Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() For videos accompanying this chapter see ExpertConsult.com . See inside cover for access details.
For videos accompanying this chapter see ExpertConsult.com . See inside cover for access details.
Nerve injury, repair, and reconstruction describes the traditional classification of nerve injuries as well as a clinical grouping of nerve injuries according to the mechanism of injury. A clinical grouping of open versus closed and crush versus traction/avulsion or penetrating injuries has implications on the severity of associated soft tissue and bony injury, and impacts diagnosis and the timing of surgical treatment. Preoperative adjunct studies are reviewed and their usefulness in surgical decision-making for different injury patterns is discussed. The clinical classifications also provide guidance to the peripheral nerve surgeon about realistic expectations for recovery.
Specifics on nerve repair techniques, usefulness of pre- and postoperative imaging and electrical studies and their role in the assessment of the nerve injury and recovery, and therapy modalities to assist in the clinical recovery are discussed.
New additions to the chapter include the appropriate use of newer technology such as acellular human nerve allografts and biodegradable conduits, a discussion of the treatment of neuromas, and an updated guideline for treatment of complex regional pain syndrome.
Nerve injury and its sequelae have posed countless challenges to surgeons and patients over the past centuries. Since the development of microsurgical techniques in the 1960s, a relative explosion of innovation and knowledge has occurred to provide surgeons with greater tools to treat nerve injury.
Nerve injury classification remains the same, and the original principles of repair (early repair of complete injury, anatomical and tension-free coaptation, use of autologous nerve grafts), are still considered the gold standard. Improved understanding of microfascicular anatomy and the internal neural topography now enables surgeons to not only perform a microfascicular repair, but has led the way to the development of nerve transfers in nerve reconstruction. Nerve transfers can restore distal function and critical sensation in difficult nerve injuries, including proximal injury or those with large nerve gaps.
Despite these innovations, the major limitation to peripheral nerve recovery is still the time it takes for the nerve to regenerate. This remains true regardless of the technique used for the repair. Without prompt motor nerve input, denervated muscle after a prolonged period of time becomes resistant to nerve regeneration. Although avoidance of tension at the neurorrhaphy site and improvements in suturing techniques have improved the process to some extent, the ultimate success is dependent on innervation time, number of neurorrhaphy sites, supply and type of donor nerves, and the condition of the surrounding tissue. At every coaptation site, a percentage of nerve fibers are lost.
New technology is being developed to assist in evaluating the nerve injury and providing alternative solutions for nerve repair. This technology includes improved nerve stimulators, nerve conduits, and acellular nerve allografts. Alternatives to standard treatment for short, small-diameter nerve gaps include commercially available nerve conduits and acellular human-processed nerve allografts. Schwann cell-lined nerve conduits and tissue-engineered substitutions are still experimental, but may offer the solution for enhanced nerve regeneration in the future. It is important, however, to remember that even the “gold standard” nerve autograft gives functional results typically deserving of a “bronze” award, and no nerve substitute to date equals the results of an autograft. Nerve transfer techniques have largely replaced nerve grafts for many proximal-level nerve injuries in our practice. A careful evaluation of the patient, nerve injury, and available options, including their limitations, is important in enabling the modern surgeon to restore function and reduce post-injury complications.
Even with new advances, post-injury neuroma and complex regional pain syndrome continue to affect patients. Ongoing research into successful management strategies can offer these patients new possibilities for improved pain control and quality of life.
Sir Herbert Seddon’s classification system was the first to classify peripheral nerve injuries and is based on gross and histological anatomical changes rather than the mechanism of injury. He described three types of nerve injuries. (a) Neurapraxia involves a local conduction block at a discrete area along the course of the nerve. Wallerian degeneration does not occur and the recovery is excellent within a “short” time period. (b) Axonotmesis implies axonal damage, while (c) neurotmesis is the equivalent of a peripheral nerve transection. In both axonotmesis and neurotmesis, Wallerian degeneration occurs distal to the site of injury. Although an axonotmetic injury will recover, a neurotmetic injury cannot. Within axonotmesis, Seddon described a degree of injury associated with scarring and less complete recovery. Within neurotmesis, he described injury of “in continuity” scar and an inability to recover.
Sunderland emphasized Seddon’s expanded description of axonotmesis and neurotmesis with a classification that emphasized five degrees of nerve injury. Sunderland’s classification system is widely used today due to the improved clarity in describing partial injuries and its ability to communicate the probability of recovery. ( Table 52.1 and Fig. 52.1 ). First-degree (neurapraxia) and second-degree (axonotmesis) injuries recover spontaneously, the latter at a rate of 1–1.5 mm/day or 1 inch/month (2.5 cm/month). Third-degree injuries have partial recovery through the fascicles that are not affected by scar. Surgical correction of third-degree injuries is challenging, as identifying scarred fibers while preserving undamaged fascicles with the potential for spontaneous recovery is technically challenging. Fourth- and fifth-degree injuries do not recover spontaneously and require surgical reconstruction. Mackinnon later described a sixth-degree injury that could include normal fascicles with various degrees of injury to other fascicles. The sixth-degree injury is the surgeon’s most technically challenging injury.
| Mackinnon | Sunderland | Seddon | Injury | Recovery | |
|---|---|---|---|---|---|
| Degree I | Degree I | Neurapraxia | Conduction block resolves spontaneously | Favorable | Fast/excellent |
| Degree II | Degree II | Axonotmesis | Axonal rupture without interruption of the basal lamina tubes | Favorable | Slow/excellent |
| Degree III | Degree III | Rupture of both axons and basal lamina tubes, some scar | Favorable | Slow/incomplete | |
| Degree IV | Degree IV | Complete scar block | Unfavorable | None | |
| Degree V | Degree V | Neurotmesis | Complete transection | Unfavorable | None |
| Degree VI | Combination of I–V ± normal fascicles | Mixed | Mixed | ||
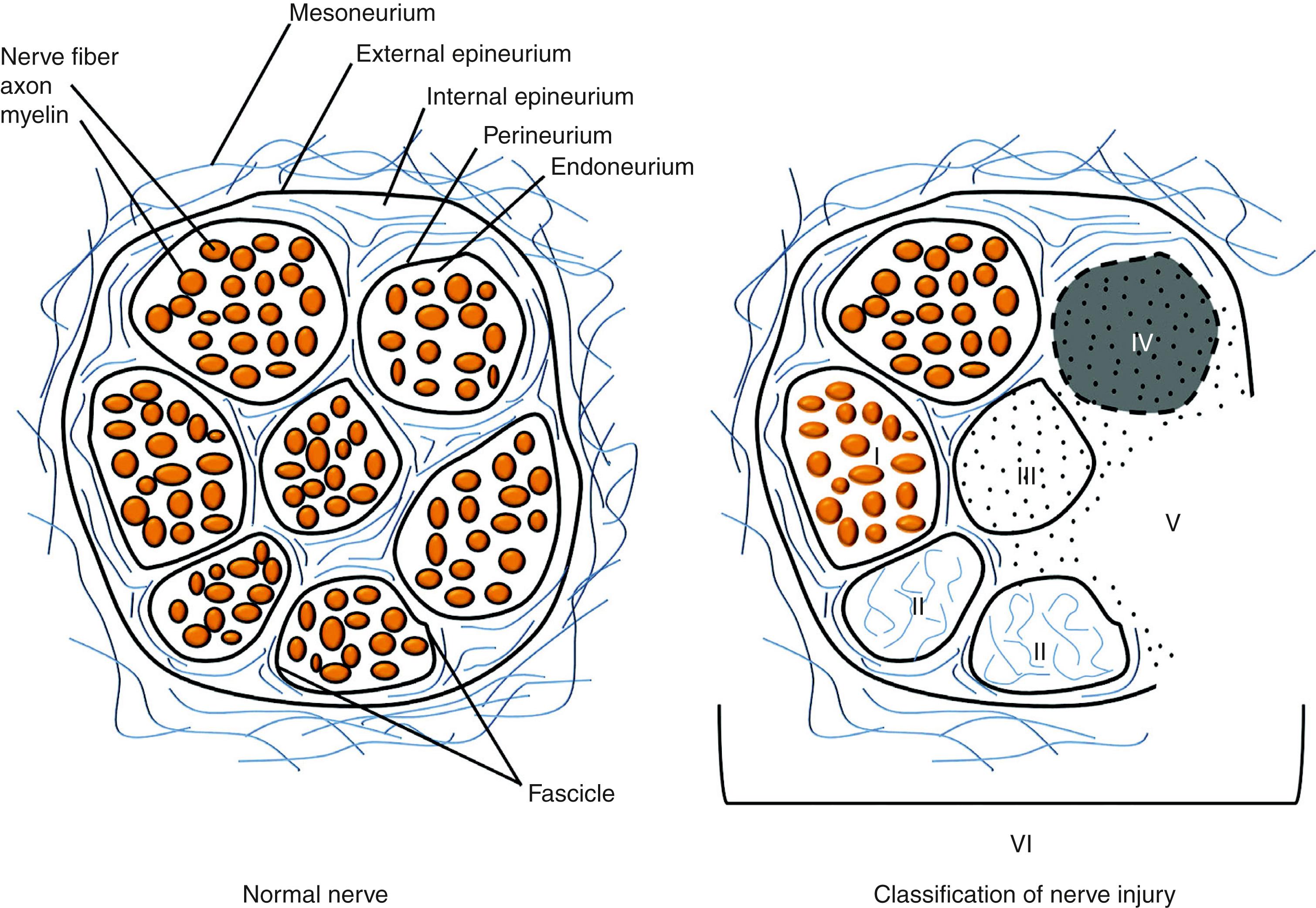
It is clinically helpful, and simpler, to classify peripheral nerve injuries into “favorable” and “unfavorable.” Favorable injuries recover fully and quickly (neurapraxia, first degree) or slowly (axonotmesis, second degree), or only partially and slowly (axonotmesis, third degree). “Unfavorable” injuries will not recover (neurotmesis, fourth and fifth degrees).
Nerve injuries may alternatively be classified by mechanism of injury or by open versus closed injury. This clinical classification is useful in determining the likelihood for spontaneous recovery, and provides an algorithm for managing these injuries. Table 52.2 lists the typical nerve injury patterns and their significance with respect to assessment and treatment. For simplicity, nerve injuries can be grouped as: (a) crush and compression injuries; (b) stretch and avulsion injuries; and (c) penetrating injuries. Although avulsion and crush injuries tend to be closed injuries, they may be open. When the skin is violated, a significant force was exerted to the body and thus it is not unusual to have associated soft tissue, vascular, and orthopedic injuries. The extent of soft tissue loss, especially muscle and skin, will ultimately influence the outcome of the nerve repair. More extensive soft tissue damage, with or without concomitant bony injuries, raises the complexity and the likelihood for a staged reconstruction. Often, the nerve reconstruction is appropriately delayed in light of more acute vascular and orthopedic injuries.
| Type | May Be Injured | Significance |
|---|---|---|
| Stretch/Avulsion Injury | ||
| Avulsion | Nerve roots | Unable to be repaired primarily |
| Nerves exiting | Indication for nerve transfer | |
| Foramen, bony fracture | ||
| Stretch | Any nerve | Mixed nerve injury (degree VI) |
| Crush and Compression Injury | ||
| Complex crush | Skin, subcutaneous tissue, muscle, nerve ± bone | Varying degree of depth, loss of function is related to amount of tissue destruction |
| Chronic compression | Nerve | Slow onset, reversible |
| Acute compression | Nerve ± muscle | Quick onset, reversible muscle ischemia, variable recovery of both muscle and nerve |
| Compartment syndrome | Nerve + muscle | Quick onset, reversible muscle ischemia if ischemia less than 6 h; no or variable recovery of muscle if released after 6 h |
| Penetrating Injury | ||
| Sharp | Skin, subcutaneous tissue, muscle, nerve ± bone. All levels | Needs surgical exploration because of high probability for nerve disruption |
| Blunt | Variable | Injury may extend further then expected |
| Blast | Variable | Injury pattern depends on ballistic makeup and velocity |
| Electrical | Variable | Neuropathy is from damage to myelin sheath and ranges from neuropathy to causalgia |
The most common peripheral nerve injury in the extremities is from external forces crushing the tissue. In the case of light compressive forces, a self-limiting neurapraxia occurs, but with increased force, the higher the probability of irreparable damage. In addition to external force, increased internal pressure from a hematoma, fracture, and local tissue edema may increase the compressive injury to the nerve. Compartment syndrome is the extreme of the spectrum and constitutes a surgical emergency. An early sign of impending compartment syndrome is a decrease in vibration sensibility. The complications of a missed compartment syndrome are so devastating that the standard of care is to err on the side of caution and release the muscle compartment if clinical suspicion is high.
Although nerves are fairly resistant to injury, especially when the surrounding tissue is not significantly damaged, a mixed nerve injury can occur. The connective tissue surrounding larger nerves can contract as it heals, causing an additional compression of the nerve as well as a tethering effect to the surrounding tissue. In addition, local soft tissue damage, especially to muscle, which is much less resilient to pressure than skin, nerve, and tendon, can lead to fibrosis. Thus a relatively minor nerve injury may produce enough muscle swelling to cause irreparable damage without clinical evidence of a compartment syndrome, leading to more extensive permanent damage than one would anticipate.
This mechanism of nerve injury is usually treated conservatively and exploration is warranted if the nerve recovery does not follow an expected pattern. Along with traction injuries, crush injuries are followed with serial exams prior to 3 months post injury, and electromyograms (EMGs) at 3 months if no clinical evidence of neurological recovery is noted.
Stretch or traction injuries to a nerve occur when the strain exerted on a particular nerve exceeds the maximum limit that nerve diameter can accept. The internal structure of the nerve becomes injured, usually without any appreciable external evidence of injury. High-velocity or high-impact injury can often lead to a proximal avulsion of the nerve or nerve root. Soft tissue attachments around joint or bony foramina act as tethering points along the nerve’s path. If arteries and veins run in parallel to the nerves, which is often the case, a severe injury can compromise the circulation.
Even at major medical centers with ample resources and personnel it is rare to see a multispecialty surgery occur in the emergency setting at the time the limb-threatening injury is addressed. The nerve injury is usually addressed at a later date.
Nerve injuries in which the proximal nerve is accessible are repaired directly or with a graft. Nerve transfer and possible tendon transfers can be performed to complement and augment the nerve reconstruction. Nerves avulsed distally at the neuromuscular junction present a different problem, as there is no distal end available for repair. For motor nerves, the proximal nerve can be directly implanted into the muscle belly. Some clinical studies show as good as Medical Research Council (MRC) grade 4 motor recovery 1–2 years after direct nerve-to-muscle neurotization; however, experimental studies do not support these findings; rather, recovery is much less than a nerve coaptation would produce and we rarely use this technique in preference to tendons or muscle transfers.
Penetrating trauma can be a result of sharp or blunt penetration, and will often have concomitant vascular and tendons injury in addition to the nerve injury. A sharp laceration, such as a hand laceration from a knife or piece of glass, necessitates exploration if a nerve deficit is present. The likelihood that the nerve is partially or completely transected is high. It is recommended to explore these injuries semi-electively within the first week. The more time that passes from the injury date, the less likely the two ends of the nerve can be mobilized to perform a primary repair. More than likely a gap will result after the traumatized nerve is trimmed to healthy nerve and a graft will be needed. In our practice, acute nerve grafting is performed if there is concern about a degree of injury that would make it difficult to reoperate in the future.
In the event of a penetrating trauma with an associated vascular injury, immediate exploration is warranted. Occasionally in proximal injuries with large arterial injuries with or without underlying fractures, the nerve injury is overlooked in the face of more urgent vascular and orthopedic injuries. Rather, the deficit is noticed postoperatively, when it is unclear if the nerve injury is from the inciting event, iatrogenic during the repair of the vascular injury, or secondary to edema or hematoma. Although a CT scan or MRI may be helpful to evaluate for the latter, internal scarring of the nerve may not always be clearly determined. As with traction injuries, a very proximal penetrating nerve injury may benefit from distal nerve transfers to augment recovery in addition to the acute repair or grafting.
Blunt penetrating and blast injuries are usually treated conservatively, similar to closed crush and stretch injuries, because they may recover spontaneously. The local tissue trauma often causes a neurapraxia that resolves; however, following the same algorithm for traction or crush injury, those injuries that do not recover clinically after 3 months should be evaluated by electrodiagnostic studies.
A thorough history and clinical examination with an understanding of anatomy is the cornerstone of evaluating nerve injuries. Mechanism of injury can raise or lower the clinical suspicion of irreversible nerve injury requiring reconstruction, as described above for crush, traction, avulsion, and sharp and blunt penetrating injuries. An open injury is easier to assess, as surgical exploration is most often justified except in the case of a blast injury. Typically, we recommend that a sharp injury with associated motor or sensory deficits be explored within 7 days if possible. Conventionally, it is accepted that primary neurorrhaphy should be possible up to 2 weeks after an injury. With injuries that present later than 3 weeks, the surgeon should be prepared to do a nerve graft repair.
Determining spontaneous sensory or motor nerve recovery in closed injuries may be difficult and intervention is guided by serial physical examination. For sensory nerve injuries, an advancing Tinel’s sign signals sensory recovery. Sensation may be tested using two-point static and moving discrimination, the Semmes–Weinstein filament test, or the “10 test.” This quick and easy screening test uses the patient’s own subjective perception to moving light touch to elicit differences in sensation between the right and left sides of the mirror digit. For example, both index fingers are stroked at the same time and patients are asked if the sensation is the same or different between the two digits. This technique is particularly useful in young children and in patients who have some underlying neuropathy. If in doubt that the patient has a nerve injury after the “10 test,” two-point discrimination is a more sensitive test to determine severity of injury.
In motor nerve injuries, serial physical examination of affected muscle groups is performed and graded using the Medical Research Council (MRC) scale. Muscle groups are tested against gravity and against resistance. Other muscle groups unaffected in the extremity by the nerve injury can also be assessed for strength and may provide options for future nerve transfer donor fascicles.
In addition to serial physical examination, electrodiagnostic studies are performed generally at 3 months post injury to evaluate for recovery as EMG changes indicating recovery precede clinical recovery.
Signs of ongoing acute denervation include positive sharp waves, fibrillations, and fasciculations on EMG. Motor unit potentials (MUPs) are the EMG equivalent of active muscle activation. There are three commonly encountered scenarios in electrodiagnostic testing at 3 months post injury:
No acute denervation, normal MUPS – This electrodiagnostic pattern corresponds to a neuropraxic injury, or Sunderland I, and will recover fully
No acute denervation, abnormal MUPS – This pattern represents a slightly higher level of injury, Sunderland II, which has begun to heal and will recover fully
Acute dennervation, some abnormal MUPS – There is ongoing denervation of the muscle but there are some recovering MUPs. These patients may benefit from distal decompression to enhance recovery (preventing the “double crush phenomenon”). Clinical recovery will be partial
Acute dennervation, no MUPS – This electrodiagnostic pattern represents a severe injury. The prognosis for recovery is guarded and surgical intervention and reconstruction is recommended.
We recommend early evaluation of nerve injury and close follow-up. This strategy enables clinical identification of those patients whose mechanism of injury warrants early exploration, and avoids delay in recognizing a nerve deficit that will not resolve spontaneously. It also enables us to order the electrodiagnostic testing to evaluate both injured nerves and potential donors if we are considering nerve or tendon transfers in the reconstruction. There are clinical investigations using newer ultrasound machines that can detect nerve pathology; however, these studies are still investigational and are highly operator-dependent.
Many factors besides technique affect nerve repair and age of the patient seems to be the most important factor. From historical data, we know that nerve repair in children and young adults yields better results than in adults. One reason is that the nerve has a shorter distance to travel. Nerves regenerate at 1–1.5 mm/day, and in a child the nerve recovery would be expected to reach the intended target organ earlier than in an adult and thus have a quicker recovery. Secondly, a child’s brain has greater plasticity and more neural activity than an adult, thus the child has an enhanced ability to process the reinnervated motor and sensory input within the brain cortex.
The type of injury, the location, and the degree or depth of the injury affect the outcome. A sharp laceration will have less associated soft-tissue trauma as a crush or avulsion injury at the same level. Proximal injuries may affect larger muscle or sensory domains. The reconstruction can be more challenging and a minor misalignment of fascicles has greater consequences than a more distal injury. Sensory nerve lacerations in the finger repaired a few days after the injury usually have excellent outcomes while sensory nerve neuromas from an original crush injury may require reconstruction with grafts months after the injury and do not recover as well. Often the internal damage is underestimated and if care is not taken to be outside the zone of injury, the repair may be suboptimal.
When considering the surgical intervention, timing, type of repair, tension and alignment of fascicles have an equally important role in the outcome of nerve recovery. Earlier repair usually results in better outcomes and the best results occur if the repair can be performed within 3 weeks. A good result can still be expected if the repair occurs before 6 months, although a nerve graft will most likely be needed. Functional recovery is directly proportional to the number of motor axons reaching the target endplate and inversely proportional to the time of denervation: “Time = muscle.”
In the case of a nerve transection, patients who are clinically stable should be taken to the operating room for exploration and repair of the nerve within the first 7–10 days. Repair within the first 72 hours is preferable in large motor and mixed nerves, as a nerve stimulator can be used to map motor fascicles in the distal segment. Nerves repaired acutely within the first 2 days are considered repaired primarily. A delayed primary repair occurs between 2 and 7 days. In either case, the proximal and distal ends of the nerve are freshened and an end-to-end coaptation performed. Nerves repaired after the first week are considered repaired secondarily. The cut-off days of 2 and 7 are arbitrary because even at 2–3 weeks from injury, the nerve can occasionally be coapted without need for a graft; however, the longer the time from injury, the less likely it is that a primary neurorrhaphy can be achieved, even with significant mobilization of the proximal and distal nerve ends.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here