Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Central nervous system disorders are a frequent cause of urologic symptoms and voiding dysfunction.
The bladder, bladder neck, external urethral sphincter, and urethra must have coordinated activity to safely store and evacuate urine.
Complications of neurologic disease or injury can affect the storage, emptying, or both phases of the micturition cycle.
Neurourologic pathology is dependent on the location of the lesion within the nervous system.
Consequences of high pressure within the urinary tract can result in urinary tract infection, stones, upper and lower urinary tract deterioration, and potential renal failure.
Treatment of neurourologic symptoms should focus on maintenance of low bladder storage pressure, prevention of incontinence, promotion of bladder emptying, and avoidance of infection.
Central nervous system disorders are a frequent cause of urologic symptoms and voiding dysfunction. Lower urinary tract symptoms and erectile dysfunction may lead to significant clinical, social, and economic costs for the patient, caregiver, and health care system. Appropriate recognition and timely management of urologic issues relevant to neurological conditions are important to avoid potentially irreversible adverse outcomes.
This chapter outlines a simplified approach to patients with urologic problems secondary to neurological conditions. A brief discussion of the relevant neuroanatomy and physiology of the urinary tract is provided, followed by a review of the neurourologic evaluation, clinical findings in patients with several common neurourologic disorders and complications, and management of related lower urinary tract symptoms.
The lower urinary tract has two basic physiologic functions: (1) low-pressure storage of adequate volumes of urine with appropriate sensation and (2) periodic, voluntary expulsion of urine from the bladder in a coordinated and complete fashion. To provide these functions, the bladder, bladder neck, external urethral sphincter, and urethra must have coordinated activity mediated by the autonomic and somatic nervous systems ( Fig. 17.1 ).
Disruption of neurotransmission at any level of the neuraxis may result in significant alterations in urinary function. The clinical manifestations of neurological conditions on the urinary tract depend on the specific location of the injury, the completeness of the injury, the age of the patient and resultant neuroplasticity, and the presence of preexisting urinary tract disease (e.g., benign prostatic hypertrophy) and other comorbidities.
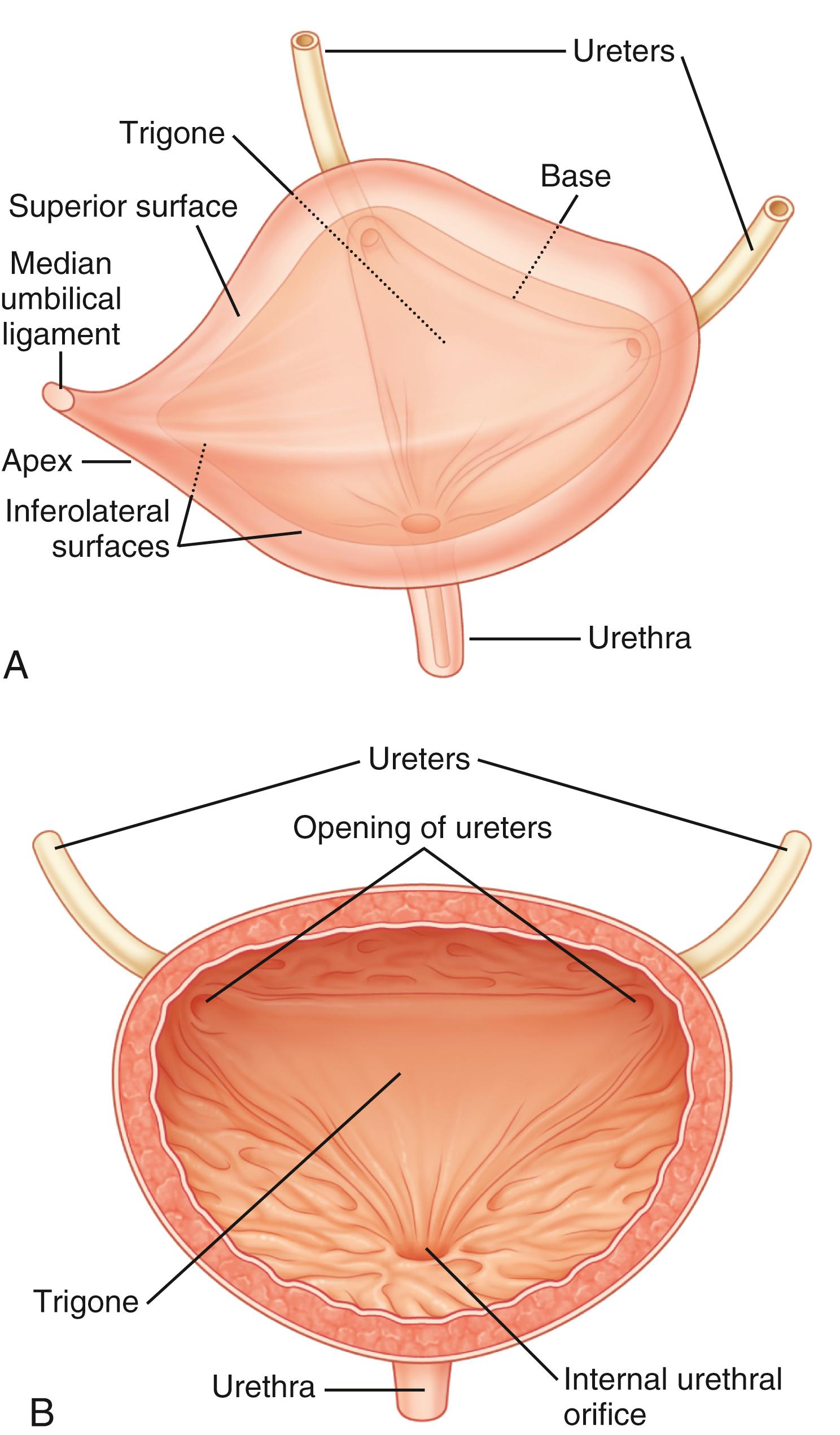
The urinary bladder is a hollow organ composed of a layer of transitional epithelium (urothelium), a layer of thick smooth muscle, and an outer adventitial layer of fat and connective tissue ( Fig. 17.2 ). The function of the bladder is related to the intrinsic properties of these tissues as well as nervous system control. Compliance, defined as the ratio of the change in intravesical pressure to bladder volume, is mostly dependent on the native viscoelastic properties of the bladder wall. The normal bladder is highly compliant and will thus maintain low intravesical pressure during filling. Bladder injury, fibrosis of the bladder wall, congenital or acquired neurological denervation, and bladder outlet obstruction can result in detrusor thickening and increased collagen content, conditions that significantly affect compliance. Low bladder compliance results in high urinary storage pressure, which if unrecognized and untreated leads to upper urinary tract deterioration and renal failure, an extremely important consideration in many, but not all types of neurological disease.
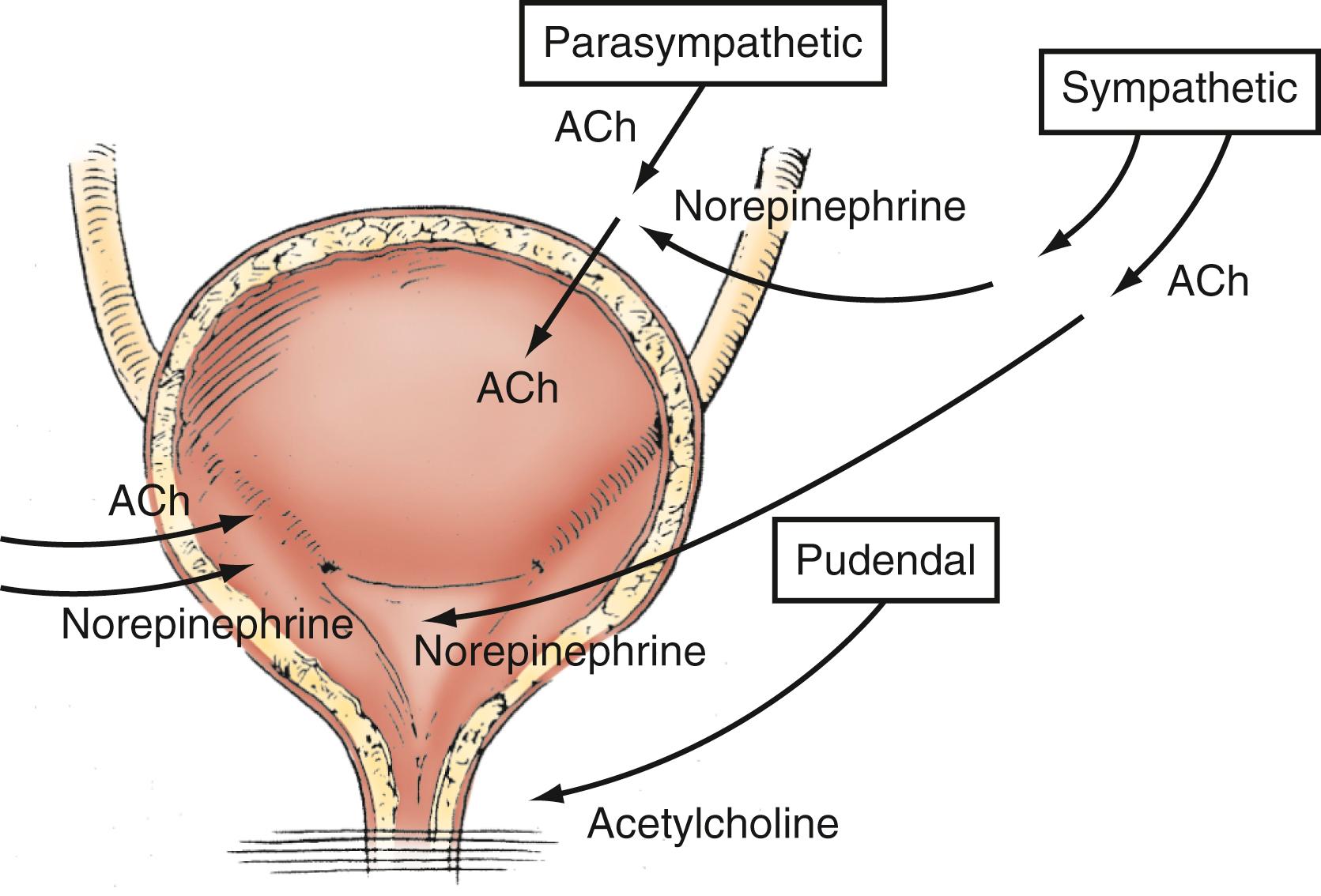
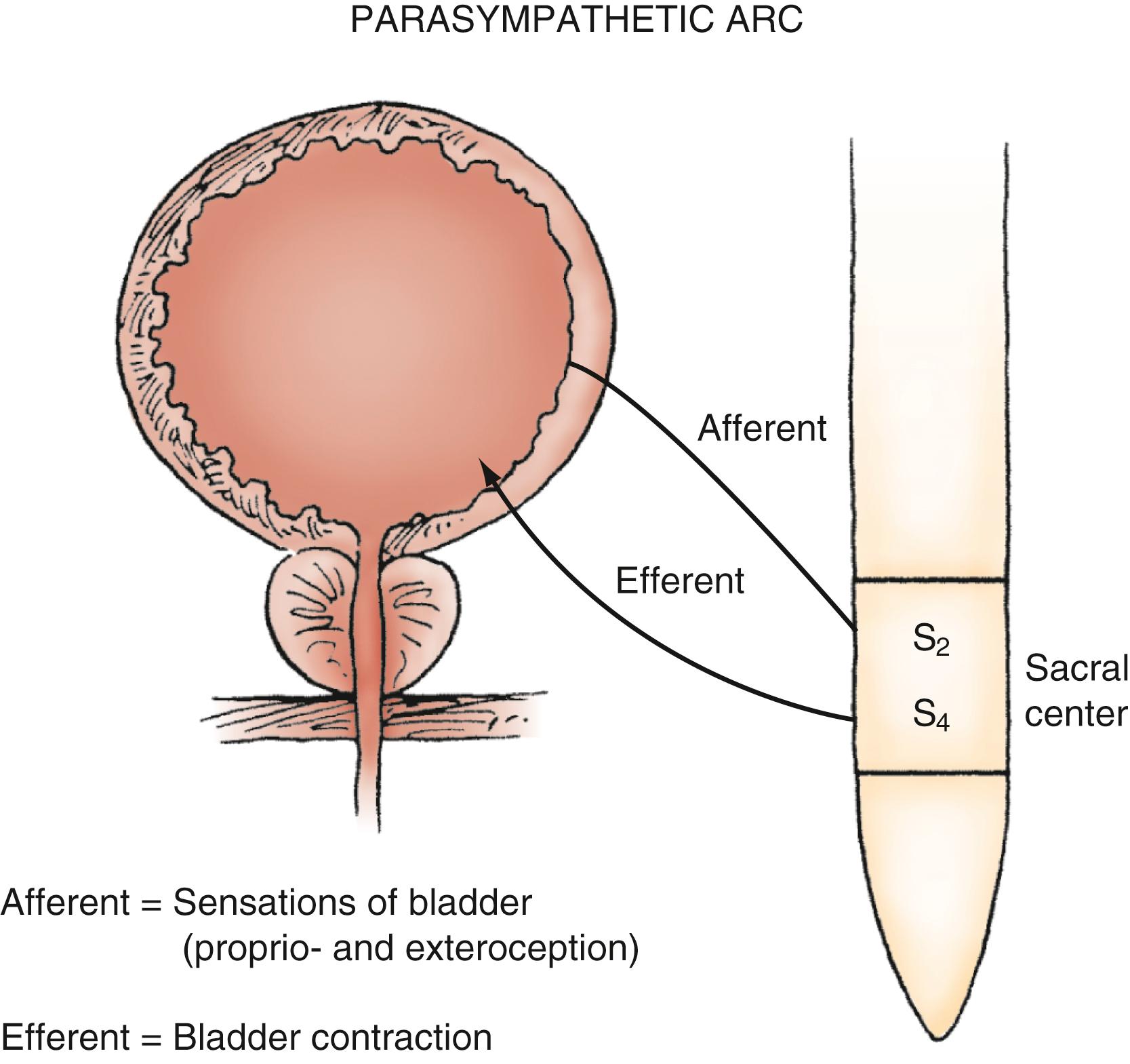
Parasympathetic innervation to the lower urinary tract originates in the S2–S4 segments of the spinal cord and provides excitatory motor input to the bladder. Cholinergic preganglionic neurons within the intermediolateral sacral cord send axons to ganglionic cells within the pelvic plexus and the bladder wall. Postganglionic neurons within the bladder wall and pelvic plexus release acetylcholine, which activates cholinergic receptors on the detrusor smooth muscle cells and initiates bladder contraction ( Fig. 17.3 ). Sympathetic pathways originate in the T11–L2 spinal segments, travel in the sympathetic chain ganglia to the prevertebral ganglia in the superior hypogastric and pelvic plexuses, and innervate the bladder via short adrenergic neurons. The bladder contains varying expression of α- and β-adrenergic receptors, and sympathetic stimulation promotes storage of urine via detrusor relaxation and contraction of the bladder neck and outlet ( Fig. 17.4 ) . Activation of β-adrenergic receptors within the wall of the bladder provides inhibition and relaxation of the detrusor muscle. Activation of cholinergic receptors stimulates the detrusor to contract, primarily through the M3 muscarinic receptor subtype. Additional neurotransmitters, including nitric oxide, adenosine triphosphate, and neuropeptides, also have a proposed modulatory role in bladder relaxation and contraction.
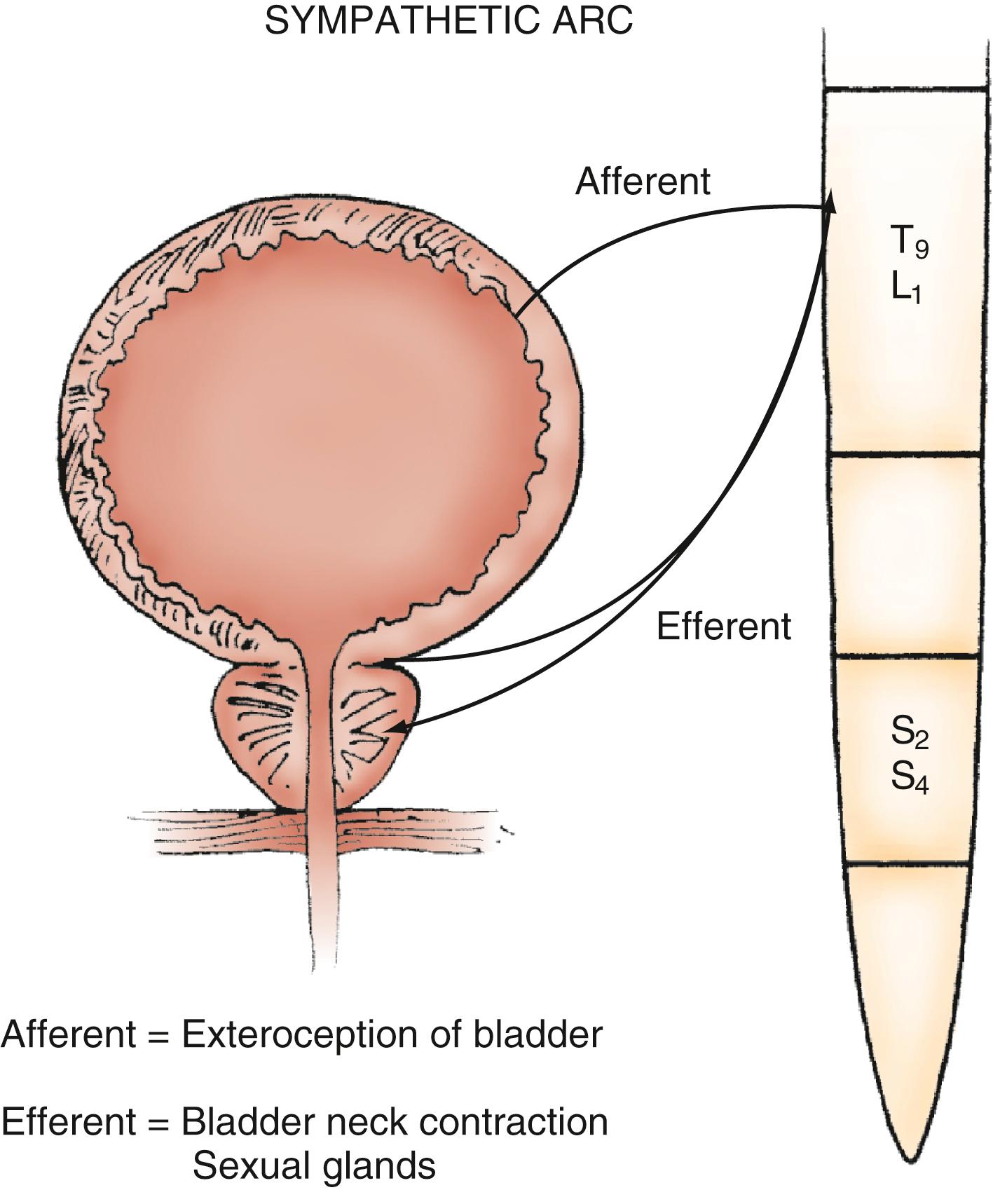
The bladder outlet is composed of the trigone, ureterovesical junction, bladder neck, and proximal urethra. α-Adrenergic receptors predominate in this area, and stimulation of postganglionic α-adrenergic receptors provides excitatory input to the trigone, bladder neck, and proximal urethra that results in increased bladder outlet closure force. The bladder neck region and adjacent proximal urethral segment are often referred to as the internal urethral sphincter or smooth muscle sphincter, and although it is not a true anatomic sphincter, it has a role in the maintenance of continence and efficient voiding.
Afferent (sensory) transmission of lower urinary tract stimuli travels via the pelvic, hypogastric, and pudendal nerves to the dorsal root ganglia of the lumbosacral spinal cord. The pelvic nerve afferents monitor the volume of the bladder and the amplitude of bladder contraction via myelinated (Aδ) and unmyelinated (C) fibers within the bladder wall. The unmyelinated fibers are also implicated in the transmission of urgency and pain. , Newer research indicates that cells within the urothelium may also exhibit sensory and signaling properties.
The urethra is a conduit for the release of urine from the bladder. The proximal 2 to 3 cm of the urethra in both males and females is primarily sphincteric. Smooth and striated muscle contribute to urethral closure forces. In males, the prostatic glandular and fibromuscular stroma tissue surrounding the proximal urethra can affect voiding through extrinsic compression and obstruction. The most proximal segment of the urethra in both sexes consists primarily of smooth muscle, which is not under voluntary control. Innervation of the proximal urethra is similar to that of the bladder neck. There is a high density of α-adrenergic receptors that, when stimulated, produce an increase in intraurethral pressure. Conversely, the use of α-adrenergic antagonists can reduce outlet resistance and facilitate release of urine. At the level of the pelvic floor in both sexes, there is a sheet of striated muscle extrinsic to the urethra as well as a discrete layer of striated muscle within the wall of the urethra. The extrinsic muscle consists of primarily fast-twitch muscle fibers and is under voluntary control. The intrinsic muscle tissue, also called the intrinsic sphincter or rhabdosphincter, consists primarily of slow-twitch fibers and provides passive urinary continence. Innervation of external sphincter and pelvic floor musculature is primarily somatic from branches of the pudendal nerve. The pudendal nerve pathway begins in the anterior horn cells of the S3 and S4 segments of the spinal cord. Afferent sensory information from the urethra and pelvic muscles also travels via the pudendal nerve ( Fig. 17.5 ). ,
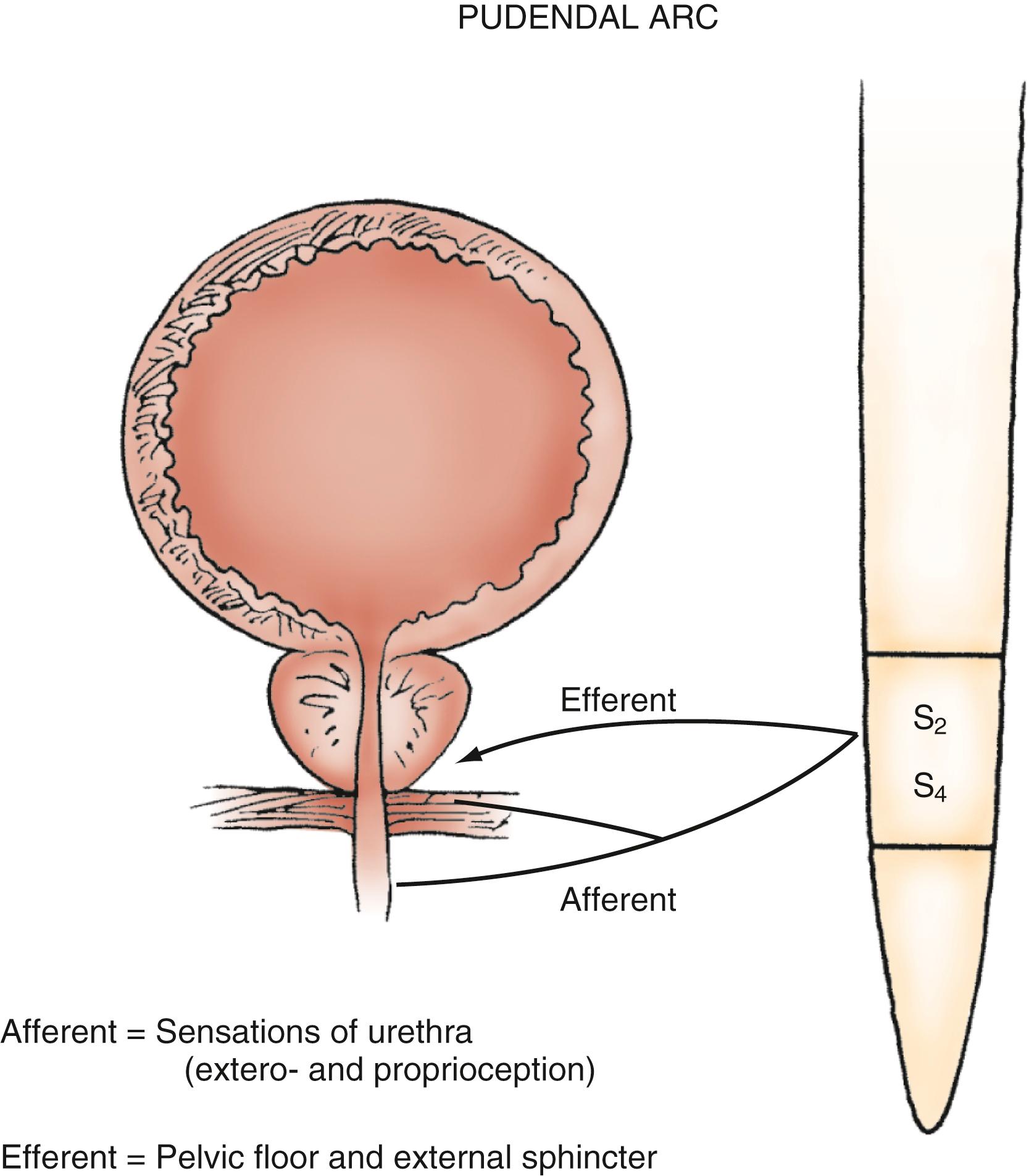
Micturition is organized as a series of “on-off” reflexes at various levels of the neuraxis. Voluntary bladder emptying is initiated at the level of the cerebral cortex. However, many of the events are reflexive and modulated by the autonomic nervous system. Voluntary voiding occurs as a result of sudden relaxation and opening of the bladder outlet and external sphincter coordinated with a detrusor contraction of adequate magnitude. The normal micturition event is coordinated at the level of the pons via ascending and descending spinal pathways to ensure opening of the bladder outlet just before the onset of voluntary detrusor contraction. In the absence of anatomic or physiologic obstruction, such as prostatic enlargement, normal bladder emptying occurs at low pressure and is complete. Disruption of the neurological pathways subserving micturition (e.g., from brain tumor, spinal cord injury [SCI], or spinal stenosis) may result in loss of coordination and control of the micturition reflex and a variety of abnormal voiding patterns. Under conditions of neurological disease or injury, detrusor or sphincter function, or both, may become overactive or underactive. Thus depending on the specific neurological lesion, voiding reflexes may be facilitated (resulting in urgency and frequency) or suppressed (resulting in urinary retention).
Storage and evacuation of urine should occur at low pressure. Consequences of high pressure within the urinary tract include urinary tract infection, stones, upper urinary tract deterioration, and potentially renal failure.
A detailed history and physical examination are essential for assessment, accurate diagnosis, and treatment of urologic conditions resulting from neurological disease. Ultimately, ongoing reassessment at regular intervals is also necessary to prevent progression of urologic disease in these patients.
Voiding abnormalities can be the initial symptom of a number of neurological disorders, can result from acute or chronic neurological disease, or can be a sentinel event for progression of neurourologic disease. Symptoms can be categorized as resulting from disorders of bladder filling/storage or voiding. This classification is somewhat artificial because symptoms can often be unreliable in defining specific disease states. It is common for patients to have a combination of symptoms or disorders of both bladder filling and voiding. Nevertheless, a history of the onset, duration, and aggravating or ameliorating factors should be noted, especially in relation to the neurological event.
Filling/storage symptoms include urinary urgency, frequency (more than eight voids per 24 hours), incontinence, and nocturia. Voiding symptoms include hesitancy, straining to void, dysuria, and double voiding. It was previously thought that filling/storage symptoms were attributable to abnormalities of the bladder and that voiding symptoms were caused by abnormalities of the bladder outlet and sphincter, but it is now clear that this correlation is not accurate. The designation lower urinary tract symptoms is now used to globally describe the spectrum of symptoms that bring the patient in for evaluation. Furthermore, it is important to recognize that cognitive function may be impaired in many patients with neurological disease. Such individuals, as well as those with afferent disorders of the lower urinary tract (resulting from spinal cord abnormalities, peripheral nerve lesions, or cortical disease) may have limited or no ability to perceive normal lower urinary tract sensation and thus have few symptomatic complaints. Nevertheless, there may be underlying significant and potentially serious pathology.
Complaints regarding sexual dysfunction can also help define a neurological disorder related to the functions of erection, ejaculation, libido, and orgasm. The process of erectile function is a complex phenomenon and is mediated by sympathetic, parasympathetic, and somatic mechanisms.
A history of additional medical, neurological, urologic, obstetric, and gynecologic problems or surgeries can provide additional insight into the neurourologic dysfunction. Frequently, the urologic sequelae of a particular disorder can be complicated by a patient’s prior urologic status. Age-related changes such as atrophic vaginitis, stool impaction or constipation, delirium or changes in mental status, and restricted mobility must be considered. The use of a voiding diary can properly document fluid intake and output over a 48-hour period, provide an idea of functional bladder capacity and patterns of voiding and leakage, and document excessive fluid intake if present.
In addition to a thorough patient history, the physical examination can provide important information regarding overall evaluation of the patient’s urologic complaints. A routine urologic examination should include an abdominal examination, inspection of the external genitalia, and palpation of the flank. In men, a digital rectal examination should be performed to evaluate prostate size, tenderness, and consistency. In women, a vaginal examination should be performed. The examination should include palpation of the urethra on the anterior vaginal wall to evaluate for discharge or a mass to rule out urethritis, a urethral diverticulum, or a tumor. Inspection and palpation of the vaginal mucosa can assess for atrophic vaginitis, which can increase the risk for infection as well as identify anatomic abnormalities such as cystocele, enterocele, uterine prolapse, and gynecologic cancers. All of these disorders can mimic the symptoms of patients with neurourologic complaints.
Sensory, motor, and reflex deficits correlate with a specific level of neurological lesion and can often, but not invariably, predict a pattern of bladder and sphincter function. Sensory examination of the anterior abdominal wall, genitalia, and lower extremities reflects the integrity of the thoracic, sacral, and lumbar nerve roots, respectively. The anterior portions of the scrotum and labia majora derive innervation from the thoracolumbar spinal cord, and the sacral nerve roots innervate the posterior portion of these organs. Sensory testing of the saddle area of the perineum can evaluate the afferent limb of the pudendal nerve. Basic motor and muscular tone should also be evaluated, specifically the tibialis anterior, gastrocnemius, and toe extensors, which are innervated by the lower lumbar and upper sacral nerves. A rectal examination to assess the external anal sphincter is important for evaluation of the pelvic floor musculature. Voluntary contraction of the external anal sphincter confirms innervation of the pelvic floor and integrity of the corticospinal tract. Preserved sphincter tone in the absence of voluntary contraction is consistent with a suprasacral lesion, whereas diminished tone is consistent with a sacral or peripheral nerve abnormality.
Neuromuscular reflexes are tested to evaluate the integrity of the sensorimotor pathways known as the segmental reflex arcs. The sacral reflexes are specialized cutaneous reflexes that permit direct evaluation of sacral spinal cord segments, as outlined in Table 17.1 . The bulbocavernosus reflex represents the function of sacral cord segments S2–S4. It is elicited by gently squeezing the glans penis in men or compressing the clitoris in women while checking for contraction of the anal sphincter. Alternatively, pulling gently on an indwelling urethral catheter provokes the afferent pathway. The reflex is mediated by pudendal or pelvic nerve afferents and pudendal nerve efferents. It may be absent in patients with sacral or peripheral nerve injury and in 10% to 15% of normal patients. The anal reflex reflects the function of sacral nerves S2–S5 and is elicited by stroking the perianal skin, which causes anal contraction. External anal sphincter function can be considered to represent all of the perineal striated musculature. The cremasteric reflex (L1–L2) can be activated by stroking the inner part of the thigh in a downward direction; it triggers elevation of the ipsilateral testicle as the motor response via activation of the cremasteric muscle. Deep tendon reflexes should also be evaluated (see Table 17.1 ).
| Reflex | Spinal Cord Segment | Afferent | Efferent | Testing Method |
| Bulbocavernosus | Sacral 2–4 | Pudendal nerve | Pudendal nerve | Squeeze the penile glans or clitoris while checking for anal contraction |
| Anal | Sacral 2–5 | Pudendal nerve | Pudendal nerve | Stroke the perianal skin while checking for anal contraction |
| Cremasteric | Lumbar 1–2 | Genitofemoral nerve (femoral branch) | Genitofemoral nerve (genital branch) | Stroke the inguinal skin while checking for elevation of the ipsilateral testicle |
When evaluating a patient with voiding dysfunction secondary to a presumed neurological abnormality, electrolyte, blood urea nitrogen, and creatinine levels should be determined, and urinalysis with culture should be performed. Serum creatinine is a measure of renal function, although it will remain at normal levels until approximately 50% of the total glomerular filtration rate has been lost. Abnormal renal function testing results can indicate high-pressure bladder storage, vesicoureteral reflux, intrinsic preexisting renal disorders, or prerenal azotemia. Urinalysis and urine culture with sensitivity testing are necessary in all patients with voiding symptoms. Cytologic evaluation of urine should be performed for patients with indwelling catheters, hematuria, and risk factors for urothelial carcinoma, such as smoking.
The most devastating complications of neurourologic diseases are related to deterioration of the upper urinary tract, which leads to progressive silent renal failure. Patients with known neurogenic voiding dysfunction or injury that could potentially compromise the lower urinary tract should be routinely screened with upper urinary tract imaging. An upper urinary tract study should be performed at the initial evaluation. Although there is no standard for routine imaging, many experts recommend yearly upper urinary tract imaging, with less frequent evaluation in patients with stable neurological disorders. , Any change in neurological symptoms or voiding habits requires reevaluation of both the lower and upper urinary tracts. Common abnormalities detected by radiologic imaging include hydronephrosis, chronic pyelonephritis or renal scarring, vesicoureteral reflux, and renal calculi.
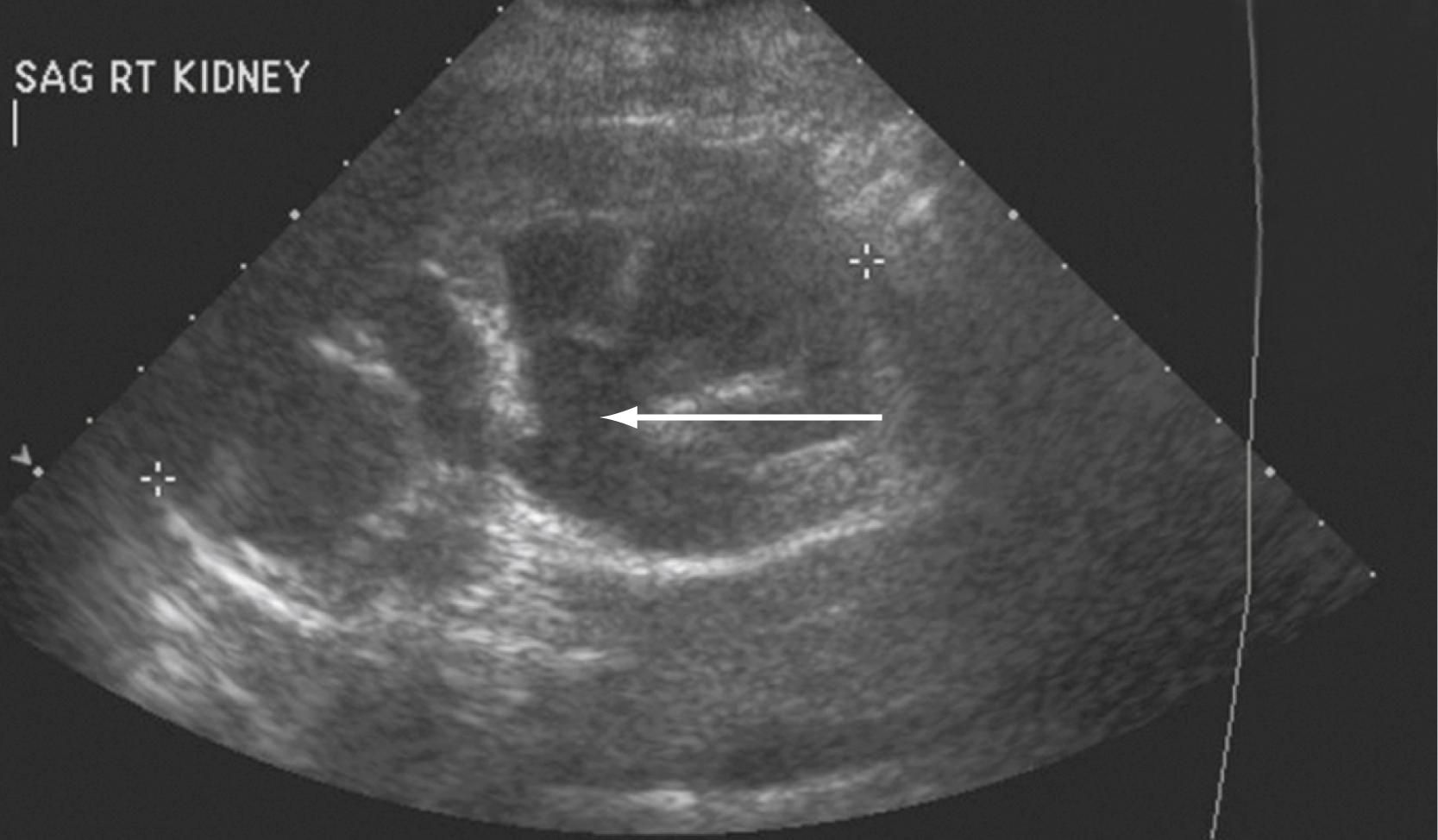
The screening modality most frequently used is ultrasonography. Although it is limited in evaluation of the ureters and provides no functional information about the kidney, ultrasonography is not invasive and does not subject the patient to radiation. It may reveal mass lesions, stones, or hydronephrosis ( Fig. 17.6 ) . Noncontrast CT, excretory urography, or intravenous pyelography can provide more information about urinary tract anatomy. Similarly, contrast-based imaging with higher-resolution CT can also be used for evaluation of renal function and renal, ureteral, and bladder abnormalities. Nuclear isotope renal scanning provides information about scarring or chronic pyelonephritis and can measure differential renal function and excretory function as it relates to obstruction of the upper urinary tract.
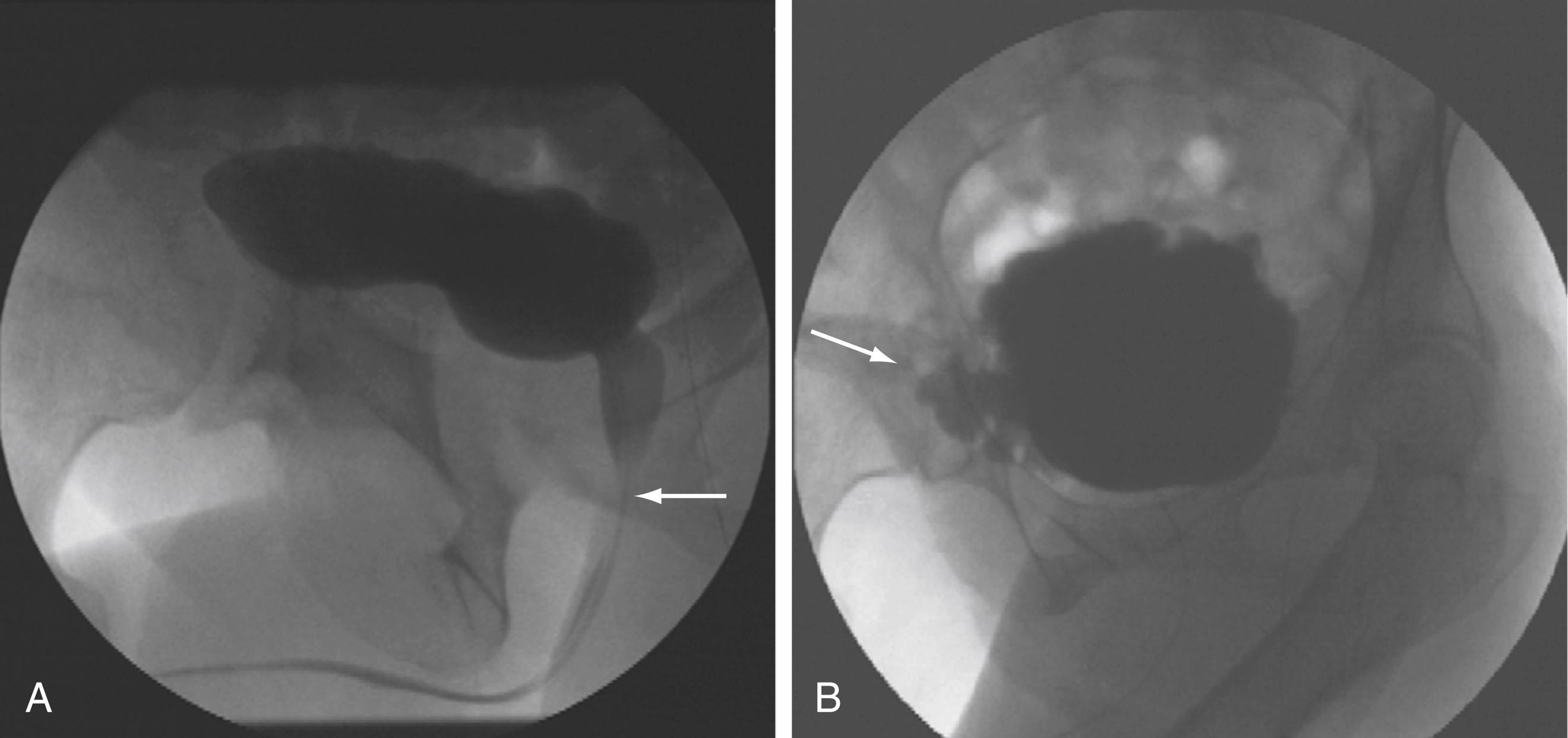
Imaging of the lower urinary tract is commonly performed by direct visualization with cystoscopy or by radiologic methods such as cystography. A voiding cystourethrogram provides information regarding the presence of vesicoureteral reflux and the morphologic characteristics of the bladder, bladder neck, proximal urethra, and striated sphincter during urine storage, bladder filling, and voiding ( Fig. 17.7 ).
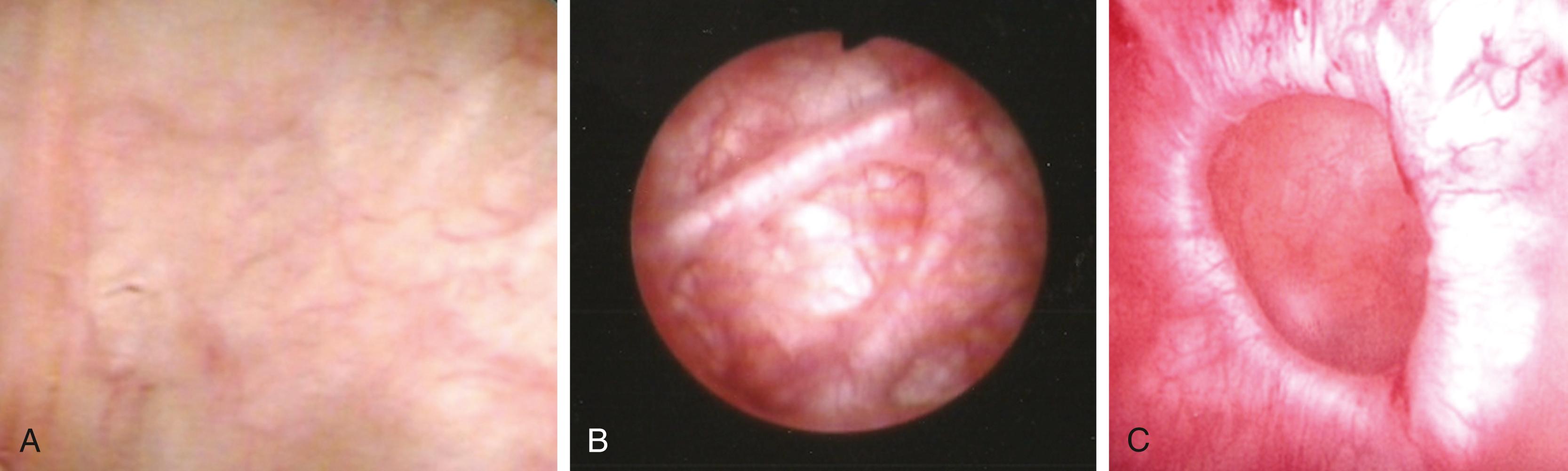
Cystoscopy allows direct visualization of the urethra and bladder surfaces and may identify tumors, inflammation, foreign bodies, or morphologic changes indicative of long-term obstruction or detrusor overactivity, such as detrusor trabeculation or diverticula. Urethral strictures and other abnormalities and prostatic obstruction can also be assessed. Patients with unexplained hematuria and risk factors for urothelial carcinoma require cystoscopy ( Fig. 17.8 ).
As mentioned previously, urinary symptoms are relatively nonspecific in the setting of neurourologic and other complicated lower urinary tract disorders. Urodynamic testing provides objective information on the function of the lower urinary tract, including the bladder, bladder outlet, and urethra. Urodynamics incorporates several studies, such as uroflowmetry, filling and voiding cystometry (pressure-flow urodynamics), electromyography (EMG) of the external sphincter, and occasionally radiographic imaging (videourodynamics). Findings from urodynamic data provide diagnostic and prognostic information and permit the formulation of a rational treatment plan.
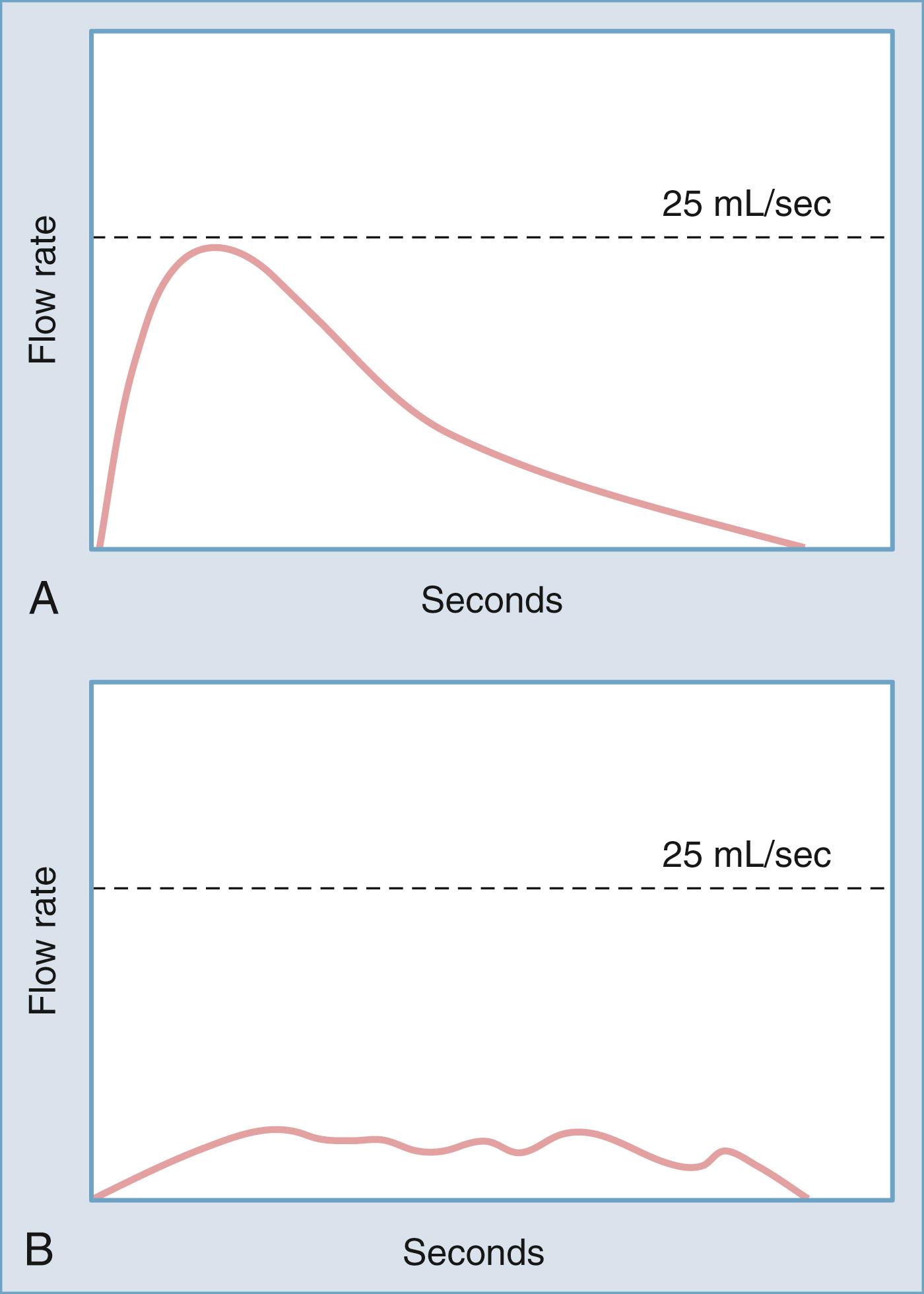
Noninvasive urodynamic testing includes assessment of postvoid residual (PVR) urine and uroflowmetry. There is no absolute volume above which PVR is universally considered to be abnormal. However, an elevated PVR indicates poor bladder contractility or bladder outlet obstruction, or both. Uroflowmetry provides data on the rate of urinary flow over time from the urethra. The pattern of urination and the mean time and maximal flow rate are important pieces of information. Age-related nomograms for males and females have been developed. Similar to an elevated PVR, a low flow rate indicates poor bladder contractility or bladder outlet obstruction, or both ( Fig. 17.9 ).
The filling cystometrogram analyzes the filling and storage function of the bladder. Two catheters, one transurethral intravesical probe and one intrarectal probe, are used to measure intravesical pressure and intra-abdominal pressure, respectively. The difference between these measured values is the calculated or subtracted detrusor pressure. Filling cystometrography provides information on the sensory function of the bladder, total capacity, accommodation during filling (bladder compliance), and the presence of involuntary bladder contraction (detrusor overactivity). Ideally, the study reproduces the symptomatology and provides a physiologic correlation for a patient’s complaints.
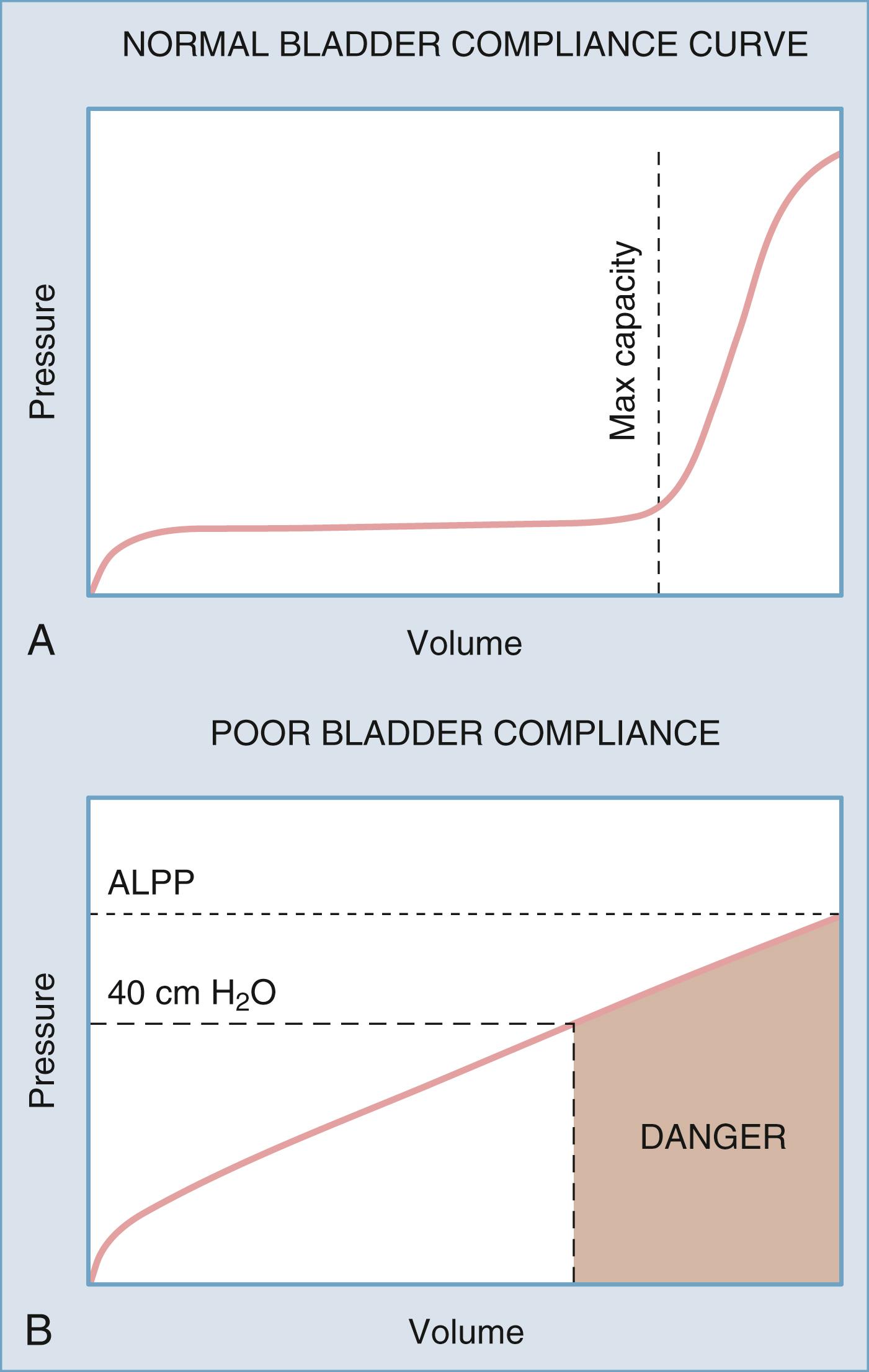
Bladder compliance, or the change in pressure divided by the change in volume during bladder filling, is determined to evaluate the ability of the bladder to fill with urine at low pressure. Normally the bladder will fill without an appreciable increase in intravesical pressure until near bladder capacity because of the innate viscoelastic properties of the bladder wall. Decreased bladder compliance, characterized by a tonic increase in intravesical pressure with ongoing bladder filling, can be secondary to multiple conditions, including neurological disease affecting the spine or peripheral nerves. Low compliance can be a significant risk factor for the development of upper urinary tract complications ( Fig. 17.10 ) .
Bladder filling can also be notable for phasic increases in bladder pressure. Such transient increases in bladder pressure are termed involuntary detrusor contractions. The presence of involuntary bladder contractions, or detrusor overactivity, can be either idiopathic or secondary to a neurological condition. Phasic detrusor overactivity is not usually associated with a risk for upper urinary tract deterioration.
The pressure-flow component of urodynamics assesses the voiding phase of the micturition cycle. Bladder pressure, intra-abdominal pressure, and urinary flow rates are simultaneously recorded during voiding. Specifically, readings can indicate the presence of bladder outlet obstruction, poor detrusor contractility (detrusor underactivity), or inadequate coordination of detrusor and sphincter function, such as in patients with detrusor-sphincter dyssynergia. Total voiding time, the use of abdominal muscles or pressure to void, and the ability to appropriately initiate and terminate micturition are also observed.
Sphincter EMG is used to record bioelectric potentials generated by the striated sphincter complex during bladder filling, storage, and micturition. EMG provides information on voluntary control of the pelvic floor musculature and coordination between the detrusor and pelvic floor ( Fig. 17.11 ) . Patch or needle electrodes are placed in the perineal area. During bladder filling, EMG activity should gradually increase and reach a maximum before voiding. This so-called guarding reflex is also present during the Valsalva maneuver, coughing, or other maneuvers that increase abdominal pressure. Loss of the guarding reflex indicates a neurological abnormality.
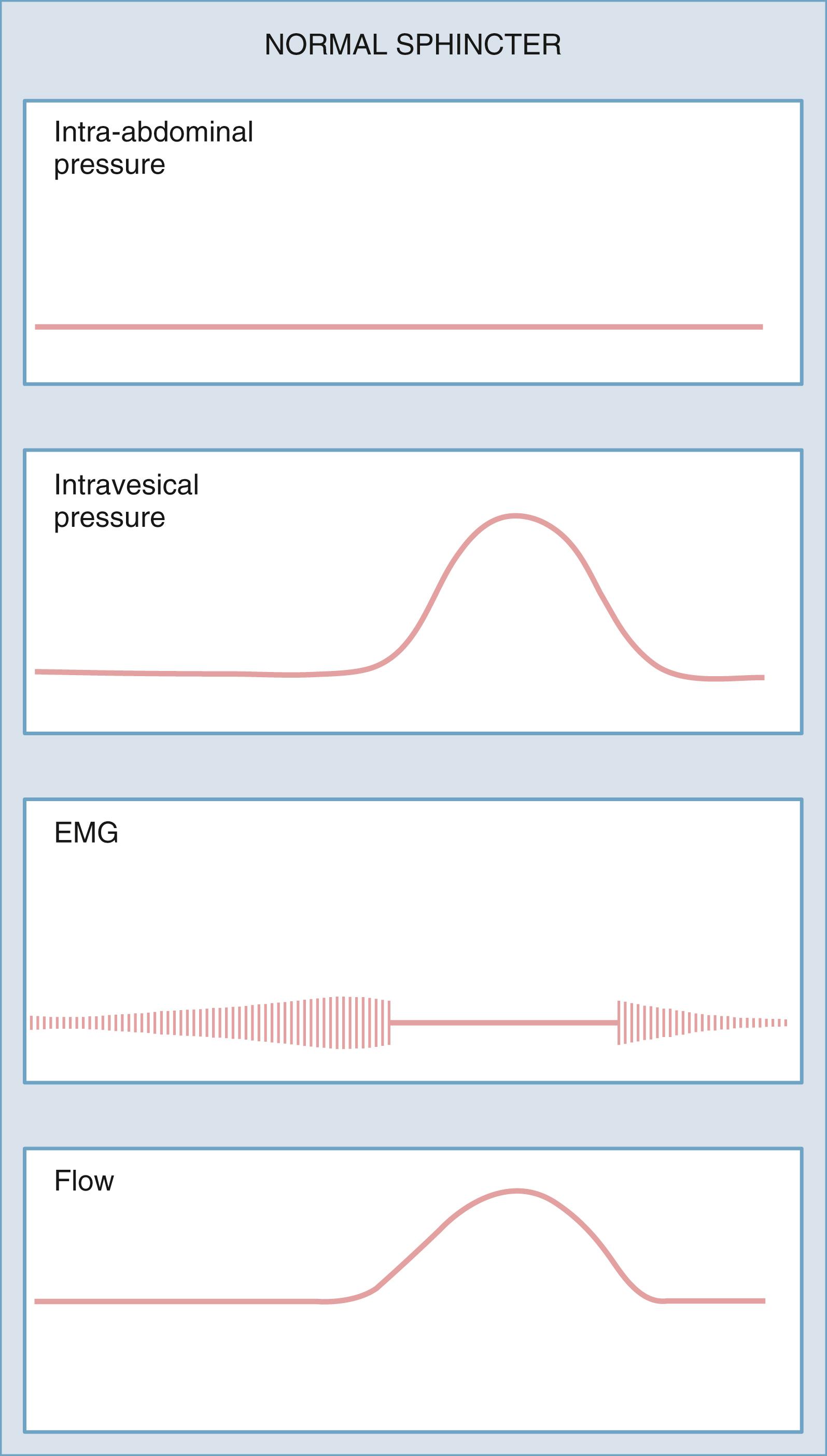
The first measurable event in micturition is a sudden change in sphincter EMG to electrical silence, which is indicative of relaxation of the pelvic floor. This is followed by a detrusor contraction. True striated sphincter dyssynergy occurs only in patients with neurological disease or injury at the level of the spinal cord and represents involuntary sphincter contraction at the time of detrusor contraction. Detrusor-sphincter dyssynergia occurs in patients with suprasacral SCI and is caused by interruption of the spinobulbar-spinal pathway that provides coordinated voiding. This should be differentiated from disorders of pelvic floor relaxation or dysfunctional voiding, which are not caused by neurological disease and are the result of failure to relax the sphincter/pelvic floor at the time of a detrusor contraction.
Performing pressure-flow urodynamics with concomitant fluoroscopic imaging of the bladder during the filling and voiding phases is termed videourodynamics . Images of the bladder neck, urethra, and bladder wall are correlated with the pressure, flow, and EMG tracings. The addition of fluoroscopy helps in the diagnosis of complex lower urinary tract dysfunction by identifying the specific location of an obstructive process, confirming normal bladder neck function, visualizing detrusor-sphincter dyssynergia, and evaluating the anatomic abnormalities in complex cases of incontinence. It is also possible to document cases of vesicoureteral reflux during the study. Because of cost and complexity, it is recommended that videourodynamics be used when traditional pressure-flow urodynamics is inadequate for diagnosis or in complex neurourologic cases.
Urinary complications of neurological disease or injury can affect the filling/storage, emptying, or both phases of micturition. The deficits are usually related to the area of the nervous system involved in the disease, the primary urologic function of the damaged area, and whether the process is irritative, degenerative, or destructive. Although significant variability exists within a given disease entity, characteristic symptom patterns and urodynamic findings are common, depending on the level or location of the lesion. It is important to recognize that the urinary tract and the neurological diseases that affect it do not exist in isolation. Other nonurinary and non-neurological processes and conditions can affect the urinary tract and result in symptoms, signs, and urinary tract compromise.
Suprapontine lesions most often result in decreased descending inhibitory input to the brainstem, which results in the symptoms of urinary frequency, urgency, nocturia, and occasionally urge incontinence. Sensation is preserved and bladder–bladder outlet interaction is coordinated. Detrusor overactivity is found on urodynamic studies, and bladder emptying is typically complete.
Cerebrovascular accidents (CVAs) result from hemorrhage, thrombosis, or occlusion and cause a broad range of subsequent physical and cognitive complications. The effects on the lower urinary tract depend on the location and extent of damage from the ensuing lesion, with lower urinary tract symptoms being more common with lesions involving the frontal lobe. Acutely following the initial neurological insult, urinary retention secondary to detrusor areflexia (poor bladder contractility) can occur and may often be overlooked during the management of more life-threatening events. Placement of a Foley catheter at initial encounter can provide information regarding fluid status while alleviating any complications from retention. Urinary incontinence occurring within 7 days of a stroke is a poor prognostic indicator. Patients will have variable degrees of lower urinary tract recovery after a period of areflexia, and a fixed deficit can develop over a period of weeks to months. The most common long-term expression of lower urinary tract dysfunction after a CVA is detrusor overactivity. , Bladder sensation is variable but generally remains intact, which leaves the patient with symptoms of urinary frequency and urgency with or without urinary incontinence. Compliance is not usually affected. The voiding dysfunction can be summarized as detrusor overactivity with coordinated smooth and striated sphincter activity. Patients with urinary retention after initial recovery from a CVA should be evaluated for other lower urinary tract abnormalities including bladder outlet obstruction.
In patients with detrusor overactivity, inhibition of involuntary bladder contractions can be achieved by forceful voluntary contraction of the striated urethral sphincter and pelvic floor muscles (Kegel exercises). After a CVA, patients instructed in these maneuvers will be able to suppress the urinary leakage associated with detrusor overactivity but will probably still have urinary frequency and urgency. If the patient is unsuccessful in performing such exercises, urge incontinence may occur. Shortly after a CVA, estimates of incontinence range from 44% to 83%, with 70% to 80% of patients regaining continence over time.
Both primary and metastatic brain tumors can cause voiding dysfunction through local compression and destruction of cortical tissues. The areas most frequently involved in brain tumor–associated bladder dysfunction are the superior aspects of the frontal lobe. Associated voiding dysfunctions are usually detrusor overactivity and urinary incontinence. Because of cognitive deficiency, the ability to suppress micturition is often impaired. Smooth and striated sphincter function during micturition is coordinated with bladder contraction, and urinary retention is unlikely.
Lower urinary tract symptoms are commonly associated with idiopathic normal pressure hydrocephalus (iNPH). Storage symptoms such as urinary frequency, urgency, and urgency incontinence are seen in the vast majority of patients. Urodynamic evaluation of individuals with iNPH and lower urinary tract symptoms almost always reveals detrusor overactivity with or without incontinence. Fortunately, neurosurgical treatment of the underlying iNPH results in stabilization and often improvement of the lower urinary symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here