Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuropathy results from functional abnormalities or structural insults to the axons (sensory, motor, or combined), the myelin, or both. The insensate neuropathic joint is liable to arthrosis, fractures, alignment deformities, abnormal function, ulcer, and infection. Predilection for skeletal location is influenced by whether the insult is central or peripheral and the degree to which sensory, motor, and/or autonomic function is insulted.
Because diabetic pedal neuroarthropathy (Charcot foot) is commonly encountered by radiologists, the presentation and pathogenesis of this entity are emphasized.
Neuropathy may be inherited, congenital, or acquired. Inherited neuropathies include hereditary sensory and autonomic neuropathy, sporadic sensory neuropathy, and Charcot-Marie-Tooth disease. Congenital insensitivity to pain (familial dysautonomia with and without anhidrosis) is a well-known but uncommon neuropathy of unknown etiology. Acquired peripheral neuropathy results from neural insult secondary to trauma; cancer; many common drugs, including corticosteroids; human immunodeficiency virus infection; nutritional deficiencies; alcoholism; syphilis; vascular and metabolic diseases, including diabetes mellitus types 1 and 2; and/or infection affecting the peripheral nerves. Central causes for neuropathy include stroke, syringomyelia, tumor, spinal cord injury, and meningomyelocele.
More than 100 types of peripheral neuropathy have been described. Charcot-Marie-Tooth disease has a prevalence of 1 in 2500. Syphilis was the disease most commonly associated with neuroarthropathy until antibiotic control in the mid-1930s. In some underdeveloped countries, Hansen disease (leprosy) persists as a major cause of neuroarthropathy (see Chapter 70 ). The longer life span of diabetic patients due to insulin treatment permits the development of chronic complications, with diabetes presently the most common cause of neuroarthropathy in developed countries. There are an estimated 20 million diabetic patients in the United States, with pedal neuropathy detectable in about 7.5% at disease onset and 30% overall. Only 0.1% to 2.5% progress to Charcot changes, which are more common in patients of advanced age, with longer disease duration, with poor glycemic control, and with ocular and renal damage.
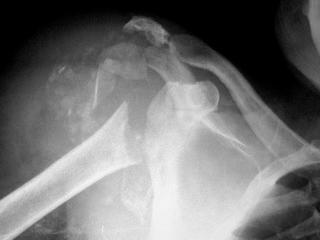
Different neuropathic conditions have a predilection for specific joints. In the upper extremity, syringomyelia is the most common cause for Charcot joint, usually of the shoulder, but, occasionally, the elbow or hand. The finding of shoulder girdle neuroarthropathy should elicit a search for cord abnormality ( Fig. 62-1 ). Congenital insensitivity to pain can involve multiple joints (lower extremity more commonly than the upper extremity) and the spine. Syphilitic and alcoholic neuropathies affect the weight-bearing joints of the lower extremity, most commonly the knee. Alcoholic pedal neuroarthropathy manifests in the forefoot. The inherited nerve disorder of Charcot-Marie-Tooth disease (type 1 demyelination, type 2 axonal) is manifested by progressive motor and sensory neuropathy due to damage to the peroneal nerve. Fatty replacement and atrophy of calf and pedal flexor and extensor muscles result in high arch and clawtoe deformities. Diabetic neuroarthropathy usually affects the ankle and tarsus and, less commonly, the metatarsophalangeal joints. Large-joint Charcot involvement does occur in diabetes, although uncommonly. The neuroarthropathy of Hansen disease commonly involves the feet or hands, resembling other conditions with sensory deficit, such as frostbite and scleroderma ( Fig. 62-2 ). Vertebral involvement is seen with cord pathologic processes, such as meningomyelocele, congenital insensitivity to pain, syphilis, and, rarely, diabetes. If the spine is fused, Charcot changes usually are seen just caudal to the lowest fused level ( eFig. 62-1 ).
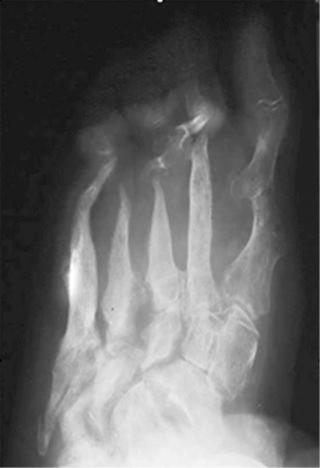
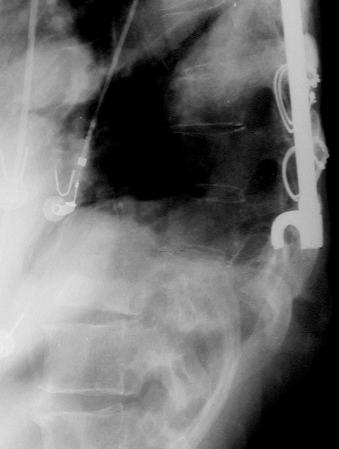
With congenital insensitivity to pain, trivial trauma may result in fracture or epiphyseal detachment. Beginning at birth, manifestations include fractures, dislocations, Charcot joints, osteonecrosis, and osteomyelitis, most commonly of femur, tibia, metatarsals, and spine ( Fig. 62-3 ). In the chronic setting, the extremities may be shortened due to premature epiphyseal fusion.
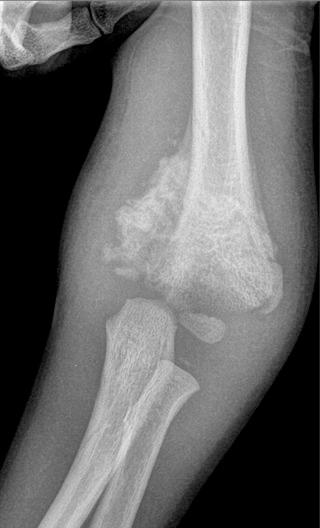
Pedal neuropathy is often asymmetric in diabetic patients, with the acutely affected foot edematous, with or without pain. Chronic motor neuropathy produces muscle atrophy, resulting in alignment deformities. If neuropathy is unchecked, Charcot changes may ensue. The acute Charcot foot will be hot and edematous with palpable pulses and is clinically worrisome for cellulitis or even osteomyelitis. The mean skin temperature increases from 2° C to 5° C in the Charcot joint compared with the contralateral foot. Rearfoot valgus, forefoot adductus, and hypermobile joints are common. Diabetic ischemic ulcers usually occur at the toes or the plantar aspect of the first and fifth metatarsal heads and are shallow, erythematous, and painful and do not bleed. In contradistinction, diabetic neuropathic ulcers are fungating and callused, may bleed, can be painless, and typically occur over midfoot and hindfoot bony protuberances.
Jean Martin Charcot posited that neuropathic arthropathy (usually luetic in the 1860s) resulted from damage to the central nervous system and trophic peripheral centers controlling the nutrition of bones and joints. His concepts are no longer in currency, but Charcot's name continues as the descriptor for neuropathic osteoarthropathy. Two theories—the neurovascular and the neurotraumatic—elucidate the pathophysiology of neuroarthropathy. According to the neurovascular theory, in the absence of neural stimulus to the extremities, sympathetic tone is compromised. This results in vasodilatation and hyperemia in the soft tissues and marrow, predisposing to osteopenic subchondral bone liable to pathologic fractures. The fractures can heal with exuberant and bizarre callus formation. Diabetic Charcot changes require intact vascularity. Therefore, diabetic persons with vasculopathy and neuroarthropathy are nearly mutually exclusive cohorts, although there are case examples of persons with vasculopathy developing neuropathy after a revascularization procedure.
The neurotraumatic theory explains neuroarthropathy as the result of diminished proprioception and loss of normal sensory feedback, with progressive joint destruction from repeated trauma. The patient may be unaware of the problem and add insult to injury by continuing to use the joint.
Most likely, both neurotraumatic and neurovascular causes are collusive in the diabetic neuropathic foot. Furthermore, hyperglycemia causes adverse effects on nerve metabolism, perineural vasoconstriction, and hypoxia. It is not understood why Charcot foot is often unilateral and occurs only in a small subset of diabetic patients.
Regarding pedal neuroarthropathy, the foot can be divided longitudinally into medial and lateral columns, with the medial column involved earlier and more frequently by neuroarthropathy. In diabetic patients, the forefoot is more often involved with ischemia, and the midfoot and hindfoot are more often involved with neuroarthropathy.
The weight-bearing line is drawn from the calcaneal tuberosity to the sesamoids on lateral radiographs taken with the patient bearing weight. If the pedal arch collapses and a bony structure extends below this line (i.e., rocker-bottom deformity), the overlying soft tissues may ulcerate ( Fig. 62-4 ).
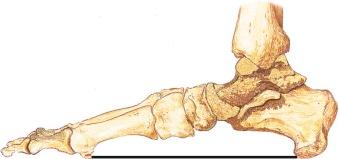
The gross Charcot foot specimen has bone fragments embedded in synovium ( eFig. 62-2 ). On histologic study, the specimen may demonstrate calcium, cartilage, and shards of bone lining the deep layers of the synovium.

This is termed detritic synovitis and may also be seen with osteonecrosis, calcium pyrophosphate deposition disease, psoriatic arthritis, and osteoarthritis. Biopsy may be performed to confirm infected Charcot foot or differentiate Charcot foot from tumor (e.g., chondrosarcoma is occasionally initially considered in Charcot shoulder), but biopsy and a histologic analysis are now infrequently performed to diagnose an uninfected Charcot joint.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here