Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
NIH grant R01DK041274 is acknowledged for financial support.
The first physiological observations suggesting the existence of a Na + /H + exchange mechanism in mammalian membranes were made by Mitchell and Moyle and by Brierley et al. in rat liver and cow heart mitochondria, respectively. These observations were soon followed by similar findings in prokaryotic plasma membrane by Harold and Papineau in Streptococcus faecalis and by West and Mitchell in Escherichia coli. In 1987, Goldberg et al. cloned the first Na + /H + antiporter from E. coli , later termed nha A. Its mammalian counterpart was cloned shortly after by Sardet et al. and reported in 1988 and 1989. Cloning of this Na + /H + exchanger (NHE), initially described as growth factor-activatable Na + /H + antiporter, and later termed NHE-1, initiated the explosion of knowledge about function, structure, and regulation of what was soon determined to be a family of proteins involved in membrane Na + /H + antiport mechanism. Families of NHE were identified in bacteria, yeast, plants, and animals.
Although the basic principle of Na + /H + exchange is consistent among species, there are also some considerable differences. In vertebrate animals, the ubiquitous plasma membrane Na + /K + -ATPase mediates the efflux of 3Na + and the influx of 2K + , a process coupled to the hydrolysis of ATP. This electrogenic Na + /K + exchange establishes a Na + gradient across the plasma membrane that is used by the cell for a number of physiological functions. These processes are largely dependent on Na + /H + exchange, a mechanism critical in the regulation of intracellular and systemic pH, cell volume, absorption, and reabsorption of sodium in the intestinal tract and kidney, respectively. This physiological need dictates the polarity of the exchange, with extracellular sodium being exchanged for intracellular proton. On the other hand, in lower organisms such as bacteria, plants, and yeast, the ability to withstand extreme hypersaline or hyperalkaline conditions results from developed Na + /H + antiport mechanisms leading to the net uptake of protons and net loss of sodium. In addition to differences in Na + /H + exchange polarity, its stoichiometry also varies. For example, while all the described mammalian NHE are electroneutral, E. coli bacteria exhibit electrogenic Na + /H + exchange, with a stoichiometry of 1Na + /2H + and 2Na + /3H + for NhaA and NhaB isoforms, respectively (see Fig. 56.1 ).
In mammalian cells, tight pH i regulation is crucial for cell function and survival, as its subtle changes may significantly affect cellular physiology. Variations in pH i mediated by NHE mechanism have been associated with basic biological processes such as proliferation, cell adhesion and migration, cell volume regulation, and transepithelial electrolyte and water transport. This chapter focuses primarily on the structure, function, and regulation of NHE expressed in the mammalian gastrointestinal (GI) tract. The reader is referred to the following publications for an overview or original articles on plants, Caenorhabditis elegans , and procaryotic Na + /H + antiport, respectively. Comprehensive reviews on mammalian Na + /H + exchange were published by Orlowski and Grinstein, Zachos et al., Donowitz et al., and by Gurney et al. with a particular emphasis on intestinal Na + /H + exchange. Phylogenetic analysis of evolutionary relations among NHE sequences from all phyla, including those sequences well characterized as well as those electronically annotated based on sequence similarity, has been published by Brett et al.
As described by Brett et al., > 550 sequence entries could be identified as putative NHE by automated annotation projects on the basis of sequence conservation within 10 transmembrane domains TM3–TM12 within the NH 2 -terminal half of proteins. The classification of membrane transport proteins remains an evolving process and is periodically updated with the new and improved evolutionary bioinformatics tools. Based on the most recent classification (Ref. http://www.tcdb.org /), monovalent cation proton antiporter (CPA) superfamily is now divided into three CPA families: CPA1 (TC# 2.A.36), CPA2 (TC# 2.A.37), and CPA3 (TC# 2.A.63). According to the classification current at the time of writing this chapter, CPA2 and CPA3 families include primarily bacterial, fungal, and plant transporter proteins, with one exception, human transmembrane and coiled-coil domain 3 (TMCO3) and a member of CPA2 family expressed in expressed in the human cornea, lens capsule, and choroid-retinal pigment epithelium. CPA1 family is the largest of the three and includes proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants and animal, including the nine mammalian NHE paralogs from the SLC9A subfamily (NHE1–9), NHA1, and NHA2 in SLC9B subfamily, and two sperm-specific NHE’s (NHE10, NHE11) from the SLC9C subfamily, which were previously categorized in the NaT-DC family. CPA2 and CPA3 families of the monovalent CPAs, as well as NHE10 (SLC9C1) and the putative NHE11 (SLC9C2) will not be discussed in this chapter. Within the CPA1 family, NHA1 (Slc9b1) was identified in the spermatozoa where it controls sperm motility beyond cauda epididymis. NHA2 (or NHE Domain-Containing protein 2, NHEDC2, Slc9b2), was described as an amiloride-insensitive Li + -NHE. In the sperm, along NHA1, it contributes to its motility, although its mouse tissue expression pattern is broad and includes the GI tract, liver, pancreas. In transfected polarized renal epithelial cells, it was primarily localized to the apical membrane. Kondapalli et al. showed recently that the endogenous NHA2 was expressed in the distal convoluted and connecting tubules where it was upregulated in response to high-Na + diet and thus postulated to play a role in salt tolerance and in the pathogenesis of essential hypertension. In the pancreas, NHA2 is expressed in insulin-producing β-cells, where it was found to play permissive roles in sulfonylurea- and secretagogue-induced insulin secretion. Beyond 5 months of age, NHE2-deficient mice develop glucose intolerance, which is further exacerbated by a high-fat diet. NHA2 was also found in osteoclasts, where it was upregulated by RANK ligand. The same group localized NHA2 expression in the osteoclast mitochondria and showed that its knockdown reduced osteoclast differentiation and resorptive function. However, with improved methods of NHA2 detection, Hoffstetter et al. showed that NHA2 colocalizes with late endosomal and lysosomal markers, is highly enriched at the basolateral membrane of polarized osteoblast, and is not expressed in the mitochondria. While upregulation by RANKL was confirmed, NHA2-deficient mice did not demonstrate any skeletal defects, and the authors concluded that NHA2 is dispensable for osteoclast differentiation and bone resorption in mice. Although NHA2 protein has been detected in the stomach, jejunum, and colon, its roles in the mammalian gut remain unknown. Intriguingly, in Drosophila gut epithelium, both Nhe1 and Nha2 were required for the protection against high luminal Na + load, despite their distinct transport properties (as H + -Cl − cotransporter and an NHE, respectively), thus implicating its role in response to osmotic stress and Na + tolerance. Since, with the exception of NHA2, all of the NHE expressed in the GI tract belong to the CPA2 family, NHE1–9 isoforms will remain the primary focus of this chapter’s discussion.
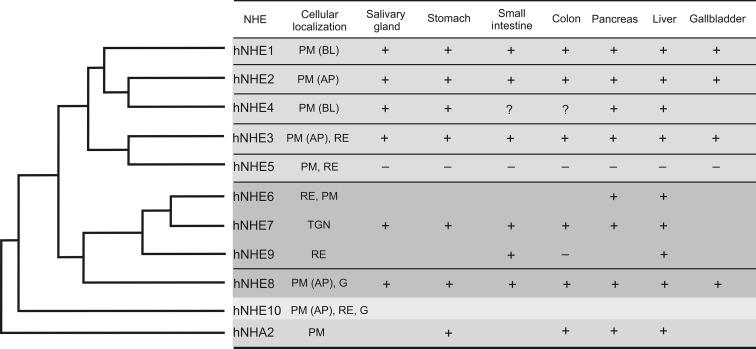
Following the cloning of NHE1, later considered a prototypical mammalian NHE, eight other isoforms from diverse species have been described by various methodological approaches. The nine proteins belonging to the SLC9A subfamily of mammalian NHE demonstrate considerable variation in their amino acid sequence, ranging from under 12% (hNHE1 vs. hNHE9) to over 70% identity (hNHE6 vs. hNHE7). Eight of them have been detected in the GI tract ( Fig. 56.2 ) with segmental differences, crypt-villus gradients of expression, and different cellular localizations, all of which determines their proven or alleged functions. An additional isoform of chloride-dependent NHE has been cloned from the rat colon. Its sequence shared 100% homology of the 375 N-terminal amino acids (aa) with NHE1, and a divergent 63aa sequence in the C-terminal portion of the protein, suggestive of an alternative splice variant of the NHE1 gene. However, while the rNHE1 gene is located on chromosome 5q36, the cDNA coding for the novel C-terminal 63aa aligns fully with chromosome 17p14, thus representing a chimeric noncolinear transcript. This cDNA may represent either a product of trans -splicing of pre-mRNA or a cloning artifact and has not been independently verified.
Although all the nine NHEs have not been systematically studied in terms of their secondary structure and membrane topology, modeling algorithms predicting hydrophobic and hydrophilic regions of the proteins suggest the same general arrangement. According to the modeling, approximately 60% of the amino-terminal of the protein is amphipathic and contains 10–12 membrane spanning α-helices, which are relatively conserved among different isoforms. Much more hydrophilic and less conserved carboxyl-terminus faces the cytoplasm (see Fig. 56.3 ). This region of NHE proteins, as determined empirically and through prediction sequence analyses, contains multiple phosphorylation sites and sites responsible for interaction with accessory proteins, all of which are believed to serve regulatory functions.
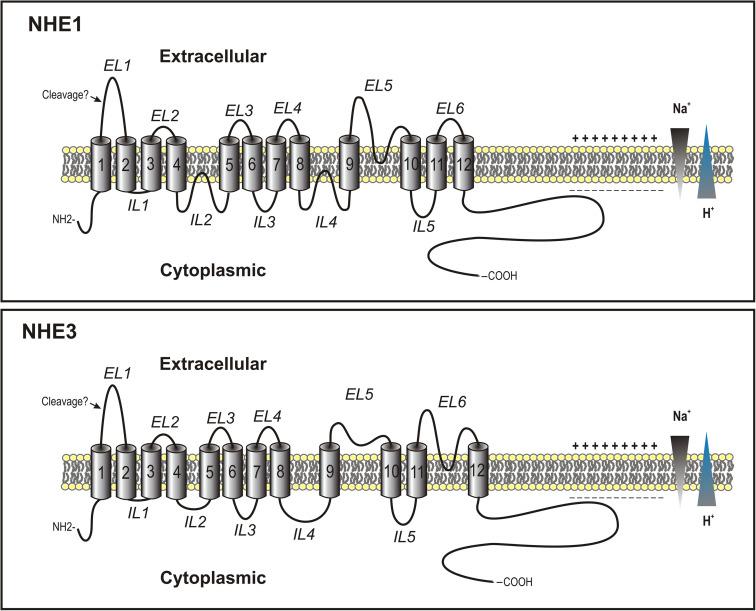
Of the nine NHEs cloned to date, two-dimensional structure of NHE1 and NHE3 has been most extensively studied utilizing a variety of experimental approaches including cysteine substitution and accessibility, C-terminal truncation, identification of glycosylation sites, proteolytic cleavage, and epitope immunolocalization. Not all of these studies confirm this general model of NHE membrane topology, particularly with regard to the C-terminal region. The notion of entirely cytoplasmic location of this domain has been contended both in NHE1 and NHE3, by Khan and Biemesderfer et al., respectively. Both studies argued that truncations or amino acid substitution may alter the natural structure and folding of the protein, and suggested that epitope immunolocalization of native proteins indicates at least some extracellular epitopes located with the C-terminal tail. This observation seems to be supported by the observations with Nhx1, a yeast homologue of mammalian NHEs, which has been shown to be glycosylated on two asparagine residues located within the C-terminus. However, considering the fact that the C-terminal domain of NHE1 or NHE3 does not appear to have hydrophobic regions of sufficient length to form a membrane-spanning domain (15–20 amino-acids), and functional analyses of regulatory domains within this region suggest multiple interactions with cytoplasmic and cytoskeletal factors, it remains unclear whether the controversy over the topology of NHE C-terminus is a result of methodological differences or whether it reflects actual variations in membrane orientation.
Due to the highly variable amino acid sequence among various NHE isoforms, the N-terminus may contain a cleavable signal peptide. In NHE1, for example, the cleavage would occur before the experimentally confirmed glycosylation site in the first extracellular loop ( Fig. 56.3 ). If this hypothetical scenario were true, the predicted topology of NHE1 and NHE3 would change to an 11-membrane-spanning domain configuration. Experimental evidence from studies on NHE1 protein utilizing cysteine substitution suggests, however, that the N-terminus is retained in the mature protein and remains in the cytosol. On the other hand, experiments with in vitro translated NHE3 point to cleavage of the N-terminal signal peptide during processing in microsomes. Based on the results of these studies, membrane topology predictions should not be generalized to all members of NHE family and secondary structures of each of the remaining NHE proteins would have to be experimentally addressed. Some of the different models of NHE1 membrane topology have been reviewed by Kemp et al.
NHE1 and NHE3 have been demonstrated to form homodimers in the cytoplasmic membrane. The formation of dimers appears to depend on protein-protein interaction within the amphipathic membrane-spanning region of the proteins, but individual subunits were found to function independently within the complex. Kinetic analyses of renal brush-border membrane Na + /H + exchange also suggested the presence of dimers and tetramers, dependent on the outside Na + concentration.
Based on the secondary structure of NHE proteins and their membrane topology, two major domains can be distinguished: an N-terminal amphipathic region including all 10–12 membrane-spanning domains, and a C-terminal hydrophilic tail. The latter segment is believed to be intracellular and confers regulation of NHE activity by direct or indirect interactions with kinase, cytoskeletal, and other proteins. Within the amphipathic region, transmembrane domain 4 (TM4) is crucial for NHE1 function, with the residues Phe161, Phe162, Leu163, and Gly173 affecting affinity for Na + and/or its resistance to inhibitors. Slepkov et al. showed that within this transmembrane helix, Pro167 and Pro168 are critical in NHE1 activity, expression, and membrane targeting. Within the seventh transmembrane domain (TM7), Glu262 and Asp267 are indispensable for NHE1 activity, with their charge and acidity being the most critical. Transmembrane domain 9 (TM9) contains a sequence conferring sensitivity to antagonists. A hybrid NHE1, in which this transmembrane helix has been replaced with analogous segment of the amiloride-resistant NHE3, was resistant to amiloride, ethylisopropylamiloride, HOE694, and cimetidine. Within this transmembrane domain, His349 may be one of the critical moiety bestowing sensitivity to amiloride compounds, as described by Wang et al. Mutation of amino acids Tyr454 and Arg458 within the eleventh transmembrane domain (TM11) has shown that both amino acids are essential in targeting NHE1 to the cell surface. TM11 and its neighboring intracellular loop (IL5) have also been implicated in pH sensing. Mutation of Gly455 or Gly456, although it did not affect the protein’s affinity for Na + or H + ions, resulted in an alkaline shift in NHE1 pH-dependence. In contrast, mutation of Arg440 in IL5 had the opposite effect. Based on these data, it has been postulated that both Arg440 in IL5 and glycine residues in the conserved segment of TM11 constitute the putative pH i sensor in NHE1. Other NHE isoforms have not been studied in such detail.
The long, hydrophilic cytosolic domain of NHE1 (amino acids 500–815), which regulates the activity of the amphipathic N-terminal domain, is a target for phosphorylation by protein kinases and participates in binding of regulatory proteins. Several protein kinases are thought to phosphorylate NHE1, including Erk1/2, p90rsk, p160ROCK, p38, and a Nck-interacting kinase. By changing NHE1 pH dependence, phosphorylation increases its activity in a more alkaline pH. A number of regulatory proteins have been demonstrated to bind to the cytosolic tail of NHE1, including calmodulin, calcineurin homologous protein (CHP), tescalcin, and carbonic anhydrase II. The reader is referred to review articles published by Putney et al., Slepkov and Fliegel, Baumgartner et al., Malo and Fliegel, and references therein for more detailed descriptions of modes of NHE1 activity regulation. The role of the cytoplasmic C-terminal domain of NHE3 functioning as a scaffold which binds multiple regulatory proteins and links NHE3 to the cytoskeleton has been reviewed in detail by Donowitz et al. and is described in a later section on posttranscriptional regulation of NHE3 activity.
The steady-state velocities of most NHE isoforms show a saturating, first-order dependence on the outside Na + o concentration (with K Na values 3–50 mM), consistent with simple, saturating, Michaelis-Menten kinetics, which is indicative of a single binding site. The kinetics of NHE4 isoform seems more complicated. Under unstimulated isotonic conditions, NHE4 appears inactive; however, under hypertonic stress, the kinetics of NHE4-mediated Na-uptake follow a sigmoidal rather than hyperbolic curve with increasing concentrations of extracellular Na + , a phenomenon characteristic of allosteric regulation. Most plasmalemmal NHE isoforms are specifically transporting Na + in exchange for H + , with much lower efficiency toward Li + or NH 4 + and essentially no affinity for K + . NHE4 is an exception to this rule, since it shows similar exchange rates for Na + and Li + , and an even higher rate for K +, suggesting that this isoform functions as a nonspecific cation/H + exchanger. Similarly, NHE8, which is described as a both intracellular and plasmalemmal isoform, is capable of K + /H + exchange when reconstituted in artificial liposomes. NHE7, an exclusively organellar isoform localized in the trans-Golgi, network, also functions primarily as a K + /H + exchanger, a feature likely shared by other organellar NHE isoforms.
Contrary to a typical first-order dependence of exchange kinetics for Na + , hydrogen concentration dependence does not follow a simple Michaelis-Menten equation which assumes no cooperativity, but rather displays characteristics typical of allosteric effect, with more than one binding site for H + . The kinetic analysis of Na + /H + exchange appears to be in agreement with structural data suggesting a presence of a H + i sensor in the 11th transmembrane domain, and an element regulation pH set point located in the intracellular loop 5 (see Section 56.2 ). The allosteric regulation of Na + /H + exchange by intracellular protons may not be a universal feature of all NHEs. As an example, NHE5 displays a simple first-order dependence on the H + i concentration, when expressed in fibroblasts, suggesting no cooperativity.
All well-characterized mammalian NHE have been classified as electrochemical-potential-driven transporters, and they have been categorized into the monovalent cation proton antiporter family (CPA1; 2.A.36), according to classification developed by Busch and Saier and endorsed by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). This classification is based on the fact that cation fluxes via NHE mechanism are driven exclusively by the transmembrane gradients of substrates and are only secondarily dependent on ATP. Although NHE proteins do not bind or consume ATP directly, cellular ATP depletion results in marked inhibition of NHE1, NHE2, and NHE3 activities, despite a maintained transmembrane H + gradient. ATP depletion affects NHE1 and NHE2 by reducing their sensitivity to intracellular pH (H + i ), while NHE3 is characterized by both impaired H + i sensing and reduced maximal velocity of transport ( V max ). Although the precise mechanism of these phenomena is not clear, a role for plasmalemmal phosphatidylinositol 4,5-bisphosphate (PIP 2 ) has been postulated. Since binding of PIP 2 to the C-terminus of NHE1 is critical in maintaining NHE1 activity and ATP depletion results in a decline in plasma membrane PIP 2 content, this could represent a potential mechanism for ATP-dependence of NHE1. It remains to be determined whether ATP depletion impairs NHE2 and NHE3 via similar mechanisms.
Chloride-dependent Na + /H + exchange has been functionally identified in the epithelium of the rat distal colon. This phenomenon was postulated to be the result of functional coupling of the chloride channel to a novel NHE isoform, later cloned as a putative Cl − -dependent NHE. Two other studies in the mouse, however, demonstrated no Cl − -dependent NHE in the colonic crypts. One explanation for this discrepancy is a possibility that not all Cl − replacements are equally inert, but this is not likely as two different anion substitutions with different permeabilities (nitrate or gluconate) produced the same results. Other explanations include the possibilities that the volume changes caused by Cl − depletion affect different cell types differently, or that coupled Cl − /anion exchange may be necessary for dissipation of the intracellular gradient in some cells. These discrepancies may also be due to species differences (rat vs. mouse), site of analysis (crypt base or midcrypt region), or simply the use of different methodological approaches. It has also been demonstrated that NHE1–3 isoforms can be Cl − dependent to a certain extent. This phenomenon is incompletely understood, but may involve impaired H + i sensing with depletion of intracellular Cl − . Overall, the described inconsistencies in descriptions of Na + /H + exchange Cl − dependence suggest that it may not be a ubiquitous function of colonic crypt epithelia.
Over the years, a number of Na + /H + exchange inhibitors have been developed, initially as an effort to inhibit NHE1 in cardiac ischemia/reperfusion injury, and later as potential adjuncts in anticancer therapy. The pharmacology of NHE inhibitors was reviewed by Masereel et al. Amiloride, a K + -sparing diuretic, was the first described NHE inhibitor, which could also inhibit a electrogenic Na + channels and the Na + /Ca ++ exchanger. NHE1 and NHE2 isoforms are the most sensitive to amiloride inhibition, whereas NHE3 and especially NHE4 are amiloride-resistant isoforms. The sensitivity of NHE8 and NHE9, two of the most recently cloned NHE isoforms, to currently known inhibitors has not been evaluated, and only limited information on sensitivity of NHE4 and NHE7 is available. Development of several pyrazine or phenyl derivatives of amiloride increased their potency toward NHEs, particularly NHE1, and more importantly increased their selectivity by eliminating the inhibitory potency toward the Na + channel and Na + /Ca 2 + exchangers. Of these molecules, DMA, EIPA, HOE-694, and HOE-642 are the most frequently used in experimental settings.
Several NHE inhibitors based on a bicyclic template have been introduced, such as zoniporide, SM-20550, BMS-284640, T-162559, or TY-12533. Other compounds not related to amiloride have also proven useful, especially S-3226, as the first NHE3-specific inhibitor. Cimetidine, clonidine, and harmaline, although not frequently used, have also been reported to act as weak and nonspecific inhibitors of Na + /H + exchange. More recently, ligustrazine (2,3,5,6-tetramethylpyrazine) and its analogs, along with several other compounds (e.g., KR-32560, KR32570, or KR-33028) have been introduced as NHE1 inhibitors. It is important to point out that the reported IC 50 values have been frequently derived from studies with forced expression NHEs in NHE-deficient fibroblasts, and significant differences in sensitivities of endogenous NHEs may not be uncommon.
Intestinal Na + absorption mediated by NHE3 has more recently become a target of commercial investigation, with the hope of developing novel treatment for hypertension and/or constipation-predominant irritable bowel syndrome (IBS-C). Two oral nonabsorbable NHE3 inhibitors have been developed, SAR218034 (SAR) and tenapanor. Pharmacokinetics studies indicated that they did not cross the intestinal barrier in biologically active doses. Tenapanor was well tolerated in phase I clinical study and both inhibitors increased fecal and reduced urinary Na + concentrations in rodents and humans. Not surprisingly, both drugs caused an increase in luminal fluid resulting from increased Na + , leading to loose stools. In a spontaneously hypertensive rat model, SAR in conjunction with NaCl-laden drinking water markedly reduced systolic blood pressure. In a rat model of chronic kidney disease model associated with hypertension, hypervolemia, cardiac hypertrophy, and arterial stiffening (salt-fed 5/6 nephrectomized rats), tenapanor reduced extracellular volume expansion, albuminuria, and blood pressure, in addition to promoting protective cardiorenal effects such as reducing left ventricular hypertrophy. Both drugs show enhanced effects if administered in conjunction with an angiotensin-converting enzyme inhibitor, which was deemed important in cases in which hypertension could not be controlled by the administration of a single medication. However, at the time of writing this chapter, the development of tenapanor and SAR, and the general concept of inhibition of intestinal Na + /H + exchange as an antihypertensive strategy, appears to have been abandoned by Ardelyx (Fremont, CA) and Sanofi (Paris, France), respectively. Ardelyx continues the investigation into the use of tenapanor and its close analogs in patients with IBS-C and for the treatment of hyperphosphatemia in end-stage renal disease patients on dialysis. In a phase 2, randomized, placebo-controlled efficacy and safety trial, 50 mg of tenapanor twice a day, response rate for IBS-C symptoms such as pain, discomfort, bloating, cramping, and fullness, was significantly higher in the tenapanor than placebo group, with diarrhea as the most frequent adverse effect. The concept behind the use of NHE3 inhibition in hyperphosphatemia is based on the described increased fecal P i excretion and reduced urinary P i excretion in NHE3 inhibitor-treated rats with chronic kidney disease with vascular calcification, in which tenapanor markedly reduced ectopic calcification and protected renal function. The mechanism of this phenomenon is not clear. However, in hemodialysis patients, tenapanor indeed moderately but significantly lowered serum phosphate level, albeit with diarrhea affecting as much as 68% of participants. It is not yet evident whether targeting NHE3 would be more efficacious and have less adverse effects that the P i binders currently used clinically.
NHE1, the first cloned mammalian NHE, remains the most extensively studied NHE isoform, although the preponderance of information on NHE1 expression, activity, and regulation comes from systems other than the GI tract, and as such may or may not be applicable to GI physiology and pathophysiology. Since the large body of knowledge about this isoform precludes us from including it in this chapter, the reader is referred to more focused reviews discussing various aspects of the biology of NHE1. Only a basic overview of this isoform is presented here, with a particular emphasis on its role in the physiology of the digestive tract.
Mammalian NHE1 is an 813–822 amino acid protein with a calculated molecular mass of ~ 91 kDa. NHE1 contains consensus sequences for both N- and O -linked glycosylation, and there is evidence that Asn-75 in the first extracellular loop of NHE1 is glycosylated, explaining the appearance of the mature 110 kDa form of NHE1 in Western blotting. Its membrane topology has been extensively studied and is schematically represented in Fig. 56.3 .
NHE1 is expressed ubiquitously in almost all mammalian cell types where it resides exclusively on the plasma membrane. Dependent on the cell type, NHE1 tends to accumulate in distinct membrane domains. In polarized epithelial cells, NHE1 is expressed on the basolateral membrane ; in cardiac myocytes, it is concentrated around the intercalated disks and t-tubules ; while in fibroblasts, it is found along the border of lamellipodia. In the rat small-intestinal epithelium, no detectable difference in segmental expression of NHE1 mRNA has been described, with only a minor decrease in expression along the crypt-villus axis in the jejunum. Similarly, no longitudinal differences in NHE1 expression have been detected in the human intestine. This relatively uniform expression of NHE1 is consistent with its perceived role as a “housekeeping isoform” participating in the regulation of intracellular pH and volume.
NHE1 serves primarily to regulate intracellular pH, and its activation is associated with a number of downstream cellular events. The transient increase in pH i induced by NHE1 participates in cell proliferation and promotes transit through the G2-M checkpoint of the cell cycle. This finding may be related to the role NHE1 plays in proliferative responses of hepatocytes and hepatic stellate cells (HSCs) as described later in this chapter. NHE1 also appears to regulate cell differentiation, since deletion or inhibition of NHE1 has been shown to impair differentiation pathways. A role for NHE1 in apoptosis regulation has also been postulated, since high NHE1 activity confers resistance to proapoptotic stimuli. Additionally, NHE1 function is important in cytoskeletal organization and cell migration. The cytoplasmic tail of NHE1 acts as an anchor for actin filaments via binding of ezrin, radixin, and moesin (ERM) proteins, and disruption of these interactions or inhibition of NHE1 activity results in inhibition of cell migration and of formation of focal adhesions. NHE1 knockout mice are viable, although they have stunted growth and reduced survival rates. They also exhibit severe neurological defects (slow wave epilepsy, ataxia, and neuronal degradation) and present with abnormalities in gastric histology (see Section 56.5.4 ).
It is not clear whether involvement of NHE1 in these mechanisms is equally critical in the cells of the GI tract. No intestinal defect was demonstrated in otherwise rapidly renewing intestinal epithelium in NHE1 −/− mice, suggesting that the role of NHE1 in the intestinal crypt cell proliferation is minor. Also the described role of NHE1 in cellular differentiation may not represent a ubiquitous mechanism since its expression along the crypt-villus axis did not correlate with the differentiation status of enterocytes.
Regulated expression of NHE1 mRNA has been described in various systems, but especially in myocardium. Human, mouse, rabbit, and pig NHE1 gene promoter have been cloned and characterized to a various extent, with mouse NHE1 promoter analyzed in more detail than other species. The activity of this promoter is largely dependent on AP-2-like transcription factors as well as a poly(dA:dT) region of the promoter interacting with yet unidentified nuclear protein. Serum and growth factors have been shown to stimulate promoter activity in cardiomyocytes and fibroblasts through more distal elements of the promoter (0.8–1.1 kb) interacting with COUP transcription factors ; however, these in vitro findings do not correlate with data obtained from transgenic mice-bearing NHE1 gene promoter reporter construct. In the latter studies, crossing these mice with AP-2α or COUP-TFI knockout mice did not change the reporter gene expression in embryonic mouse tissue.
Regulation of NHE1 gene expression in GI tissues has not been extensively studied, although a limited amount of available data suggests that, consistent with its housekeeping role, NHE1 is not regulated at the mRNA level in situations where other NHE isoforms are. Examples of such circumstances are metabolic acidosis, microvillous inclusion disease, small bowel resection, glucocorticoid administration, or postnatal development.
The cytoplasmic C-terminal regulatory domain is associated with a number of functionally distinct signaling molecules, including phosphatidylinositol 4,5-bisphosphate (PIP 2 ), calcineurin homologous protein (CHP)1, and actin-binding proteins of the ezrin, radixin, moesin (ERM) family. In the distal C-terminal region, NHE1 contains a number of serine residues phosphorylated by ERK-regulated kinase p90RSK and Ste20-like Nck-interacting kinase (NIK) upon activation of growth factor receptors and by Rho kinase 1 (ROCK1) upon activation of integrin receptors and G protein-coupled receptors for thrombin and lysophosphatidic acid (LPA). Phosphorylation of C-terminal serine results in increased NHE1 activity, whereas phosphorylation of Ser703 by p90RSK promotes direct binding of the multifunctional adaptor protein 14-3-3, which conceivably serves as a focal point for the assembly of other signaling molecules. Additional proteins such as calmodulin (CaM), heat shock protein HSP70, and carbonic anhydrase II have also been shown to bind to this regulatory domain of NHE1. The latter interaction is particularly intriguing, as it may explain the ultimate changes in NHE1 exchange activity observed upon phosphorylation. It has been postulated that serum-induced phosphorylation within the last 178 amino acids of the C-terminus facilitates binding of carbonic anhydrase II, which through catalysis of CO 2 hydration causes local acidification resulting in the increase in NHE1 activity. The signaling molecule scaffolding at the C-terminus of NHE1 has been exhaustively overviewed by Baumgartner et al., and the reader is referred to this article and the references therein for more detail. It is not known whether all the described mechanisms of posttranslational modifications of NHE1 activity are ubiquitous to all cell types, and whether they have functional consequences in the digestive tissues. Na + /H + activity at the basolateral membrane of enterocytes increases with age, despite unchanged expression of NHE1 mRNA. This may represent age-dependent changes in NHE1 activity mediated by one or more of the abovementioned mechanisms, especially since another potential basolateral isoform NHE4 has not been detected in the small-intestinal epithelium.
Two studies implicated NHE1 in the pathophysiology of inflammatory bowel disease (IBD). In a rat model of acetic acid or trinitrobenzenesulfonic acid (TNBS)-induced colitis, Khan et al. described an induction of NHE1 mRNA in the colonic mucosa. Also in vitro, in Caco-2 and HT-29 human intestinal epithelial cells, inhibition of Na + /H + exchange with amiloride and other unrelated NHE inhibitors has been shown to reduce IL1β-, TNFα-, and LPS-stimulated IL-8 production, IL-1β-induced NF-κB activation, and phosphorylation of ERK (extracellular signal-regulated kinase). In the latter study, amiloride administered in vivo to DSS-treated rats resulted in attenuated symptoms of colitis and decreased neutrophilic infiltration in the colonic mucosa. The interpretation of these results is complicated by that fact that IC 50 for inhibition of IL-8 production by amiloride was ~ 30-fold higher than IC 50 required to inhibit NHE1 and NHE2, the two isoforms likely to be expressed in the selected cells used under culture conditions. Plasma concentration of amiloride in DSS-treated rats was not evaluated. It is possible, therefore, that the observed effects may represent nonspecific effects of the selected inhibitors. Moreover, recent analysis of NHE1 gene expression in human IBD is not consistent with the data obtained from rodent models of colitis. Khan et al. demonstrated a decreased mRNA expression in colonic biopsies from Crohn’s disease (CD) and ulcerative colitis (UC) patients, as compared to healthy colon. Similarly, Sullivan et al. found the expression of NHE1 to be decreased in the sigmoid mucosa of CD and UC patients. Consistently, Magro et al. described acute or chronic inhibitory effects of IFNγ on the activity of NHE1 in Caco-2 cells. Contrary, a recent study by Farkas et al. showed elevated expression and activity of NHE1 in the colons of patients with active UC. Therefore, the involvement of NHE1 in the pathogenesis of IBD and its potential as a therapeutic target is still unclear. In a rat model of necrotizing enterocolitis (NEC), the observed decrease in expression and activity of NHE1 was ascribed to cellular acidification, which was postulated to participate in the failure of the epithelial barrier and consequently in the pathogenesis of NEC. The plausibility of NHE1 involvement in liver cirrhosis through activation of stellate cells, as well as in hepatic tumorigenicity, is discussed in Section 56.5.2 .
In the rabbit and rat esophagus, NHE1 is the only plasma membrane NHE and is allosterically activated by reduced pH i in a protein kinase C (PKC)-dependent mechanism. The same mechanism of NHE1 activation along with Ca 2 + /calmodulin-dependent pathway mediates the cytoprotective effects of salivary epidermal growth factor (EGF) in acid-exposed cells. Loss of this mechanism in patients with low salivary EGF levels increases susceptibility to severe esophageal damage in gastroesophageal reflux disease (GERD) and contributes to the overall risk for the development of Barretťs esophagus. NHE1 expression is increased in GERD patients and in Barretťs esophagus, where it likely represents a cellular defensive mechanism to manage the acute and chronic acid overload. Bile acids present in reflux chyme reduce the ability of the cells to control their pH i by nitric oxide-mediated NHE1 inhibition, thus leading to increased DNA damage and potentially to mutations and cancer progression.
However, NHE1 has diverse physiological roles extending well beyond pH i and cell volume control, including cell proliferation, growth, migration, and apoptosis, and contributes to pathologic processes such as cancer cell invasion and heart failure. In a Barretťs adenocarcinoma cell line, acid pulse-induced NHE1 activity correlated with increased proliferation, which could be reduced by inhibition of NHE1 or PKC. This finding leads to a somewhat paradoxical proposal that while pathophysiological NHE1 inhibition in GERD may be detrimental to cell function, genomic integrity, and progression to Barretťs esophagus, pharmacological inhibition of NHE1 may be of therapeutic value in preventing progression from Barretťs to cancer, or in esophageal cancer therapy.
NHE2 was first cloned from rat and rabbit intestinal cDNA libraries by Collins et al. and Wang et al. and by Tse et al., respectively. Human NHE2 was cloned by Ghishan et al. and later corrected by Malakooti et al. Among the members of the human Scl9a family of NHE, NHE2 protein shares the most similarity with NHE4, especially within the cytoplasmic C-terminus. Interestingly, in the human, rat, and mouse, the Slc9a2 and Slc9a4 genes cosegregate on chromosomes 2, 9, and 1, respectively. The adjacent chromosomal location of the two NHEs in all three species strongly suggests that they arose by gene duplication early in the evolution. The predicted molecular weights of NHE2 protein in rat, rabbit, and human are ~ 91 kDa, although its mobility on SDS-PAGE gels does not confirm these calculations. Mature rabbit NHE2, when expressed in PS120 fibroblasts, was shown to be an O -linked sialoglycoprotein. In these studies, neuraminidase shifted the mobility of NHE2 protein from 85 to 81 kDa, and O -glycanase further shifted the mobility of the 81 kDa protein to 75 kDa. Incubation of PS120/NHE2 cells with an O-glycosylation inhibitor benzyl N -acetyl-alpha-D-galactosaminide reduced the size of the 85 kDa protein to 81 kDa, although this was without consequence for the initial rate of Na + /H + exchange in these cells.
NHE2 is expressed in the epithelia of all digestive organs, with particularly high expression in the proximal colon. Outside of the GI tract, NHE2 activity and/or expression has been described in the kidney (cortical thick ascending limb of the nephron, macula densa, distal convoluted tubules, and connecting tubules), endometrium and placenta, chondrocytes, inner ear, heart, testes, and adrenal glands. Expression of NHE2 in the individual digestive organs is described below in a later section on physiological roles of Na + /H + exchange in the digestive tract. With the exception of gastric epithelium, NHE2 was unambiguously demonstrated on the apical membrane of polarized epithelial cells. Since NHE2 has an exclusive ability to be activated by elevated extracellular pH (pH o ), it has been speculated that NHE2 may be the NHE isoform that mediates Na + /H + exchange activation by an increase in interstitial HCO 3 – concentration during acid secretion in gastric epithelium, a hypothesis that assumes basolateral localization of this isoform. Immunohistochemical evidence for this assumption is lacking as yet. In the intestinal epithelium, expression of NHE2 along the crypt-villus axis shows some species-dependent differences. In rabbits, NHE2 is present in the brush-border of the entire villus of the small intestine, in colonic surface cells, and in the apical membrane of the upper half of the crypt. In the mouse colon, however, NHE2 is predominantly expressed in the crypt cells, suggesting a role for this isoform in crypt pH i and volume homeostasis.
Despite a relatively wide expression of NHE2, its physiological role remains elusive. NHE2 stably transfected in NHE-deficient Chinese hamster ovary (CHO) cells (AP-1) showed a relatively high affinity for amiloride and its analogues, with potencies in decreasing order of EIPA (IC 50 = 79 nM) > DMA (IC 50 = 250 nM) > amiloride (IC 50 = 1.4 μM) > benzamil (IC 50 = 320 μM). Nonamiloride compounds also inhibited NHE2 with the following order of potency: clonidine (IC 50 = 42 μM) > harmaline and cimetidine (both with IC 50 = 330 μM). Kinetic analyses showed that NHE2 Na + o dependence followed simple, saturating Michaelis-Menten kinetics with an apparent affinity constant for Na + (K Na ) ~ 50 mM. Intracellular H + activated NHE2 by a positive cooperative mechanism with an apparent half-maximal activation value of p K 6.90. Li + and H + acted as competitive inhibitors of NHE-mediated Na + influx, while extracellular K + had no effect on NHE2 activity.
The information provided by the analysis of NHE2 −/− mice suggests a role in muscarinic stimulation of salivary secretion, as well as in gastric physiology (see discussions below). The involvement of NHE2 in gastric parietal cell homeostasis seems particularly significant since NHE2 gene ablation leads to a reduced number of parietal and chief cells, loss of net acid secretion, and progressive inflammation in the form of diffuse corporal gastritis. Other roles for NHE2 in the physiology of digestive organs, presumed from the expression and/or functional studies, are discussed in more detail in Section 56.4.2.2 of Na + /H + Exchange in the Digestive Tract. Overall, the results of the available reports suggest that NHE2 plays a negligible role in net Na + or fluid absorption in the mouse digestive tract. The disparity between these results and the functional studies demonstrating contribution of NHE2 to various cellular functions (especially intestinal Na + absorption) remains unresolved; however, unidentified compensatory mechanisms may help explain the significance of this gene in the physiology of intestinal and renal epithelium.
Rat and human NHE2 promoters have been cloned and characterized. Both proximal promoters lack canonical TATA and CAAT boxes, are highly GC rich, and share about 59% homology with a number of conserved, predicted, regulatory elements. Only rudimentary analysis of the human NHE2 promoter has been performed, with prediction analyses indicating putative binding sites for the following trans -acting factors: Sp1, AP-2, Egr-1, p300, NF-κB, Oct-1, zinc finger protein-1, MyoD, two caudal-related homeobox (Cdx) family members, CdxA and Cdx-2, glucocorticoid receptor (GRE), thyroid hormone receptor, a CACCC site, and several polyoma viral enhancer 3 sites. Of all these sites, only Sp1, AP-2, CACCC, NF-κB, and Oct-1 were conserved in human and rat NHE2 promoters. A minimal promoter of the rat NHE2 was identified and found to be regulated by Sp transcription factors, with Sp1 acting as an activator, and Sp3 and Sp4 playing inhibitory roles in transfected renal epithelial cells. Regulation of both rat and human NHE2 promoter in intestinal epithelial cells also involves Sp1 and Sp3 transcription factors, although both of them appear to be stimulatory. NHE2, like NHE1, can be activated by serum and by EGF. There also appears to be a transcriptional component to the mechanism by which NHE2 is activated by EGF. In suckling rats, parenterally administered EGF increased expression of NHE2 mRNA in the small intestinal epithelium but not in the kidney. This finding was confirmed in EGF-treated RIE cells, which also showed activation of the rat NHE2 promoter in transient transfection experiments. In adult mice, however, neither exogenous EGF nor salivarectomy affected NHE2 mRNA expression in the small intestine, suggesting that this regulation may be species and/or age dependent. The response to growth factors induces the production of 1,2 diacylglycerol (DAG), an activator of protein kinase C (PKC). Phorbol 12-myristate 13-acetate (PMA), a DAG structural analog activates NHE2 activity (see Section 56.4.2.4 ), but also stimulates its mRNA expression. PMA was later shown to trigger phosphorylation of nPKCδ, activation of extracellular signal-regulated protein kinase-1 and -2 (ERK1/2), and subsequent nuclear translocation of Egr-1 transcription factor. Egr-1 directly binds to human NHE2 promoter and promotes NHE2 gene transcription. It remains unknown whether this mechanism of NHE2 transcriptional regulation is related to the differential effects of EGF during growth/aging.
Similar bimodal regulation of NHE2 by osmolarity has been described. In PS120 cells, hyperosmolarity inhibited NHE2 activity, but other reports showed activation of NHE2 in mouse inner medullary collecting duct (mIMCD-3) cells, AP-1 cells, and colonic crypt cells. In the case of renal mIMCD cells, mRNA expression was also induced by hyperosmotic stress. A TonE-like element and a novel cis -element, termed OsmoE, were identified in the rat NHE2 promoter as being responsible for the increased transcription of the NHE2 gene induced by hyperosmolarity, with both elements acting in concert to provide maximal transcriptional induction. The transcription factors interacting with these elements have not been identified, and at this point it is also not known whether the same mechanism is present in the colonic crypts.
During postnatal development, expression and activity of NHE2 in the rat small intestinal epithelium dramatically increase around the time of weaning. This increase is due to transcriptional activation of NHE2 gene, as shown by nuclear run-on assay and by reporter gene analysis in transgenic mice bearing − 2.4 kb of the rat NHE2 promoter (Kiela et al., unpublished observations). Interestingly NHE2 expression in the rat kidney follows a reciprocal pattern, with highest expression in the suckling period and a decline toward adulthood, implying tissue-specific mechanisms regulating postnatal changes in NHE2 expression.
NHE2 protein has a relatively short half-life (~ 3 h) compared to other NHE isoforms (NHE1—24 h, NHE3—14 h) and is subject to lysosomal degradation, as determined in PS120 fibroblasts and Caco-2 cells. This suggests that changes at the level of gene transcription or translation may be more critical for NHE2 regulation than for other isoforms with long half-lives. NHE2 is a residual plasma membrane protein and unlike NHE3 does not undergo endosomal recycling. Glycosylation of NHE2 may affect its cellular localization, since unglycosylated 75 kDa rabbit NHE2 was found predominantly intracellularly, although it is not clear whether this represents a regulatory mechanism or is simply related to the maturational stage of NHE2 protein synthesis. Of the two well-characterized apically expressed NHE isoforms, NHE2 and NHE3, NHE2 activity is considered relatively stable and is not regulated by many factors. Extracellular alkalinization activates NHE2, which is believed to propel increased proton extrusion in gastric parietal cells during secretagogue-stimulated acid secretion (see Section 56.5.4 ). The maximal rate of exchange ( V max ) mediated by NHE2 was shown to be stimulated by serum, fibroblast growth factor (FGF), and protein kinase C activator PMA in PS120 fibroblasts. Intracellular ATP depletion reduced the NHE2 activity by a dramatic decrease in H + affinity as well as V max , with virtual elimination of the allosteric effect of H + . ATP depletion also eliminated the stimulatory effect of serum, suggesting that growth factor-stimulated NHE2 activity is mediated via its pH-sensing mechanism. Thrombin increased NHE2 V max without altering the Hill coefficient, although it is not clear if this could be attributed to increased intracellular Ca ++ ascribed to thrombin-treated fibroblasts. In the same study, thrombin also increased NHE3 activity, whereas it was shown later that elevation of intracellular Ca ++ by thapsigargin in Caco-2/bbe cells inhibited NHE3.
NHE2 has a particularly well-documented role in the gastric epithelium, although alterations in NHE2 expression or activity in gastric disorders have not been documented. Downregulation of NHE2 activity and gene expression has been documented in rats and Caco-2/bbe cells treated with interferon γ, implicating a role for NHE2 in inflammation-associated diarrhea. The lack of absorptive defect in the intestine of NHE2 −/− mice, however, suggests that cytokine-mediated changes in NHE2 function may not be critical for electrolyte absorption in the inflamed intestinal mucosa. Surprisingly, enteropathogenic E. coli invasion of intestinal epithelial cells significantly increased NHE2 activity via a PKC ε -mediated mechanism, while it inhibited activities of NHE3 and Cl − /OH – exchange. The authors speculated that NHE2 activity might represent a potential compensatory response to increased luminal fluid resulting from inhibition of NHE3 activity, disruption of tight junctions, inflammatory response, or alterations in anion exchanger activity. On the other hand, TNF inhibits expression of NHE2 through an NF-kB-dependent mechanism, a phenomenon postulated to contribute to inflammation-associated diarrhea in IBD. Although our group has not demonstrated any differences between wild-type or NHE2 −/− mice in their susceptibility to DSS-induced mucosal injury, Moeser et al. showed that NHE2-deficiency prolongs recovery from mesenteric ischemia with increased mucosal permeability and disruption in the localization of the tight junctions proteins occludin and claudin-1.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here