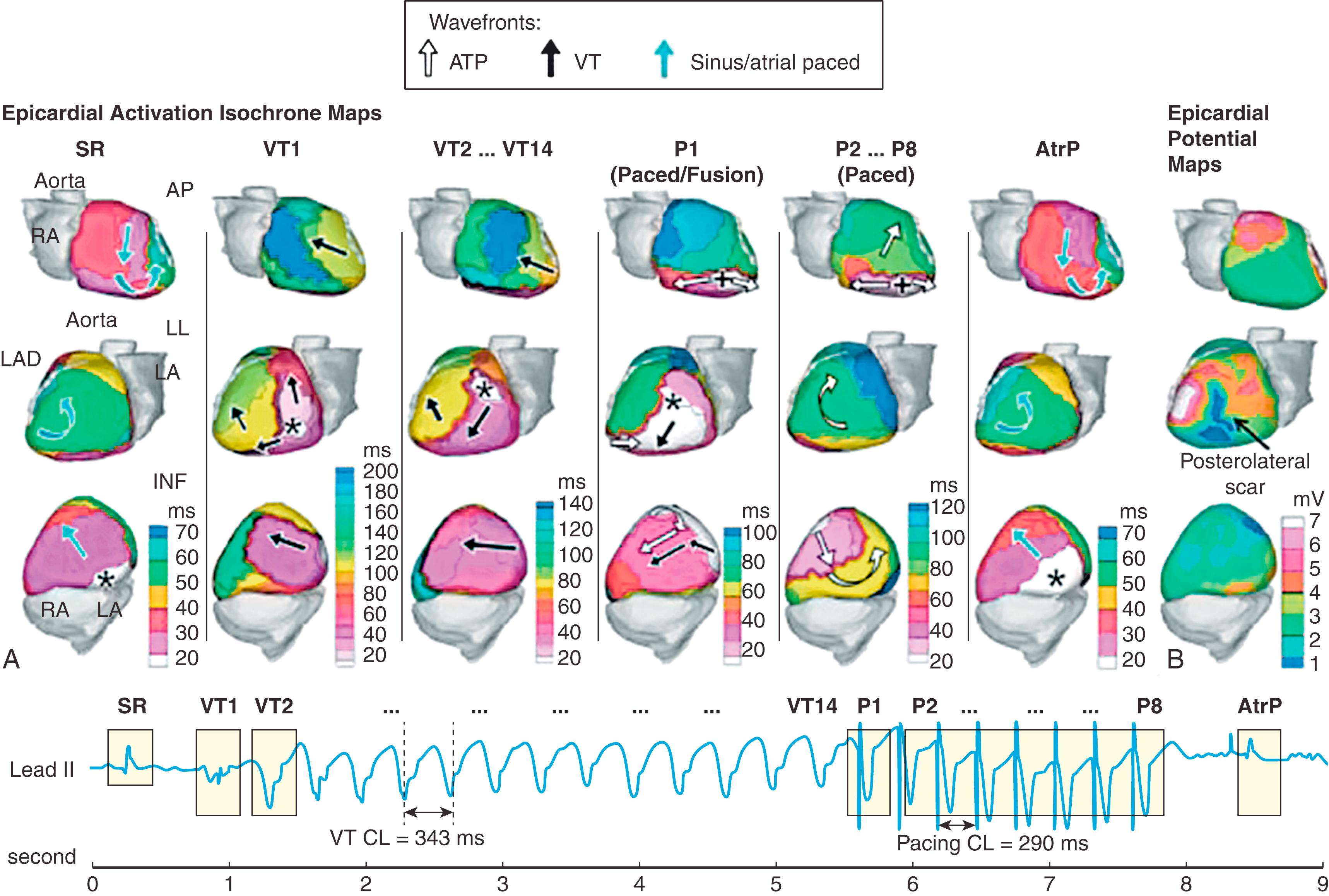
Fig. 68.8A shows ECGI activation maps during initiation, continuation, and termination by antitachycardia pacing (ATP) of spontaneous VT in a 40-year-old patient with nonischemic cardiomyopathy (LV ejection fraction of 35%). The patient experienced 248 VT episodes terminated by ATP over 14 days. shows a continuous ECGI reconstruction of one such event, as summarized in Fig. 68.8 . The left column of Fig. 68.8A shows the activation pattern during SR, before spontaneous initiation of VT. The next beat (VT1) originated from the inferolateral base (asterisk) and triggered the VT. The next VT beat (VT2) and all the VT beats that followed (VT2 to VT14) were monomorphic and started from a different location (superolateral LV base, asterisk) versus the triggering beat (VT1). VT cycle length was 343 ms. ATP pacing started after 14 beats of VT, at 85% of the VT cycle length. The first ATP beat (P1) consisted of fusion between the VT wavefront (starting from asterisk) and the wavefront generated by the RV pacing (originated at plus sign). The next ATP beats (P2–P8) successfully captured the myocardium, and the activation was because of RV pacing alone (wavefront originating and spreading from plus sign exclusively). The beat after the last ATP beat (atrial paced [AtrP]; right column of Fig. 68.8A ) was identical to the SR beat before VT onset. provides dynamic images of the entire event. Fig. 68.8B shows ECGI epicardial potential maps with low voltages in the posterolateral region (dark blue), indicating the presence of scar where VT originated.
The accuracy of ECGI depends strongly on the exact methodology used and details of its execution. Therefore the results of the many studies summarized here apply to the method and details of its application in this lab; they cannot be extrapolated and generalized to other laboratories. Moreover, even for the same general approach (e.g., the Boundary Element Method with Tikhonov Zero Order regularization), results depend on details such as measurement noise, signal conditioning (removal of baseline drift; filtering), values of regularization parameter, accuracy and resolution of segmentation and meshing of the heart and torso surfaces, and more. Consequently, one can evaluate accuracy of the ECGI procedure within the same lab, but comparison between labs and generalization of results is limited. This appendix summarizes many validation studies of ECGI as implemented and practiced in this lab over many years.
The torso-tank setup consists of an accurate human-shaped torso, with a beating dog heart placed in the correct anatomic position for a human. It allows for precisely controlled experiments. In these experiments, body surface potentials and epicardial potentials over the entire heart are simultaneously recorded. The body surface potentials provide the input for the ECGI reconstruction, and the recorded epicardial potentials provide a gold standard for evaluation. It accurately represents the geometric relationship between the heart and torso surfaces in the human anatomy (the proportion of size between the dog heart and the tank that was molded on the torso of a 10-year-old boy are representative of an adult human). This relationship determines the transfer matrix that is used in the ECGI computations. Recordings were conducted with 256 or 384 body surface electrodes, 490 or 64 electrodes in a nylon sock placed over the heart, and 184 electrodes on rod tips pushed against the heart. , Advantages of this experimental setup include the following: (1) there is consistency of the torso volume conductor between measurements and computations (homogeneous in both, so the evaluation of the ECGI computation method is not affected by a volume-conductor inconsistency); (2) epicardial electrodes cover the entire heart; and (3) the heart can be manipulated during the experiment—for example, necrosis was produced so that its effect on ECGI reconstruction could be evaluated by comparing electrograms from the same locations before and after necrosis formation. Also, accessibility of the heart during the experiment provides tight control over electrode contact, placement, and so on.
An anterior pacing site was reconstructed 7 mm from the measured site and a posterolateral site within 4 mm of the measured site. Two other sites, between these two extreme ones, were reconstructed 0 mm and 10 mm from the measured pacing sites. Average distance between reconstructed and measured sites was 5 mm (range, 0–10 mm). The reconstructed pacing sites were determined from the reconstructed epicardial potential maps (not isochrones). In this method, the initiation site is determined at the center of a quasielliptical negative potential minimum on the epicardium during early activation (this is based on the properties of wavefront propagation in the anisotropic myocardium). Two simultaneous pacing sites 52 mm apart (anterior and posterolateral) were reconstructed 7 mm and 4 mm from the actual locations. Two anterior simultaneous sites were reconstructed at their exact positions. Two posterolateral sites only 17 mm apart were reconstructed with 5 mm and 4 mm errors of position.
Correlation coefficients (CCs) between reconstructed and measured EGMs were greater than 0.9 for 72% of all epicardial electrodes (54% with CC >0.95). Some outliers (only 5% of electrodes) had poor CC (<0.5).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here