Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The autonomic nervous system plays a significant role in the genesis and maintenance of ventricular arrhythmias (VAs) and powerfully modulates the underlying substrate in a dynamic manner. Blockade of the sympathetic nervous system, whether through medications or neuraxial modulation, has been extensively studied and associated with a reduction in the risk for sudden cardiac death (SCD) and burden of VAs, whereas pharmacologic sympathetic blockade is standard-of-care for atrial fibrillation (AF). Vagal modulation is currently under investigation for treatment of VAs, given that parasympathetic dysfunction contributes to occurrence of VAs and increases risk for SCD after myocardial infarction and in heart failure. Various methods for vagal modulation have also shown promise for AF. In this chapter the anatomy of the cardiac sympathetic nervous system and the pathologic changes associated with neural remodeling in the setting of scar and cardiomyopathy are briefly discussed. Subsequently, methods beyond medical therapy for neuraxial modulation of AF and ventricular tachyarrhythmias (VT) are presented.
The preganglionic sympathetic neurons that innervate the heart reside in the intermediate zone of the thoracic spinal cord. These preganglionic sympathetic nerves pass through the white rami communicantes, enter the sympathetic trunk, and terminate in the cervicothoracic ganglion as well as the T2 to T4 ganglia. Of note, preganglionic neurons may synapse on neurons within the ganglia at the same thoracic level or may travel within the sympathetic chain and synapse on neurons of ganglia at other spinal levels. The preganglionic neurotransmitter within the ganglia is acetylcholine. The cervicothoracic ganglia, which are often a fusion of the C8 and T1 ganglia, are called the left stellate (LSG) and right stellate (RSG) ganglia, respectively. The LSG and RSG, along with the ganglia of T2 to T4, give rise to postganglionic axons that target organs including the heart, the esophagus, trachea, and the head and neck ( Fig. 137.1 ).
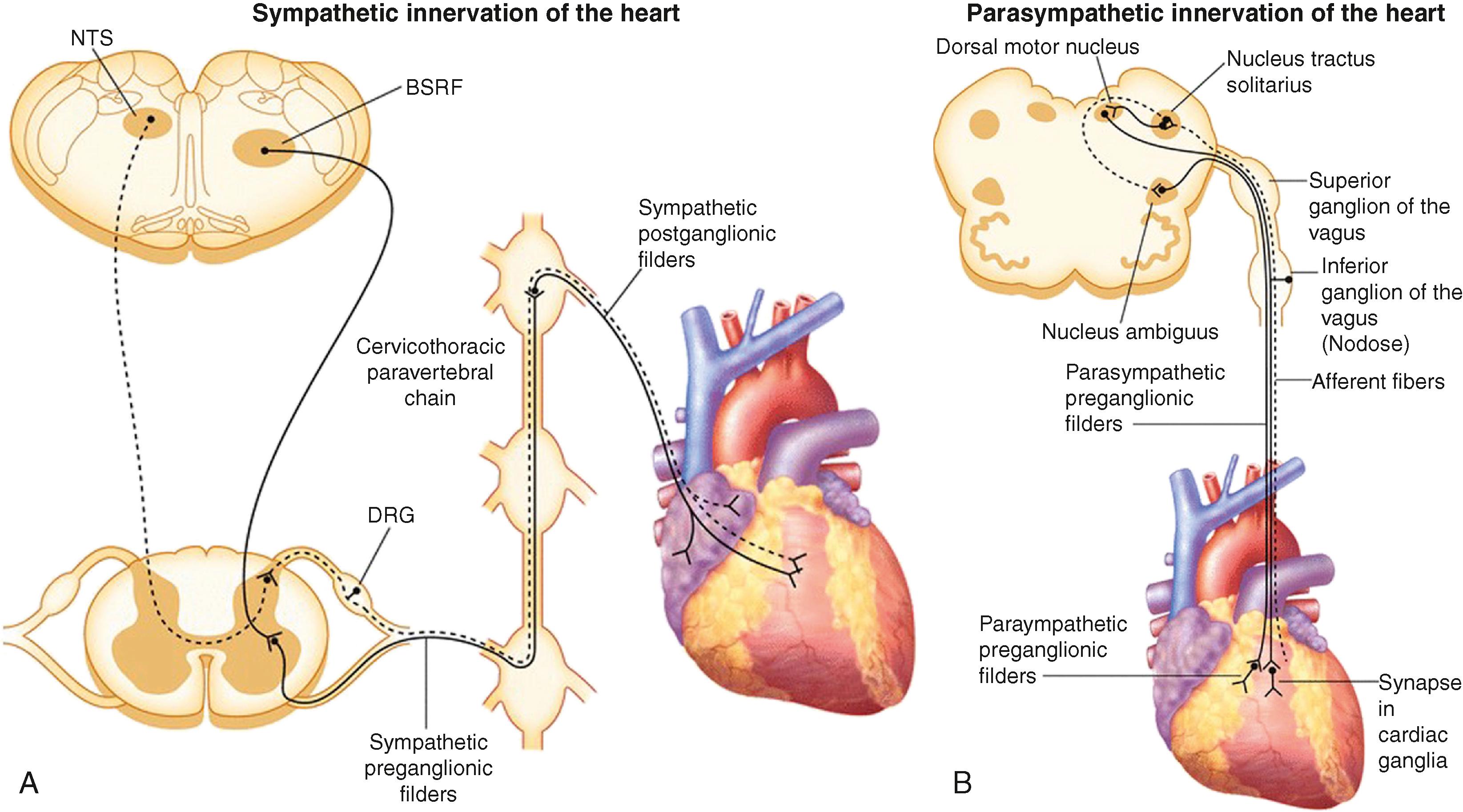
The vagal preganglionic fibers arise from dorsal motor nucleus and nucleus ambiguus of the brainstem and synapse on postganglionic parasympathetic neurons, part of the intrinsic cardiac nervous system of the heart (see Fig. 137.1 ). , Of note, postganglionic sympathetic nerve fibers from the stellate ganglia join the branches of the preganglionic vagus nerve to form the left and right cardiac (cardiopulmonary) nerves that are destined for the heart. , These cardiac nerves form a network of neurons on the epicardium called the cardiac plexus, consisting of multiple intrinsic cardiac ganglia located at the posterior aspect of the left atrium, at the aortopulmonary window, at the superior and posterior aspect of the right atrium, and at the junction of the inferior vena cava and the dorsal inferior surface of the right atrium. Postganglionic sympathetic nerve fibers run along the cardiac vessels and innervate myocardium. Similarly, postganglionic parasympathetic fibers from the intrinsic cardiac ganglia also innervate both the atria and ventricles as well as the conduction system.
It is important to note that cardiovascular afferent nerves, transmitting information from the heart to the central nervous system (CNS) travel within cardiopulmonary nerves and via the vagal and sympathetic highways to the nodose and dorsal root ganglia, respectively. Processing of afferent information at multiple levels, including the intrinsic cardiac nervous system, extracardiac intrathoracic ganglia, spinal cord, brainstem, and higher centers, subsequently alters sympathetic and parasympathetic efferent output to the heart, maintaining normal rhythm and cardiac function.
Myocardial infarction (MI) and heart failure, in addition to leading to scar formation, cause remodeling of cardiac nerves. Initial denervation, followed by nerve-sprouting and transdifferentiation of sympathetic nerves, leads to heterogeneity in repolarization and can modulate reentry.
MI causes death of sympathetic fibers within the scar and loss of efferent sympathetic innervation at noninfarcted apical sites. This process is followed by attempts at reinnervation of the infarcted areas by the peripheral neurons of the stellate ganglia. In this regard, localized areas of nerve sprouts have been noted in border zone regions of myocardial infarcts in patients with cardiomyopathy. , Norepinephrine levels in animal models of MI have been noted depleted in dense scar regions, are predominantly decreased in border zone regions (with possible exception local areas of nerve sprouts), and can be increased in basal noninfarcted regions. In response to LSG stimulation, viable myocardial sites apical to an infarct show evidence of heterogeneity in innervation; certain viable sites shorten their repolarization, whereas others show no response. All denervated areas showed denervation supersensitivity, defined as an exaggerated response to norepinephrine infusion in a canine model of MI. A similar phenomenon was noted in patients with ischemic cardiomyopathy undergoing VT ablation. Activation recovery interval (ARI) measurements, a surrogate of action potential duration, show a reduced response to indirect sympathetic stimulation via nitroprusside in dense scar and in the viable periinfarct myocardium, suggesting denervation. These sites demonstrate an exaggerated response to isoproterenol infusion, suggesting the presence of denervation supersensitivity in humans. Further, the response to sympathetic stimulation is extremely heterogeneous, with a greater than twofold increase in dispersion in ARI with nitroprusside infusion. The cellular mechanisms for this response do not involve differences in the β-adrenergic receptor or the α-subunit of stimulatory G protein density. , Heterogenous sympathetic reinnervation appears largely because of production of chondroitin sulfate proteoglycans in scar regions, that by binding to their receptor, tyrosine phosphatase receptor sigma, prevent normal sympathetic reinnervation. Modulation of this receptor or its absence in a mouse infarct model restores normal sympathetic reinnervation after axonal injury and reduces risk for VAs. These studies demonstrate that transmural MI and heart failure not only alter the myocardial substrate for arrhythmias but also disrupt innervation to histologically viable myocardium, leading to denervation supersensitivity and a nonuniform electrophysiologic response to sympathetic stimulation ( Fig. 137.2 ). This contributes to the genesis of VAs in both acute and chronic MI.
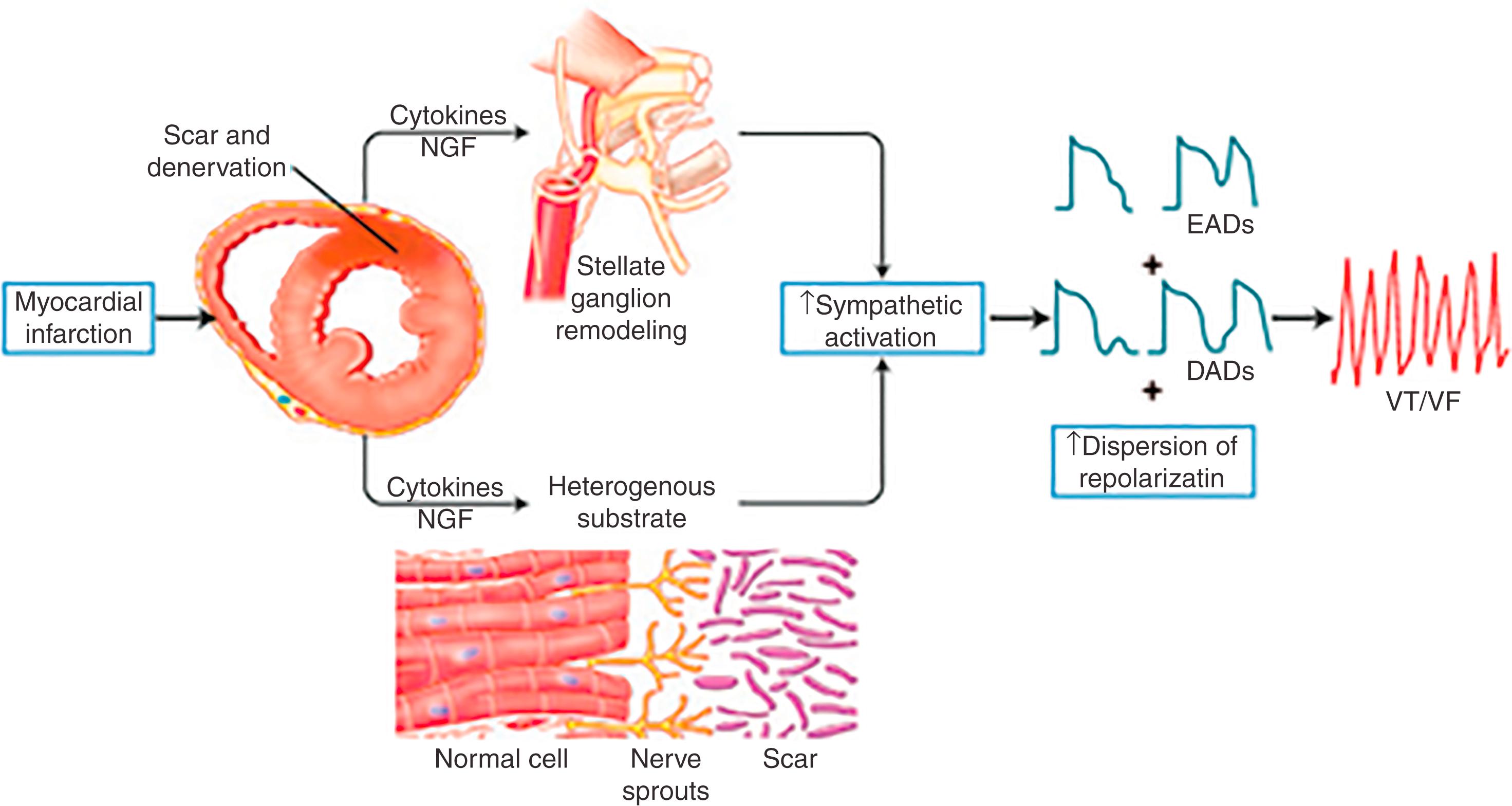
In addition to cardiac neural remodeling, structural remodeling of both the RSG and LSG in the setting of MI and heart failure has been described. Increased nerve density, along with increased mRNA levels of NGF and GAP-43 in the LSG of dogs with MI and nerve sprouting, has been reported. Similarly, in human cadavers with evidence of cardiac scar, the neuronal number in the LSG is increased compared with cadavers with normal hearts. In a different study, compared with cadavers with normal hearts, the stellate ganglia of cardiomyopathy patients with VT demonstrate increased neuronal size and synaptophysin density, as well as evidence of oxidative stress. These results provide a possible mechanistic link between neural remodeling (nerve sprouting within the myocardium) and remodeling of the stellate ganglia and VAs. Interaction between areas of denervation, regional nerve sprouting and transdifferentiation (neural remodeling), electrical remodeling because of heart failure, and structural and functional remodeling of the stellate ganglia all combine to create a substrate that can be conducive for VAs and SCD (see Fig. 137.2 ).
In 1978, Coumel and colleagues suggested that either limb of the autonomic nervous system may generate AF. The effects of sympathetic stimulation have been studied in both animal models and humans. Sympathetic activation increases calcium transients and atrial ectopy, often from the pulmonary veins, which can trigger AF. Sympathetic stimulation further reduces the atrial effective refractory period (ERP), predisposing to AF. In addition, sympathetic stimulation can cause nonuniform electrophysiologic changes, leading to heterogeneity that sets the stage for multiple reentry. Wang and Nattel showed that normal atrial tissue contains at least three types of atrial myocytes with distinct repolarization characteristics. Each cell type may show dramatic differences in action potential duration and ERP when exposed to interventions that augment outward currents, increasing regional heterogeneity and creating the substrate for multiple reentry. Subsequent studies have suggested that rather than being triggered by either vagal or sympathetic overactivity, AF may be associated with simultaneous activation of both limbs of the autonomic nervous system. Simultaneous spontaneous stellate ganglia discharge and vagal nerve activity have been observed to precede the onset of paroxysmal AF in a canine AF model of rapid atrial pacing and heart failure. , , Cryoablation of bilateral stellate ganglia and of the superior cardiac branches of the left vagus nerve eliminated all episodes of paroxysmal AF. In orthotopic heart transplant patients, who have both vagal and sympathetic inputs removed and therefore have hearts that are decentralized from the CNS, AF is rare and often only associated with rejection or significant transplant vasculopathy.
Many studies have suggested that sympathetic nerve stimulation can predispose to VAs. Greater increases in the amplitude of early afterdepolarization have been observed with LSG stimulation. Priori et al. showed that LSG stimulation caused delayed afterdepolarizations in vivo in cat hearts, suggesting triggered activity as the mechanism of ventricular arrhythmogenesis. Further, an increase in dispersion with sympathetic stimulation, both during ischemia and in normal canine and porcine hearts, has been observed. In the porcine heart, LSG stimulation increased ARI dispersion by fourfold and caused VF in 25% of the animals. In open-chest dogs, electrical stimulation of LSG, the left middle cervical or left caudal pole of the cardiopulmonary nerve, or the ventrolateral nerve caused VT in 13 of 22 normal dog hearts, with isochronal mapping showing the earliest electrical excitation occurring on the posterior aspect of the ventricles. Electrical stimulation of the left ansa subclavia in open-chest dogs during left circumflex occlusion increased the incidence of VF from 35% to 73%. The incidence of induced VAs in dogs with myocardial ischemia increased from 54% to 68% and 63% with LSG and bilateral stellate stimulation, respectively. RSG stimulation had no significant effect on the incidence of arrhythmias in this study. Further, by measuring local VF intervals, Opthof et al. showed that LSG stimulation can increase dispersion in refractoriness by shortening refractoriness across nonischemic sites while either not changing or increasing refractoriness at ischemic sites during coronary occlusion, thereby increasing dispersion across the ischemic border by 14% to 59%. Finally, subthreshold LSG stimulation has been shown to increase nerve sprouting in dogs with MI. These dogs have a greater number of episodes of VT compared with controls, suggesting that additional mechanisms underlie the increase in dispersion seen in infarcted hearts.
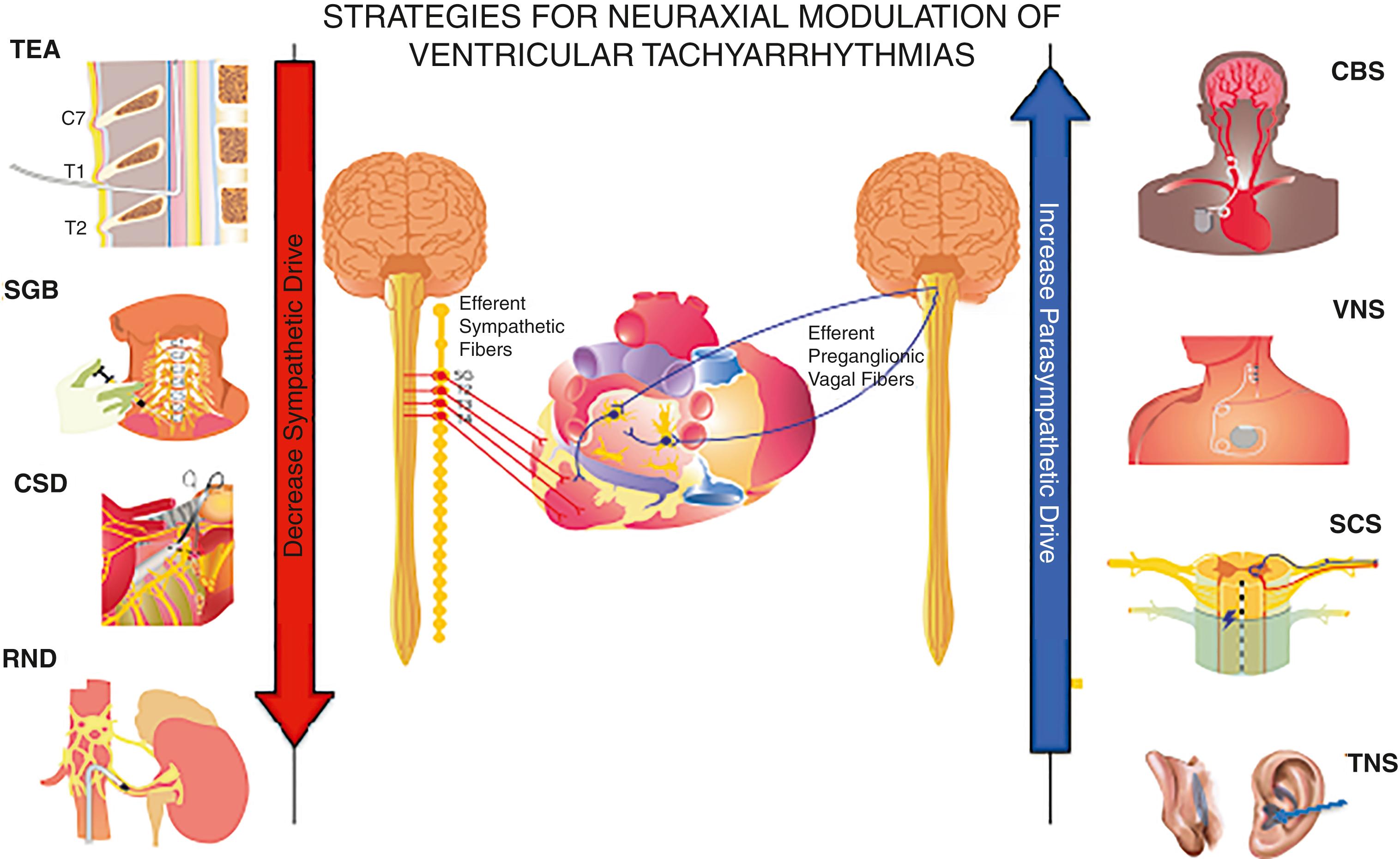
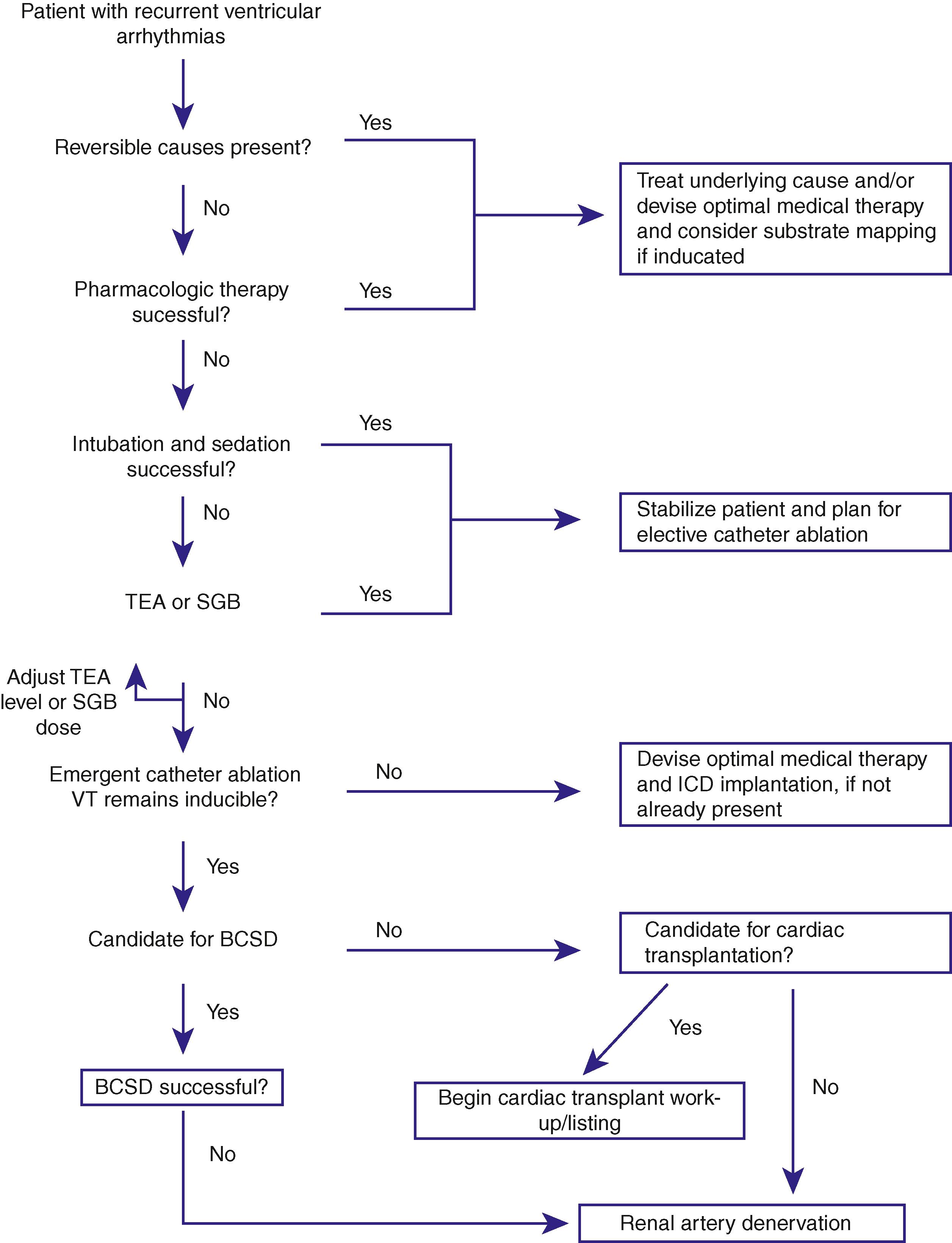
Nonpharmacologic treatment of atrial arrhythmias and VAs is predominantly aimed at reducing sympathetic tone. Various neuromodulatory therapies including thoracic epidural anesthesia, stellate ganglion blockade, cardiac sympathetic denervation, and renal denervation have been tried with some success ( Fig. 137.3 ). Given that refractory or resistant VAs pose a life-threatening problem and a therapeutic challenge, a flow chart suggesting a step-wise approach to incorporation of various neuromodulatory therapies in the treatment of patients with refractory VT is presented in Fig. 137.4 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here