Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Incidence is rising, likely because of increased detection; the majority are found in the gastrointestinal (GI) tract and pancreas.
Peak incidence is in sixth to seventh decade of life.
Histologic: well differentiated (previously known as carcinoid tumors) versus poorly differentiated (G3) (previously known as neuroendocrine carcinomas)
Well-differentiated neuroendocrine tumors (NETs) can be further subgrouped into low (G1) and intermediate grade (G2) by mitotic rate (<2 mitoses/10 high-power field [hpf] vs. 2–20 mitoses/10 hpf) or Ki67 labeling index (<3% vs. 3%–20%).
Majority originate from enterochromaffin cells in GI tract.
Other locations include bronchopulmonary tract, larynx, pancreas (islet cell tumors), biliary tract, thymus, ovary, kidney, and skin.
This varies depending on physical characteristics, origin, and if hormones are produced.
Ninety percent of patients are hormonally asymptomatic; hormonal products must obtain direct access to the systemic circulation (hepatic metastases, significant retroperitoneal disease, primary tumor not draining to portal venous circulation) to be active and not cleared by liver.
Mass of tumor can cause intussusception or obstruction (e.g., small bowel obstruction [SBO], jaundice, appendicitis, pancreatitis).
Hormone secretion can cause carcinoid symptoms, such as:
Flushing—bradykinin, hydroxytryptophan, prostaglandins
Diarrhea—serotonin
Cramping—serotonin
Endocardial fibrosis—serotonin
Pellagra—depletion of niacin stores due to serotonin
Bronchospasm—bradykinin, histamine, prostaglandins
Telangiectasia—vasoactive intestinal peptides, serotonin, prostaglandins, bradykinin
Glucose intolerance—serotonin
Arthropathy—serotonin
Hypotension—serotonin
Symptoms can be induced by consumption of ethanol, chocolate, or blue cheese and by exertion and surgery.
Metastatic potential is related to grade, location, and size.
Urinary 5-hydroxyindoleacetic acid, serum chromogranin A, and substance P are most commonly measured.
Chest computed tomography (CT) scan—bronchial NET
Endoscopy/endoscopic ultrasound (EUS)—ability to biopsy gastric, duodenal, distal small ileal NETs, colorectal carcinoids, and pancreas
Multiphasic CT scan and/or CT enterography—liver metastases, retroperitoneal masses, and carcinomatosis
Nuclear medicine scans (pentetreotide indium-111, gallium Ga-68 Dotatate positron emission tomography [PET] scan, and MIBG [metaiodobenzylguanidine I123])
Angiography/selective venous sampling—may be useful in difficult cases
Appendiceal
Less than 1 cm, low grade without mesoappendix invasion and negative margin—appendectomy
One to 2 cm—controversial because risk of metastases is rare, particularly if smaller than 1.5 cm
Greater than 2 cm or other higher risk factors for lymphatic metastases in good-risk patient—right hemicolectomy
Small bowel (more likely to metastasize)
Multicentric in 20%–40%—must examine all of small bowel and colon
Resection of involved small bowel and mesentery; can consider cholecystectomy if somatostatin analogs are likely to be used in future
Rectal
Less than 1 cm, T1, low grade—endoscopic/transanal excision
One to 2 cm, low grade controversial
Greater than 2 cm, T2 or other higher-risk factors, low anterior resection (LAR) or abdominoperineal resection (APR) to resect and clear draining nodes
Gastric
Type 1 associated with chronic atrophic gastritis and achlorhydria: endoscopic resection of small tumors and surveillance, antrectomy for progression to reduce gastrin secretion or more radical resection for more advanced disease
Type 2 associated with gastrinoma: treat primary
Type 3 sporadic NET not in association with chronic atrophic gastritis: partial or total gastrectomy and regional node resection
Metastatic disease
Observation in asymptomatic patients with very slow progression
Systemic therapy
Somatostatin analogs, both for symptoms and for tumor growth
Molecular therapies targeting mechanistic target of rapamycin (mTOR), thymidine kinase (TK), or vascular endothelial growth factor (VEGF), such as everolimus, sunitinib, bevacizumab
Interferon-α
Radiolabeled somatostatin analogs
Cytotoxic chemotherapy, particularly for high-grade or rapidly growing cancers
Surgical intervention
Obstruction, perforation, bleeding, or other tumor-related symptoms
Potentially resectable disease
Severe carcinoid symptoms for which sufficient debulking anticipated with low morbidity
Regional intraarterial therapy
Radiofrequency or stereotactic body radiation therapy (SBRT) ablative therapies
This is found in 0.1% of individuals with duodenal ulcers and 2% of individuals with ulcers refractory to medical therapy.
Twenty-five percent of gastrinomas are associated with multiple endocrine neoplasia syndrome type 1 (MEN1).
Gender distribution is 60% male, 40% female.
Mean onset of symptoms is before sixth decade of life.
Eighty-five percent of gastrinomas are located in the gastrinoma triangle, more commonly in duodenum than in pancreas itself ( Fig. 52.1 ).
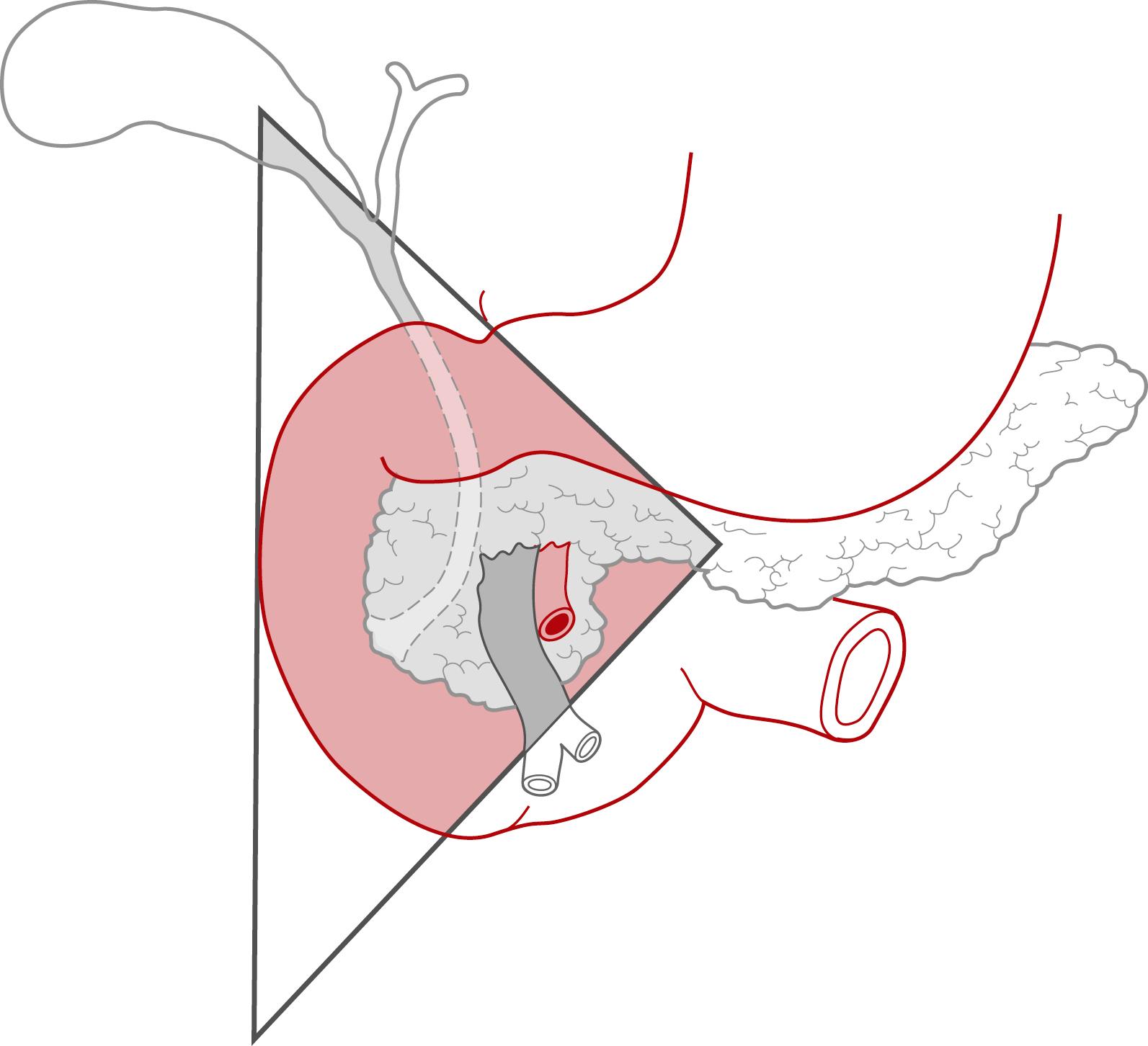
Ectopic locations include splenic hilum, gastric wall, mesentery, and liver.
Symptoms are secondary to hypergastrinemia; increased gastrin leads to acid hypersecretion by parietal cells and peptic ulceration.
Abdominal pain and diarrhea are most common symptoms.
It may be caused by ulcers that 90% of individuals have endoscopically confirmed in the upper GI tract.
Ulcers may also lead to bleeding (30%–50%) and perforation (5%–10%).
Diarrhea presents as only symptom in 20% of cases. It is caused by acid hypersecretion, injury to small bowel mucosa, malnutrition caused by inactivated enzymes, and increased motility.
Gastroesophageal reflux
Serum gastrin level
Increased in 90% of patients (reference range, 100–200 pg/mL)
Fasting serum gastrin level greater than 1000 pg/mL in presence of gastric acid diagnostic
For serum gastrin levels 200–1000 pg/mL, secretin stimulation testing can distinguish gastrinomas from G-cell hyperplasia
Localization
Endoscopy/EUS, multiphasic CT, or magnetic resonance imaging (MRI)
Somatostatin receptor scintigraphy or Ga-68 Dotatate PET/CT
Most gastrinomas have somatostatin receptors.
False-negative rate for small duodenal wall gastrinomas is high.
Angiography with selective arterial secretin stimulation and venous sampling
Intraoperative
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here