Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the author: ![]() Video 16.1.
Video 16.1.
In the otorhinolaryngologic subspecialty of otology, neurotology, and lateral skull base surgery, the focus is on the anatomy, physiology, and abnormalities of the three cranial nerves that traverse the temporal bone—the cochlear, vestibular, and facial nerves—as well as specific tests of their functions.
There are two major reasons for conducting measures of auditory system function: to provide information to help determine the anatomic site of a lesion, from which the underlying cause of the patient’s symptoms can be inferred, and to assess the receptive communication capabilities of the patient.
Vertigo is an illusion of movement in any plane or direction. Affected patients feel themselves moving or see abnormal movement of their surroundings. With rotational receptor asymmetries, patients experience a true rotational or spinning movement (e.g., Ménière disease, benign positional vertigo, or vestibular neuronitis). With gravitational receptor asymmetries, patients have a gravitational receptor dysfunction type of vertigo; they often describe a “rocky,” “wavy,” or “tilting” sensation. Other descriptors include a sensation as “being on a moving boat,” “the floor falling out from underneath,” and “flipping.”
Cervical vestibular evoked myogenic potential (cVEMP) tests saccular function, and ocular vestibular evoked myogenic potential (oVEMP) tests utricular function; thus the cVEMP reflects inferior vestibular nerve function, while the oVEMP reflects superior vestibular nerve function. Clinically, these neurophysiologic differences can be used to differentiate vestibular schwannomas (acoustic neuromas) of superior and inferior vestibular nerve origins.
cVEMP and oVEMP studies are particularly useful in the diagnostic testing of patients with suspected third window syndrome, such as superior semicircular canal dehiscence. With these patients, there is typically an increased amplitude in oVEMP and cVEMP responses on the affected side and also a reduced threshold (less acoustic stimulation [volume] required to illicit the response) measured on the affected side using cVEMP testing.
When vertigo is a major complaint, one of the earliest differentiations that must be made is between an abnormality of the peripheral and central components of the vestibular system. With a peripheral lesion, vertigo of any significance is accompanied by nystagmus, and the two are usually directly proportional. With central lesions, the vertigo and nystagmus are not usually proportional. The patient may have gross nystagmus without complaining of vertigo. Because of the location and progression of most central lesions that cause vertigo, patients with vertigo generally have other symptoms and signs that indicate the correct diagnosis.
The rationale for unilateral deafferentation of the vestibular labyrinth is that the CNS is better able to compensate for complete loss of vestibular function than for fluctuating partial loss. The peripheral vestibular disorder that most commonly necessitates vestibular neurectomy is Ménière disease, although in most cases it is successfully managed with medical therapy. Vestibular neurectomy is rarely needed with contemporary medical management.
The fundamental goal of a cochlear implant is to replace a nonfunctional inner ear hair cell transducer system by converting mechanical sound energy into electrical signals that can be delivered to the cochlear nerve in severely or profoundly deaf patients (i.e., individuals who derive little or no benefit from hearing aids).
Candidacy criteria for children include 9 months of age and older, severe to profound bilateral sensorineural hearing loss, limited benefit from hearing aids, no medical contraindications, family support and appropriate expectations, and an educational program that emphasizes auditory skill development. Candidacy criteria for adults include severe or profound bilateral sensorineural hearing loss, limited benefit from hearing aids, no medical contraindications, appropriate expectations, and family support. Cochlear implantation for single-sided deafness has emerged into mainstream clinical practice with one manufacturer securing US Food and Drug Administration (FDA) clearance for this application.
For patients who do not have an intact cochlear nerve and have bilateral deafness (or in whom bilateral deafness is expected to develop with neurofibromatosis type 2), auditory brainstem implants represent an option for hearing rehabilitation. The FDA-approved auditory prostheses have the same sound processors, but they have electrode arrays that are designed to be placed directly on the brainstem cochlear nuclei.
In the otorhinolaryngologic subspecialty of otology, neurotology, and lateral skull base surgery, the focus is on the anatomy, physiology, and abnormalities of the three cranial nerves that traverse the temporal bone—the cochlear, vestibular, and facial nerves—as well as specific tests of their functions. In 2004, the Accreditation Council for Graduate Medical Education implemented the certification process for neurotology. Surgical management of intractable vertigo is not reviewed in this chapter; however, the indications for superior canal dehiscence repair are briefly introduced in the context of vestibular assessment and discussion of various vestibular disorders. Facial nerve injury secondary to temporal bone fracture is discussed in this chapter only in the context of the cochlear and vestibular nerves, although more extensive reviews related to treatment are discussed in Chapter 391, Chapter 393, Chapter 398 and offered in other publications. Skull base anatomy, including the temporal bone, is also reviewed in Chapter 3 .
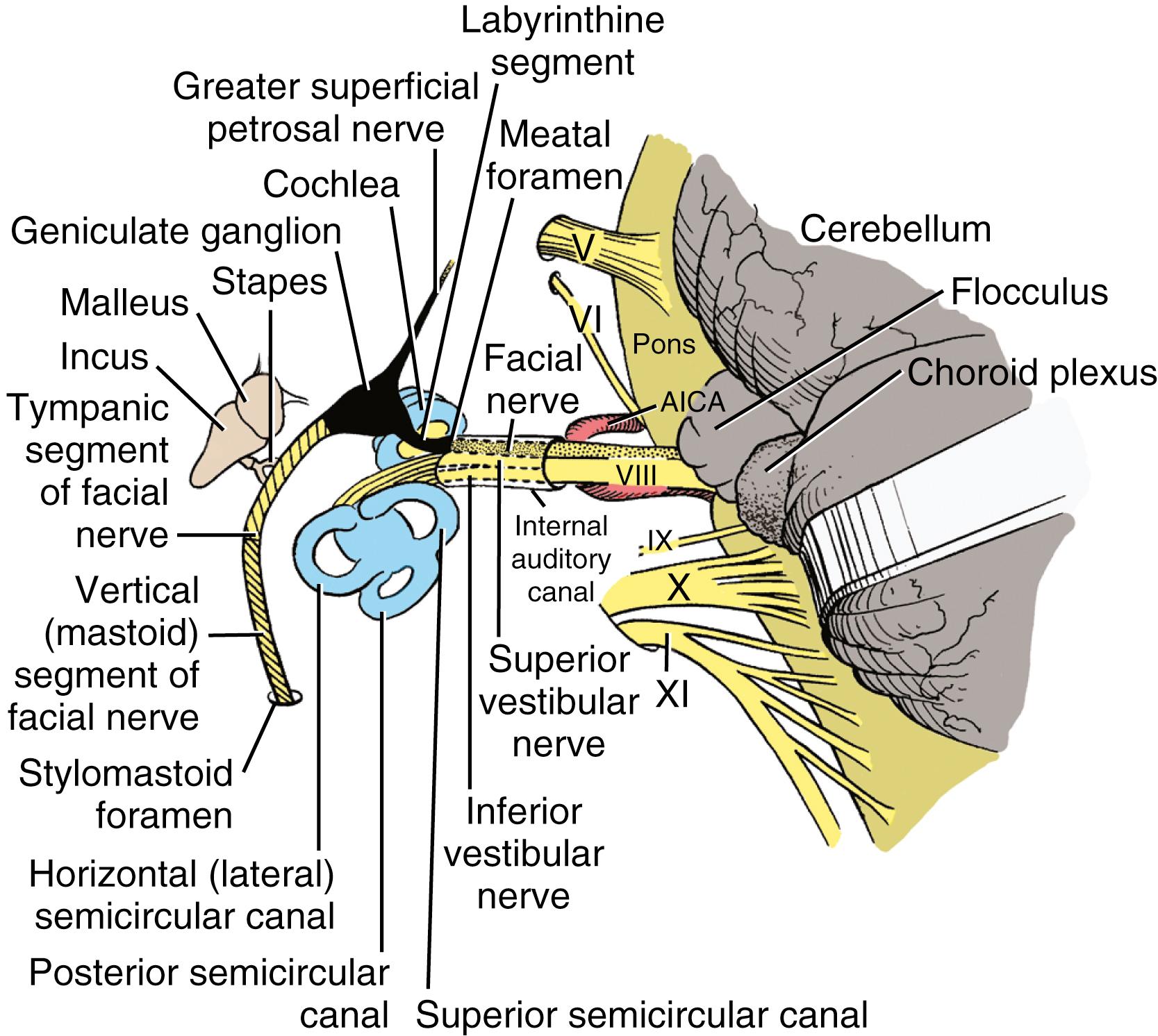
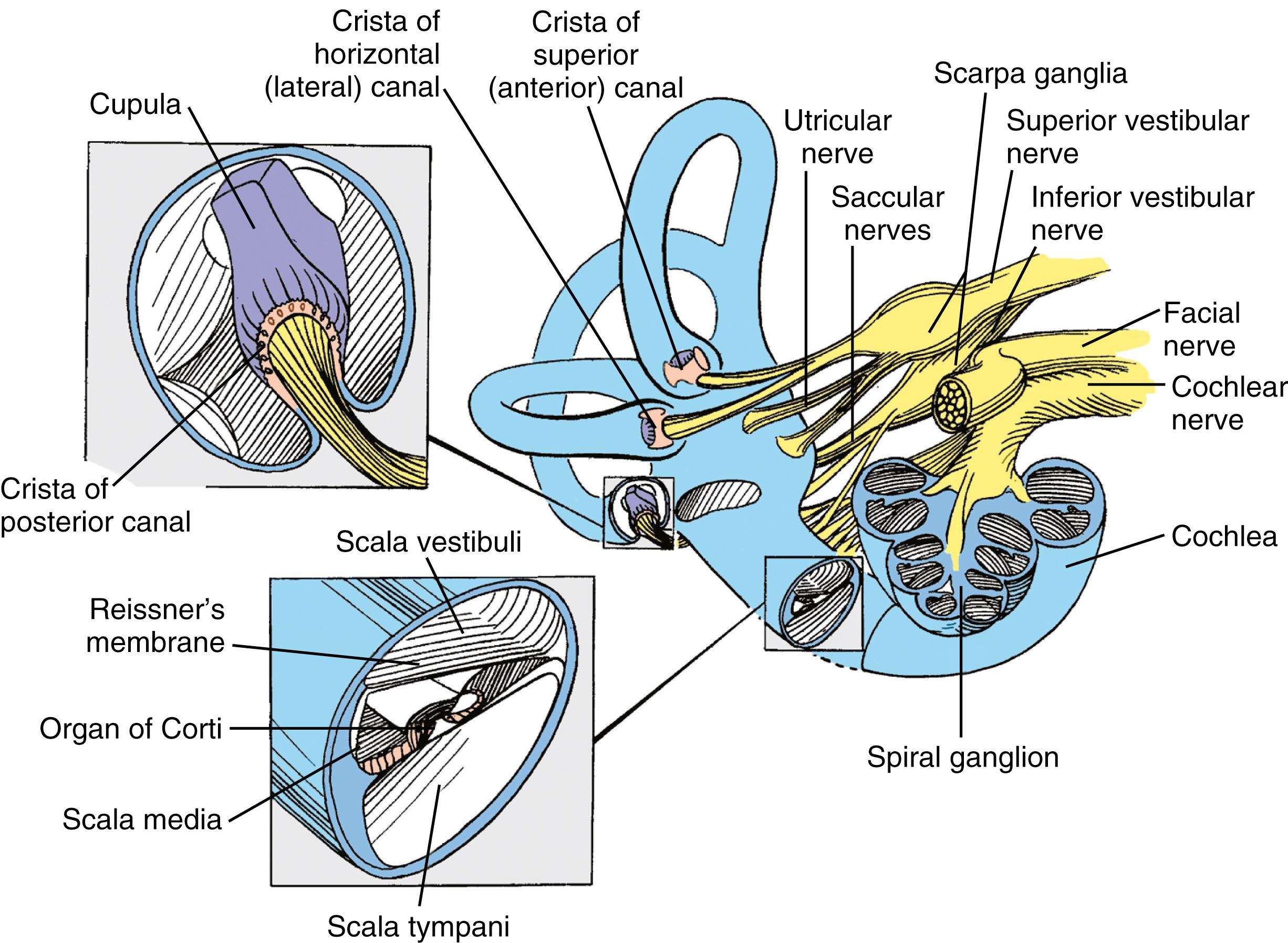
The eighth cranial (vestibulocochlear) nerve (cranial nerve [CN] VIII) consists of two distinct components: the cochlear and the vestibular nerves. The neurons of these primary afferent nerves are bipolar; their dendrites synapse on the inner and outer hair cells within the cochlea and on the type I and type II hair cells within the cristae and maculae of the vestibular end-organs. Understanding the functions of the primary afferent neurons constituting CN VIII requires knowledge of the complex structures of the peripheral vestibular and auditory systems.
During the embryonic stage, the neurosensory portions of the vestibulocochlear system are derived from ectoderm. With growth and development, these neurosensory epithelial structures become incorporated within the petrous portion of the temporal bone and are encased in the otic capsule. Concurrently, the middle ear space develops as an invagination from the first pharyngeal pouch and comes to lie lateral to the otic capsule. Meanwhile, the external ear develops from the overlying epithelium, and the fundus of its canal abuts the middle ear space at the tympanic membrane.
The vestibulocochlear labyrinth consists of a continuous complex of bony spaces encased in the otic capsule and within the petrous bone; this complex is called the bony labyrinth ( Figs. 16.1 to 16.4 ). It is filled with perilymph (biochemically similar to cerebrospinal fluid), and suspended within it is an almost exact miniature replica of the bony spaces: the membranous labyrinth. This labyrinth is filled with endolymph (see Fig. 16.2 ). The cochlea is located anteriorly and is divided from the vestibular labyrinth by the vestibular, cochlear, and facial nerves within the internal auditory canal (IAC). The vestibular portion of the bony labyrinth lies posterior to the cochlea and the IAC (see Figs. 16.3 and 16.4 ).
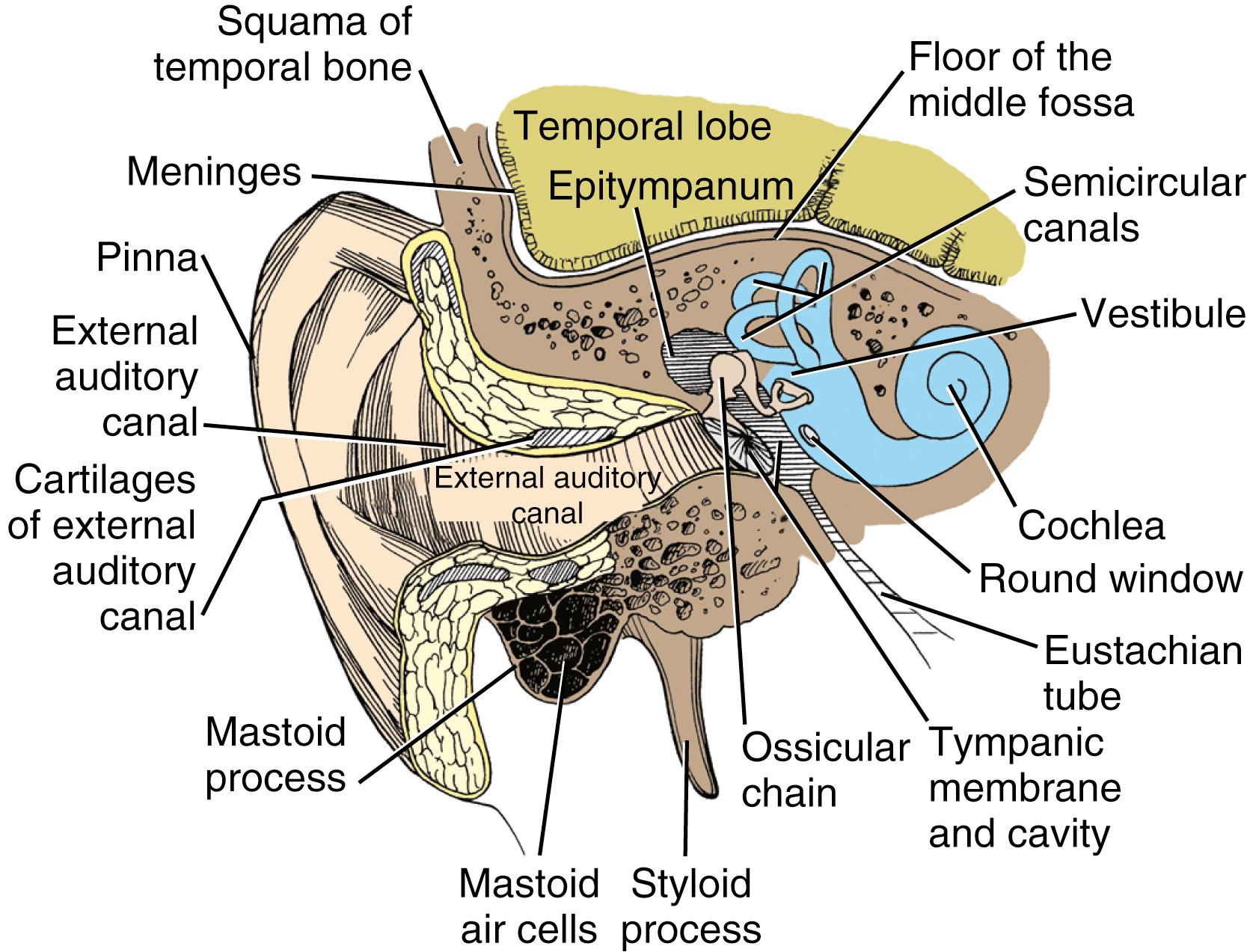
The cochlea (from the Greek word for “snail”) is made up of a hollow 33-mm tube that spirals 2.5 times. The bony center, around which the spiral is coiled, assumes the shape of a tapered screw (from the Latin word modiolus ) if the outer parts of the bony spiral are removed. The long axis of the modiolus has a sloping anterolateral orientation in the head, with its base abutting the anterior part of the fundus of the IAC. Fine canals (habenula perforata) within the modiolus house the dendrites of the cochlear nerve and their bipolar somata, which constitute the spiral ganglion.
The basal turn of the cochlea forms a distinct bony promontory on the medial wall of the mesotympanum (i.e., the middle ear) and is the only portion of the cochlea that is visible during middle ear surgery. The remaining turns of the cochlea are enclosed within the petrous bone. At the posterior aspect of the promontory are two fibrous barriers to communication with the inner ear: the oval window superiorly, which faces laterally and houses the footplate of the stapes, and the round window inferiorly, which faces posteriorly and is contained within the bony round window niche (see Figs. 16.1 and 16.2 ).
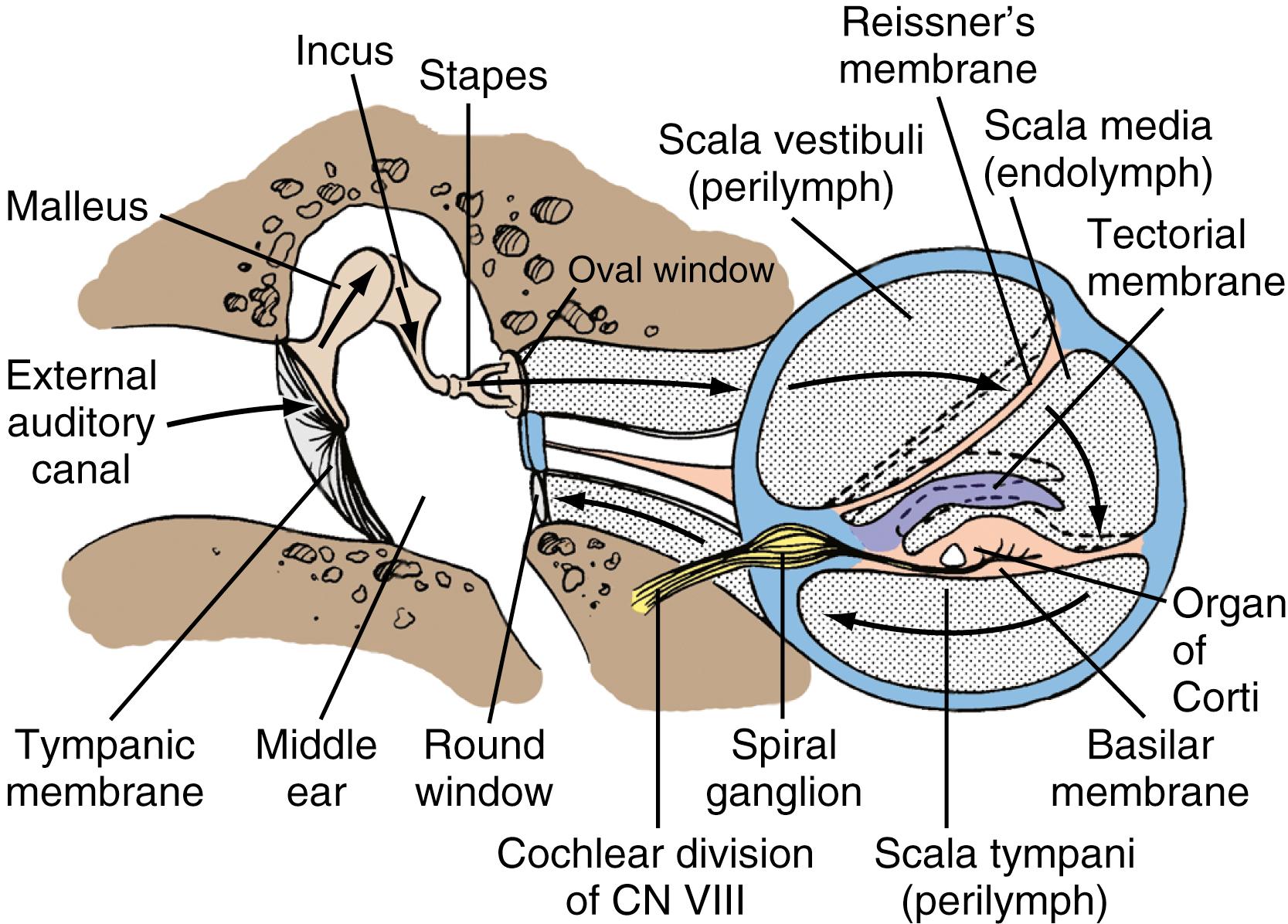
A transverse sectional view through the cochlea shows that it is composed of three compartments: the scala vestibuli, scala media, and scala tympani (see Figs. 16.2 and 16.4 ). The scala media, which contains endolymph, is separated from the scala vestibuli and scala tympani by the Reissner membrane and the basilar membrane, respectively. The scala vestibuli and scala tympani contain perilymph, and these two compartments communicate with each other at the cochlear apex through an opening at the tip of the basilar membrane called the helicotrema. At the base of the upper compartment, called the scala vestibuli, is the oval window, and at the base of the lower compartment, called the scala tympani, is the round window.
The basilar membrane has a complex structure resting on it that is best appreciated in cross-section. The basilar membrane and the thin, sloping Reissner membrane form a tube that ends blindly and is sealed at the helicotrema. This is the scala media, or cochlear duct, and it contains endolymph (see Figs. 16.2 and 16.4 ). Extending along the entire basilar membrane and spiraling with the cochlea is the organ of Corti. It contains the structurally complex sensory epithelium innervated by the cochlear nerve. As viewed from above, the basilar membrane is widest near the helicotrema and narrowest at the base. Maximal high-frequency vibration of the basilar membrane occurs at the base, and maximal low-frequency vibration occurs at the apex, thereby resulting in hair cell transduction of high frequencies at the base and of low frequencies at the apex.
The organ of Corti consists of a single row of inner hair cells and three rows of outer hair cells. The inner and outer hair cells slope toward each other to form a triangular canal (the tunnel of Corti ) between them . The tectorial membrane extends in a medial-to-lateral direction within the scala media and above the hair cells, where the hair cell stereocilia are embedded.
For an understanding of the various types of hearing loss, it is important to review the mechanism of transmission of sound to the neural receptors (see Fig. 16.2 ). Air vibrations impinge on the tympanic membrane and cause it and the malleus to vibrate. This physical vibration is transmitted through the incus to the stapes and by way of its footplate to the fluids of the labyrinth. The fluid vibrations produce a stimulus along the basilar membrane that activates the organ of Corti. Impulses are transmitted through the cochlear nerve endings to the cochlear nuclei in the pontomedullary junction. The impulses then continue to the auditory areas of the cortex.
The pars tensa of the tympanic membrane and its attached malleus are set in motion by sound; the motion is then continued to the oval window. Because the effective vibratory area of the tympanic membrane is about 17 times as large as the area of the footplate of the stapes and because the manubrium of the malleus is 1.3 times as long as the long process of the incus, the ratio of amplification from the tympanic membrane to the stapedial footplate is about 22:1.
Because fluid is incompressible, the round window membrane acts as a compensating membrane to accommodate the vibrations of the stapes footplate (see Fig. 16.2 ). If sound vibrations were to reach the oval and round windows at the same time, a certain amount of the sound would be canceled. This does not normally take place because of the phase difference between the windows, which is facilitated by several factors. The intact tympanic membrane protects the round window from direct sound impingement; the tympanic membrane is connected to the oval window through the ossicular chain, which makes direct transmission by this route faster; and the round window membrane faces backward, at right angles to the plane of the tympanic membrane, and is recessed within the niche. These factors delay the impingement of sound onto the round window membrane, which produces the phase difference.
As a consequence of these phenomena, a sizable perforation of the tympanic membrane results in a hearing loss of 30 to 35 dB by air conduction, whereas dislocation of the ossicular chain with an intact tympanic membrane produces an air conduction loss of about 55 to 60 dB (i.e., maximal conductive hearing loss).
Cochlear signal transduction of high frequencies occurs near the basal portion, and transduction of low frequencies occurs near the apical regions of the basilar membrane and the organ of Corti. Detailed mechanisms of sound transduction and perception are beyond the scope of this chapter but can be found in other publications.
There are two major reasons for conducting measures of auditory system function: to provide information to help determine the anatomic site of a lesion, from which the underlying cause of the patient’s symptoms can be inferred, and to assess the receptive communication capabilities of the patient. Because reduced auditory sensitivity is common to most auditory disorders of peripheral origin, an estimate of the magnitude of the loss of sensitivity provides the basis of any differential diagnostic auditory test battery.
The purpose of this section is to describe the current primary measures of auditory system function. Some of these measures depend on the subjective response of the patient and are referred to as subjective measures of hearing. Other measures require no subjective response from the patient, but the patient must be quiescent and cooperative. Such objective measures of auditory system function can be conducted when the patient is alert and cooperative or when the patient is sedated or anesthetized.
An initial question in the diagnosis of auditory system dysfunction is whether the patient’s hearing deviates in sensitivity from normal hearing. Before development of the clinical audiometer, assessment of hearing was typically conducted with tuning fork tests. Tuning forks continue to be an integral part of the initial assessment of patients with hearing loss, particularly at the patient’s bedside or in the clinic.
Each tuning fork emits a pure tone of a particular frequency, depending on the physical characteristics (i.e., mass and inertia) of the fork. An experienced practitioner can activate the fork by striking it with a “standard” blow. By comparing the length of time that the patient can hear the slowly damping intensity of the fork with that of the practitioner (assuming that the practitioner has normal hearing), it is possible to determine the patient’s relative magnitude of hearing loss at a particular frequency. Because of problems in reliably striking a standard blow and the noise levels in most clinical examination rooms, tuning forks are more often used in a qualitative manner to assess the type of hearing loss. A calibrated audiometer is the instrument of choice for more precisely determining the magnitude and configuration of hearing loss as a function of frequency.
In the Rinne test, the patient’s responses to air conduction and bone conduction sensitivity are compared. For patients with normal hearing and those with sensorineural hearing loss, the tuning fork is heard longer by air conduction than by bone conduction because of the advantage provided to the air conduction signal by the normal middle ear system. However, when the patient hears bone conduction longer than air conduction, a conductive (middle ear) hearing loss is suggested. This ensues when the air conduction route of transmission is no longer the more efficient route to the cochlea.
The Weber test is an assessment of the ear to which the auditory signal is referred: the tuning fork is placed at the center of the patient’s forehead, and the patient is asked in which ear the signal is heard. When both ears are normal or symmetrically abnormal, the auditory signal is localized to the center of the head. If there is unilateral middle ear (conductive) loss, the sound is lateralized to the ear with the conductive loss. If there is unilateral sensorineural loss, the sound is lateralized to the ear with the better sensorineural sensitivity.
The tuning fork tests provide the examining physician with an initial impression of the probability of hearing loss and the possible site of the auditory lesion affecting hearing sensitivity. However, tuning forks do not provide definitive information on the magnitude and configuration of the hearing loss, and they provide virtually no information on potential differential sites of lesions in patients with sensorineural hearing loss. Such information is available from more formal measures of auditory system function.
Pure-tone threshold hearing sensitivity is the subjective procedure by which auditory sensitivity is determined. In the United States, the American National Standards Institute (ANSI) has established standards for the calibration of clinical audiometers. The output sound pressure level for standard circumaural or inserted earphones, or both, is specified when measured in a standard coupler, referred to as an artificial ear. The artificial ear simulates the impedance characteristics of the average human ear at the plane of the tympanic membrane. The decibel levels used in audiometers for the normal threshold for air conduction can be found in other publications.
To assess hearing loss by air conduction, the examiner determines the magnitude (in decibels) by which the patient’s hearing deviates from the 0-dB hearing level (i.e., normal hearing). To determine hearing loss, hearing sensitivity is assessed at octave frequencies between 250 and 8000 Hz. There is increasing interest in assessing hearing between 8000 and 16,000 Hz, but testing in the ultra-audiometric range (10 to 20 kHz) is not routine.
In summary, pure-tone air conduction testing is the initial and critical measurement for subjective hearing loss. The measure provides an indication of the magnitude and configuration of the hearing loss as a function of frequency. However, little differential diagnostic information can be obtained from this description of audiometric configuration because auditory system dysfunction at various anatomic sites may result in similar patterns of loss of sensitivity. Other hearing tests have been developed for the purpose of distinguishing among the various sites of auditory dysfunction.
The primary audiologic tests used to distinguish conductive from sensorineural hearing loss are the comparative measures of air and bone conduction thresholds. The procedure for measuring bone conduction thresholds is similar to that for measuring air conduction thresholds, except that a vibrotactile stimulator transduces the signal, usually coupled to the mastoid of the ear being tested. The diagnostic utility of the difference between air and bone conduction sensitivity is based primarily on two assumptions: (1) that the air conduction threshold is a measure of the function of the total auditory system, both conductive and sensorineural components, and (2) that the threshold for bone conduction is primarily a measure of the integrity of the sensorineural auditory system and is not significantly influenced by the functional status of the external or middle ear. It has been demonstrated, however, that the external ear and middle ear do provide minor, but important contributions to the bone conduction threshold in the normal auditory system. Consequently, some conductive disorders do cause minor, but significant alterations in bone conduction sensitivity because of changes in the contribution of the middle ear to the bone-conducted signal reaching the cochlea.
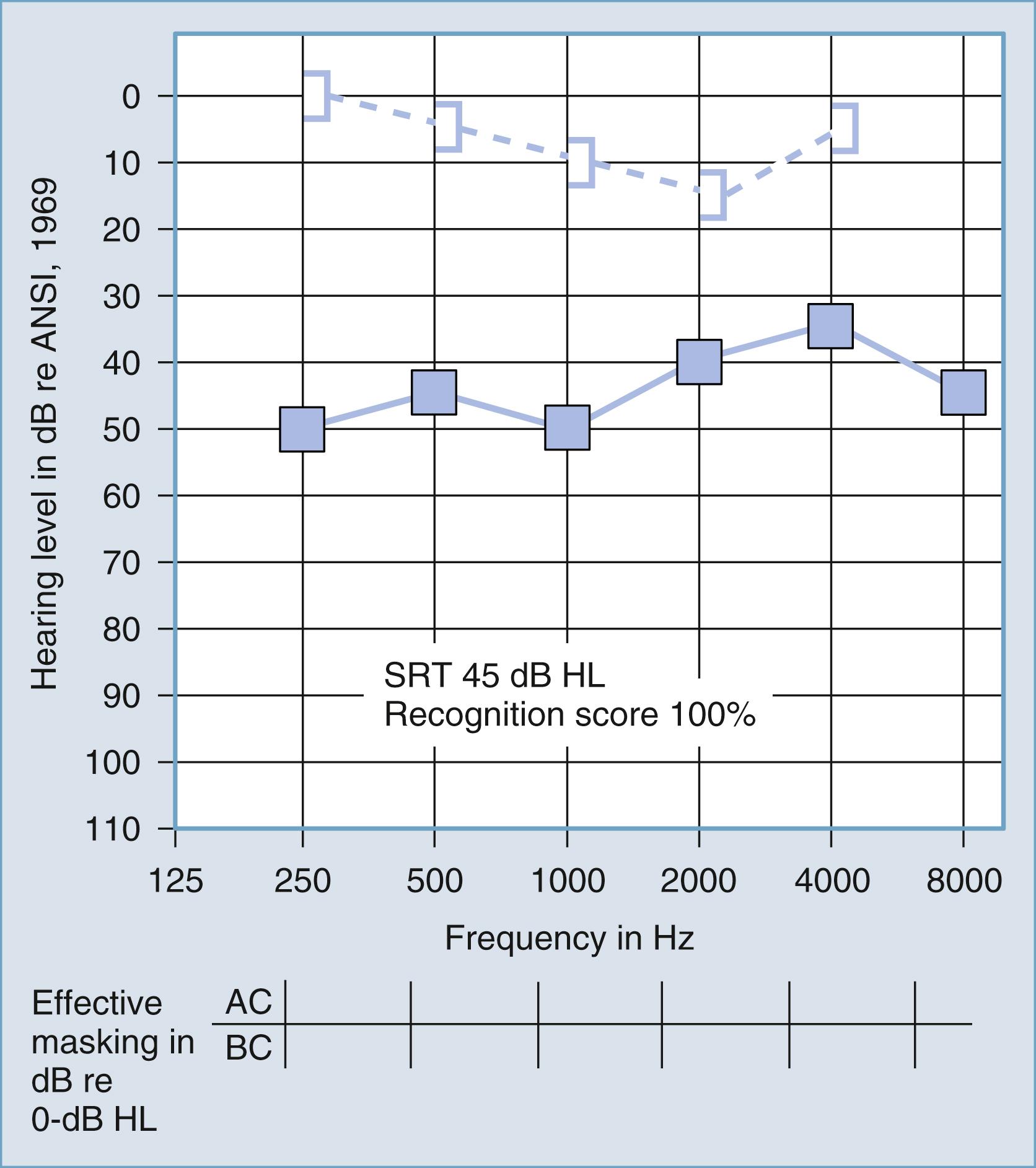
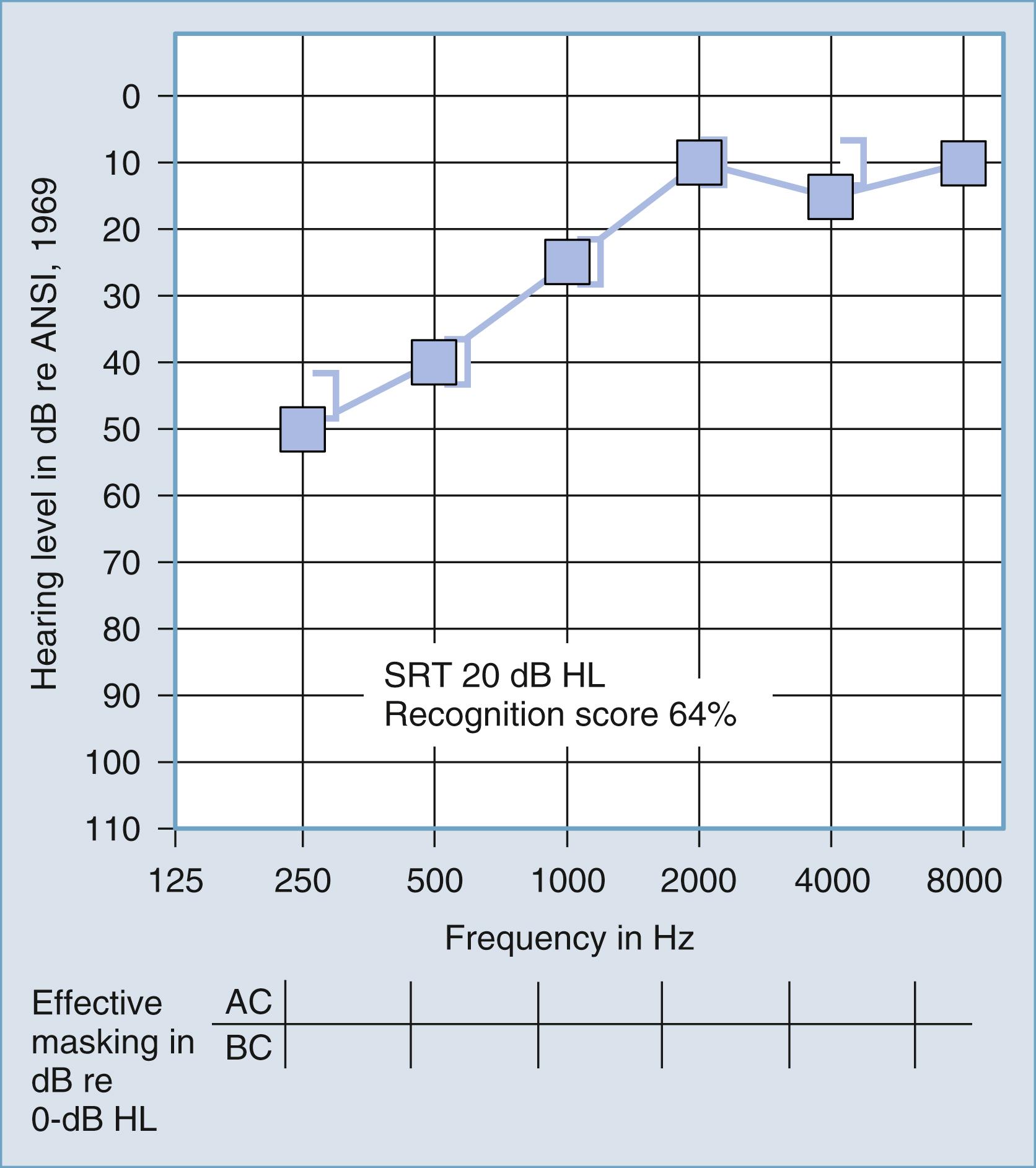
Despite this limitation, the difference between air and bone conduction pure-tone thresholds provides the most definitive indication of the effect of disorders in the external and middle ear on threshold sensitivity. Dirks provided a thorough review of the clinical principles of bone conduction testing. Examples of conductive and sensorineural hearing loss are plotted in Figs. 16.5 and 16.6 , respectively. Notice that in conductive hearing loss (see Fig. 16.5 ), hearing sensitivity by air conduction deviates from normal hearing (0-dB hearing level), but bone conduction sensitivity is within normal limits. In sensorineural hearing loss (see Fig. 16.6 ), hearing sensitivity by both air conduction and bone conduction deviates equally from normal hearing (0-dB hearing level). Combinations of sensorineural and conductive hearing loss are called mixed hearing loss.
When a patient has a substantial difference in hearing sensitivity between the two ears, it is necessary to rule out the potential participation of the better hearing ear when testing the poorer hearing ear. Masking is defined by ANSI as the amount by which the threshold of audibility of a sound is raised by the presence of another (masking) sound. As early as 1940, Fletcher observed that a restricted bandwidth of frequencies contained within a broadband noise was sufficient to mask a pure-tone threshold. Most clinical audiometers contain narrow bands of noise that encompass the critical band of frequencies necessary to mask frequency-specific stimuli.
The process of clinical masking can be rather complex, especially in patients with bilateral conductive hearing loss. The problem arises because the masking stimulus is presented by air conduction but must be intense enough to reach and raise the elevated threshold by air conduction. In overmasking, the masking stimulus from the nontest ear crosses intracranially to the test ear to raise the threshold of that ear. The procedures developed for masking must take into consideration the air and bone conduction thresholds of both ears of the patient.
In some circumstances of severe bilateral conductive hearing loss, it may be impossible to obtain a threshold for bone conduction (or possibly air conduction) without overmasking. Fortunately, as described later in this section, acoustic immittance studies can be performed without regard to “masking dilemmas” and can provide additional diagnostic information on the functional status of the middle ear. Studebaker described the rules for clinical masking in detail.
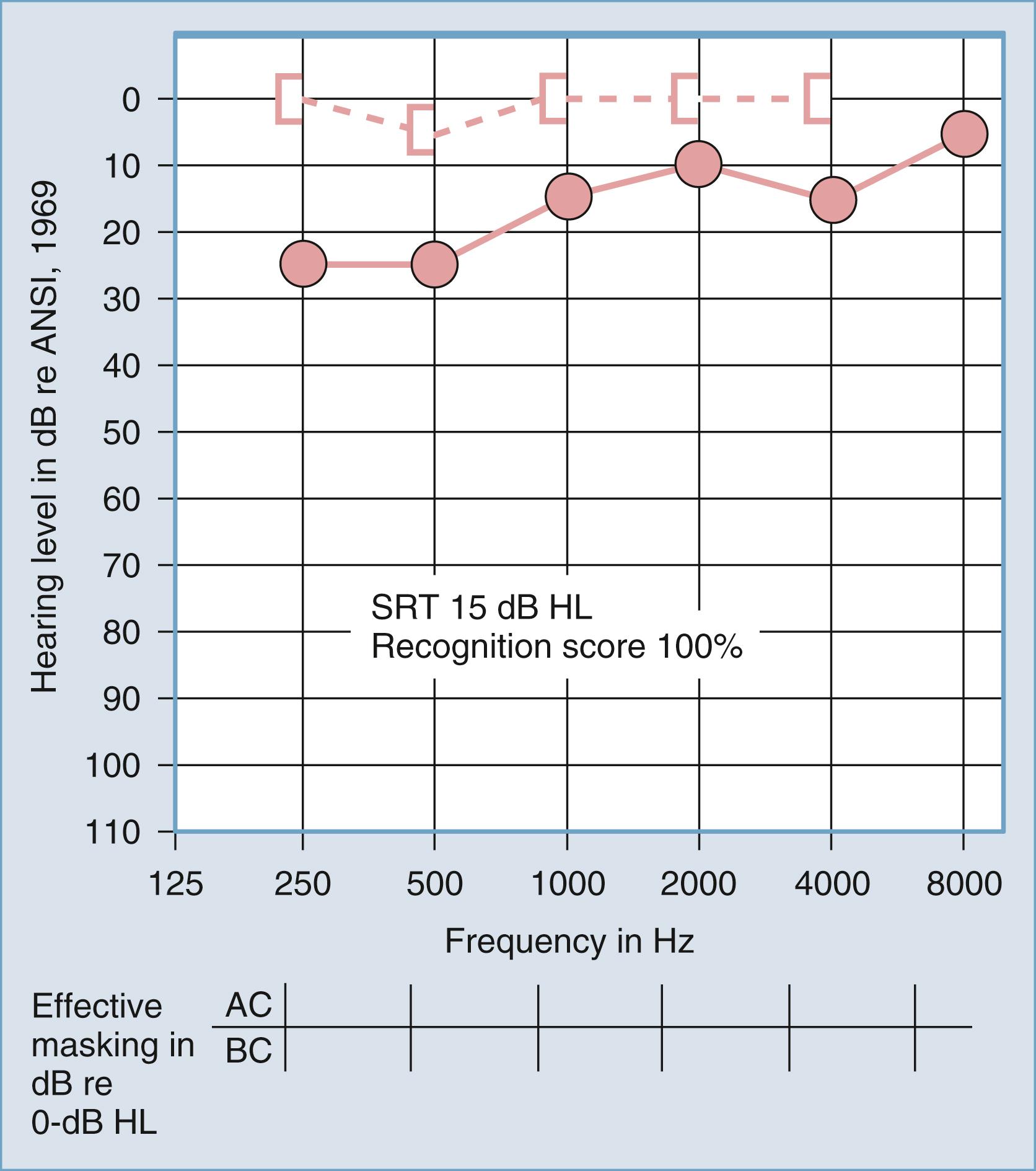
Reduced speech recognition is among the most difficult problems faced by persons with hearing loss. Reduced speech recognition also provides differential diagnostic information on the probable site of the auditory lesion. Speech audiometry is therefore used to assess the receptive communicative ability of the patient and to predict the site of an auditory lesion. Two tests are performed in a standard auditory battery: (1) measurement of the sensitivity for speech, referred to as the speech recognition threshold (SRT), and (2) measurement of the recognition (discrimination) ability at suprathreshold levels.
Traditionally, the SRT is measured with the use of spondaic words: that is, two-syllable words in which equal stress is placed on each syllable, such as baseball, cowboy, and sidewalk . No standardized method for presentation of the words has been accepted, although practical means for standardization have been suggested. The SRT is reported as the decibel hearing level below which the patient cannot successfully recognize the two-syllable words. The SRT is expected to approximately equal the average hearing loss for pure tones in the midfrequency region (500 to 2000 Hz), regardless of the type of hearing loss (conductive or sensorineural). The SRT has little differential diagnostic significance, except in cases of pseudohypacusis, but it is used to provide a descriptive measure of hearing loss for speech and to confirm the pure-tone air conduction sensitivity measures.
Measurement of speech recognition at suprathreshold levels is conducted with standardized lists of words or sentences. Standardized material has been chosen to meet specific criteria that enable comparison with everyday speech. The material available for use includes monosyllabic word lists, nonsense syllables, and sentences. The results are reported as the percentage of correct scores at a specified level above the SRT.
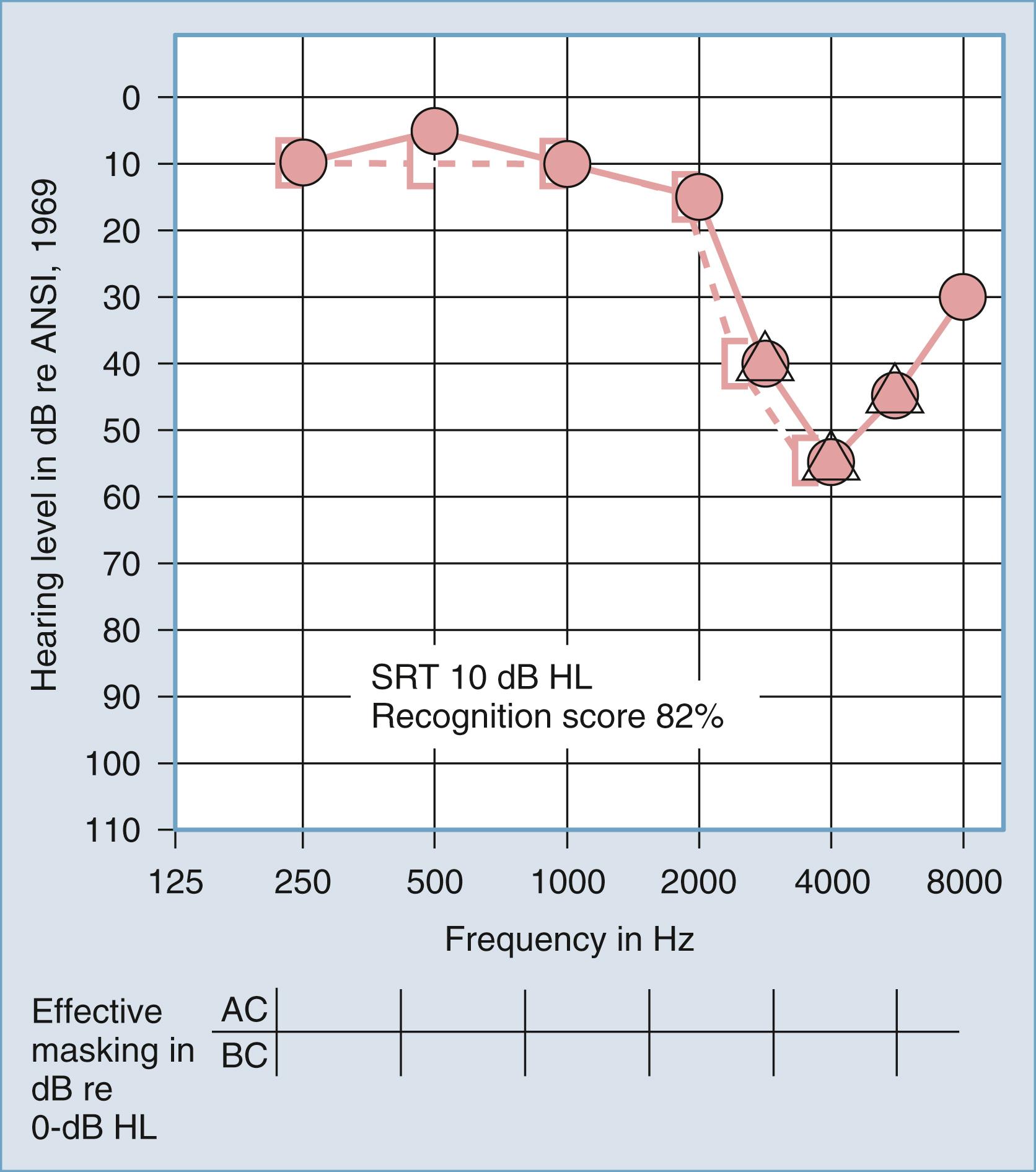
Patients with conductive hearing loss typically score high with these materials, whereas those with sensorineural hearing loss show decreased discrimination, depending on the magnitude and configuration of the sensorineural hearing loss and the site of the auditory lesion (i.e., cochlear or retrocochlear). Recognition of isolated speech segments is unaffected by conductive hearing loss (if the materials are presented at suprathreshold levels) because the encoding mechanisms of the cochlea and CN VIII are normal. When the presentation level overcomes the threshold sensitivity loss, the ability to understand speech segments is excellent; however, when the conductive mechanism is normal but lesions of the auditory system affect the cochlear or retrocochlear structures, the ability to understand the consonant elements of speech is affected. When the cochlear structures are normal but CN VIII or low-brainstem structures are affected by a space-occupying lesion, speech recognition can be severely affected. One of the early diagnostic signs of lesions of CN VIII or the low brainstem is severely reduced speech recognition scores in the presence of mild or moderate pure-tone hearing loss.
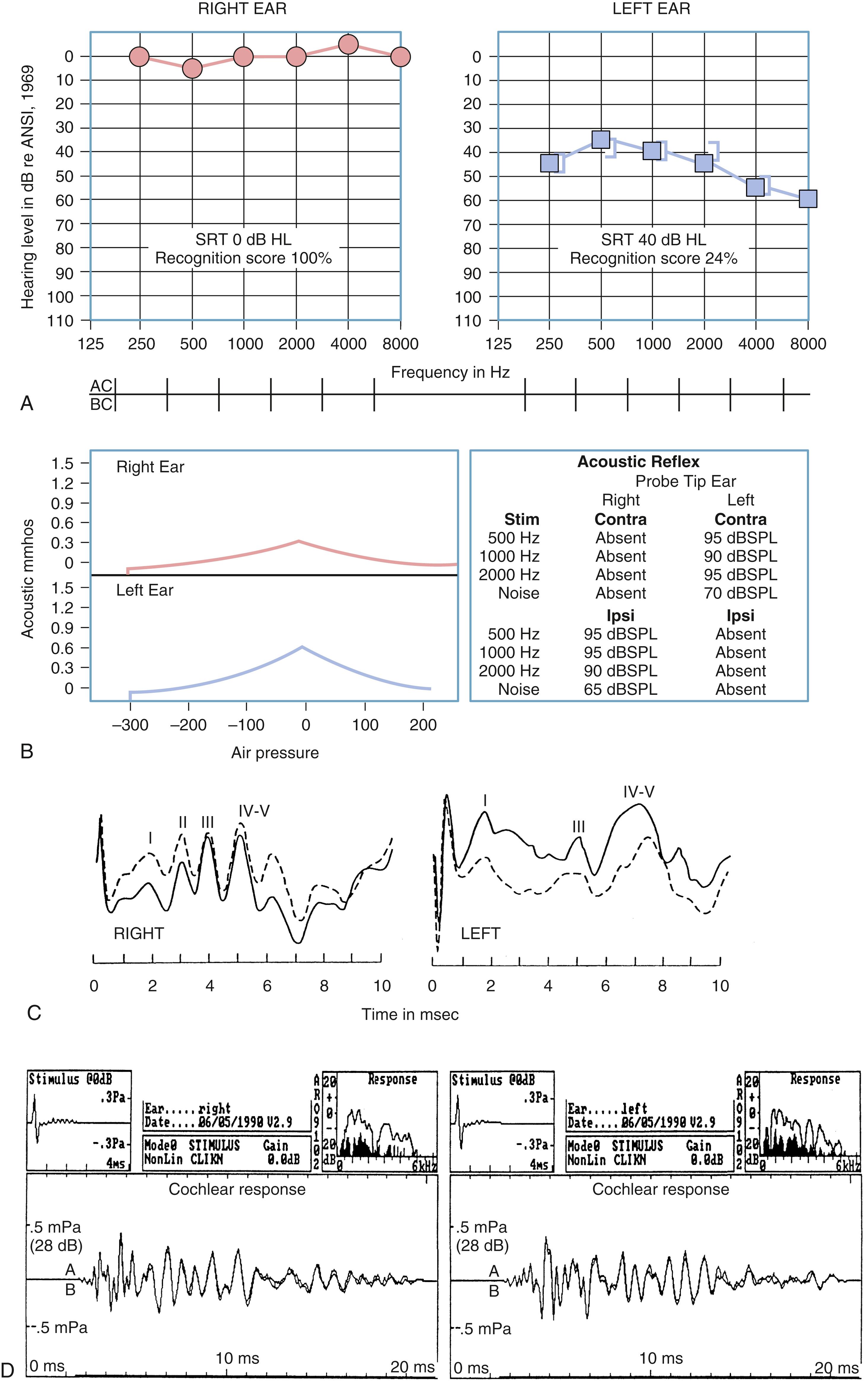
Figs. 16.7 and 16.8 provide examples of the effects of conductive and sensorineural hearing loss, respectively, on the SRT and speech recognition scores of two patients. Figure 16.7 is a plot demonstrating conductive hearing loss secondary to otosclerosis. Notice the elevated pure-tone air conduction threshold and SRT, together with normal bone conduction sensitivity and excellent speech recognition score. Fig. 16.8 is a plot demonstrating sensorineural hearing loss secondary to Ménière disease (discussed later). Notice the low-frequency sensorineural hearing loss, the mildly elevated SRT, and the diminished speech recognition score (64%). This example reveals the potential effect of a cochlear lesion site on speech recognition ability. Early in the course of Ménière disease, it is unusual for speech discrimination ability to be abnormal. Figs. 16.5 and 16.6 reveal other ways in which conductive hearing loss from chronic otitis media (see Fig. 16.5 ) and sensorineural hearing loss from industrial noise trauma (see Fig. 16.6 ) affect speech and pure-tone results.
Among the most significant advancements in the differential diagnosis of middle ear impairments and one that provides definitive information on CN VIII and low-brainstem function is measurement of acoustic immittance. This procedure requires no active participation by the patient, provides objective evidence of middle ear function, and is a means of testing the integrity of the acoustic reflex arc. The two procedures included in immittance studies are tympanometry and acoustic reflex measures.
Tympanometry provides evidence of the relative change in impedance (or its reciprocal, admittance) with a change in ear canal air pressure at the plane of the tympanic membrane. The tympanogram provides indirect evidence of the mechanical integrity of middle ear structures when changes in ear canal air pressure are introduced. When pathologic conditions such as middle ear effusion, ossicular chain fixation, or ossicular chain discontinuity occur, concomitant changes in admittance at the plane of the tympanic membrane take place. Such changes in admittance affect the efficient transmission of acoustic energy across the middle ear space to the cochlea and introduce hearing loss. The changes in transmission characteristics can also be measured objectively by direct measures of changes in relative admittance. Certain clinical instruments can introduce changes in ear canal air pressure while simultaneously measuring the effects of the changes in air pressure on transmission of energy through the middle ear to the cochlea. In a normal middle ear system, negative and positive changes in air pressure (in relation to atmospheric pressure) produce predictable decreases in the relative transmission of energy through the middle ear space. In pathologic conditions such as middle ear effusion and ossicular chain fixation, the relative changes in admittance decrease; this finding is indicative of high impedance (low admittance) of the middle ear system. In ossicular chain discontinuity and some disorders of the tympanic membrane, the effect is decreased impedance (increased admittance) of the middle ear system. These measures of relative change in impedance with alterations in ear canal air pressure can also provide evidence of tympanic membrane perforations and the functional integrity of pressure equalization tubes that may have been placed in the tympanic membrane. The tympanogram provides objective evidence of the integrity of the middle ear system and differential diagnostic information on the underlying middle ear source of any resulting conductive hearing loss that may have been demonstrated on the pure-tone audiogram.
The acoustic reflex is the reflexive contraction of the stapedius muscle on delivery of an acoustic stimulus. The stapedius muscle contracts reflexively and bilaterally on presentation of an acoustic stimulus. The muscle contraction results in a concomitant increase in impedance in the middle ear, as measured at the plane of the tympanic membrane. Unfortunately, when middle ear disorders introduce changes in middle ear impedance, it is not possible to measure evidence of further changes in impedance that may be introduced by contraction of the stapedius muscle. Only when tympanometry reveals the middle ear system to be functioning normally is it possible to test the integrity of the acoustic reflex arc.
When tympanometry has revealed the middle ear system to be functionally normal, two types of acoustic reflex measurements can be made: acoustic reflex threshold measures and acoustic reflex adaptation measures. The same equipment used to obtain the tympanogram can be used to measure the integrity of the acoustic reflex arc.
Constant air pressure is maintained in the external auditory canal, and impedance or admittance is monitored over time. The intensity of a reflex-inducing acoustic stimulus is increased until a change in impedance or admittance is observed. The lowest intensity at which the reflex-inducing acoustic stimulus results in a change in acoustic impedance or admittance is specified as the acoustic reflex threshold. Typically, lesions in cochlear sites produce a change in this threshold only for wideband noise stimuli, not for pure-tone stimuli, until the hearing loss exceeds approximately the 60-dB hearing level. When the hearing loss is of cochlear origin and the loss exceeds 60 dB, there may be an increase in the threshold of the acoustic reflex, even for pure-tone stimuli. The measure of acoustic reflex threshold can be used in cases of sensorineural hearing loss to provide differential diagnostic information on the site of the sensorineural hearing loss.
In patients with a retrocochlear site of the lesion (CN VIII and low brainstem), the acoustic reflex may be elevated or absent. Abnormal elevation or absence of the acoustic reflex in the presence of hearing loss at a less than 60-dB hearing level is audiologic evidence of a retrocochlear site of the lesion. ,
Acoustic reflex adaptation is measured by introducing (into the ear contralateral to the reflex-measuring tip) a 10-second, pure-tone stimulus at 10 dB above the acoustic reflex threshold for that particular stimulus. Acoustic reflex adaptation is defined as a decrease in impedance or admittance that exceeds 50% of the nominal impedance or admittance observed at the onset of the 10-second stimulus. This adaptation is not a function of the inability of the stapedius muscle to maintain contraction throughout a 10-second period; rather, it is an adaptation to the 10-second, continuous pure tone presented to the contralateral (test) ear. The test ear in acoustic reflex adaptation is the ear receiving the acoustic stimulation, not the ear in which the acoustic impedance or admittance is being measured. Acoustic reflex adaptation is typically observed only in ears in which a retrocochlear (CN VIII or low brainstem) disorder is present.
Fig. 16.9 is an example of audiometric data from a patient with a left acoustic neuroma within the cerebellopontine angle. The pure-tone results reveal mild, left-sided sensorineural hearing loss, and the speech recognition score is very poor (24%). The tympanograms were normal bilaterally, but no acoustic reflex was identifiable with acoustic stimulation of the left ear. When the measuring tip was in the right ear, evidence of stapedius muscle contraction was observed only with ipsilateral stimulation. When the measuring tip was in the left ear, the stapedius muscle contracted only when the acoustic stimulus was presented contralaterally. As evidenced by acoustic reflex measures, this is the classic audiometric result in a patient with a left acoustic neuroma.
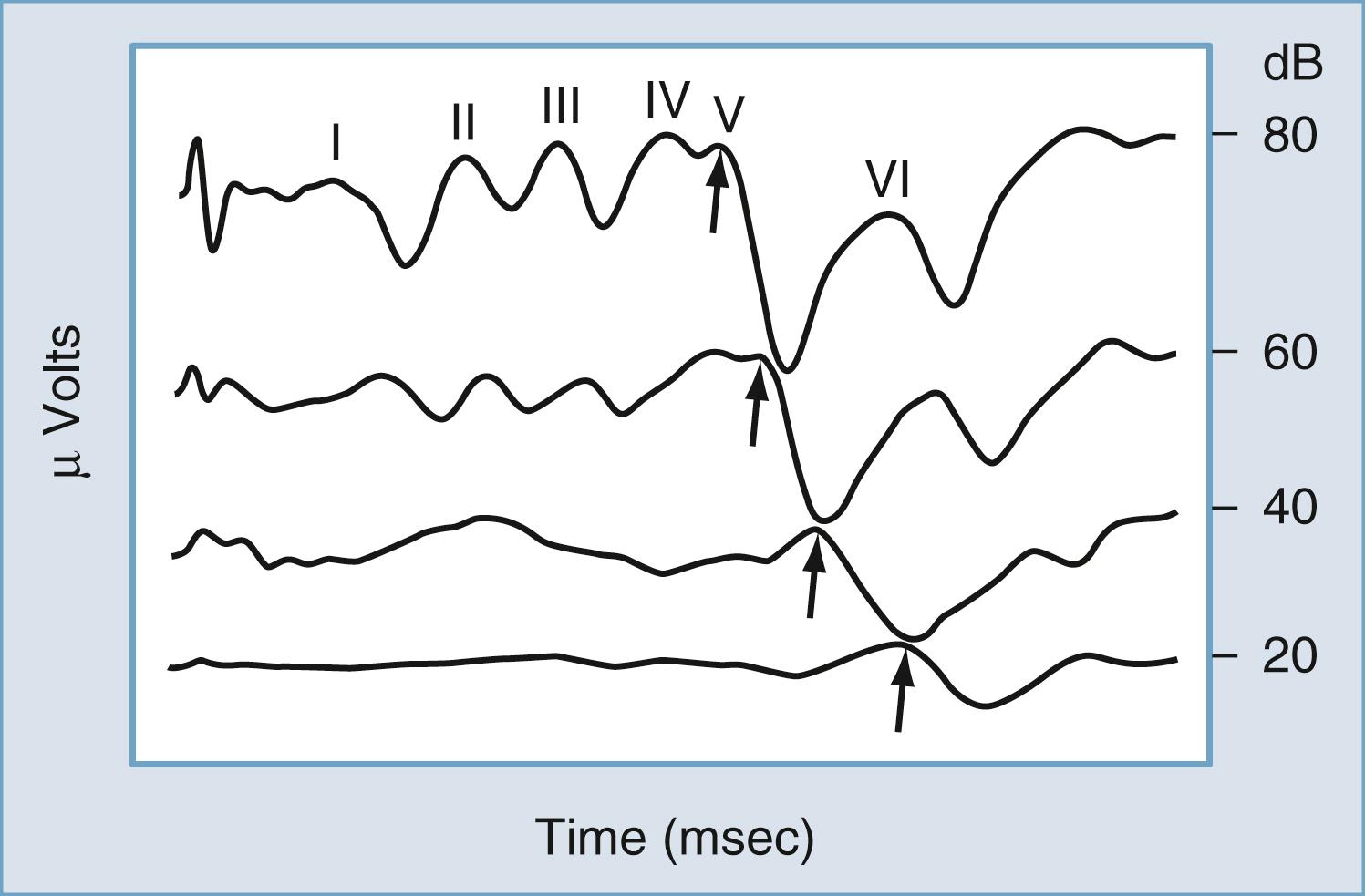
Possibly the most powerful audiologic test currently available in differentiating between cochlear and retrocochlear lesions is measurement of the auditory brainstem response (ABR). The ABR is one of several clinically useful auditory evoked potentials and the one most often assessed in site-of-lesion testing. The ABR is typically evoked with a short-duration pulse delivered to the ear at a predetermined intensity. At high-intensity levels, the acoustic stimulus evokes as many as five amplitude peaks. The peaks were first identified and categorized by Jewett and Williston. Three of these peaks (waves I, III, and V) are the major peaks and are generally accepted as corresponding to firing of the first-order neurons of CN VIII (wave I), the superior olivary complex (wave III), and the inferior colliculus (wave V). These three major waves are present at approximately 2-msec intervals (in children and adults with normal hearing) after the onset of the acoustic stimulus at high-intensity levels ( Fig. 16.10 ). As revealed in Fig. 16.10 , as the intensity of the stimulus is decreased, the amplitude of all peaks decreases, the latency of each peak increases, and the replicability of the early waves (I and III) decreases. Only wave V is identifiable at threshold levels.
Starr and Achor were among the first researchers to use ABR results to describe a diverse set of patients with neurological disorders. Patients with cortical problems had normal ABR values, whereas patients with acoustic nerve and low-brainstem disorders had abnormal ABR results. This early evidence has been corroborated by numerous other reports in which ABR values were shown to be abnormal in patients with CN VIII and low-brainstem disorders. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here