Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the neonatal period the most frequently encountered spinal pathologies are of developmental nature. Spinal tumors or infections are exquisitely rare in neonates. Spinal trauma may occasionally be encountered and typically result from a complicated/traumatic delivery. This chapter will consequently focus on spinal malformations.
The development of the spinal canal and its contents is highly complex and involves multiple “programmed” anatomic and functional developmental and maturational processes. Malformations of the spinal canal and cord may be an isolated anomaly involving “only” the neuroaxis (brain and spinal cord/canal) or may be part of a complex syndrome or malformation (e.g., cloacal malformation). Next to the primary developmental etiology, the malformed spinal canal and cord may be secondarily injured because of prenatal, perinatal, or postnatal factors (e.g., long-standing exposure of the neural tissue to the amniotic fluid, mechanical injury during delivery, or postnatal infection). Detailed knowledge about normal neonatal spine imaging, as well as malformations, is essential to recognize malformations early (preferably prenatally) to counsel the parents during pregnancy, to plan possible intrauterine interventions, to make decisions about the mode of delivery, to optimize postnatal care, and to predict functional outcome.
In both the prenatal and postnatal period, ultrasound (US) and magnetic resonance imaging (MRI) give highly sensitive and specific information about the full extent and character of the spinal anomaly. Conventional radiography and computed tomography (CT) are rarely indicated and should be avoided to limit radiation exposure. In the following paragraphs the most common spinal malformations will be discussed based on their most apparent clinical findings.
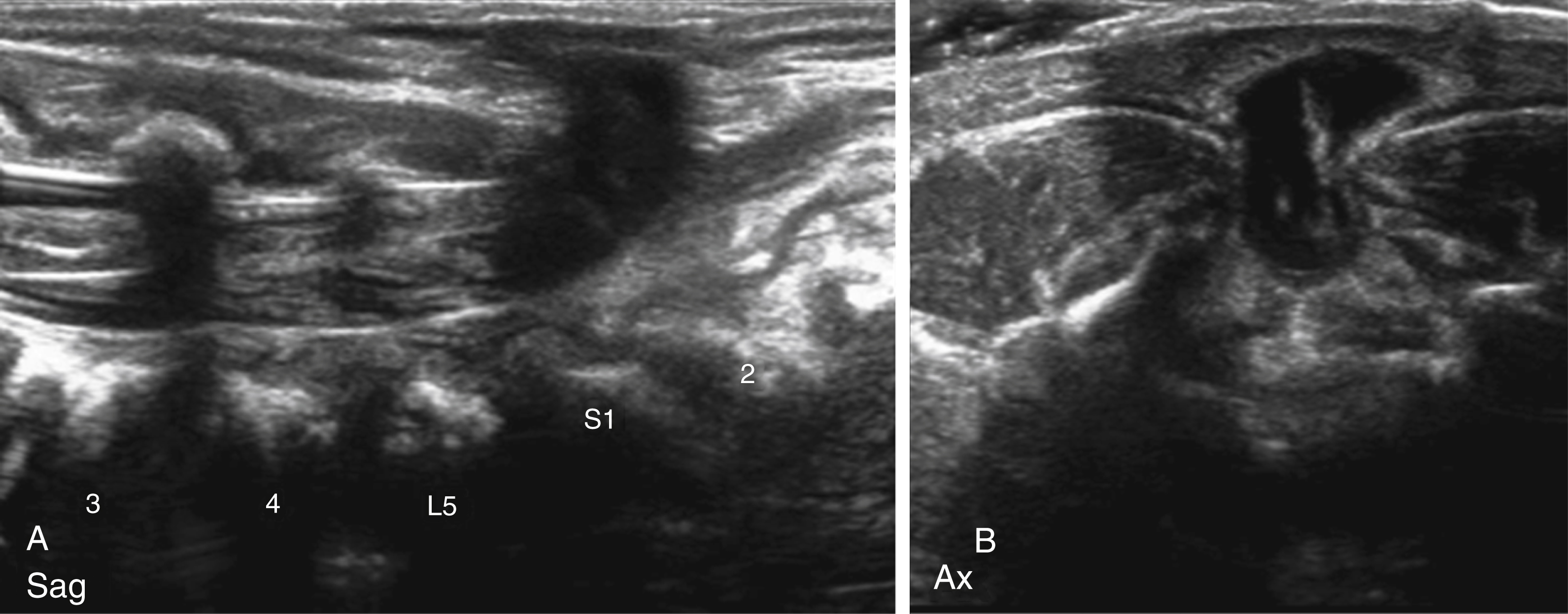
This is a common history seen in neonatal spinal sonography. Physical examination features that are more correlative with spinal abnormalities are dimple locations higher along the gluteal cleft or at lumbar level, larger or draining pits, and skin findings such as hairy patches and vascular anomalies. Sonography is an excellent initial evaluation for the spinal canal in the first few months of life because of the lack of ionizing radiation, relative ease of performing the examination, and presence of dorsal cartilage allowing sonographic window into the canal. Often, the sonographic findings are reassuring for normal spinal development. Features that are used to determine normal development are normal location of the conus medullaris at/above L2, normal appearance of a thin filum terminale, and normal pulsation of the cauda equina nerve roots. Pulsation of the conus can be documented with cine imaging or M-mode interrogation. The sonographic numbering of the vertebral bodies can be performed by counting caudal to cranial or by looking for the lumbosacral angle at L5-S1. Variants of normal that are frequently seen include a mildly prominent distal central canal (also known as ventricularis terminalis) and/or a filar cyst just below the conus.
If there is no skin covering a spinal defect, the neural placode is exposed to the surface and is termed an open neural tube defect . If the neural placode is level with the adjacent skin, the lesion is classified as a myelocele (MC); if the neural placode is pushed outside of the hypoplastic/malformed osseous spinal canal, the neural placode appears raised in relation to the adjacent skin and will “pull out” the attached meninges, appearing as a bulging fluid-filled sac. Consequently, this lesion is classified as a myelomeningocele (MMC). Both lesions result from an incomplete or segmental defective closure of the neural tube in which the resultant neural placode did not detach from the adjacent surface ectoderm. The exposed surface of the neural placode should have become the inside of the neural tube, is consequently covered by ependyma, and leaks cerebrospinal fluid (CSF). The neural placode itself is believed to be less functional because of multiple complex primary and secondary processes, including the failure of closure itself with resulting deranged neuroarchitecture but also because of chronic injury as a result of the long-lasting exposure of the neural tissue to the amniotic fluid. Adjacent bone, muscle, and skin are also deficient in various degrees of severity. These malformations most frequently occur at the lumbosacral levels; however, the thoracic or cervical spinal cord may also be involved.
Furthermore, nearly all neonates with an open spinal dysraphism will have an associated Chiari type II malformation. It is believed that the chronic leakage of CSF at the level of the spinal dysraphia during the early second trimester is causative for the occurrence of an associated Chiari type II malformation. In several fetal centers around the world, based on this hypothesis, open spinal dysraphias are closed during intrauterine life with the goal to limit the severity of the Chiari type II malformation and to reduce the degree of associated hydrocephalus and possible need for ventriculoperitoneal shunt.
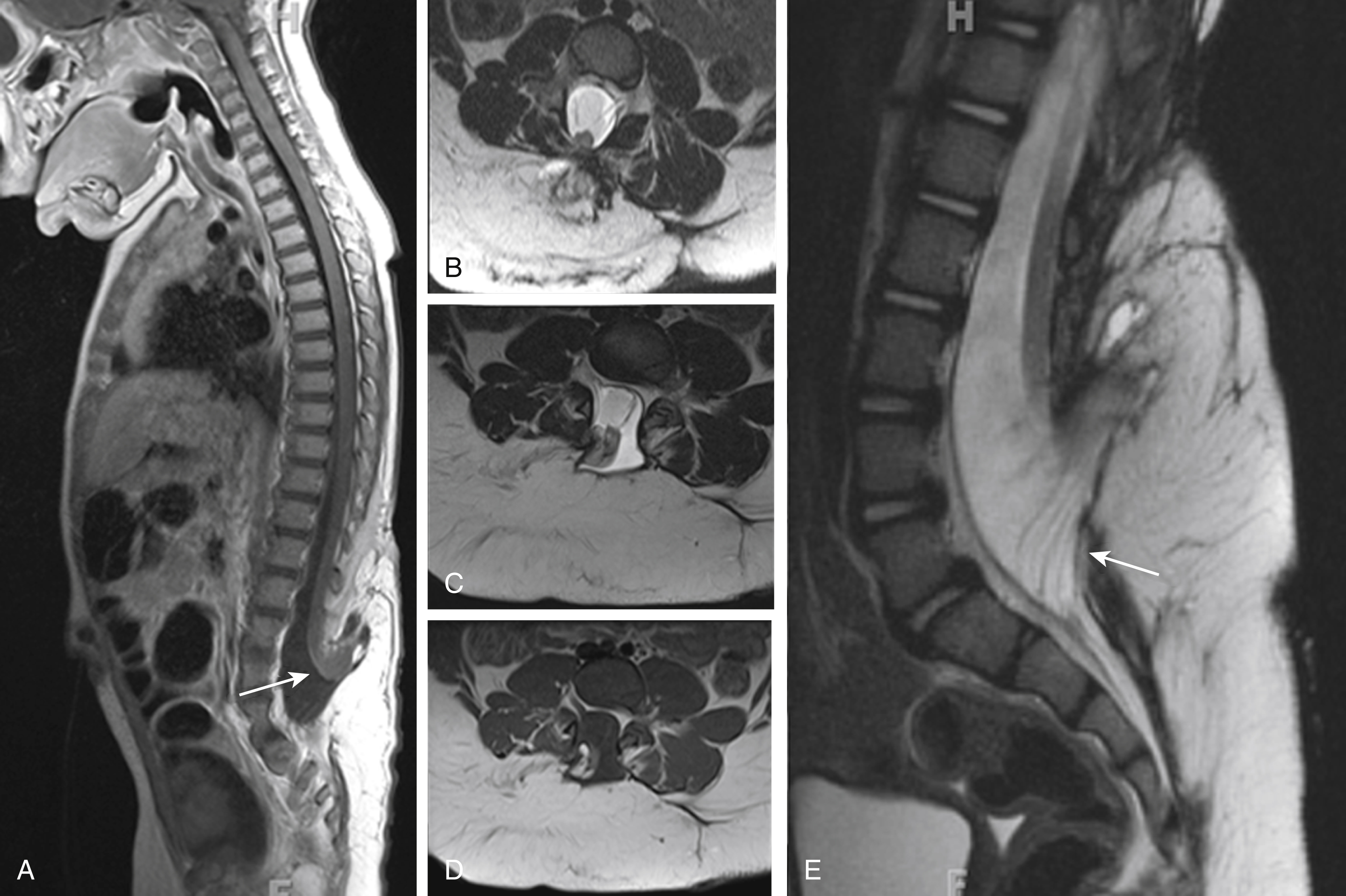
Identification of an open spinal dysraphism is often done on prenatal imaging (US and MRI) ( Figs. 29.1–29.3 ). A midline neural placode is seen either in level with the adjacent surface ectoderm (MC) or protruding like a bubble above the level of the surface ectoderm (MMC). Nerve roots appear along the anterior surface of the neural placode and, depending on the degree of neural placode protrusion, will appear “stretched” in their course toward the neural foramina. In MMC malformations, various degrees of meningeal structures will be encountered herniating lateral to the neural placode. The spinal canal is usually widened with absent or hypoplastic lateral and dorsal musculoskeletal structures. Infrequently, the neural placode may protrude asymmetrically outside of the malformed spinal canal. In addition, various degrees of hydromyelia may be encountered in the intact spinal cord superior to the spinal dysraphia. Within the cranial vault the typical stigmata of a Chiari type II malformation are encountered. Prenatal US typically identifies the spinal dysraphism and Chiari type II malformation, and fetal MRI is typically used to increase the level of anatomic detail and should focus on evaluating additional malformations or complications ( Fig. 29.4 ). Fetal MRI is especially helpful for the complete diagnostic workup of the Chiari II malformation (see Fig. 29.1 ). The associated findings often determine the long-term motor and neurocognitive outcome.
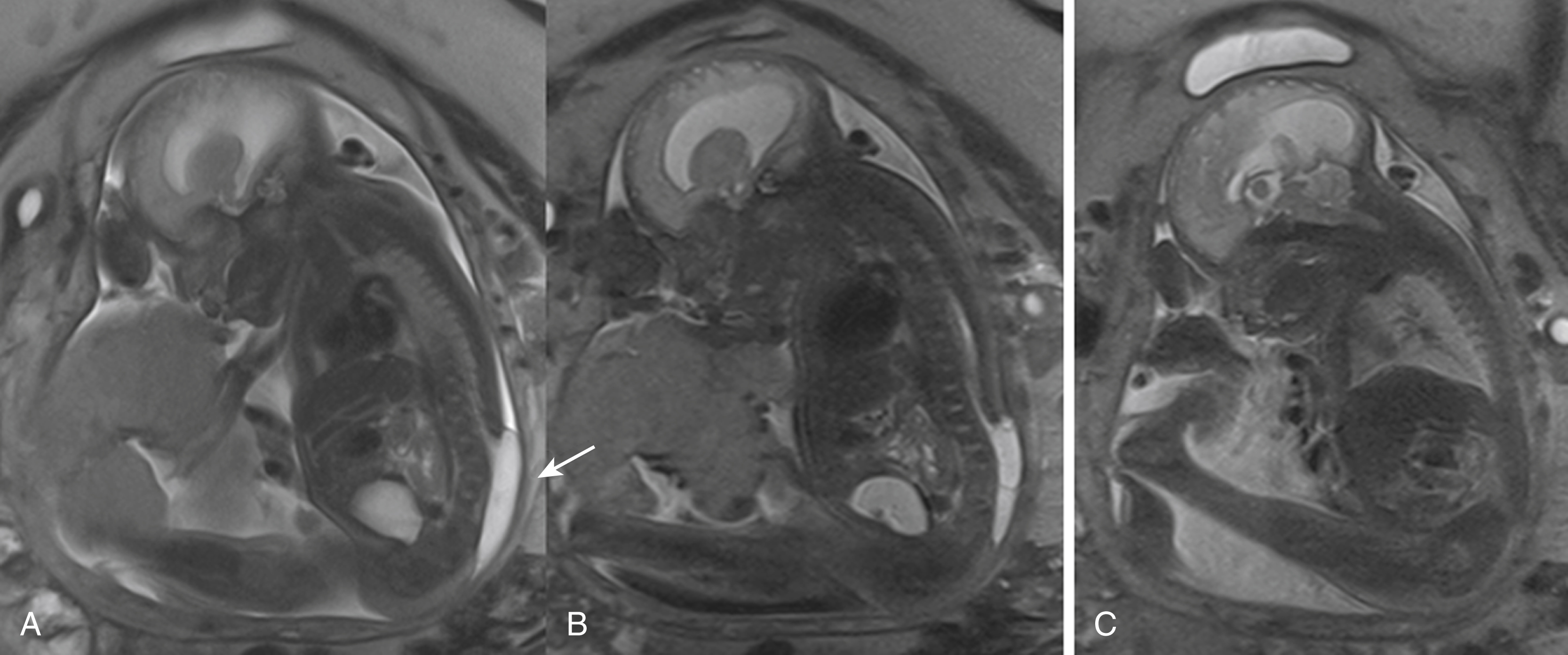
Skin-covered focal bumps/lumps along the midline of the neonatal back have a significantly better functional and neurocognitive prognosis than the non-skin-covered spinal dysraphias. In skin-covered dysraphias (closed neural tube defects) the neural placode is covered/protected by skin and subcutaneous tissue, limiting injury during intrauterine life. In addition, no leakage of CSF is observed; consequently, Chiari type II malformations are typically not seen. Depending on the degree of herniation of the neural placode outside of the malformed/hypoplastic osseous spinal canal and the size of the associated subcutaneous lipoma, these spinal dysraphias are categorized as myeloschisis with intradural lipoma, lipomyelocele (LMC), or lipomyelomeningoceles (LMMCs).
These skin-covered spinal dysraphias are believed to result from a premature dysjunction of the neural tube from the adjacent surface ectoderm before the neural tube has completely closed. The associated lipoma attached to the neural placode is believed to result from the interaction of adjacent mesodermal cells with the inner lining of the nonclosed neural tube during development, which induces the development of excessive amounts of fat. The resultant lipoma is consequently in close contact to the neural placode. Depending on the amount and extension of the fat, variant size lipomas occur that may be located exclusively intradural or may extend through the osseous defect into the subcutaneous region. On clinical inspection, significant lumps/bumps may be seen. Furthermore, cutaneous stigmata, including hairy tufts or focal skin discolorations, may be seen overlying the dysraphia. LMCs and LMMCs most frequently occur in the lumbar region.
Most children have a normal neurocognitive development but may require urological, orthopedic, and gastroenterological assistance. Their long-term morbidity consists mostly of neurogenic bladder dysfunction with possible renal damage if not treated appropriately.
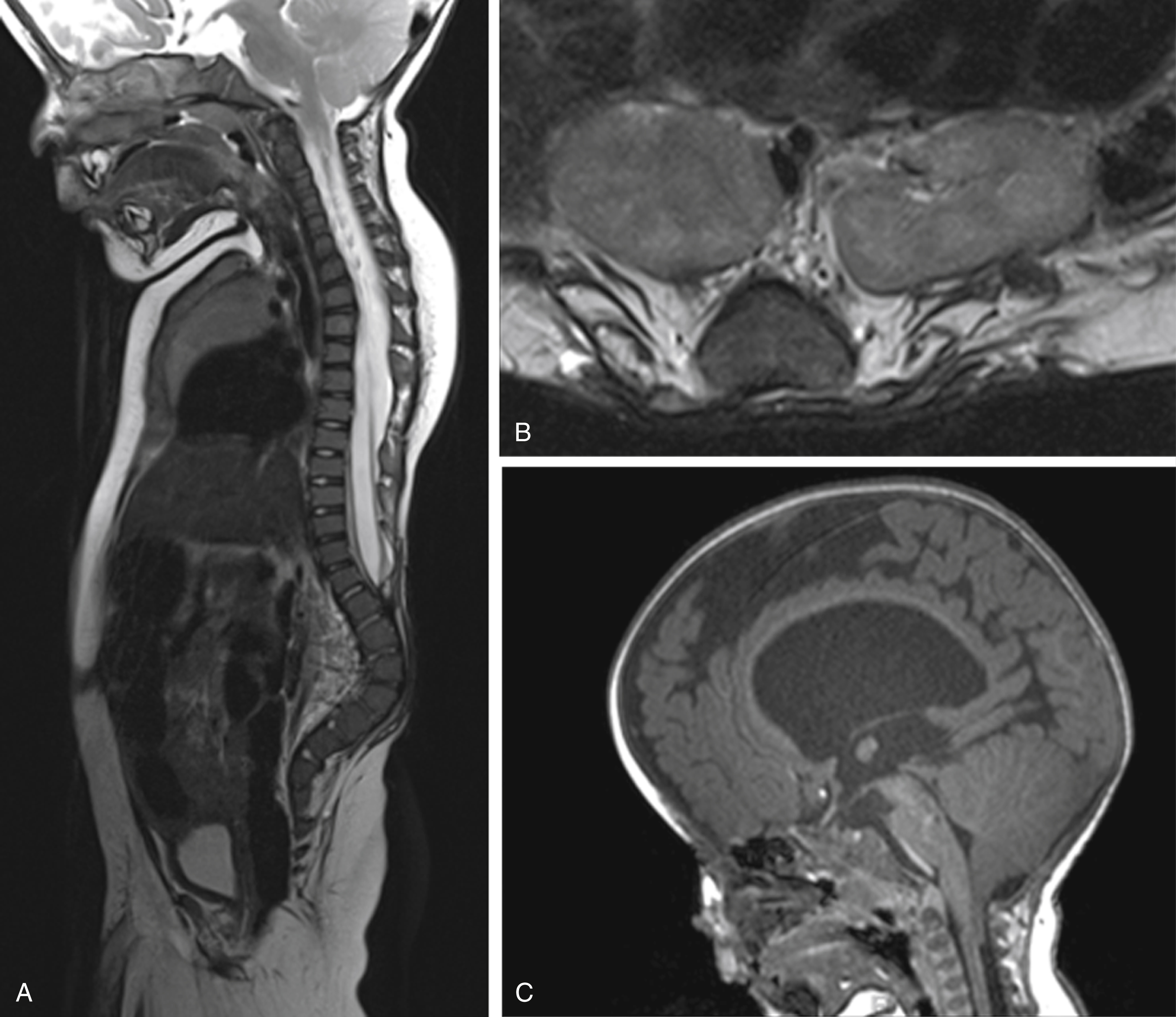
Prenatal and postnatal neuroimaging (US and MRI) rely on the identification of the osseous defect with a fat-covered neural placode within the level or dorsally of the osseous defect ( Figs. 29.5 and 29.6 ). Similar to the open spinal dysraphias, the closed malformations are classified depending on the amount of tissue that is protruding outside of the spinal canal. The spinal cord is often tethered. If no significant lipoma is present, the skin-covered spinal dysraphias may be easily missed by prenatal US. Fetal MR usually displays the skin-covered spinal dysraphia in better detail but may also occasionally fail to identify the anomaly. T1-weighted MR sequences may be helpful for identification of the T1-hyperintense lipoma. Chiari type II malformations are exquisitely rare in skin-covered spinal dysraphia. The size of the lipoma can vary significantly and may pose a large cosmetic issue for the child, especially as the child grows older. Surgical reduction of the lipoma is frequently performed later in life. A precise identification of the interface between the lipoma and the neural placode is essential for guiding surgical correction. A large lipoma may exert massive mass effect on the neural placode, even resulting in partial rotation of the neural placode with asymmetric protrusion of meninges outside of the level of the spinal canal. Finally, evaluation for additional associated malformations is important, for example, urogenital or anorectal malformations.
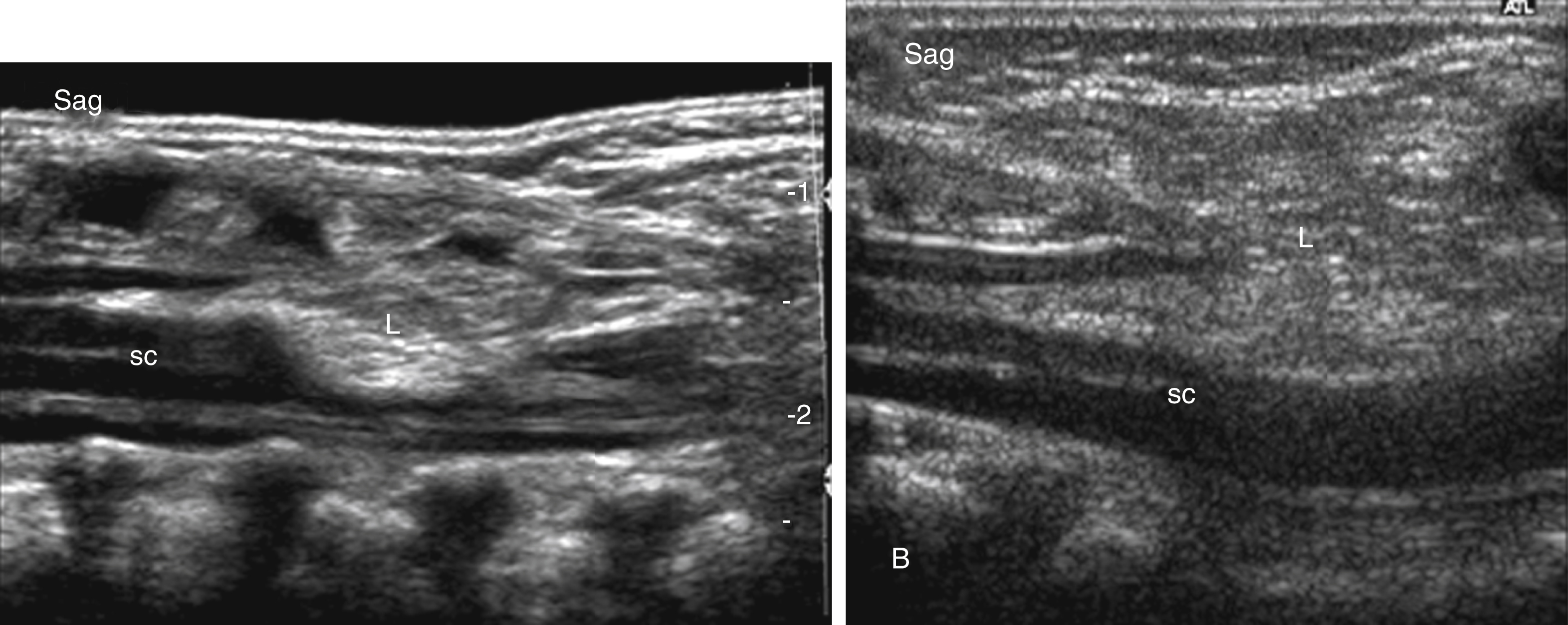
Dorsal dermal sinuses usually become apparent as a midline dimple or small pin point opening from which clear CSF may intermittently leak. In addition, a focal skin discoloration or hairy tuft may be seen in close proximity to the skin dimple. The CSF leakage is frequently missed by the parents because the neonatal diapers absorb the leaked CSF. Dorsal dermal sinuses consist of an epithelium-lined fistulous canal or tract extending from the neonatal back through the adjacent subcutaneous tissues, toward the spinal canal, where it courses through the dural sac to reach the adjacent spinal cord. In the majority of cases an associated intradural lipoma is noted that is adherent to the spinal cord. Based on their embryology the fistulous tract may extend into the central canal of the spinal cord. Dorsal dermal sinuses are believed to result from a focal or incomplete disjunction of the neuroectoderm from the surface ectoderm while the skin is closing dorsally to the neural tube. During embryological neural tube/spinal cord migration from the surface into the developing spinal canal, a persistent connection between ectoderm and neural tube can occur, resulting in a fistulous tract. Most dorsal dermal sinus lesions are in the lumbar region; however, thoracic and cervical cases have been described in the literature. They represent a port of entry for infections and meningitis and may result in a caudal syndrome with extensive inflammation of the cauda equina nerve roots.
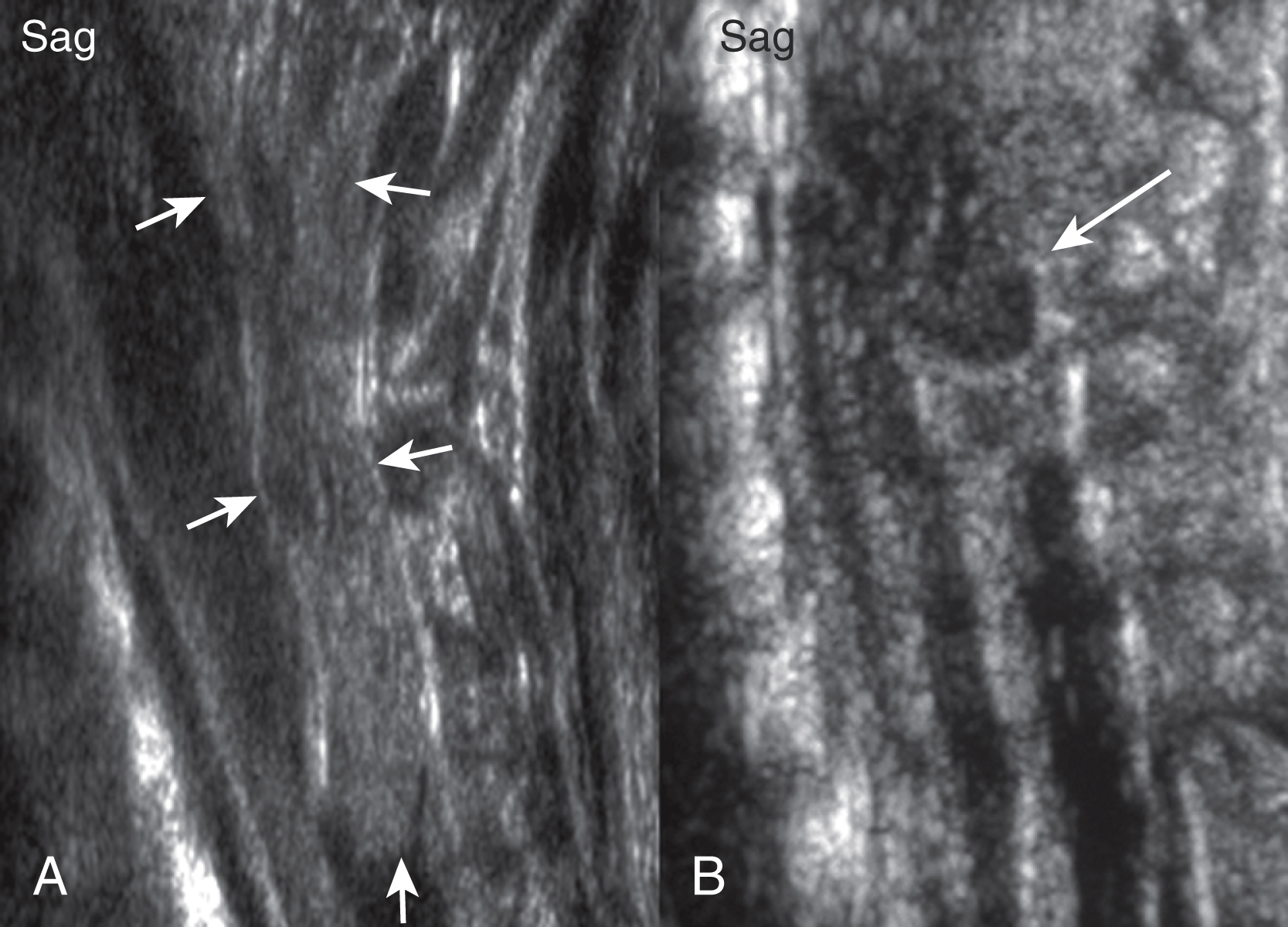
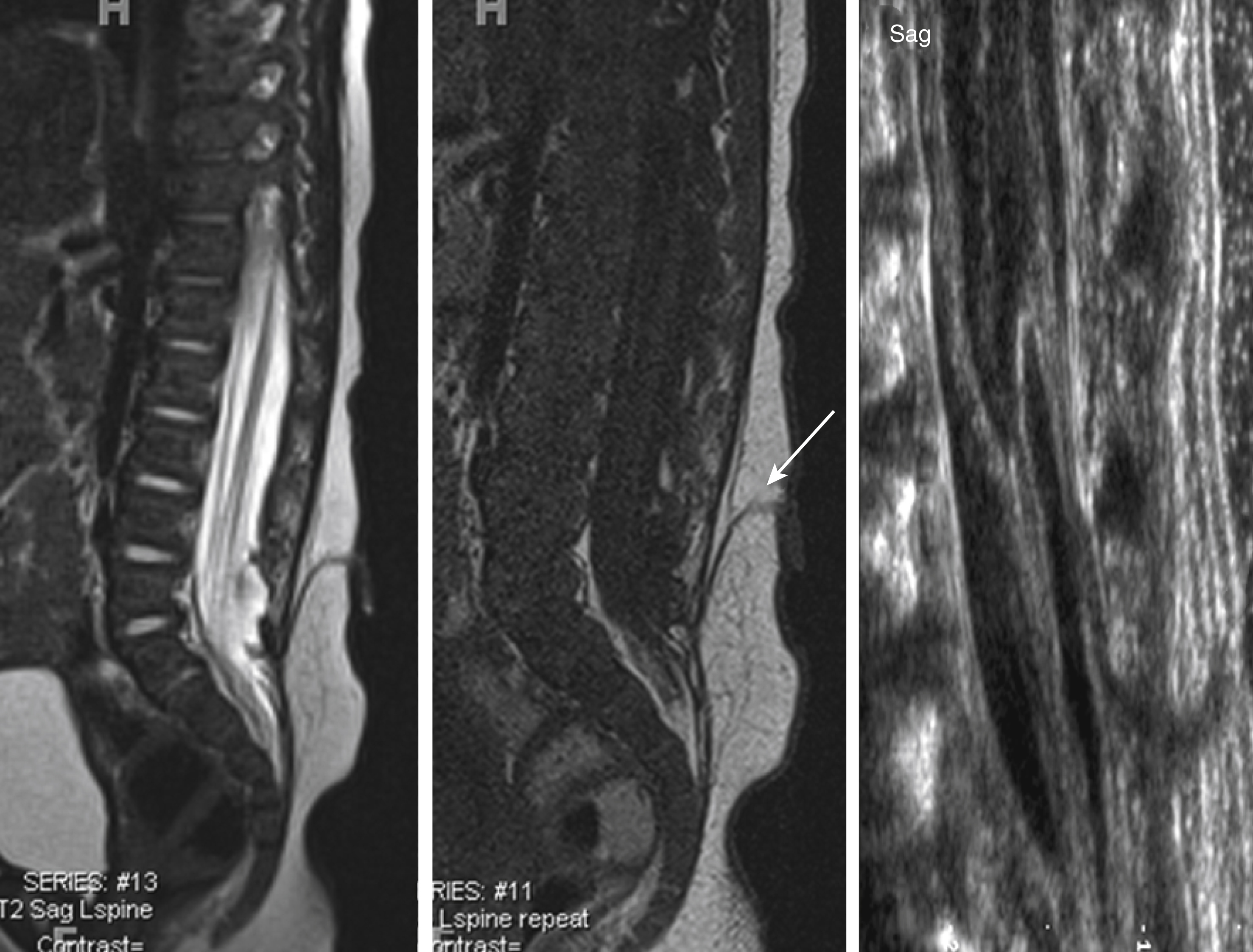
Dorsal dermal sinuses may be recognized on prenatal US based on a cord tethering and/or by the simultaneous identification of the adjacent intradural, perimedullary lipoma. The fistulous tract is rarely seen on prenatal US. Prenatal MRI usually correctly characterizes the malformation. In contrast with an MMC, the spinal cord appears closed, no neural placode is noted, and most importantly the spinal cord below the lipoma is intact. No associated Chiari type II malformation is seen. A mild dilatation of the central canal may be seen. Furthermore, the tip of the spinal cord may be low ending. Postnatal US and in particular neonatal MRI easily identify the malformation ( Fig. 29.7 ). The fistulous tract is well seen on thin-sliced, high-resolution T1- or T2-weighted images as a T1-hypointense, T2-hypointense, or hyperintense linear streak extending from the cutaneous dimple toward the spinal cord. The tract is usually well seen on non-fat-saturated sequences because the adjacent subcutaneous fat renders a good lesion contrast. The tract is most frequently directing upward/cranially and will end in up to 50% of cases in an intradural lipoma, which is adherent to the involved segment of the spinal cord. On very thin sliced, heavily T2-weighted long-echo imaging the fistulous tract may be directly identified coursing between the nerve roots of the cauda equina. Contrast-enhanced sequences may be considered if inflammation is suspected. CT is rarely indicated.
Differential diagnosis includes a sacral dimple, which is known as a focal depression of the skin just above the neonatal buttocks. Sacral dimples are a common variant, occurring in up to 5% of the neonates. The lower the dimple, the less likely that is connected to the spinal canal/cord. If clear fluid can be discharged from the dimple, if a hairy tuft is associated, or if there are clinical features of lower extremity or bladder dysfunction, US or MRI should be considered to rule out a spinal malformation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here