Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
* URL referenced in this chapter includes: Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ .
Elevation of the serum bilirubin level is a common, if not universal, finding during the first week of life and has been reviewed elsewhere. This can be a transient phenomenon that will resolve spontaneously or can signify a serious or even potentially life-threatening condition. There are many causes of hyperbilirubinemia and related therapeutic and prognostic implications. Independent of the cause, elevated serum bilirubin levels can be potentially toxic to the newborn infant. This chapter will review perinatal bilirubin metabolism and address assessment, etiology, toxicity, and therapy for neonatal jaundice. The diseases in which there is a primary disorder in the metabolism of bilirubin will be reviewed regarding their clinical presentation, pathophysiology, diagnosis, and treatment.
In 1864 Städeler used the term bilirubin, derived from Latin ( bilis, bile; ruber, red), for the red-colored bile pigment. Bilirubin is formed from the degradation of heme-containing compounds ( Fig. 4-1 ), the largest source of which is hemoglobin, and other sources are the cytochromes, catalases, tryptophan pyrrolase, and muscle myoglobin.
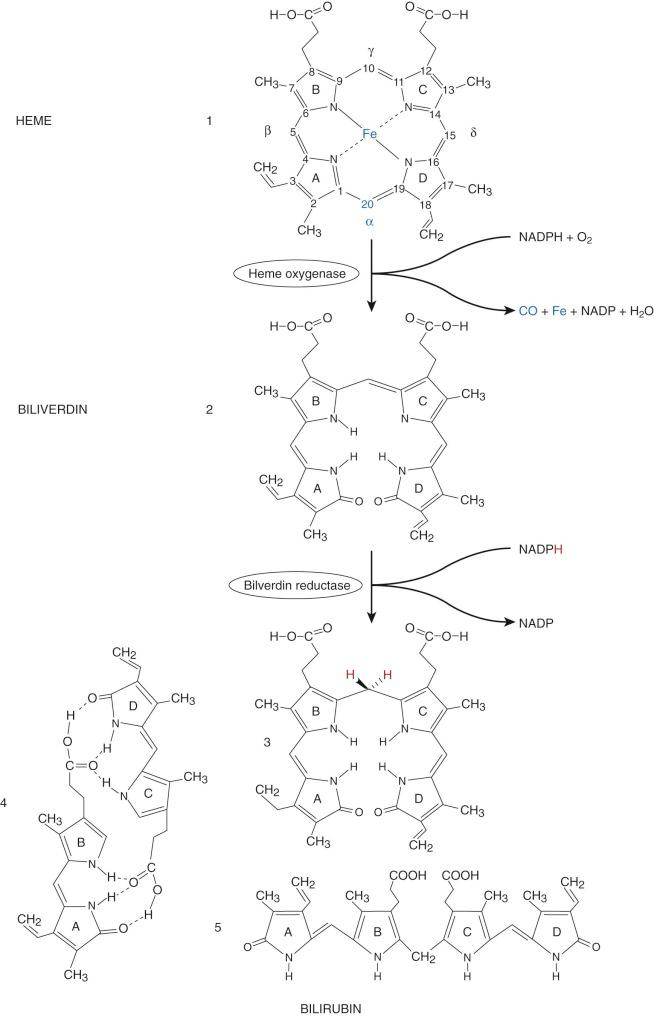
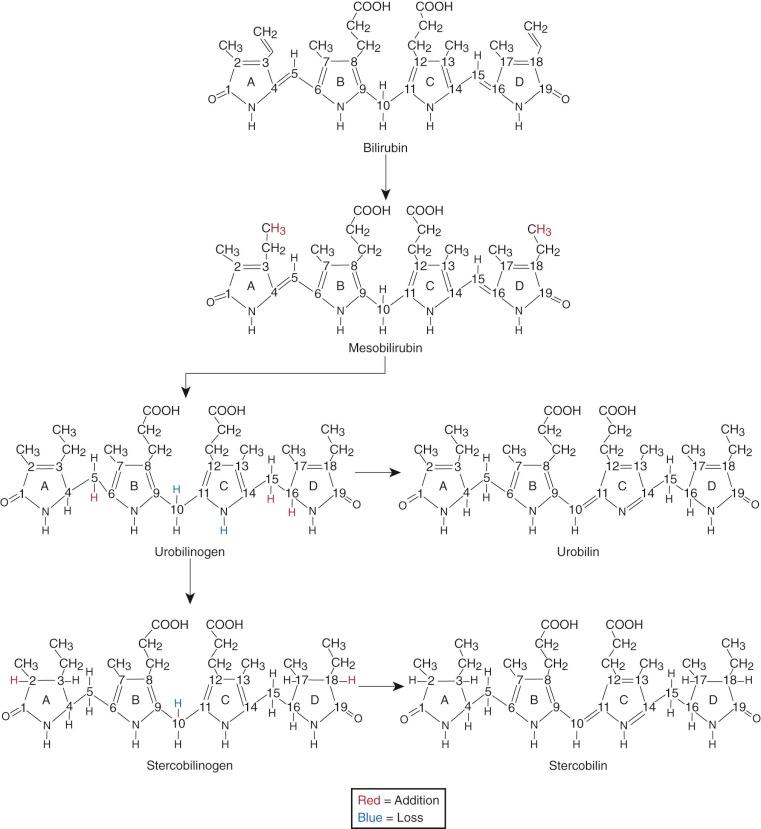
The formation of bilirubin is initiated by cleaving the tetrapyrrole ring of protoheme (protoporphyrin IX), which results in a linear tetrapyrrole (biliverdin). The first enzyme system and rate limiting step in bilirubin synthesis is microsomal heme oxygenase (HO). Two major forms of HO have been identified. HO1, the inducible form, is located in the spleen, liver, and bone marrow. HO2, the constitutive form, is located in testes, central nervous system (CNS), vasculature, liver, kidney, and gut.
HO results in reduction of the porphyrin iron (Fe III to Fe II ) and hydroxylation of the α methine (=C-) carbon. This α carbon is then oxidatively excised from the tetrapyrrole ring, yielding carbon monoxide (CO); this is the only physiologic source of CO. This excision opens the ring structure and is associated with oxygenation of the two carbons adjacent to the site of cleavage. The cleaved α carbon is excreted as CO, which has numerous biological effects, including neurotransmission, vasodilation as well as mediation of apoptosis and anti-inflammatory processes. The iron released by HO can be reutilized by the body. HO produces a linear tetrapyrrole, biliverdin IXα. The IX designation is a result of Fischer's grouping of the protoporphyrin isomers, group IX being the physiological source of bilirubin. In utero, bilirubin IXβ is the first bile pigment seen and can be found in bile or meconium by 15 weeks' gestation. Small amounts of bilirubin IXβ are also found in adult human bile. The central (C-10) carbon on biliverdin IXα is then reduced from a methine to a methylene group (-CH 2 -), thus forming bilirubin IXα. This is accomplished by the cytosolic enzyme biliverdin reductase. The proximity of this enzyme results in very little biliverdin ever being present in the circulation.
Bilirubin formation can be assessed by measurement of CO production. Such assessments indicate that the daily production rate of bilirubin is 6 to 8 mg/kg/24 hr in healthy term infants and 3 to 4 mg/kg/24 hr in healthy adults. In mammals, 80% to 85% of bilirubin produced daily originates from hemoglobin. Degradation of hepatic and renal heme appears to account for most of the remainder, reflecting the very rapid turnover of certain of these heme proteins. Although the precise fate of myoglobin heme is unknown, its turnover appears to be so slow as to be relatively insignificant.
Catabolism of hemoglobin occurs very largely from the sequestration of erythrocytes at the end of their life span (120 days is adult humans, 90 days in newborns). A small fraction of newly synthesized hemoglobin is degraded in the bone marrow. This process, termed ineffective erythropoiesis, normally represents less than 3% of daily bilirubin production but may be substantially increased in people with hemoglobinopathies, vitamin deficiencies, and heavy metal intoxication. Infants produce more bilirubin per unit of body weight because of their greater red blood cell mass and shorter red blood cell life span. Additionally, hepatic heme proteins represent a larger fraction of total body weight in infants.
Although bilirubin has long been thought of solely as a waste product, data suggest that some mild degree of hyperbilirubinemia may be helpful because of the anti-oxidant capacity of bilirubin and its potential role as a free-radical scavenger and cytoprotectant.
Bilirubin is poorly soluble in aqueous solvents and thus requires biotransformation to more water-soluble derivatives for excretion from the body. This poor solubility is related to the structure of bilirubin. Rather than being linear (structure 5, Fig. 4-1 ), bilirubin undergoes extensive internal hydrogen bonding (structure 4, Fig. 4-1 ). This shields the polar propionic acid side chains and makes bilirubin very nonpolar and lipophilic. The carbon–carbon double bonds at positions 4-5 and 15-16 can assume two different configurations (similar to cis and trans ) depending on whether the higher priority atoms or groups (based on atomic number) are on the same ( Z, zusammen, German: together ) or opposite ( E, entgegen, opposite ) sides of the double bond. The naturally occurring form of bilirubin, 4Z,15Z-bilirubin IXα, can be represented by any of the three structures (3-5) depicted at the bottom of Figure 4-1 . Knowledge of this stereochemistry is important in understanding phototherapy, which will be discussed later.
Bilirubin's poor aqueous solubility necessitates a carrier molecule, albumin, for transport from sites of production in the reticuloendothelial system to the liver for excretion ( Fig. 4-2 ). Each albumin molecule possesses a single high-affinity (K a =7 × 10 7 M -1 ) binding site for one molecule of bilirubin. A binding affinity of this magnitude implies that, at normal serum bilirubin levels, all bilirubin will be transported to the liver bound to albumin, with negligible amounts free to diffuse into other tissues. Secondary binding sites of lesser affinity also exist on albumin. Albumin also serves as a carrier for other compounds such as xenobiotics and fatty acids.
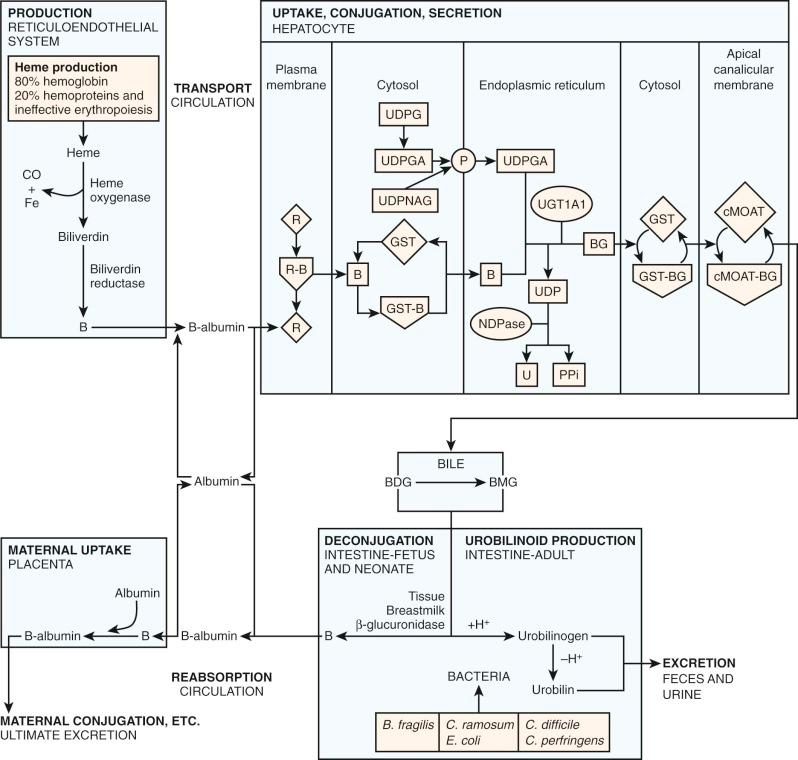
The structure of the liver is well suited for the uptake of bilirubin by individual hepatocytes. Cords of hepatocytes are arranged radially so that adjacent sinusoids border all hepatocytes. The flow of blood through the sinusoids is slower than that of other capillary beds because it is generated by portal venous pressure rather than arterial pressure. There is easy passage of albumin-bound bilirubin from the plasma into the tissue fluid space (space of Disse) between the endothelium and the hepatocyte because the sinusoidal endothelium of the liver lacks the basal laminae, which are found in other organ capillary systems. The pores of the endothelium allow direct contact with the plasma membrane of the hepatocyte.
A hepatocyte with a schematic illustration of bilirubin metabolism is shown in Figure 4-2 . In the first step, bilirubin dissociates from its albumin carrier and enters the hepatocyte via a membrane-receptor carrier, which facilitates entry into the hepatocyte. Carrier-mediated transport into the hepatocyte has been demonstrated for several organic anions including bilirubin, bromsulfophthalein (BSP), and indocyanine green (ICG), though bilirubin has also been shown to be able to pass through membranes by simple passive diffusion. Evidence suggests that bilirubin, BSP, and ICG share the same hepatocyte-receptor carrier because they exhibit competitive inhibition when injected simultaneously. This finding cannot be explained by subsequent intrahepatic metabolism, because these anions are handled differently by the hepatocyte: Bilirubin is conjugated with glucuronic acid in the endoplasmic reticulum (ER), BSP is conjugated with glutathione in the cytosol, and ICG is excreted directly without biotransformation. Data from rat hepatocytes suggest that the anion binding–receptor carrier is a dimeric protein with subunit molecular weight of 55,000. Antibody studies confirm the expected location in the plasma membrane and demonstrate blocking of uptake. Organic anion transporting polypeptide 2 (OATP2, recently named OATP1B1 under new nomenclature ) has shown high-affinity uptake of bilirubin in the presence of albumin and is a member of the OATP family, transporter symbol SLC21A.
Carrier-mediated transport of bilirubin into the hepatocyte is necessary because of differences in protein-binding inside and outside the hepatocyte. Outside the hepatocyte, bilirubin is bound to albumin (affinity constant ~10 8 , concentration: 0.6 mM). Inside the hepatocyte, bilirubin is bound to glutathione S-transferase B (GST), historically known as ligandin or the Y protein (affinity constant ~10 6 , concentration 0.04 mM). GST constitutes a family of proteins that exhibit important functions both as enzymes and as intracellular binding proteins for nonsubstrate ligands such as bilirubin. Carrier-mediated uptake helps generate a concentration gradient for bilirubin uptake despite the difference in affinity between albumin and GST. GST is important in the intracellular storage of bilirubin and bilirubin conjugates and reduces efflux from the hepatocyte back into plasma.
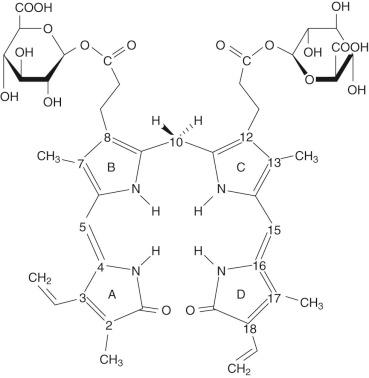
Inside the hepatocyte, bilirubin is conjugated with glucuronic acid within the ER (microsomes). The glucuronic acid donor is uridine diphosphate glucuronic acid (UDPGA). The conjugation results in an ester linkage formed with either or both of the propionic acid side chains on the B and C pyrrole rings of bilirubin ( Fig. 4-3 ). The enzyme responsible for this esterification is bilirubin UDP-glucuronosyltransferase (BUGT; Online Mendelian Inheritance in Man [OMIM] *191740). BUGT is distinct from the other glucuronosyltransferase isoforms which catalyze the conjugation of thyroxine, steroids, bile acids, and xenobiotics. BUGT (which is the same as UGT1A1, see below) is embedded in the lipid environment of the microsomal membrane, and perturbations of this environment greatly affect in vitro measurements of BUGT activity. Since BUGT is located on the interior of the ER, the existence of a permease has been hypothesized to facilitate the transport of UDPGA from the cytosol across the lipid layers of the ER. The permease has been proposed because uridine diphosphate glucose (UDPG) is present in the cytosol in higher concentrations, yet UDPGA serves as the preferred donor for bilirubin conjugation. Uridine diphosphate N-acetyl glucosamine (UDPNAG) is considered to be a natural regulator of BUGT because UDPNAG increases in vitro BUGT activity threefold. The mechanism for this is unknown and could possibly involve facilitation of the permease UDPGA transporter. After providing glucuronic acid for conjugation, UDP is converted to uridine and inorganic pyrophosphate by a nucleoside diphosphatase (NDPase) that is also located on the interior of the ER and prevents the reverse reaction.
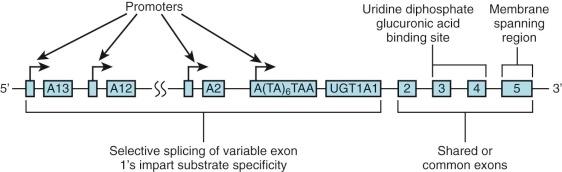
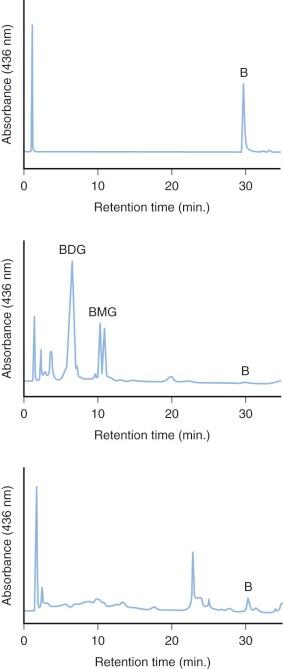
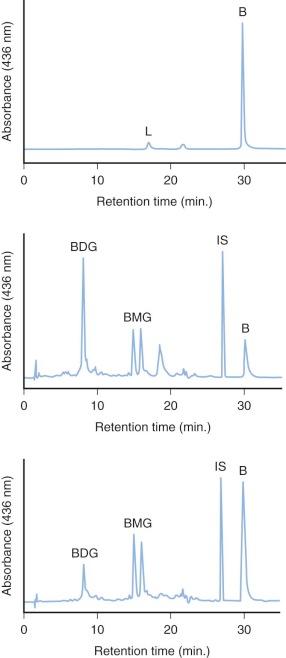
The specific isoform responsible for bilirubin conjugation is UGT1A1 (trivial name HUG-Br1 or BUGT, EC 2.4.1.17). This is part of the UDP glycosyltransferase superfamily of enzymes encoded by the UGT gene complex on chromosome 2 that is involved with metabolism of many xenobiotic and endogenous substances. The UGT1 gene encodes several isoforms and has a complex structure consisting of four common exons (2-5) and 13 variable exons encoding different isoforms ( Fig. 4-4 ). At least 30 different UGT1 mutant alleles have been described that cause Gilbert's syndrome and Crigler-Najjar (CN) syndromes I and II. UGT1A1 catalyzes the formation of both bilirubin mono- and diglucuronides but also metabolizes other hormones and drugs, so mutations could be involved in carcinogenesis and adverse drug reactions. In normal adult humans the majority of bilirubin conjugates are excreted in the bile as bilirubin diglucuronides (approximately 80%) ( Fig. 4-5 , middle panel). Lesser amounts of bilirubin monoglucuronides (approximately 15%) are also excreted along with very small amounts of unconjugated bilirubin (UBIL) and other bilirubin conjugates (e.g., glucose, xylose, and mixed diesters). In infants, because there is lower UGT1A1 activity, bile contains less bilirubin diglucuronide and more bilirubin monoglucuronide than the adult ( Fig. 4-6 , middle panel).
Following conjugation, bilirubin conjugates are excreted against a concentration gradient from the hepatocyte through the canalicular membrane into the bile. The ATP-dependent transporter responsible for bilirubin glucuronide passage from the hepatocyte through the canalicular membrane is canalicular multispecific organic anion transporter (cMOAT). cMOAT is a member of the ATP-binding cassette (ABC) transporter superfamily and is homologous to the multidrug resistance–associated protein (MRP2). It is also known as ABCC2 because it is encoded by the ABCC2 gene. cMOAT/MRP2/ABCC2 is involved with ATP-dependent transport across the apical canalicular membrane of a variety of endogenous compounds and xenobiotics including both bilirubin mono- and diglucuronide. cMOAT has previously been described as the nonbile acid organic anion transporter, the glutathione S-conjugate export pump, or the leukotriene export pump. Genetic mutations which alter these ABC transporters cause diseases which include cystic fibrosis, hyperinsulinemia, adrenoleukodystrophy, multidrug resistance, and, as will be discussed later in this chapter, Dubin-Johnson syndrome. This mechanism can be saturated with increasing amounts of bilirubin or bilirubin conjugates. Many other organic anions (e.g., BSP, ICG), share this same canalicular membrane excretion mechanism. Simultaneous infusions of BSP and ICG will decrease the maximal canalicular excretion of bilirubin and vice versa. The canalicular excretion mechanism for bilirubin and BSP are different from that of bile salts. Biliary excretion of conjugated bilirubin and BSP is decreased in individuals with the Dubin-Johnson syndrome, though bile salt excretion in not impaired. However, bile salt and bilirubin conjugate excretion by the canalicular membrane are not completely independent since infusion of bile salts does increase the maximal excretion of bilirubin conjugates. A similar effect in seen with phenobarbital. Conversely the maximal excretion of bilirubin conjugates can be decreased by cholestatic agents like estrogens and anabolic steroids.
Under normal conditions there is evidence that bilirubin conjugates equilibrate across the sinusoidal membrane of hepatocytes resulting in small amounts of bilirubin conjugates being present in the systemic circulation. If there is diminished hepatic glucuronidation of bilirubin (e.g., in the neonate), there will be a decreased amount of bilirubin conjugates present in the serum. Data show that in full-term newborns there is an increase in the serum level of bilirubin diconjugates (0.55 ± 0.25% on days 2 to 4 to 1.62 ± 0.99% on days 9 to 13) which are consistent with the maturation of bilirubin glucuronidation. In contrast, in premature infants less than 33 weeks' gestation, bilirubin diconjugates were very low and remained so, suggesting a more severe immaturity of the glucuronidation process.
In many pathologic circumstances, bilirubin mono- and diglucuronides are not excreted from the hepatocyte fast enough to prevent significant reflux back into the circulation. The resulting elevation of serum bilirubin conjugate levels results in the transesterification of bilirubin glucuronide with an amino group on albumin, producing a covalent bond between albumin and bilirubin. This product is formed spontaneously and is known as delta bilirubin or bilirubin-albumin. Similar nonenzymatic reactions have been demonstrated between albumin and various drugs. Delta bilirubin is not formed in hyperbilirubinemic conditions unless there is elevation of the conjugated bilirubin fraction. Both delta bilirubin and bilirubin conjugates are direct reacting, which explains a situation that has long confounded clinicians. The direct bilirubin may continue to be elevated in patients who otherwise are recovering from a hepatic insult because the delta bilirubin formed lingers due to the long (~20 day ) half-life of albumin.
When bilirubin conjugates enter the intestinal lumen (see Fig. 4-2 ), several possibilities for further metabolism arise. In adults, the normal bacterial flora hydrogenate various carbon double bonds in bilirubin to produce assorted urobilinogens ( Fig. 4-7 ). Subsequent oxidation of the middle (C-10) carbon produces the related urobilins. Because there are a large number of unsaturated bonds in bilirubin, there are many compounds formed by reduction and oxidation of these bonds. This large family of related reduction–oxidation products of bilirubin is known as urobilinoids and excreted in the feces and urine. Bacteria capable of producing urobilinoids include Clostridium ramosum, Escherichia coli, Bacteroides fragilis, Clostridium perfringens, and Clostridium difficile. The conversion of bilirubin conjugates to urobilinoids is important because it blocks the intestinal absorption of bilirubin known as the enterohepatic circulation. Neonates lack an intestinal bacterial flora and are more likely to absorb bilirubin from the intestine. This difference in bile pigment excretion between adults and neonates is demonstrated by comparing Figures 4-5 and 4-6 (lower panels).
Bilirubin conjugates in the intestine can also act as substrate for either bacterial or endogenous tissue β-glucuronidase. This enzyme hydrolyses glucuronic acid from bilirubin glucuronides. The UBIL produced is more rapidly absorbed from the intestine. In the fetus, tissue β-glucuronidase is detectable by 12 weeks' gestation and is believed to play an important role in facilitating intestinal bilirubin absorption that enables bilirubin to be cleared via the placenta. Following birth, increased intestinal β-glucuronidase can increase the neonate's likelihood of experiencing higher serum bilirubin levels. The ability of endogenous tissue β-glucuronidase to deconjugate bilirubin glucuronides has been demonstrated in germ-free animals. Breastmilk can contain high levels of β-glucuronidase, and it has been suggested that this is one factor related to the higher jaundice levels seen in breastfed infants. Feeding specific nutritional ingredients that inhibit β-glucuronidase, such as hydrolyzed casein or L-aspartic acid have been shown to result in increased fecal bilirubin excretion and lower levels of jaundice.
Jaundice and icterus both refer to the yellow discoloration of the tissues (skin, sclerae, etc.) caused by deposition of bilirubin. Jaundice, from the French jaune means yellow. Icterus is derived from the Greek word for jaundice (ikteros). Jaundice is a sign that hyperbilirubinemia exists (i.e., total serum bilirubin greater than approximately 1.4 mg/dL after 6 months of age, 1 mg/dL = 17 µmol/L). The degree of jaundice is directly related to the level of serum bilirubin and the amount of bilirubin deposited in the extravascular tissues. Hypercarotenemia, occasionally mistaken for jaundice, can impart a yellow hue to the skin, but the sclerae remain white. There are many conditions associated with neonatal jaundice. Some of these states are so commonly recognized as to be termed physiologic. Alternatively jaundice can be a sign of severe disease.
Measurement of the total serum bilirubin concentration allows quantitation of jaundice. Such measurements are very common in the newborn nursery and in one study were made at least once in 61% of term newborn infants. Two components of total serum bilirubin can be routinely measured in the clinical laboratory: conjugated bilirubin (“direct” reacting, because in Van den Bergh's test, color development takes place directly without adding methanol), and UBIL (“indirect” fraction). Although the terms direct and indirect are often used equivalently with conjugated and UBIL, this is not quantitatively correct because the direct fraction includes both conjugated bilirubin and albumin-bound delta bilirubin. Elevation of either of these fractions can result in jaundice. There is a long history of undesirable variability in the measurement of serum bilirubin fractions. One problem is the ditaurobilirubin (DTB) content of standards set by the College of American Pathologists (CAP). The DTB influences the result variably because of protein matrix differences related to the specific bilirubin measurement used. This has prompted the suggestion that standards consist of human serum enriched solely with UBIL rather than bovine serum containing a mixture of UBIL and DTB. The automated laboratory methods now used to measure serum bilirubin have been reviewed elsewhere. The Jendrassik-Grof procedure is the method of choice for total bilirubin measurement, though this method also has problems. When the total serum bilirubin level is high, factitious elevation of the direct fraction has been reported. Three newer methods have been developed which can more accurately determine the various bilirubin fractions (unconjugated, monoconjugated, diconjugated, and albumin-bound or delta): high-performance liquid chromatography (HPLC), multilayered slides, and use of bilirubin oxidase. HPLC analysis is superior and used as the gold standard, but it is too expensive and time consuming for the clinical laboratory. HPLC analysis of serum from normal human neonates in the first 4 days of life showed unconjugated and conjugated bilirubin levels rose in parallel with the conjugated fraction, making up only 1.2% to 1.6% of total pigment (3.6% in adults). Although the absolute concentration of conjugates was 2 to 6 times higher in neonates, only 20% were diconjugates (54% in adults). These sensitive HPLC data are consistent with the increased bilirubin production and relatively deficient glucuronidation seen in the neonate. Analysis with automated multilayered slide technology (VITROS, Johnson & Johnson) used in many clinical laboratories allows measurement of specific conjugated and UBIL fractions without inclusion of delta bilirubin. The conjugated bilirubin measurement is an earlier indicator of relief from biliary cholestasis than is direct bilirubin because of the long half-life of delta bilirubin. A comparison of the bilirubin oxidase method concluded that determinations of total bilirubin in neonatal serum were not advanced by this method.
Newer methods of total bilirubin measurement (Twin Beam, Ginevri, Rome, Italy; ABL 735, Radiometrer, Copenhagen, Denmark; Roche OMNI S, Roche Diagnostics, Graz, Austria) using nonenzymatic photochemical analysis offer the convenience of bilirubin quantitation outside of a core lab setting (i.e., blood gas analyzer in the nursery) using a smaller blood sample than traditional serum analysis. However, these measurements must be interpreted with caution because the instruments tend to underestimate the total bilirubin concentration at greater than 15 mg/dL.
There are conflicting data regarding the accuracy of capillary versus venous serum bilirubin levels. However, as Maisels has pointed out, the literature regarding kernicterus, phototherapy, and exchange transfusion is based on bilirubin measurements in capillary samples.
Noninvasive methods to measure jaundice levels have been shown to be useful in neonates. Current commercially available methods include the BiliChek (Respironics, Pittsburgh, Pennsylvania) and the Minolta/Hill-Rom Air-Shields Transcutaneous Jaundice Meter 103 (Air-Shields, Hatboro, Pennsylvania). The technique utilizes principles of reflectance spectrophotometry, has been validated against both HPLC and clinical laboratory measures, and is advocated by the American Academy of Pediatrics (AAP). The device is touched to the skin in a painless manner with the immediate resulting point-of-care measurement of transcutaneous bilirubin (TcB). Significant TcB levels (e.g., greater than 13 mg/dL) should prompt measurement of a serum or plasma bilirubin level. Some suggest that the yellow color of the skin is a better risk indicator of bilirubin-dependent brain damage than is the serum bilirubin level. A less expensive method useful in assessing jaundice utilizes a plexiglass color chart pressed against the baby's nose (Ingram icterometer, Thos. A. Ingram and Co. Ltd, Birmingham, England).
Reviews of neonatal bilirubin toxicity have been published elsewhere. Kernicterus (German: kern : nucleus ; Latin: icterus : yellow) is the neuropathologic finding associated with severe unconjugated hyperbilirubinemia and is named for the yellow staining of certain regions of the brain, particularly the basal ganglia, hippocampus, cerebellum, and nuclei of the floor of the fourth ventricle. Acute clinical findings associated with kernicterus, termed acute bilirubin encephalopathy, include sluggish Moro reflex, opisthotonos, retrocollis, hypotonia, vomiting, high-pitched cry, hyperpyrexia, seizures, paresis of gaze (“setting sun sign”), oculogyric crisis, and death. Long-term findings (chronic bilirubin encephalopathy) include spasticity, choreoathetosis, dental enamel dysplasia of the desiduous teeth, and sensorineural hearing loss. Milder forms of bilirubin encephalopathy include cognitive dysfunction and learning disabilities. In 17-year-old males, the risk of having an IQ score less than 85 was found to be significantly higher among full-term subjects with neonatal serum bilirubin levels above 20 mg/dL. The mechanisms of bilirubin cytotoxicity are complex and have been reviewed elsewhere. Though the neonatal period is the most common time for bilirubin-related brain damage, the neurotoxicity of bilirubin has also been documented in older children and adults with CN syndrome, type I.
The absolute level of serum bilirubin has not been a good predictor of the risk of bilirubin encephalopathy. However, it has long been known that kernicterus is likely with serum UBIL levels greater than 30 mg/dL and unlikely with levels less than 20 mg/dL. In one study, 90% of the patients who had a bilirubin level greater than 35 mg/dL either died or had cerebral palsy or physical retardation. Alternatively, no developmental retardation was found in 129 infants with bilirubin levels less than 20 mg/dL. Albumin concentration is an important variable because of the high affinity binding with bilirubin, and the ratio of bilirubin to albumin is a risk factor that has been included in the most recent AAP guidelines. Drugs and organic anions also bind to albumin and can displace bilirubin, thereby increasing the free bilirubin which can diffuse into cells and cause toxicity, such as when sulfisoxazole was given to premature infants. Walker has reviewed neonatal bilirubin toxicity and drug-induced bilirubin displacement. In recent years there has been a reemergence of kernicterus. While it has been acknowledged that total serum bilirubin may not be the most important factor related to risk, there are, at present, no other generally accepted tests (e.g., albumin saturation, reserve bilirubin binding capacity, or free bilirubin concentration) which are more helpful in identifying infants at risk for bilirubin encephalopathy. Recently the basics of bilirubin–albumin binding and neonatal jaundice have been reviewed by Ahlfors and Wennberg. Additionally, Ahlfors and colleagues have recently described how to use zone fluidics (the precisely controlled physical, chemical, and fluid-dynamic manipulation of zones of miscible and immiscible fluids and suspended solids in narrow bore conduits to accomplish sample conditioning and chemical analysis) to measure free bilirubin and overcome some of the existing problems with unbound (free) bilirubin measurement. Wennberg and colleagues speculate that enhanced sensitivity and specificity of newer methods of free bilirubin measurement will provide for the establishing of improved risk threshold for kernicterus and therefore reduce unnecessary aggressive intervention (i.e., exchange transfusion) and its associated cost and morbidity.
Another approach aimed at measuring early changes in the CNS caused by bilirubin has assessed brainstem auditory evoked potentials (BAEP). Abnormalities in the BAEP have been demonstrated in jaundiced infants and shown to improve after exchange transfusion. Data have shown that even moderate hyperbilirubinemia (mean ± SD: 14.3 ± 2.8 mg/dL) affects the BAEP, specific components of the Brazelton Neonatal Behavioral Assessment Scale, and cry characteristics.
Another reported toxicity of neonatal jaundice relates not to the CNS of the jaundiced infant, but rather to the attitude of that infant's parents. Mothers of jaundiced infants more frequently exhibit behavior suggesting the “vulnerable-child syndrome,” including inappropriate visits to the physician because of unrealistic perceptions of illness, separation difficulties, and early termination of breastfeeding. Another study showed, however, that hyperbilirubinemia and/or phototherapy during the neonatal period are not associated with impaired mother–child attachment after the first year of life.
The term physiologic jaundice has been used to describe the frequently observed jaundice in otherwise completely normal neonates. However, physiologic jaundice is merely the result of a number of factors involving increased bilirubin production and decreased excretion.
Jaundice should always be considered to be a sign of a possible disease and not routinely explained as physiologic. Specific characteristics of neonatal jaundice to be considered abnormal until proven otherwise include: (1) development before 36 hours of age; (2) persistence beyond 10 days of age; (3) serum bilirubin greater than 12 mg/dL at any time; (4) elevation of the direct bilirubin (direct bilirubin greater than 1 mg/dL if the total bilirubin is less than 5 mg/dL, or direct bilirubin greater than 20% of the total bilirubin if the total bilirubin is greater than 5 mg/dL ). The physiologic immaturity of premature infants is associated with both higher peak serum bilirubin levels and pathologic conditions.
In general, infants are not jaundiced at the moment of birth, due to the impressive ability of the placenta to clear bilirubin from the fetal circulation. However, within the next few days most if not all infants will develop elevated serum bilirubin levels (greater than 1.4 mg/dL), with clinical jaundice evident when the total bilirubin level exceeds 2 to 5 mg/dL. As the serum bilirubin rises, the skin becomes more jaundiced in a cephalocaudal manner. Kramer found the following serum indirect bilirubin levels as jaundice progressed: head and neck: 4 to 8 mg/dL; upper trunk: 5 to 12 mg/dL; lower trunk and thighs: 8 to 16 mg/dL; arms and lower legs: 11 to 18 mg/dL; palms and soles: greater than 15 mg/dL. Hence when the bilirubin was greater than 15 mg/dL, the entire body was icteric. However, darker skin tones can make jaundice difficult to estimate visually. Jaundice is best observed by blanching the skin with gentle digital pressure under well-illuminated (white light) conditions. At least one third of infants develop visible jaundice. A combined analysis of several large studies involving thousands of infants during the first week of life showed that moderate jaundice (bilirubin greater than 12 mg/dL) occurs in at least 12% of breastfed infants and 4% of formula-fed infants, while severe jaundice (greater than 15 mg/dL) occurs in 2% and 0.3% of these respective feeding groups.
There are a number of epidemiologic risk factors related to neonatal jaundice that have been reviewed elsewhere. Risk factors include male gender, low birth weight, prematurity, polycythemia, certain ethnicities (Asian, Native American, Greek), maternal medications (e.g., oxytocin, promethazine hydrochloride), premature rupture of the membranes, increased weight loss after birth, delayed meconium passage, breastfeeding, and neonatal infection. Delivery with the vacuum extractor increases the risk of cephalohematoma and neonatal jaundice. Data suggest pancuronium is associated with an increased risk of hyperbilirubinemia. There is a significant correlation between umbilical cord serum bilirubin level and subsequent hyperbilirubinemia. Maternal serum bilirubin level at the time of delivery and transplacental bilirubin gradient also correlate positively with neonatal serum bilirubin concentrations. Factors associated with decreased neonatal bilirubin levels include African race, exclusive formula feeding, gestational age 41 weeks, maternal smoking, and certain drugs given to the mother (e.g., phenobarbital).
Breastfeeding has been clearly identified as a factor related to neonatal jaundice, and this subject has been reviewed elsewhere. Breastfed infants have been shown to have significantly higher serum bilirubin levels than formula-fed infants on each of the first 5 days of life, and this unconjugated hyperbilirubinemia can persist for weeks to months. Jaundice during the first week of life is sometimes described as breastfeeding jaundice in order to differentiate it from breastmilk jaundice syndrome, which occurs after the first week of life. The former is frequently associated with inadequate breastmilk intake, whereas the latter generally occurs in otherwise thriving infants. There is probably overlap between these conditions and physiologic jaundice. Early reports linking breastmilk and neonatal jaundice with a steroid (pregnane-3(α),20(β)-diol) in some milk samples have not been confirmed by more recent larger studies utilizing more sensitive methods. There are also conflicting data regarding efforts to attribute this jaundice to increased lipase activity in the breastmilk, resulting in elevated levels of free fatty acids, which could inhibit hepatic glucuronosyltransferase. It has been suggested that the enterohepatic circulation of bilirubin can be facilitated by the presence of β-glucuronidase or some other substance in human milk. Other factors possibly related to jaundice in breastfed infants include caloric intake, fluid intake, weight loss, delayed meconium passage, intestinal bacterial flora, and inhibition of bilirubin glucuronosyltransferase by an unidentified factor in the milk. Lascari suggests that a healthy breastfed infant with unconjugated hyperbilirubinemia, normal hemoglobin concentration, reticulocyte count, blood smear, no blood group incompatibility, and no other abnormalities on physical examination may be presumed to have early breastfeeding jaundice. Because there is no specific laboratory test to confirm a diagnosis of breastmilk jaundice, it is important to rule out treatable causes of jaundice before ascribing the hyperbilirubinemia to breastmilk. Some infants with presumed breastmilk jaundice exhibit elevated serum bile acid levels suggesting mild hepatic dysfunction or cholestasis, though in general this is not the case. Breastfed infants who are fed specific nutritional ingredients, such as L-aspartic acid, that inhibit β-glucuronidase excrete more fecal bilirubin and have lower levels of jaundice than breastfed infants who receive no supplements. No commercial preparations of these specific ingredients are presently available.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here