Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgment: Special thanks to Amy Moore, MD, who coauthored the seventh edition of Green’s online chapter on nerve transfers—much of which was incorporated into this updated chapter—and to Rolfe Birch for his extraordinary contributions to this chapter in the fifth, sixth, and seventh editions.
The permanent sequelae of peripheral nerve injury can range from complete recovery to profound and debilitating functional loss. Regardless, many injury patterns are associated with a protracted convalescence, and both acute and chronic neuropathic pain can be more problematic than any associated sensory or motor loss. For injuries in-continuity, spontaneous recovery is certainly possible, though for more severe injury patterns, surgical intervention offers the only hope for functional return.
While general treatment principles for nerve injuries are widely accepted, many interventions depend on a great deal of experience-based judgment. The specific technical details of how to best satisfy these principles are still the subject of rigorous debate. Reporting bias may also help explain the inconsistencies and disparities that appear in the clinical outcomes literature. Factors including time from injury, genetic or systemic patient differences, and wide zones of injury remain factors that are largely out of the surgeon’s control. Nerve surgery can be alternatingly one of the most rewarding and most frustrating procedures, and treating physicians must be adept at patient education, surgical techniques, decision making, emotional counseling, and chronic pain control. Despite a growing appreciation of the complex interplay between cells, growth factors, guidance cues, and genetics, most major recent advances have come in the form of shifting treatment strategies such as an increased emphasis on the use of nerve transfers.
Peripheral nerve injuries in general are quite common and are associated with 1% to 3% of all traumas. , Severity and the need for intervention is often associated with the mechanism of injury. Although there are exceptions, penetrating and lacerating injuries that immediately preceded a neurologic deficit can be assumed to have resulted in nerve transection, while nerve deficits from crush injuries, fractures, and dislocations are more likely to be associated with nerve injuries in-continuity. Many of these can be treated expectantly and only a smaller number eventually require surgery. Regardless, when considering the consequences of undertreatment, population-based “rates of injury” and the percentage chance of “needing surgery” may be useful for patient counseling but in truth offer limited guidance, as assessment and treatment plans must be individualized to each patient.
The peripheral nervous system is made up of neurons, supportive glial cells (Schwann cells), connective tissue, and end targets including muscle and sensory organelles. Motor neuron cell bodies reside in the anterior horn of the spinal cord (and are referred to as anterior horn cells), and sensory neuron cell bodies are found in dorsal root ganglia, which run parallel but outside the spinal cord. Cellular projections, known as axons, emanate from both types of cell bodies and extend into the periphery to connect with corresponding end targets—sensory organelles or motor endplates. Though pain is experienced in the cut finger after a paring knife accident severs a digital nerve, the cell body that has suffered the injury actually is located approximately 36 inches proximally along the spinal column. The neuron produces cellular proteins, neurotransmitters, and growth factors carried via molecular fast and slow transport to the axonal endbulbs.
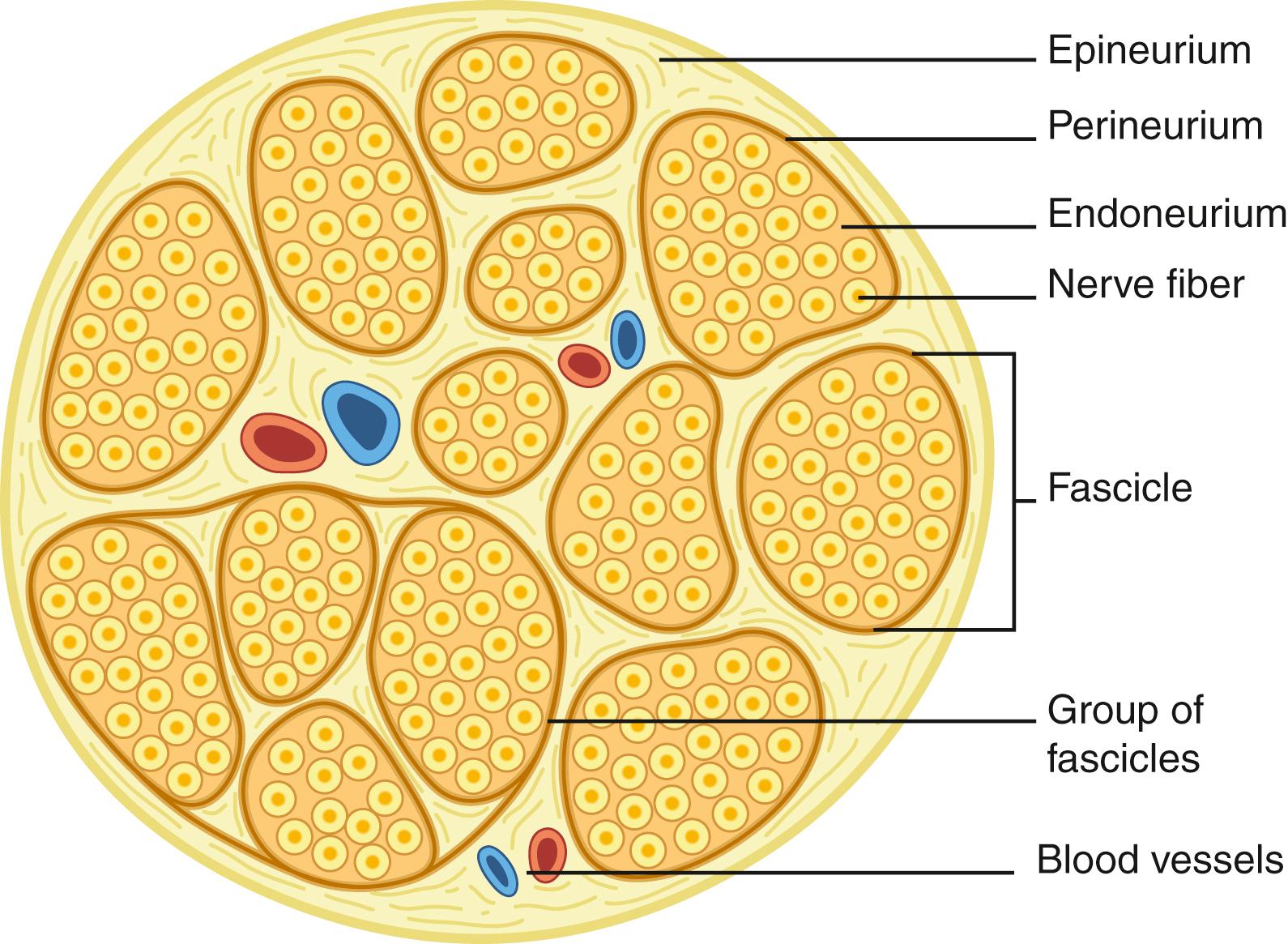
The nerve trunk consists of axonal extensions packaged in layers of specialized connective tissue: the endoneurium encases axons on the most microscopic level, the perineurium envelopes axons grouped together into macroscopically distinct fascicles, the inner epineurium cushions and protects the fascicles, and the outer epineurium encompasses and forms the surface of the entire nerve ( Fig. 30.1 ). The inner epineurial padding varies with in situ mechanical compressive and tensile stresses (e.g., so it is increased at joints), and thickenings within the inner epineurium organize and group certain fascicles together. The tough perineurium provides tensile strength to the nerve and is an extension of the blood-brain barrier from the central nervous system. The increased pressure within the central nervous system is maintained within this layer and is the reason why cut fascicles bulge from a proximal nerve stump. Multiple redundant arcades of blood vessels run along the surface and within these different structural layers to meet the high metabolic demands of peripheral nervous tissue. Though historically the intraneural plexus was felt to be poorly organized and random, more recent work has suggested a more organized and predictable pattern. ,
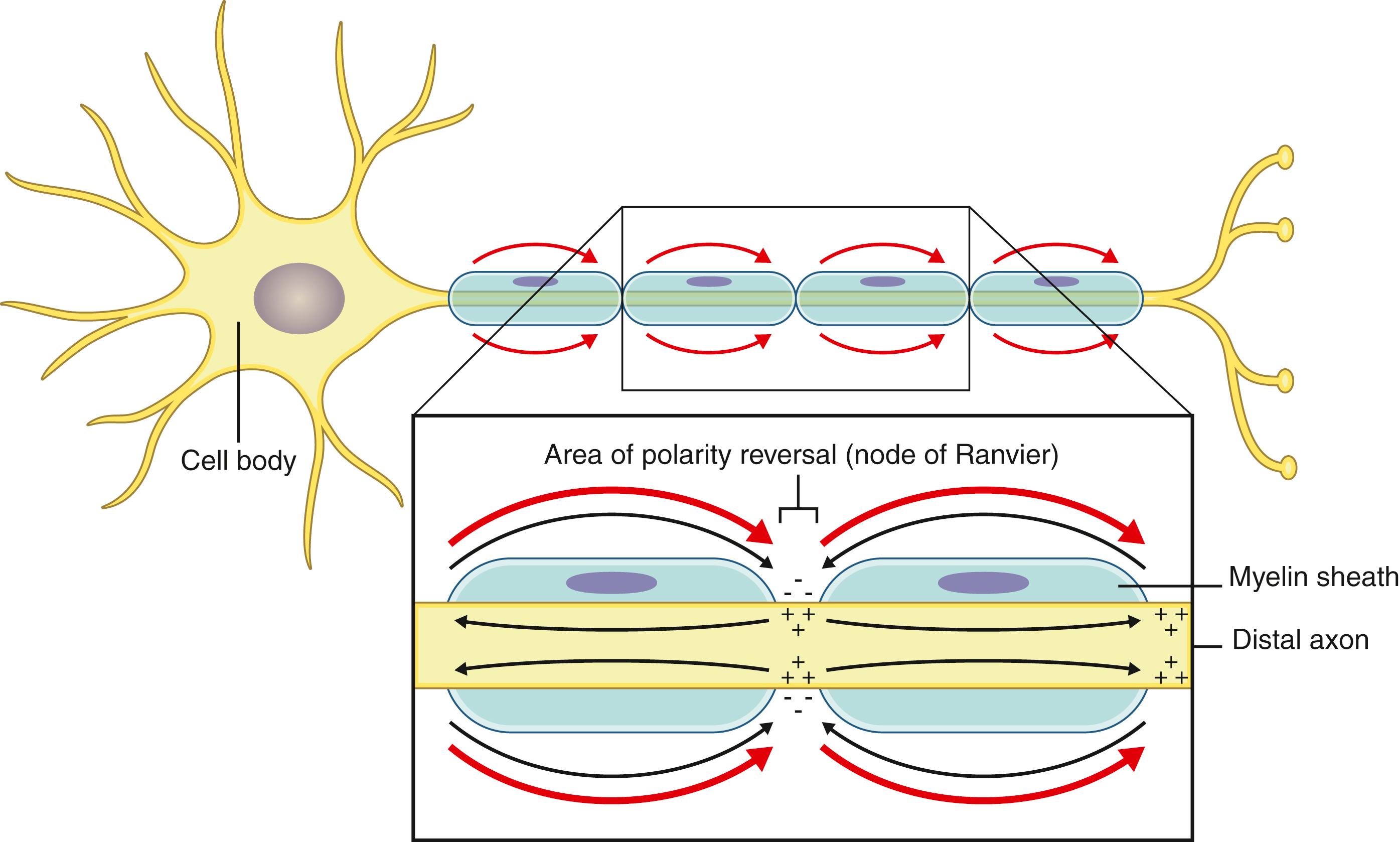
As the quintessential support unit, the pluripotent Schwann cell has roles in peripheral nervous system development, nerve regeneration, neuron maintenance, and signal conduction. Multiple small nonmyelinated nerve fibers—involved in pain, the autonomic system, nonsomatic sensory and motor fibers—are supported by shared Schwann cells. Myelinated axons, such as large motor and sensory fibers, however, are completely entubulated by columns of individual Schwann cells. The Schwann cells wrap around the axon forming tightly linked segments of a lipid-rich insulating sheath. The microscopic gaps between these segments, nodes of Ranvier, are not insulated, and this combination of alternating insulated and noninsulated areas are essential for normal signal transmission ( Fig. 30.2 ). Macrophages, mast cells, and fibroblasts are other prominent cells within the nerve.
Motor axons entering the muscle belly form focal connections at specialized synapses referred to as motor endplates. These endplates consist of neurotransmitter-rich terminal boutons on the axonal side, a microscopic intervening cleft, and a neurotransmitter receptor–rich segment of muscle lamella. Terminal Schwann cells, in yet another physiologic role, isolate and protect the motor endplates. A single motor axon has multiple branches and will innervate multiple muscle fibers (though each motor fiber is only innervated by one axon), and this population of motor fibers is referred to as a motor unit. In normal anatomic scenarios, the muscle fibers within the same motor unit are dispersed and intermixed with muscle fibers innervated by other axons.
The median, ulnar, and radial nerves represent the major peripheral nerves of the upper extremity distal to the brachial plexus. The medial brachial and medial antebrachial sensory nerves branch off the medial cord of the brachial plexus and should be familiar to the peripheral nerve surgeon since they offer potential graft material, can form painful neuromas from iatrogenic or traumatic injury, and need to be differentiated from other vital structures when exploring the medial brachium. The lateral antebrachial cutaneous nerve is a continuation of the musculocutaneous nerve that emerges from between the biceps brachii and brachialis muscle bellies to innervate the skin on the lateral aspect of the forearm.
Nerves are remarkably specialized cells uniquely designed to carry and transmit signals. Sodium-potassium intramembranous exchange pumps, as well as precise membrane permeabilities, create an ion concentration differential between the inside and immediate microenvironment outside the axon membranes (axolemma). Since ions are charged, this results in an electrical gradient between −60 and −70 mV, known as the resting potential. When a neuron is stimulated, ion gates within the proximal axonal membrane open, allowing a rapid influx of positively charged ions, which depolarize the membrane creating an electrical current. This current, known as the graded potential (since it degrades with time and distance), spreads almost instantaneously to neighboring intramembranous voltage-gated ion channels. As a specific voltage threshold is reached, these gates burst open, allowing the influx of sodium ions, which depolarize this segment of membrane wall. The generated current (known as an action potential) spreads to the next resting segment and, in this manner, the signal becomes self-propagating. The sodium influx is replaced by a potassium outflux that not only quickly repolarizes the membrane but also overshoots to temporarily hyperpolarize the membrane. The momentary hyperpolarization quickly stabilizes back to the resting potential, at which point it is capable of generating the next action potential when restimulated.
Though the generation of an action potential takes mere milliseconds, this is still relatively slow compared with a passive (or graded) current transmission. Analogous to a copper wire, passive current conduction is further enhanced with increasing diameter of the conductor (the axon) and its insulation (myelin sheath). The essential speed and efficiency of passive impulse transmission is combined with the self-propagation quality of action potentials to maximize both transmission speed and distance. Action potentials are only generated at the uninsulated nodes of Ranvier between the myelin segments. The combination of lightning-quick (passive) impulse conduction under the myelinated segments with the inception of fresh action potentials at the nodes is referred to as saltatory conduction (based on the imagery of a signal “jumping” between the nodes of Ranvier) (see Fig. 30.2 ).
When the impulse reaches the synaptic bulb at the end of a motor axon, the depolarization of the terminal membrane triggers a calcium influx releasing acetylcholine-filled vesicles into the synaptic cleft of the neuromuscular junction. The neurotransmitter diffuses across the cleft to activate nicotinic receptor-linked ion channels embedded in the sarcolemma (muscle fiber membrane). Analogous to the physiology within the axolemma, once a voltage threshold is reached, voltage-gated sodium potassium channels open, allowing an exuberant influx of sodium and depolarizing the muscle fiber membrane. The underlying voltage-sensitive sarcoplasmic reticulum release calcium ions, which influence the actin-myosin relationship, allowing these proteins to shift and slide past each other. The cumulation of millions of microscopic protein reactions results in the macroscopic muscle contraction, allowing functional skeletal movement. Contraction intensity is proportional to the number of stimulated motor axons, and the force generated is proportional to the cross-sectional area and volume of the muscle.
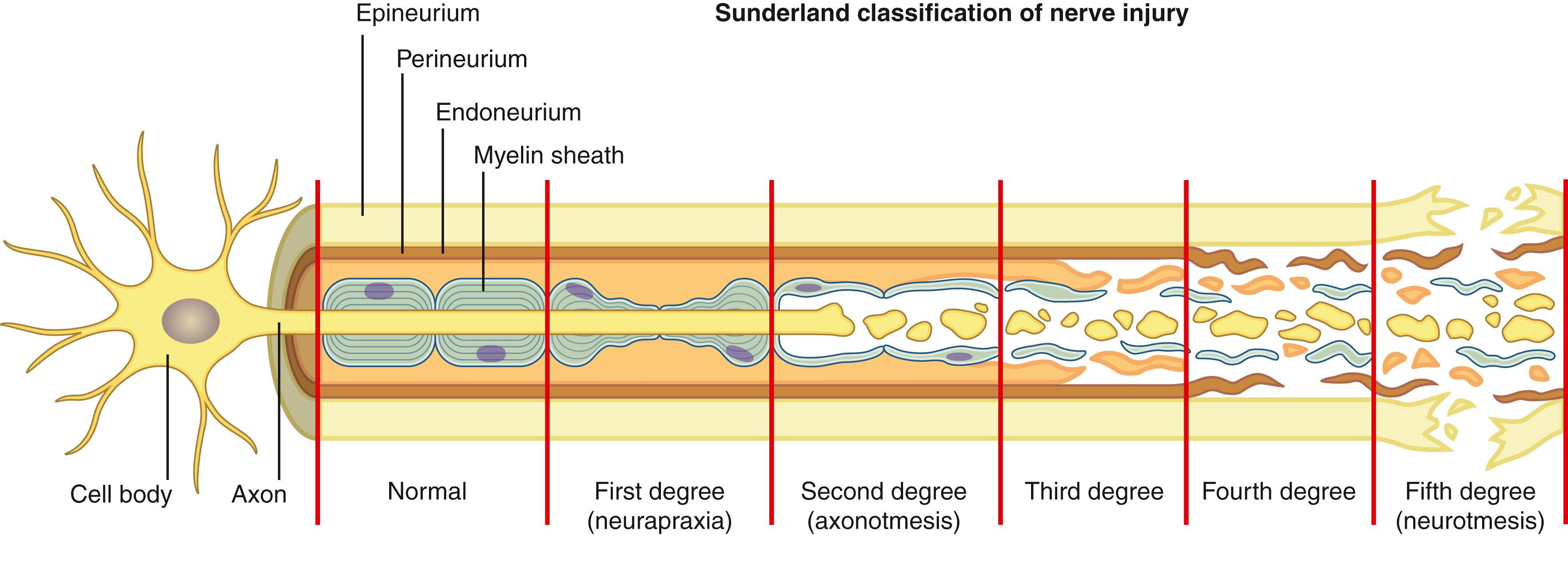
Like any biologic tissue, structural disruption and cellular response is related to the mechanism of injury and the intensity of the inflicted trauma. Peripheral nerves can be cut, pulled, or crushed, resulting in a spectrum of injury patterns ranging from focal ischemia to partial or complete rupture, as characterized by the descriptive Seddon and more detailed Sunderland classification schemes introduced 7 decades ago ( Fig. 30.3 ). , Conceptualizing nerve injuries based on a predictable and progressive sequence of physiologic and microstructural disruption is useful in predicting healing responses and appreciating diagnostic and surgical hierarchies. Mackinnon modified the classification by adding a sixth category to reflect the common incidence of combined injury patterns within the same nerve trunk.
Sunderland I (Seddon neuropraxia): The least severe injury classification implies a temporary conduction block caused by blunt trauma, stretch injury, compression, or vascular impediment. Interstitial edema, contusing, or disruption of neural metabolism can interfere with the membrane’s ability to maintain the physiologic resting potential so that, at least focally, an action potential cannot be generated. Most people have experienced a mild form of ischemic neuropraxia when sitting with their legs crossed. A more severe or prolonged form of neuropraxia involves focal degradation or loss of myelin. Depending on the cause and severity, neuropraxic dysfunction can last anywhere from a few minutes to days, weeks, or months. However, even in the most severe neuropraxic injuries, normal physiology is restored within 3 or 4 months and the prognosis for clinical recovery is excellent.
Sunderland II (Seddon axonotmesis): Associated with more severe trauma, the Sunderland II injury pattern involves disruption of the axon but preservation of its surrounding endoneurium. When divided, essential metabolites within the axoplasm cannot be transported within or across the zone of injury and the entire distal segment of the transected axon undergoes the well-characterized process of Wallerian degeneration, along with the axon immediately proximal to the injury (at least to the first node of Ranvier). An influx of extracellular calcium triggers a cascade of events including microtubule depolymerization, cytoskeleton breakdown, and axolemma disintegration resulting in cellular fragmentation and leaving disorganized cellular debris in place of the axon. , As the neuron shifts into a “damage control” mode, the cell body response is marked by marginalization of its nucleus and dissolution of the Nissl bodies, known as chromatolysis. Despite the upregulation of neuroprotectants such as heat-shock protein-27 (HSP27), up to 20% to 50% of sensory neurons do not survive. Schwann cells undergo a metamorphosis from a supportive cell to a reparative phenotype and release cytokines and neurotrophic factors to attract circulating macrophages, which enter the intraneural environment via nearby capillaries. Both the Schwann cells and macrophages participate in phagocytosis of the cellular debris, leaving endoneurial tracts cleared to accept regenerating axons. , The macrophages may remain within the nerve tissue, migrate to the lymphatic system, or undergo apoptosis. ,
Sunderland III (Seddon axonotmesis): This represents a partial internal rupture of the nerve trunk in which the degree of trauma is sufficient to disrupt the endoneurial connective tissue layer surrounding the ruptured axon. With this, the protective axonal pathways are lost, and spontaneous regenerative potential is severely limited. Additionally, the increased intraneural bleeding and inflammatory response associated with this more significant injury pattern introduces fibroblast-induced scarring that further diminishes axonal elongation.
Sunderland IV (Seddon axonotmesis): This is a complete intraneural rupture. With the loss of the longitudinally robust perineurial layer, the outer epineurium is the only connective tissue maintaining nerve trunk continuity. The resultant extreme internal derangement and fibrosis impedes all spontaneous regenerative efforts.
Sunderland V (Seddon neurotmesis): All connective tissue layers are involved, and the nerve has completely ruptured. Spontaneous regenerative potential to the motor and sensory targets is nil, and without surgical intervention, there is little hope of functional recovery.
Sunderland/Mackinnon VI: Most likely representing a large proportion of nerve injuries, this category represents mixed injuries in which, for example, some endoneurial tubes are maintained while others are completely ruptured, creating the clinically difficult scenario of partial but often functionally unsatisfactory axon regeneration.
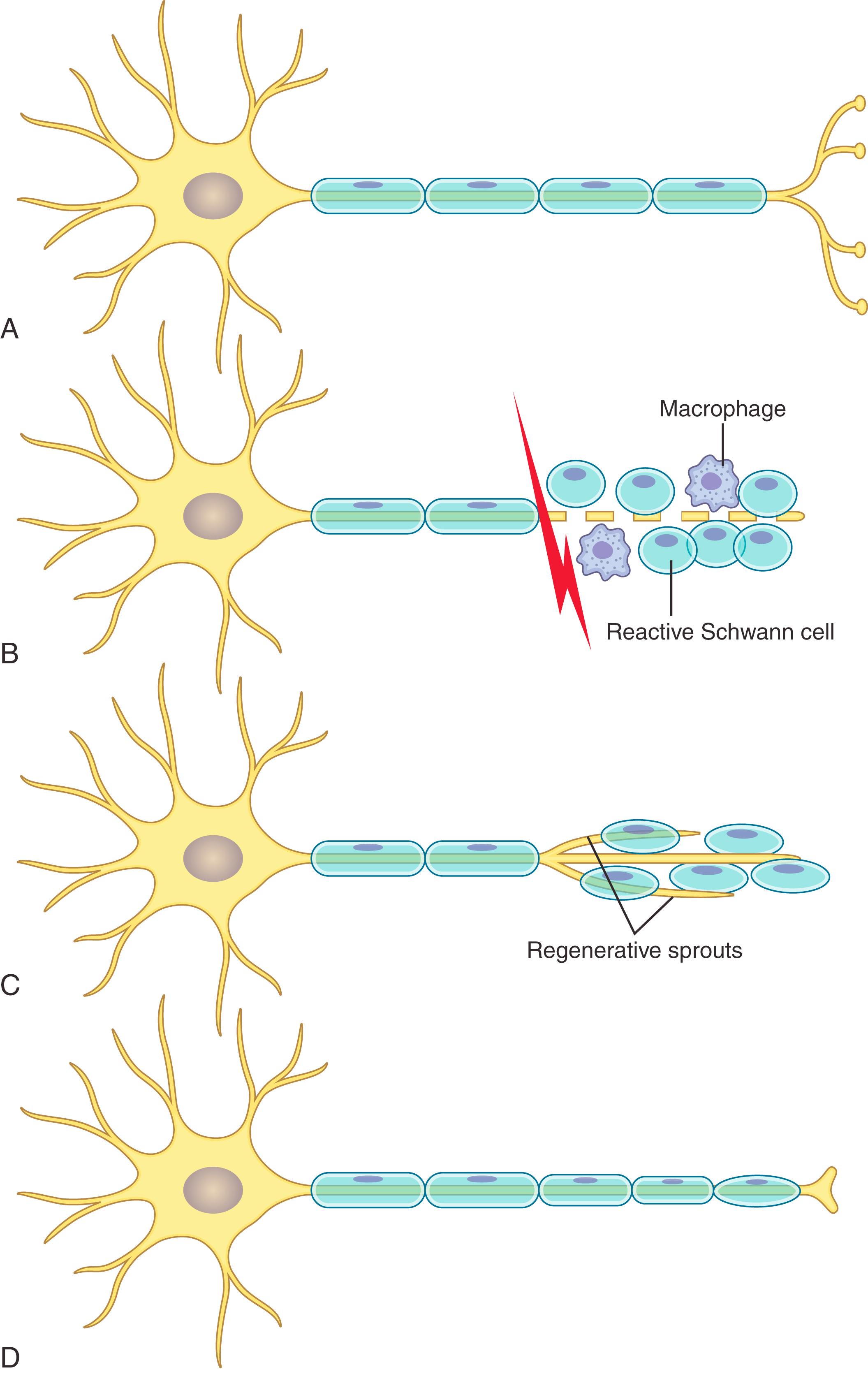
The Schwann cell’s essential role in orchestrating nerve regeneration by both stimulating and guiding regenerating axons cannot be overemphasized. Following Wallerian degeneration, the Schwann cells undergo yet another phenotype metamorphosis and, in a new role, align in columns (known as bands of Büngner) within the now unfettered endoneurial space. The Schwann cells create channels of basal lamina, which are anchored via fibronectin connections to the underlying connective tissue. Rich with glycoproteins such as laminin, these tracts will direct and guide subsequent elongating axons. Schwann cell–derived neurotrophic factors additionally provide an important stimulant to drive axonal regeneration as well. ,
Triggered by the loss of end target–mediated trophic feedback as well as a complex concoction of cytokines and neurotrophic factors released by local Schwann cells and macrophages, tubulin, actin, and lipid production are all upregulated within the cell bodies and transported to the axon stumps to support regeneration. , Growth cones form quickly after the injury and generate small finger-like filopodia to explore the local microenvironment and seek potential pathways for elongation. , Ultimately each axon will send out multiple branches in a phenomenon known as sprouting, and unimpeded axons provided appropriate pathways will regenerate at the frustratingly slow rate of 1 to 3 mm (average 1 mm) per day. In Sunderland II injuries, this unobstructed pathway is maintained, and regeneration is straightforward and predictable ( Fig. 30.4 ).
With greater degrees of intraneural derangement, fibroblast-induced scarring, or surgical scarring, the regenerative process becomes both more complex and less predictable. , Though the propensity for axons to grow toward nerve tissue (versus nonnerve tissue) was established several decades ago, more precise physiologic guidance toward appropriate type-specific end targets (i.e., motor axons growing toward motor endplates and sensory axons growing toward sensory organelles) is less well understood and less potent. The leading theory is that the axons display “preferential motor reinnervation” in which the motor axons tend to selectively enter motor endoneurial tubes, while the sensory axonal elongation progresses more randomly to enter any available tracts. Perhaps related to differences in guidance cues or Schwann cell phenotypes, the axonal guiding mechanism is far from perfect and many axons elongate toward the wrong targets. With successful reinnervation, the misguided axons prune back in what can only be considered a remarkably inefficient regenerative process.
Once the axons reach target muscles, the axons still need to grow into the muscle, reestablish contact with motor endplates, and undergo a maturation process. Additionally, expected axon regeneration rates are not precise, are inversely affected by age, and differ based on the level of injury (more proximal repairs regenerate faster). Following reinnervation, terminal axonal sprouting provides axonal branch innervation to many additional neighboring muscle fibers. Motor units are typically larger (up to five times larger according to small animal data ) and more compact and delineated than preinjury innervation patterns (in which motor units overlap and muscle fibers of that motor unit are more dispersed). This phenomenon can be appreciated on electrodiagnostic studies by the presence of wide, large electromyography (EMG) signals known as polyphasic potentials. Anticipated timing of clinical evidence of muscle recovery should be considered a range rather than a precise timepoint.
Several factors can compromise axonal regeneration with definite clinical implications. Axonal elongation only occurs if axons are able to span the zone of injury, which means that endoneurial tubes need to be intact (Sunderland II) or in very close proximity. Growth cone generated proteases and plasminogen activators can penetrate through some tissue barriers, , though extensive intraneural fibrosis as well as persistent internal derangement obstructs and prevents axonal regeneration so that with extensive or complete disruption, elongating axons become trapped in scar tissue. The ensuing bulbous mass of undirected, swirling axons is known clinically as a neuroma (either “end bulb” or in-continuity). This is not to be confused with the bulbous mass that forms at the proximal end of an unrepaired distal nerve stump ( Fig. 30.5 ). This “glioma” represents a Schwann cell and fibroblast-induced response but does not contain axons. With nerve repair, the scarred, deranged, or neuromatous tissue is resected and “close endoneurial tube proximity” is obtained surgically. Intuitively, poorly aligned macro nerve elements (i.e., fascicles) must mean that the micro elements are poorly aligned as well. Regardless of how well the ends are aligned, nerve coaptation must be maintained without excessive tension. Tension at the repair site has been shown to be detrimental to axonal regeneration, intraneural vascularity, , and Schwann cell activity. Following repair, nerve connective tissue healing is relatively quick and 50% to 65% of native nerve tensile strength is returned within a few weeks (based on small animal data).
The biologic consequences of a chronic nerve injury are seen in the cell body, the proximal nerve stump, the distal nerve stump, and the target organs (especially muscle). Fu and Gordon have demonstrated that with prolonged axotomy, axonal regeneration is compromised. More specifically, in a rodent model, repairs delayed greater than 6 months resulted in a 65% degradation in axonal regeneration. The relative contribution of the distal nerve stump and the chronically denervated muscle to overall recovery is not clear. The distal nerve stump suffers a time-dependent loss of neurotrophic potential related to a combination of loss of guidance cues, Schwann cell senescence, and endoneurial tube degeneration. , , With senescence, the Schwann cells irreversibly downgrade into a dormant inactive state, the bands of Büngner disappear, and the cells no longer support axonal regeneration. With very proximal injuries, the distal nerve tissue may suffer these negative changes despite prompt repair simply due to the slow axonal regenerative rate.
Muscle, deprived of its neurotrophic feedback, undergoes predictable and well-described changes involving the endplates, muscle fibers, and terminal axonal pathways. , The endplates rapidly degenerate and the entire muscle fiber surface temporarily forms acetylcholine receptor clusters, manifested by increased insertional activity and spontaneous fibrillations on EMG. The muscle fibers become catabolic with a general degradation of contractile proteins, loss of nuclei, and profound loss of mass and muscle fiber size (cross-sectional area)—up to 50% within 2 to 3 months. The normally self-replicating population of reparative satellite cells residing within the muscle basement membranes can preserve muscle fiber viability for many months, though this pool is eventually exhausted. With end-stage denervation atrophy, occurring at an average of 18 months in humans, the scar tissue walls off the potential endplates, the acetylcholine receptor clusters degrade, and the muscle becomes permanently electrically and functionally silent. Eventually, the muscle tissue is replaced by fibrotic adipose tissue.
Other factors affecting functional nerve regeneration and recovery include age, systemic disease processes, and other toxin exposures. Advancing age is one of the most consistently potent negative influencers of neural regenerative potential. Pediatric patients not only benefit from innate superior neural regeneration and shorter regeneration lengths (shorter arms mean shorter nerves), but also enhanced central nervous system plasticity allows rapid remodeling and adoption of even aberrant reinnervation patterns. , Older patients, by contrast, have a distinctly diminished regenerative potential and even with physiologic reinnervation are unable to adapt to the new innervation patterns or regain strength in reinnervated muscles. Systemic neuropathy related to diseases such as diabetes, or exposure to neurotoxins in certain chemotherapies, alcohol, or tobacco, compromise normal nerve physiology and purport a poor prognosis for functional recovery. ,
The mechanism of injury can have direct and indirect repercussions on nerve regeneration and clinical outcome. Tearing, avulsing, and crushing traumas create large zones of injury, which may induce greater intraneural and perineural hemorrhage and scarring—both of which can impede neural regeneration ( Fig. 30.6 ). Injuries resulting in segmental nerve loss may require grafting procedures, and, even though the intraneural plexus characterized by fascicular interconnections is not as complex as once theorized, the larger the intervening gap, the more difficult to reestablish functional axonal pathways. Finally, collateral damage to neighboring soft tissues may compromise nerve perfusion (or the diffusion necessary to support autografts) as well as ultimate functional recovery. The lack of a stable skeletal platform, contractile muscle tissue, or a pliable mobile soft tissue envelope have obvious functional implications.
As denoted by the detrimental consequences of prolonged axotomy and denervation, nerve exploration, repair, or reconstruction should be performed as soon as practical. While some scenarios offer clear indications for urgent surgery, the timing of intervention is often more complex, and observation in many circumstances is appropriate. Initial assessment of a peripheral nerve injury should include anatomic localization, extent of injury, expected natural history, and prognosis with or without surgical intervention. These goals may require serial examinations, though prolonged observation may diminish the likelihood of a favorable outcome if spontaneous recovery does not occur.
Immediate: Isolated nerve injuries are not considered emergent, but when associated with vascular injury or limb or life-threatening injuries, they can be treated concurrently.
Urgent: Any acute, sharp laceration with anatomically correlated neurologic deficits should be explored within a few days of injury. While the biologic advantages of early nerve repair as previously elucidated include improved axonal regeneration and decreased atrophy, practical benefits include decreased retraction, avoidance of fibrosis (limiting nerve elasticity and stretch tolerance), and avoidance of the formation of neuromas and gliomas (requiring additional nerve resection).
Short delay: Nerve ruptures should be identified early and may require a short period of equilibration (approximately 3 weeks) to allow demarcation of the zone of injury.
From 3 to 6 months: Closed nerve injuries in continuity may be neuropraxic (Sunderland I) or axonotmetic (Sunderland II) and, as such, have an excellent prognosis for spontaneous recovery. Alternatively, they may be associated with greater degrees of internal rupture (Sunderland III and IV), which are unlikely to recover without surgical intervention. The 3- to 6-month waiting period will allow neuropraxic injuries and some axonotmetic injuries to declare. Serial electrodiagnostic examinations will demonstrate those nerves capable of regenerating. After 3 to 6 months, intraoperative nerve assessment will be necessary to identify the remaining axonotmetic injuries that are regenerating despite the lack of clinical or electrodiagnostic evidence. Greater delays may compromise final recovery.
From 6 to 12 months: Most explorations during this time period are related to delays in diagnosis and referral or are related to nonclinical factors (socioeconomic or lack of access to quality medical care). Repairs and reconstructions are still possible during this time period, though results may be compromised, especially in older individuals or very proximal injuries.
From 12 to 18 months: Though there are reports of successful nerve transfers during this time period (especially for sensory nerves), most nerve surgery at this point is primarily focused on pain relief. Alternative strategies such as tendon transfer or free functioning muscle transfer should be considered.
Greater than 18 months: Muscle denervated past this point has a statistically low chance of regaining function. Alternative strategies for reconstruction should be considered. Most nerve surgeries after 18 months are for pain management.
Though seemingly self-evident that acute trauma patients would report sensory and motor disturbances, this is not always the case. Any open wound, penetrating wound, or even closed injury that could cause nerve damage should be treated with a high index of suspicion. Nerves running in close proximity to disrupted blood vessels should be presumed injured until proven otherwise. Interestingly, “numbness” or “I can’t feel anything” are actually very uncommon presenting complaints. More commonly, patients with significant nerve damage initially describe tingling, burning, electric shocks, or the sensation of the limb being “asleep.” Patients who present later often emphasize the associated neuropathic pain as well as functional and more complete sensory deficits. Broad and exaggerated complaints (“I can’t feel anything” or “my arm doesn’t work”) can be focused down to specific deficits during the physical exam. Associated bone, joint, and vascular collateral damage must be recognized and taken into account when trying to accurately delineate the extent of peripheral nerve injury. Finally, traumatic loss of nerve function occurs at the time of nerve injury and alternative explanations such as malingering should be considered for the unusual case of gradual or delayed development of (especially) subjective neuromuscular complaints.
In acute injuries, the exam should focus on identifying open wounds and vascular injury. With blunt or high-energy trauma, early radiographic imaging of the injured spine, chest, or extremity can help direct a more detailed physical exam and minimize unnecessary discomfort to the patient. A well-documented and detailed examination including strength grading and objective sensory testing will be essential (especially when clinically apparent improvement does not match the patient’s memory). Strength testing of all muscle groups of the affected extremity should be meticulously documented utilizing the (1974) modified British Medical Research Council (MRC) grading scale ( Table 30.1 ) recognizing that other injuries, pain, and anxiety may all confound this initial examination. Initial sensory testing must consist of more than the question, “Can you feel this?” Patients can experience proprioceptive feedback from neighboring intact sensory territories. “Does this feel normal?” or “Does this feel the same as the [contralateral extremity]?” or “Does this feel tingling or like it’s asleep?” are all better questions for identifying a nerve injury. Remaining suspicions can be most easily confirmed or alleviated by assessing sharp-dull discrimination using either a paper clip, broken wooden cotton swab stick, or tongue depressor. Multiple commercially available devices are available for static and moving two-point discrimination (2PD) testing in the dermatomes of the hand but are not reliable elsewhere. 2PD assesses axonal density, while Semmes-Weinstein monofilament testing is a threshold test that assesses the function of the existing axons and is more useful for compressive than traumatic neuropathies. Sensibility and recovery can be documented utilizing the MRC sensory grading scale (see Table 30.1 ).
| Grade | Outcome | Sensibility | Static 2PD | Moving 2PD |
|---|---|---|---|---|
| S0 | Poor | None | >15 mm | >7 mm |
| S1 | Deep pain | |||
| S1+ | + superficial pain | |||
| S2 | + some touch sensitivity | |||
| S2+ | As above with overresponse | |||
| S3 | Pain and touch sensitivity | |||
| S3+ | Good | S3 with improved location | 7–15 mm | 4–7 mm |
| S4 | Excellent | Normal | 2–6 mm | 2–3 mm |
| Grade | Outcome | Strength Across a Full Arc of Motion | ||
| M0 | Poor | Nil | ||
| M1 | Trace contraction | |||
| M2 | Active movement with gravity eliminated | |||
| M3 | Fair | Active movement against gravity | ||
| M4− | Good | Movement against slight resistance | ||
| M4 | Movement against moderate resistance | |||
| M4+ | Movement against strong resistance | |||
| M5 | Excellent | Full strength | ||
Follow-up serial examinations of strength and sensation can confirm or refute initial impressions and reveal nerve recovery. Other helpful clinical findings include the observation of atrophy to confirm muscle denervation, and, while not always present, some patients develop dry skin in the distribution of skin denervation. Due to the loss of sympathetic innervation, denervated skin does not sweat or produce oils like normal skin, and the dry skin pattern can be eerily geometric with sharply defined dermatomal borders. Finally, the electric shock elicited with tapping along the anatomic course of a nerve (Tinel sign) signifies regenerating sensory axons. For maximal utility, the test should always begin at the tip of the affected extremity and progress proximally to the repair site. With axonal regeneration (either from spontaneous regeneration or following a nerve repair), this percussion sign should progress distally from the zone of injury at approximately 1 inch per month. Failure to progress may indicate either failed axon regeneration or the formation of a neuroma. Though described as an accurate and prognostic sign for reinnervation, I have not found it as reliable. Many patients maintain Tinel sensitivity at the repair site even with successful regeneration, some patients seem to have percussion signs diffusely, and some patients (perhaps due to neuropathic pain-modulating drugs) never seem to exhibit one.
Though some institutions use imaging modalities such as diffusion tensor imaging (DTI) to study axonal regeneration, most medical centers have not translated this technology to clinical practice. However, high-resolution ultrasound (US) and magnetic resonance imaging (MRI) can often demonstrate complete nerve rupture or fascicular disruption from traction or projectile injury, and they should be considered in high-energy closed trauma. , US has the advantage of being able to scan over long distances, as well as attain an immediate comparative examination of the opposite limb, while MRI may have increased spatial resolution. In some multilevel injuries, nerve injury localization can be challenging. However, the author has seen a neuroma-in-continuity misinterpreted as nerve swelling associated with entrapment neuropathy; body habitus, nerve depth, nearby implants, and the radiologist’s skill or experience can all affect the reliability of these modalities. In many situations, neural imaging cannot differentiate nerve injuries with the potential for spontaneous regeneration versus those requiring repair.
Nerve conduction studies (NCS) and EMG are the most commonly utilized diagnostic modalities but also suffer from technical and practical limitations. A common error is to prematurely obtain these studies before the completion of Wallerian degeneration (7 to 10 days) and the establishment of the characteristic EMG profile of denervated muscle (around 2 to 3 weeks). After this time frame, NCS can differentiate conduction blocks (neuropraxia) from axonotmetic injuries. While both will fail to conduct across the injury site, a neuropraxia will maintain a recordable signal when stimulation and recordings are both distal to the zone of injury, while axonotmesis will not conduct secondary to Wallerian degeneration of disrupted axons. Axonotmetic injuries with the potential for recovery can also not be differentiated from more severe injuries, until subsequent examinations demonstrate nascent recovery potentials.
By combining diagnostic modalities, pure neuropraxic and gross neurotmetic injuries can be identified. However, this still leaves a subset of incompletely characterized nerve injuries whose treatment depends on whether or not axons can regenerate across the zone of injury. Serial exams provide the opportunity for viable axonotmetic axons to regenerate and allows previously undetected neuropraxic injuries to remyelinate. Not uncommonly, follow up examinations will reveal function not previously apparent. These situations likely represent a mixed injury pattern with a small population of neuropraxic axons or axons below the threshold of electrodiagnostic detection (also known as sampling error). Terminal collateral sprouting may expand the motor unit size and generate detectable muscle contraction and/or recordable EMG signals. These encouraging findings indicate the strong potential for further recovery.
However, excessive delay clearly worsens the prognosis if nerve reconstruction is ultimately deemed necessary. The principle of achieving reinnervation as rapidly as possible is balanced against the unnecessary excision of and reconstruction of regenerating axons by waiting the minimal amount of time necessary to make this decision. Within 3 to 6 months, neuropraxic axons will have recovered and early axonal regeneration should be detectable.
Ultimately, intraoperative nerve assessment can incorporate direct nerve-to-nerve conduction testing. Regenerating axons should have regenerated 1 inch per month of delay so that a 3- to 6-month observation period would allow 3 to 6 inches of regeneration—an adequate length to enable the placement of stimulation and recording electrodes for intraoperative nerve conduction testing. Though sound in principle, enthusiasm for this strategy has been tempered by inconsistent results and technician-dependent reliability. While the author utilizes this strategy, visualization and palpation of the damaged nerve often provide equivalent or superior information, and based on similar observation, other authors have advocated for even earlier exploration (in lieu of intraoperative NCS).
Nerve injuries can be missed. A high index of suspicion should be maintained when assessing traumatized extremities until objective evidence of normal nerve function has been obtained.
Neuropraxic and some axonotmetic nerve injuries typically associated with stretch or crush mechanisms have a better prognosis and can be treated initially with expectant observation.
Functional recovery will not be achieved following axonotmetic injuries that fail to demonstrate axonal regeneration and neurotmetic injuries (transection or rupture) without surgical intervention.
Electrophysiologic studies and soft tissue imaging can identify neuropraxic and some neurotmetic injuries.
Axonotmetic injuries with unknown prognoses can be observed for signs of recovery; exploration for absent recovery should not be delayed beyond 3 to 6 months postinjury.
For many nerve injuries, expectant observation is the definitive treatment. Reassurance, pain management, and therapy to maintain joint flexibility, however, must be individualized to each patient’s needs. The economic and emotional impact of major peripheral nerve injuries should not be underestimated; evaluation by a social worker or psychologist can be instrumental in uncovering and managing the ripple effects of an incapacitating nerve injury on occupation, family life, independence, and self-esteem.
Pain management can often be one of the most challenging aspects and should focus on nerve-modulating drugs. Practically, these fall into two categories: antiseizure and antidepressants. Amitriptyline (a tricyclic antidepressant) prescribed at a subclinical dose for depression can improve neuropathic pain, with the beneficial side effect of improving sleep when taken at night. Sleep deprivation is common with neuropathic pain and may exacerbate diurnal pain and deplete effective coping skills. Unfortunately, its sedating effects often spill over to daytime activities to an extent that many patients find unacceptable. Gabapentin (an antiseizure medication) and pregabapentin (marketed for neuropathic pain) are effective, but they also have sedating, disorienting, and vertiginous side effects that seem to lessen with prolonged use but which many patients cannot tolerate. Finally, as a second-line medication to modulate neuropathic pain in recalcitrant cases, duloxetine (trade name Cymbalta, a serotonin-norepinephrine reuptake inhibitor) can be useful, but its side effects include dry mouth, fatigue, loss of appetite, nausea, and constipation. Narcotics have profound addictive qualities and tend to lose efficacy very quickly. Tramadol, while still having the potential for abuse, in the author’s experience, tends to be slightly more effective (for nerve pain) and can be sparingly incorporated into the overall pain management strategy. Nonsteroidal antiinflammatory drugs (NSAIDs) and acetaminophen can provide additional pain relief but are associated with cardiovascular, hepatic, and gastrointestinal risks, especially in patients with comorbidities. Though many other medications have been utilized for neuropathic pain, the author typically refers his patients for a pain management consultation when first- and second-line pain-modulating drugs are not successful or tolerated.
Therapy generally consists of a combination of supervised exercises and an intensive home program to maintain joint mobility during recovery. Compression stockings and other therapist-applied modalities can be helpful with swelling, stiffness, pain, identification and management of trophic signs, and development of compensatory and coping mechanisms. Transcutaneous electrical stimulation (TENS) units seem to only provide modest benefit but have little downside other than expense and may be worth trialing. Transcutaneous galvanic muscle stimulation has been used successfully in animal models to delay atrophy, , though it is often not well tolerated and lacks convincing data of clinical efficacy.
Surgical intervention may be indicated when physical or electrodiagnostic evidence of nerve regeneration has not occurred by 3 to 6 months. The zone of injury is explored, and the damaged nerve is exposed for visual, tactile, and potentially intraoperative nerve conduction testing ( Fig. 30.7 ). Obvious full-thickness fibrosis, a nonconducting neuroma-in-continuity, or nerve rupture will need resection and reconstruction. Soft, well-vascularized and pliable nerve tissue with easily identifiable fascicles will typically recover. Distal muscle contraction with a low amperage (0.05 to 2.0 milliamps [mA]) proximal nerve stimulation (utilizing a commercially available nerve stimulator) can be left alone with the expectation of eventual recovery. For many nerve injuries, regenerative potential cannot be confidently determined by surgical observation. More aggressive external and even internal neurolysis, typically performed under operating scope magnification, may remove scar tissue to allow the identification of any intact and viable fascicles. As described above, electrodiagnostic testing along the nerve trunk or specific isolated fascicles may be helpful. Demonstration of a compound action potential conducting across the zone of injury indicates ongoing successful axonal elongation and a favorable prognosis for continued recovery. Occasionally, a benign appearance will conflict with a negative electrodiagnostic assessment at surgery. In injuries less than 6 months old, the option of further observation with potential future reexploration may be considered.
Although clinical recovery following exploratory surgery has been attributed to the therapeutic benefit of neurolysis, this conclusion lacks scientific support. Though there may be cases in which axonal regeneration is inhibited by perineurial scarring, this is very hard to demonstrate, and the procedure should be conceptualized as a strategy to assess the nerve’s potential for continued recovery before the treatment window of opportunity is lost. Therapeutic neurolysis for posttraumatic neuropathy (in the absence of demonstrable entrapment or traction neuritis) is unpredictable and controversial.
There are three primary principles to nerve repair: adequate debridement of damaged nerve, precise end-to-end alignment, and atraumatic coaptation.
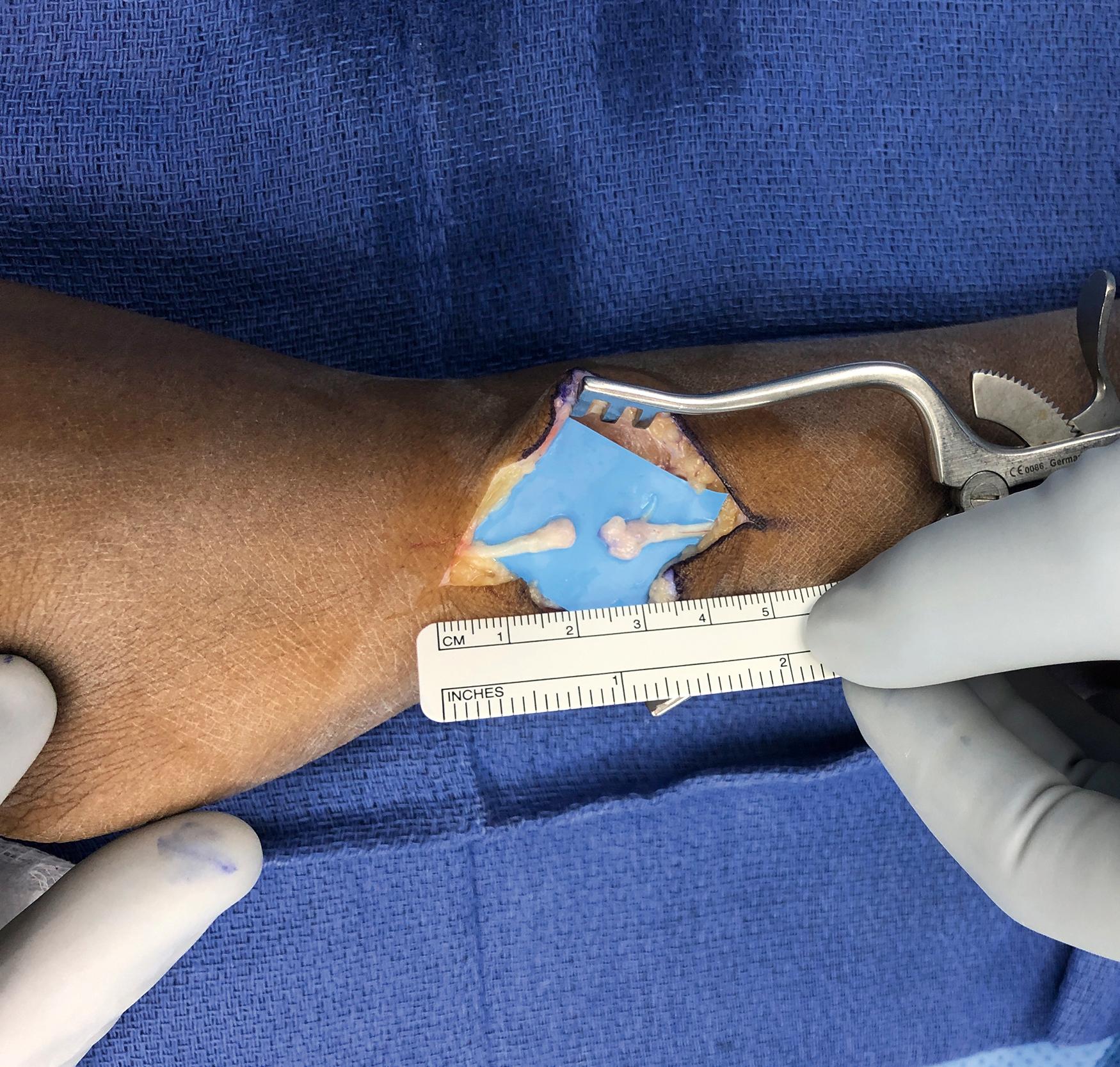
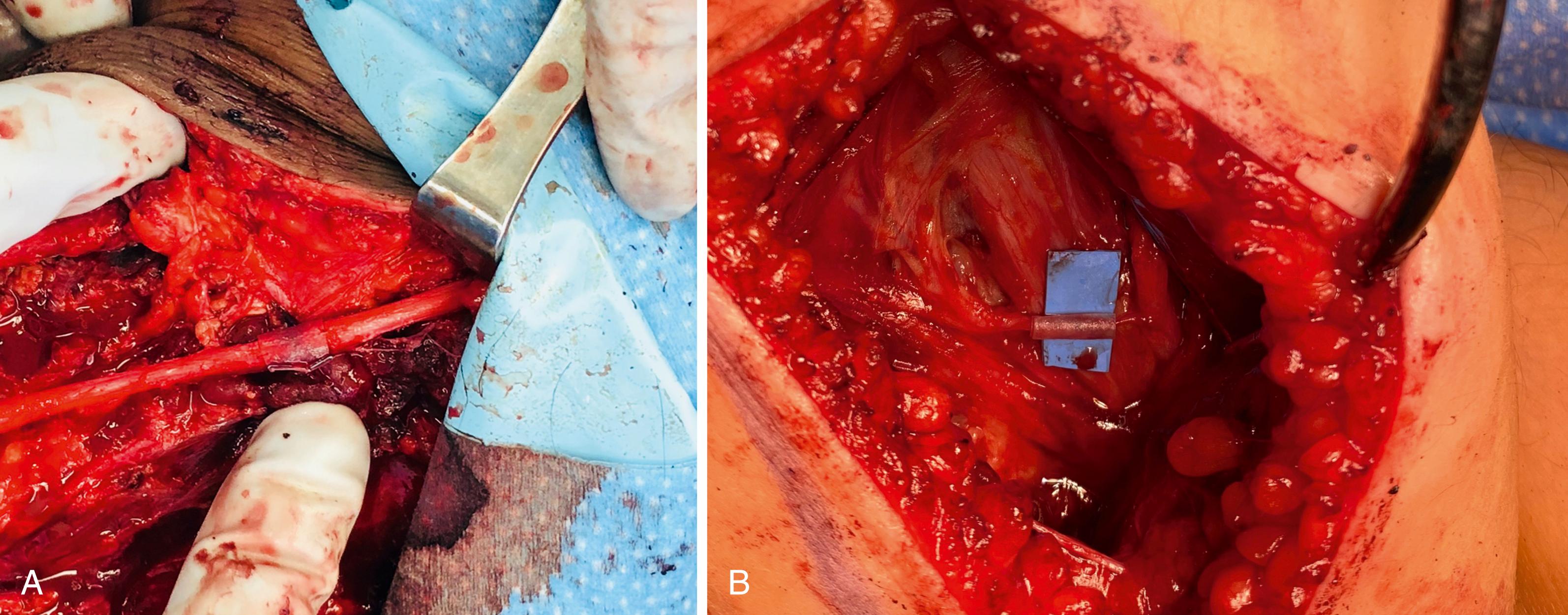
Debridement: Depending on the mechanism of injury, the zone of damaged nerve may extend well beyond the point of rupture. Repairing damaged nerve to damaged nerve will not juxtapose healthy endoneurial tubes that are capable of supporting axonal regeneration, and these repairs will be doomed to failure. A variety of techniques and tools have been described to facilitate controlled, sharp slices through the damaged nerve tissue to minimize crushing while efficiently progressing toward healthy nerve tissue. Commercially available nerve-cutting guide forceps with a straight razor, a scalpel applied to a tongue depressor, “supersharp” carbon scissors, and bipolar electrocautery all have their advocates, and none have been found to be superior. Resection should proceed until a clear fascicular pattern (with fascicular pouting from the proximal side) and healthy bleeding are apparent ( Fig. 30.8 ). Real-time, intraoperative histologic verification of at least 75% viable axons has shown promise in one study, though further validation of this strategy will most likely need to precede widespread adoption.
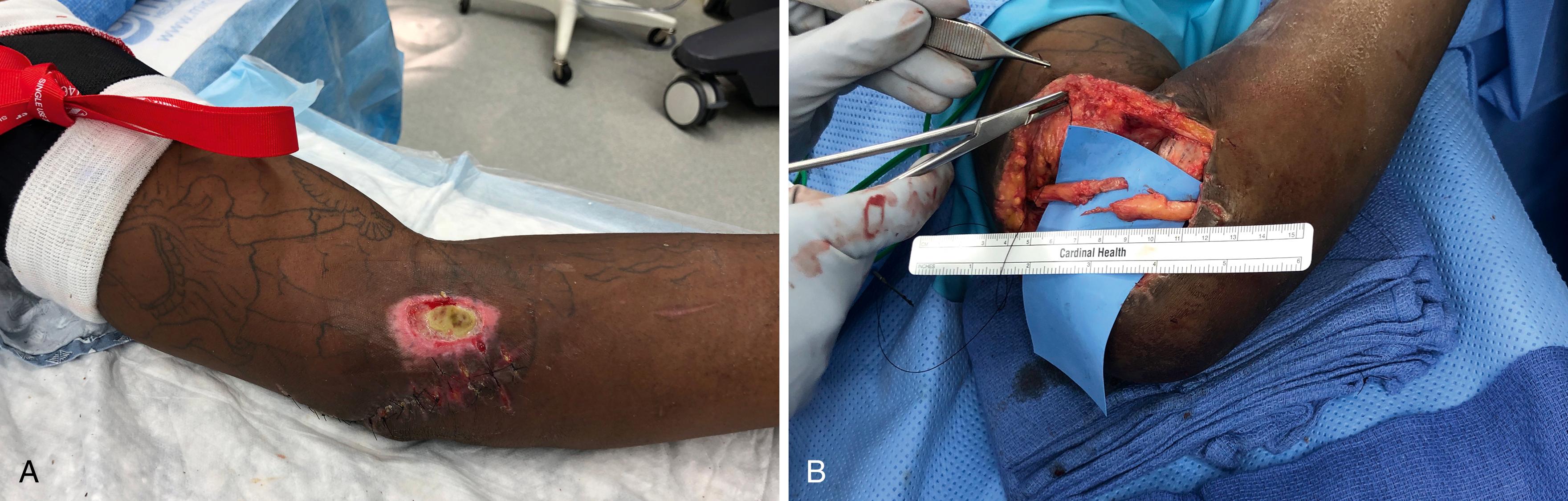
Alignment: Though most reports on alignment focus on proper orientation to optimize successful motor and sensory reinnervation, two cadaveric studies demonstrated that end-to-end fascicular alignment may not be achieved in up to 40% of repairs ( Fig. 30.9 ). , Surface markings (such as blood vessels) and fascicular patterns can facilitate proper orientation, though optimal end-to-end fascicular approximation is probably most dependent on the surgeon’s technical skill and experience.
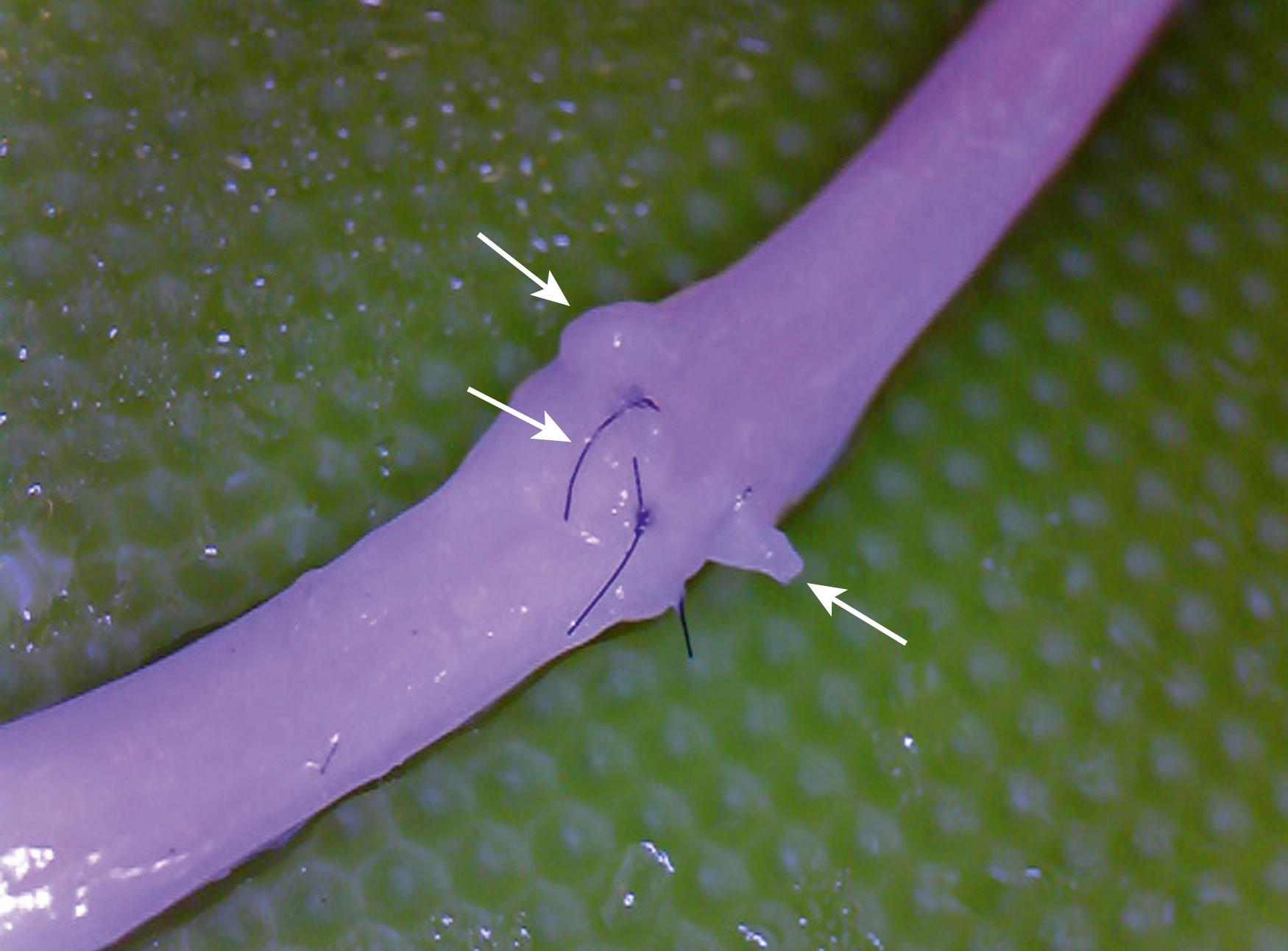
Coaptation: A variety of techniques have been described to accurately repair nerves. Microsutures are the gold standard and can be placed through the outer epineurium (epineurial repair), the internal epineurium (grouped fascicular repair), and, rarely, the perineurium (fascicular repair) in an attempt to maximize axonal alignment. The increased manipulation and suture material used to gain alignment must be balanced against the risk of iatrogenic intraneural damage and foreign body burden. In most situations, simple epineurial sutures, supplemented by a nerve wrap or fibrin glue, are adequate. For very distal nerves with well-known motor/sensory topography such as the median and ulnar nerves at the wrist, a grouped fascicular repair may be more efficacious. Suture caliber has been studied primarily as a determinant of resistance to tension. Based on rodent model data, one or two 8-0 or 9-0 suture (depending on the size of the nerve) should be able to maintain nerve stump approximation. Well-done suture neurorrhaphy is technically demanding, time consuming, and resource heavy.
Once the nerve ends have been debrided, microsutures should be placed under optical magnification. Though several reports indicate that nerve repairs can be adequately performed under loupe magnification, Bernstein et al. demonstrated in a cadaver study that the technical quality of nerve repairs was better when performed using the operating microscope. The smallest caliber and the fewest number of sutures necessary to achieve stable coaptation should be used. Care should be taken to place these sutures as atraumatically as possible by avoiding squeezing, crushing, or unnecessarily manipulating the fascicles. Microforceps can stabilize the nerve tissue by grasping the outer epineurium. Fascicular alignment in an epineurial repair is achieved by matching the fascicular and perineurial vascular patterns, precise needle penetration depth and position, and avoidance of overtightening so that the fascicles are barely touching ( Fig. 30.10 ). If using double-throw surgeon knots, care must be taken to avoid overtightening. While tension may be relieved for convenience during the first few sutures by extreme joint positioning, one should be certain that nerve approximation is maintained with one or two sutures in neutral posture. Nonphysiologic tension across a neurorrhaphy diminishes local vascular flow and risks disruption during the postoperative period.
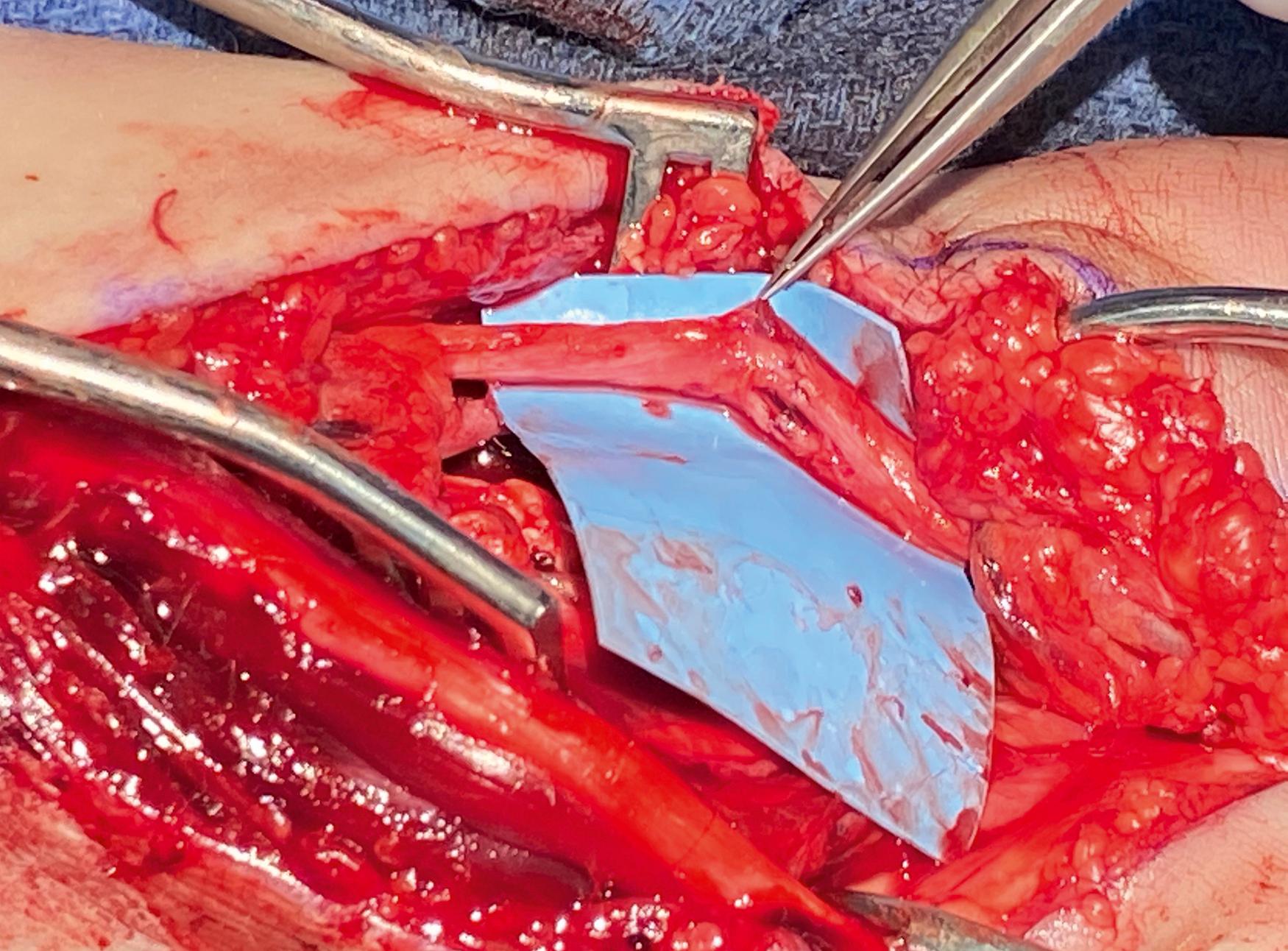
For larger diameter nerves, a grouped fascicular repair can be performed by placing a few deep sutures through the internal epineurium or superficially through the perineurium of a few fascicles to optimize the internal fascicular alignment ( Fig. 30.11 ). Alternatively, the inner epineurium can be stripped away to allow direct manipulation and individual coaptation of each fascicle. Though improved alignment is possible with this technique, if alignment is off by only a few degrees, every axon will be “misdirected,” and even if perfect, the increased intraneural trauma and scarring may negate any benefits. Fascicular repairs are much more technically challenging and time consuming, and they have not been shown to improve clinical outcomes. ,
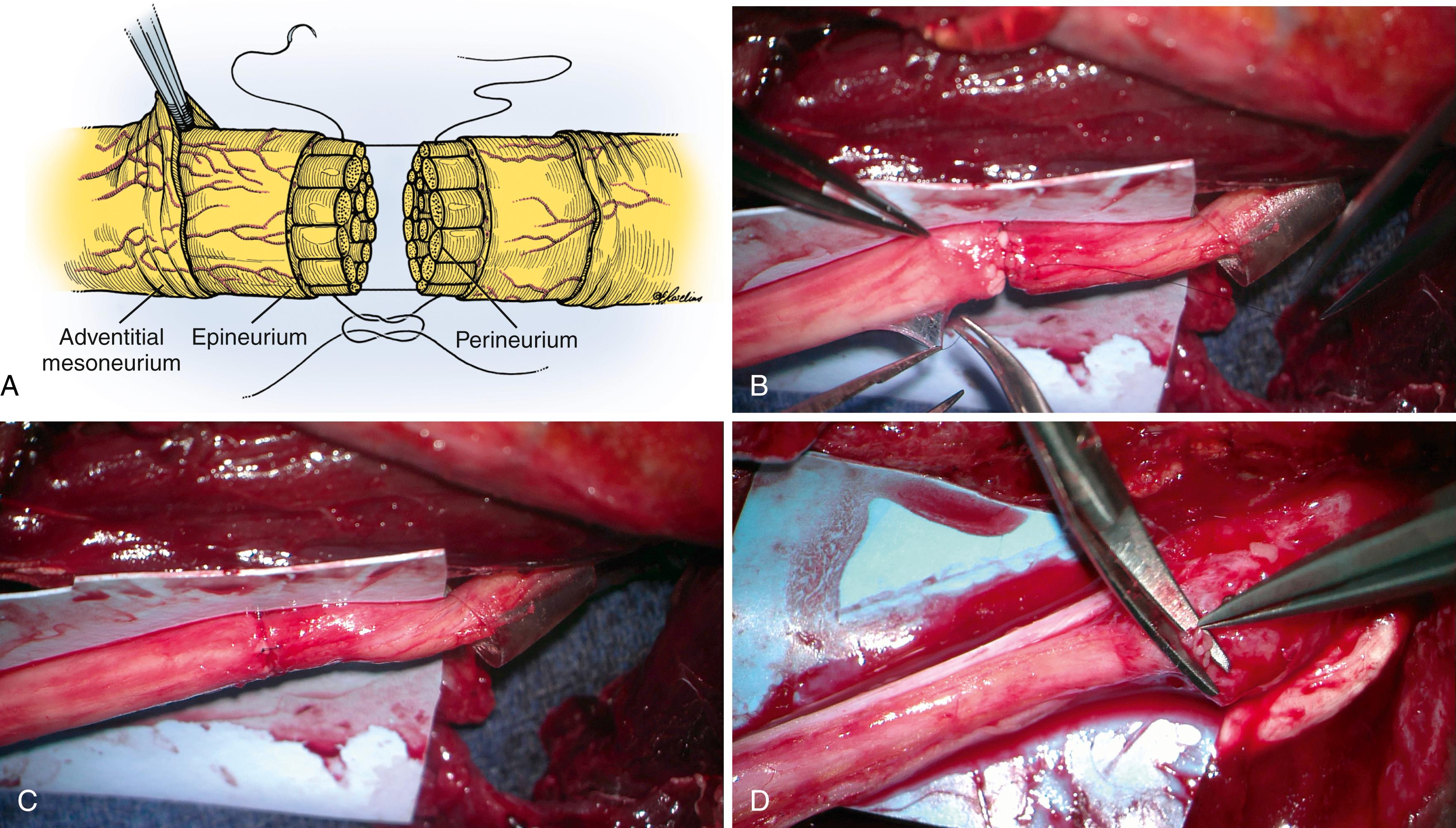
Primary repair of digital or small sensory nerves in adults generally results in good but not perfect recovery in 50% to 70% of repairs. One large study of 93 digital nerve repairs with 3½-year follow reported an average static 2PD of 10.6 mm (compared with 4 to 6 mm as normal), restoration of sharp-dull and temperature discrimination in 66 and 83% of the repairs (respectively), and only a 2% incidence of painful neuroma formation.
Major peripheral nerve primary repairs do not fare as well as smaller diameter nerves but still have a better prognosis than grafted repairs. A thorough analysis of the clinical literature is difficult due to inconsistencies in outcomes measures, stratification of injury mechanisms, and elapsed time since repair. Primary repairs are often combined with the outcomes of grafted repairs, and variables such as patient age, duration from injury to repair, and collateral damage all further confound interpretation. Sixteen of 18 primary midhumeral radial nerve repairs achieved M4 or 5 wrist and digit extension. Emamhadi et al. reported 90% good neurologic recovery in 93 cases of direct ulnar nerve repair, but they did not subclassify these results based on the level of injury, and their outcome scale deemphasized intrinsic muscle recovery. Post et al. conversely reported only modest intrinsic recovery and no good sensory recovery out of their 15 patients who underwent primary high ulnar nerve repairs. In one study of combined median and ulnar nerve repairs, functional motor recovery was noted in 23 of 26 (with tactile sensation in 12 median and 7 ulnar nerves, and protective sensation in an additional 11 ulnar and 7 median nerve repairs). In a second study focused on median and ulnar nerve repairs, 51 of 65 primarily repaired nerves achieved good or excellent recovery.
Prolonged observation compromises final recovery due to time-sensitive nerve and target muscle degenerative changes.
When possible, direct end-to-end nerve repair is preferable and is associated with improved outcomes.
The key technical aspects to repair are adequate debridement of damaged nerve tissue or neuromata, avoidance of nonphysiologic tension, and precise fascicular alignment.
Fibrin glue is a coaptation aid to augment or, in some cases, replace microsutures. Though autologous fibrin glue can be prepared, this is time consuming, requires specialized equipment, and demonstrates inferior biomechanical holding properties compared with commercially available alternatives. The commercially available fibrin glues are provided as separate solutions of fibrinogen and thrombin that when mixed form a gel-like “white” clot, which lacks the red blood cells found in physiologic clot.
A cadaveric biomechanical study demonstrated that fibrin glue as an augment to sutures resisted gapping but did not add to the ultimate tensile strength of the repair. Early dehiscence is highlighted in several rodent studies, though if the repair holds for 1 to 2 weeks, the risk of rupture lessens and equivalent axonal regeneration can be expected. Contrary to popular belief, fibrin glue inadvertently interposed between the nerve stumps will not block axonal regeneration. However, maintaining orientation and alignment during fibrin glue application can be challenging when used without one or two aligning sutures. Limited clinical evidence suggests at least equivalent efficacy to suture repairs.
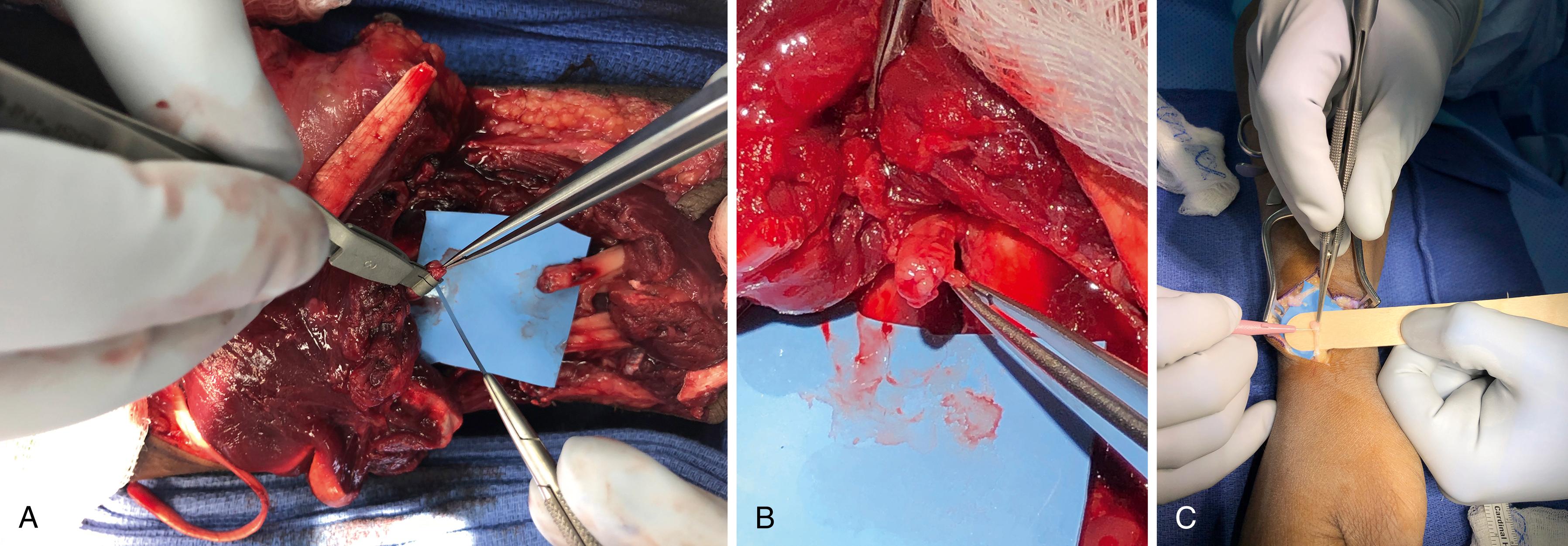
Nerve stumps can be manually approximated or preliminarily secured with microsutures. A flexible radiopaque backing can be placed behind the nerve to catch and shape the fibrin glue mixture slowly dripped around the coaptation. The backing is wrapped around the congealing fibrin glue mixture (like a taco) to shape the clot into an adhesive cylinder. This is maintained for 3 minutes to allow initial set-up, the Esmarch removed, and any excess fibrin glue excised ( Fig. 30.12 ).
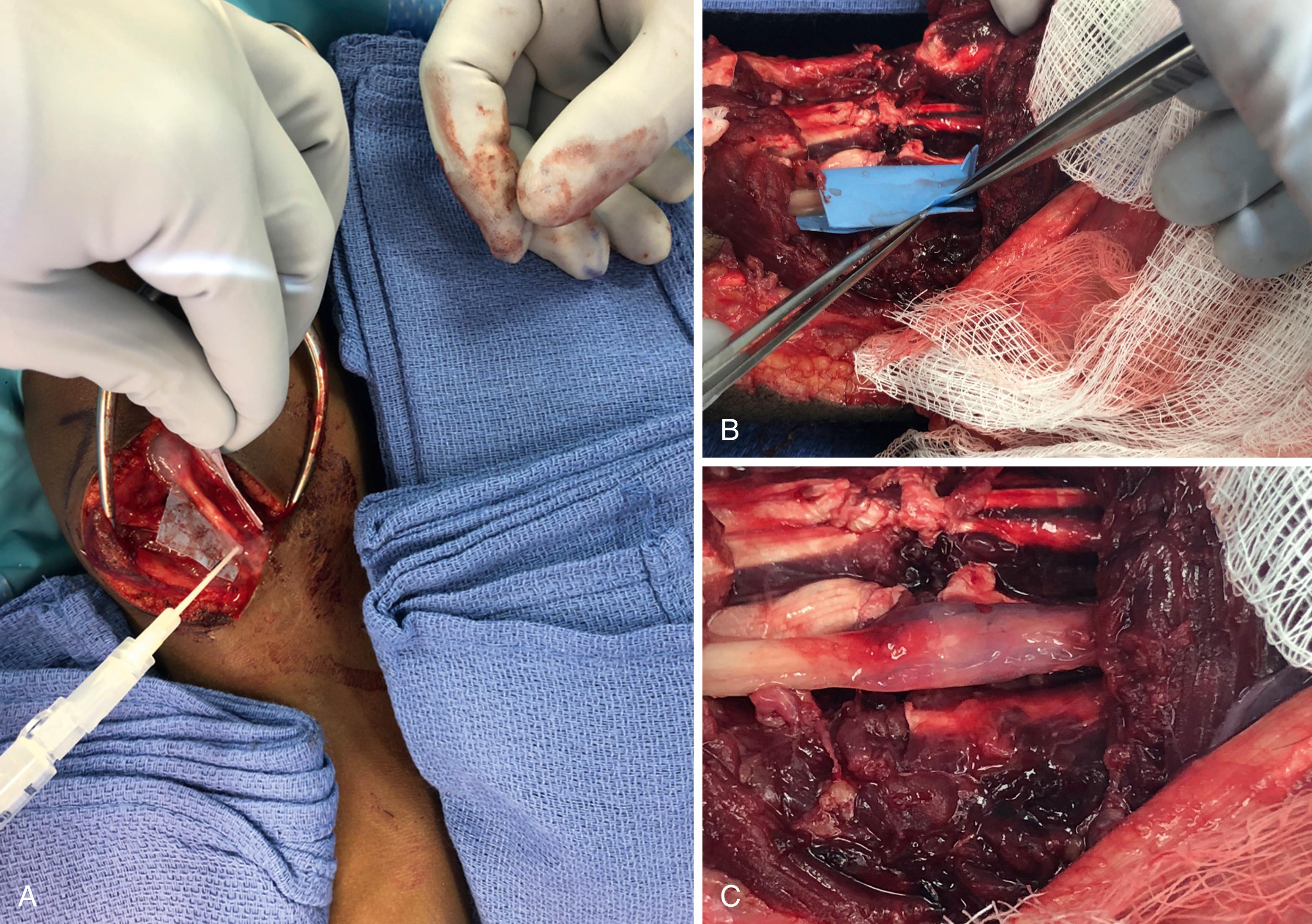
Short conduits or “nerve cuff” wraps applied around the approximated nerve ends is an alternative coaptation strategy that may offer additional biologic and practical benefits. In a sequestered protected microenvironment of a conduit, a rich milieu of neurotrophic and growth factors accumulates, and scar tissue is inhibited from invading into and around the coaptation. Whether the same applies to an externally applied cuff that is slid over a nerve repair cannot be stated with certainty. A review of several clinical studies suggested less pain at the nerve repair site when wrapped with vein or commercially available conduits or scar barriers. Mechanical neuropathic pain at the coaptation is theorized to be related to escaping axons, and alleviation of this discomfort suggests a potential for mitigation of this effect.
For less-experienced surgeons, a conduit-assisted repair technique may offer advantages of fascicular orientation, as demonstrated in a recent cadaver study compared with conduits or sutures alone. Neither this (nor the theoretical biologic advantages outlined above) have translated to substantial improvement in clinical outcomes. Potential benefits of a nerve cuff must be weighed against its increased cost and the potential for iatrogenic compression at the repair site associated with postoperative swelling.
Nerve cuff assisted repair requires a short conduit or tube of slightly greater diameter than the prepared nerve stumps. The tube is slid several centimeters proximal to one of the prepared nerve ends prior to suturing. A few microsutures are placed in the epineurium to obtain alignment and loose coaptation of the nerve ends. The conduit is then slid over the repair and either left alone, sutured in place (microsutures passed through the connector edge and the epineurium), or secured using fibrin glue. If sutures are placed, these may “unload” tension at the repair site, which is theoretically helpful, , though care must be taken to not overapproximate these repairs so that the fascicles overlap. If the connector is too big, microclips or microsutures can be used to take up the slack and ensure that the connector is splinting and directing the nerve ends toward each other. However, the fit should not be tight or even snug (since nerve ends do swell after repair). Alternatively, the connector can be slit and wrapped around the loose suture repair. Either additional sutures or fibrin glue can maintain the wrap in position ( Fig. 30.13 ).
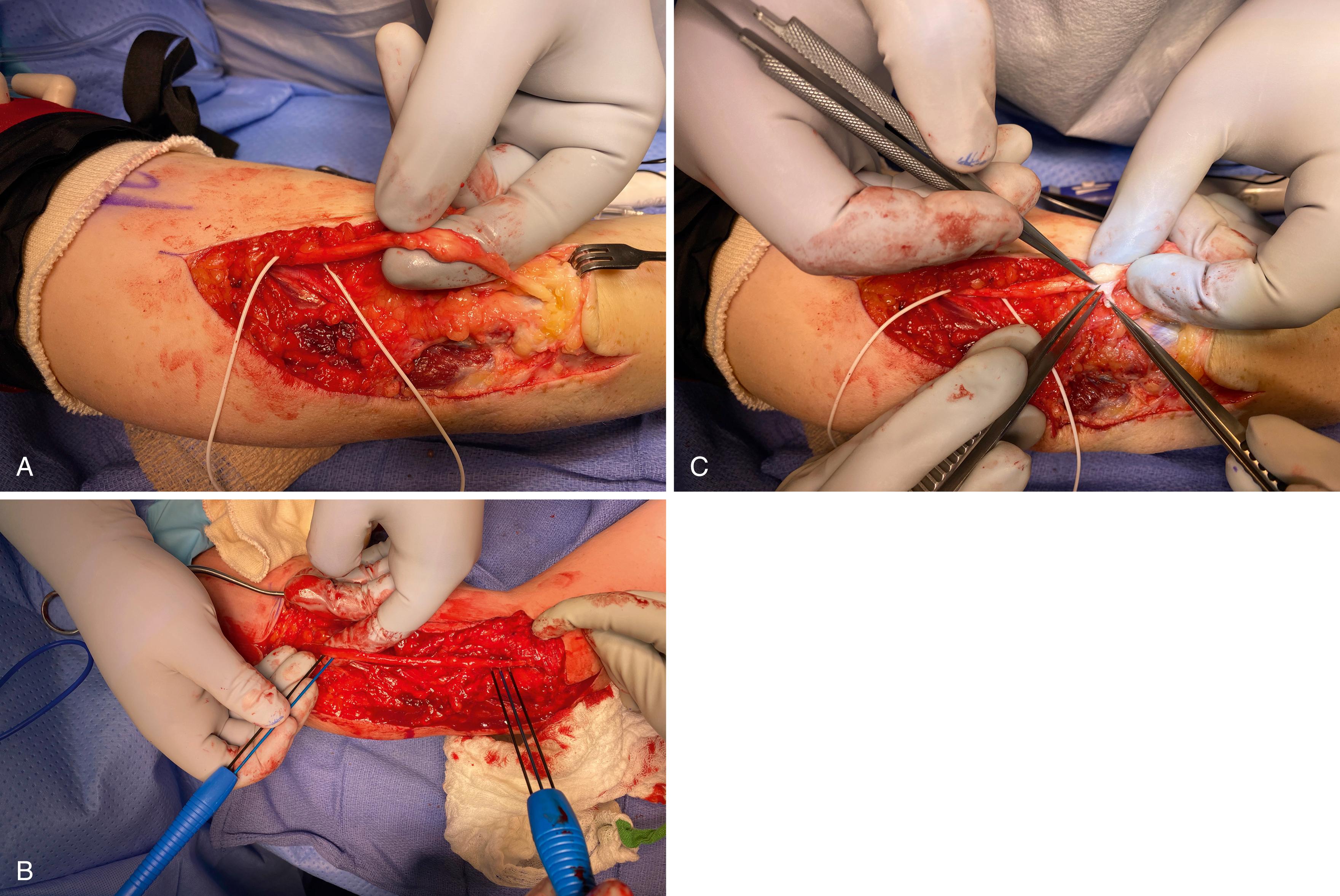
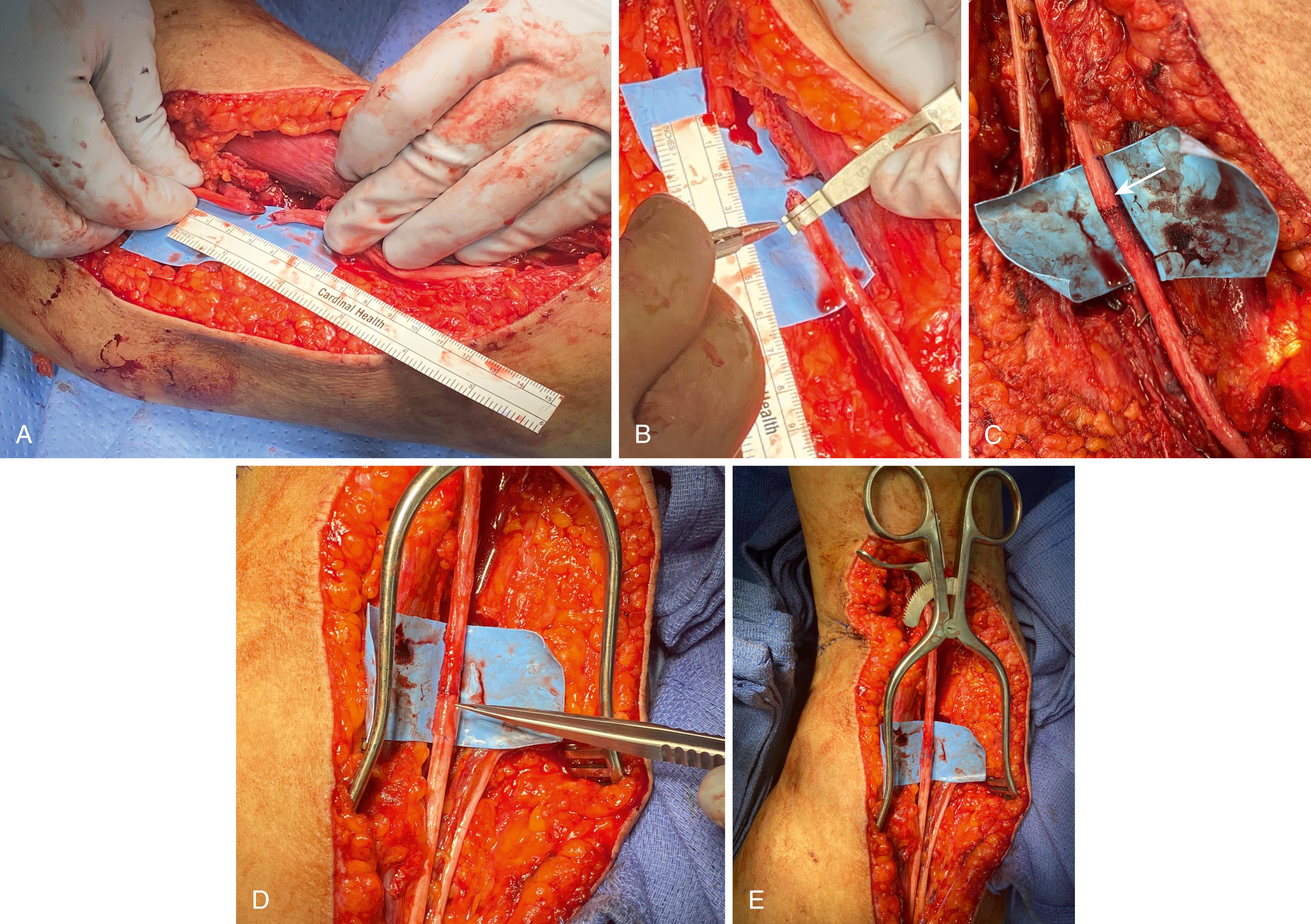
Lacerations and sharp puncture wounds are explored acutely to increase the likelihood of primary repair. A commercially available nerve cutting system coupled with a razor blade produces a clean, fresh transection (see Fig. 30.8A ). Fresh injuries are often maintained in at least a grossly oriented position, and this can be fine-tuned based on fascicular patterns and surface markers such as blood vessels. The nerves are mobilized by proximal and distal dissection to facilitate direct approximation. If nerve loss is minimal and retraction appears related to soft tissue swelling and innate nerve elasticity, mild to moderate joint positioning is acceptable. Illustrative of this principle, knee flexion can overcome sciatic nerve gaps of up to 8 cm to allow direct repair, followed by immobilization in the flexed position. The functional recovery obtained after delayed and gradual extension is markedly better than obtainable with autogenous nerve graft. Bleeding from both the external and internal epineurial vascular systems can impair visualization. Epinephrine-soaked pledgets can be applied to the cut nerve surface, or microtip bipolar electrocautery can be used on the lowest applicable setting and with copious irrigation to carefully cauterize. The ulnar and median nerves in the forearm have particularly consistent topography and the motor and sensory components for both nerves are readily defined. By repairing groups of fascicles, alignment is maximized while minimizing suture burden. Sutures are primarily placed through the epineurium; though, if necessary to obtain alignment, a superficial pass through the perineurium is acceptable. Larger nerves should maintain approximation with one or two 8-0 nylon sutures, while smaller sensory or digital nerves are generally repaired with 9-0 or 10-0 nylon sutures. Additional sutures are added to improve fascicular alignment and stabilize the coaptation. A spanning 6-0 monofilament (placed as a horizontal mattress suture through the epineurium) can “protect” the repair when any incorporated joint positioning is gradually relieved in the postoperative period. The approximated ends should be barely touching. The tendency to overtighten the repair must be avoided as this leads to overlapping fascicles or fascicles protruding from the perimeter of the repair. If this occurs, the offending fascicles can be trimmed back. At the surgeon’s discretion, hollow conduits can be slit and placed around the coaptation to splint, stabilize, and augment the repair. Commercially available fibrin glue is dripped over the connector to secure this in place and further stabilize the repair. The neighboring joints are positioned to alleviate tension at the repair site and a protective splint applied for 3 weeks before gradually reintroducing motion ( Fig. 30.14 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here