Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Opportunistic fungal pathogens have emerged as common causes of invasive disease in the compromised host.
Histopathologic examination of deep tissue is a major means used for the rapid detection and recognition of fungal pathogens causing invasive infection.
Micromorphologic and phenotypic methods continue to be the general processes used in the clinical laboratory for the identification of fungal pathogens in culture.
Molecular methods have advanced as techniques used for the confirmed identification of fungal pathogens from culture and are evolving as future methods for the rapid identification of fungal pathogens directly in clinical material.
Candida albicans continues to be the most common fungal species associated with human disease; however, non- albicans Candida species are becoming more widespread in causing invasive disease with increased resistance to standard antifungal treatments.
Dimorphic fungal pathogens are frequent causes of mild infection in people living in endemic areas; however, invasive disease, whether recurrent or primary, is becoming more common owing to the increase in immunocompromised hosts.
Recent introduction of new antifungal agents and progression of resistance among molds and yeasts have added importance to the development and standardization of antifungal susceptibility testing.
Medical mycology is the science devoted to the study of fungi and their relationship to human disease. This scientific discipline encompasses single-celled yeasts and filamentous molds as agents of disease ranging from superficial skin infection (cutaneous mycosis) to disseminated deep-seated visceral disease (systemic mycosis). Fungal agents include the historically pathogenic fungi (true pathogens), as well as the saprophytic fungi elevated to the status of pathogen by modern therapies and diseases (opportunistic pathogens). Modern therapies such as high-dose chemotherapy to treat cancer and solid organ transplantation, as well as immunosuppressive infection such as acquired immunodeficiency syndrome (AIDS), have served as the impetus to allow fungi to emerge as major causes of human disease ( ; ; ; ; ; ).
Although medical mycology education for the medical profession in general has been lacking, pathologists who have been nurtured in the surgical pathology suite have acquired a general understanding of the recognition of fungal pathogens. Overall, the general lack of education in this area is troublesome because most fungi are identified by skilled human observation rather than by machines.
The goal of this chapter is to present the fundamentals of medical mycology with an emphasis on practical issues and a description of those fungal pathogens encountered regularly in the laboratory. For a comprehensive atlas of the histopathology of fungal infections, refer to the book by and the articles by and . Additionally, although not included in the fungal kingdom, infections caused by achlorophyllous algae are also discussed in this chapter because the characteristics of the algae and the diseases they produce resemble fungi more than other infectious agents.
Since this chapter reports specifically on fungal mycoses, other types of fungal diseases are not discussed. Reviews of these other diseases are available and include the topics of noninvasive allergic responses to fungi ( ; ; ), poisoning by the ingestion of poisonous fungi (mushroom poisoning or mycetismus) ( ), intoxication by ingestion of fungal toxins (mycotoxicosis) ( ), and psychoactive drugs of fungal origin ( ).
The first hurdle for the beginning mycologist consists of nomenclature and its handmaiden, taxonomy. Members of the fungal kingdom (Eumycota) are eukaryotic cells that have the capability to reproduce by both sexual and asexual methods. Fungi are classified into phyla by the nature of their sexual reproductive structures or lack thereof ( ), with fungi that have recognized sexual reproductive structures (perfect fungi) classified into the phylum Zygomycota (now split into the phylum Glomeromycota with four additional subphylums of uncertain placement), Ascomycota , or Basidiomycota based on the type of sexual reproductive structure produced ( ). Fungi that lacked recognized sexual reproductive structures were classified in the phylum Deuteromycota, also called the imperfect fungi , on the basis of their asexual reproductive structures. This classification, however, has complicated the naming and taxonomic positioning of fungi due to dual nomenclature to have two scientific names, mainly a species name for those producing asexual (anamorphic) structures and another species name for those with sexual (teleomorphic) structures. A major change in fungal systematics occurred at the July 2011 International Botanical Congress, where it was voted to abolish the dual system of fungal nomenclature and to rename the code regulating the naming of fungi the International Code of Nomenclature for Algae, Fungi, and Plants (previously called the International Code of Botanical Nomenclature ) ( ; ). These new rules meant that after January 1, 2013, one fungus can only have one name. Although many challenges remain with respect to acceptance of what constitutes a valid name for those fungal species that had this dual nomenclature, a regulatory mechanism is now in place to allow for addressing these challenges of naming fungi ( ). However, which names to use have not always been straightforward ( ).
Historically, fungi were identified to species using a combination of morphologic and physiologic characteristics. Important terms, which are still commonly used today in the clinical laboratory to describe these characteristics of fungi, are displayed in Table 60.1 . More recently, the development of molecular methods to identify fungi has led to the discovery of fungal species that are morphologically indistinguishable and described as cryptic species. In light of these recent developments, a polyphasic approach has been suggested for the identification of fungal species. This approach includes a combination of molecular sequencing (using at least two genomic targets) or proteomic analysis (i.e., matrix assisted laser-desorption ionization time-of-flight mass spectrometry [MALDI TOF MS]), morphologic features, and physiologic characteristics in the identification process. However, since many clinical microbiology laboratories may still rely on morphologic features for identification of species, recommendations now suggest using the name species complex when the identification is based on morphology or other means that cannot distinguish between closely related species ( , ). In this chapter, a simplified conceptual approach is used to recognize the pathogens most commonly encountered in the clinical laboratory. For practical purposes, a few general characteristics shown in Table 60.2 serve as the basis for identification of fungi in the laboratory.
| Term | Definition |
|---|---|
| Aerial mycelium | Hyphae produced above the surface of the agar media |
| Anamorph | Asexual form of fungal sporulation (imperfect state) |
| Arthroconidium | Conidium derived from the fragmentation of specialized hyphae |
| Ascocarp (ascoma) | Fruiting structure that contains asci (several types exist) |
| Ascospore | Sexual spore formed within an ascus following meiosis |
| Ascus (pl., asci) | Saclike structure that contains ascospores, characteristic of Ascomycetes |
| Basidiospore | Sexual spore formed as an outgrowth of a basidium |
| Basidium | Structure that contains basidiospores (e.g., mushroom) |
| Blastoconidium (pl., blastoconidia) | Asexual spore formed by budding of the hyphal, pseudohyphal, or yeast cell |
| Chlamydospore | Thick-walled resting or survival structure (also called chlamydoconidia ) |
| Cleistothecium (pl., cleistothecia) | Enclosed ascocarp, composed of layers of hyphae that contain randomly dispersed asci |
| Collarette | Funnel-shaped structure at the apex of a phialide |
| Columella | An extension of the sporangiophore into the base of the sporangium |
| Conidiogenous cell | Cell that produces conidia |
| Conidiophore | Specialized hyphal structure that carries the conidia |
| Conidium (pl., conidia) | Asexual reproductive structure that forms on the side or the end of a hypha or conidiophore |
| Dematiaceous (phaeoid) | Pigmented conidia, spores, or hyphae due to the presence of melanin |
| Dimorphic | Displaying two morphologic types, one environmental (mold) and one in vivo (yeast) |
| Glabrous | Smooth, referring to colonial morphology |
| Heterothallic | Sexual reproduction requiring the interaction of two different thalli (mating strains) |
| Holomorph | Whole fungus; the anamorphic plus the teleomorphic state of the fungus |
| Homothallic | Sexual reproduction can take place within one thallus |
| Hyaline | Clear, colorless, or transparent |
| Hypha (pl., hyphae) | Septate or aseptate vegetative unit of a mold |
| Intercalary | Borne within a hypha |
| Macroconidium | Larger of two types of conidia produced by a mold |
| Microconidium | Smaller of two types of conidia produced by a mold |
| Mold | Filamentous fungus that reproduces by sexual or asexual means |
| Mycelium | Mass of hyphae that make up the thallus of a mold |
| Perithecium | Enclosed ascocarp with a pore at the top through which the ascospores are discharged |
| Phialide | Cell with opening through which conidia are produced |
| Pseudohyphae | Connected yeast cells (blastoconidia) that resemble a hypha but contain areas of constriction between adjacent cells |
| Sclerotic body (pl., sclerotia) | Thick-walled, dematiaceous, muriform, rounded cells found in tissue diagnostic for chromoblastomycosis |
| Septum (pl., septa) | Cross-wall in a hypha |
| Spherule | A round structure containing endospores characteristic of Coccidioides species in tissue |
| Sporangiophore | Stalk bearing the sporangium |
| Sporangiospore | Asexual spore produced within a sporangium |
| Sporangium | Saclike structure in which the asexual sporangiospores develop |
| Sterigmata | Slender outgrowth of a cell bearing conidia |
| Teleomorph | Sexual form of a fungal sporulation (perfect state) |
| Terminal | Borne at the end of a hypha |
| Thallus (pl., thalli) | Vegetative growth of a fungus; includes an interwoven mass of hyphae |
| Thermophilic | Fungi that grow at a high temperature |
| Vegetative mycelium | Hyphae produced on the surface or extending into the agar media |
| Vesicle | Enlarged or swollen cell, often at the end of a conidiophore or sporangiophore, which may also be within hyphae |
| Yeast | Single-cell fungus that reproduces by budding or by fission |
| Zygospore | A mucoraceous spore formed sexually by the union of two similar cells |
| Characteristic | Examples |
|---|---|
| Type of macroscopic growth | Colonies of unicellular organisms (yeast) |
| Colonies of filamentous organisms (molds) | |
| Morphology of yeast | Budding |
| Budding with pseudohyphae | |
| Round yeast with capsules | |
| Budding yeast with collarets | |
| Morphology of filamentous structures | True septate hyphae |
| True nonseptate hyphae (or sparsely septate) | |
| Pigment of hyphae | Hyaline (lightly pigmented or nonpigmented) |
| Dematiaceous or phaeoid (darkly pigmented) | |
| Morphology of asexual reproductive structures | Conidia |
| Spores | |
| Morphology of sexual reproductive structures | Ascospores |
| Cleistothecia | |
| Perithecia | |
| Growth rate | Slow (>10 days) |
| Medium (4–10 days) | |
| Fast (<4 days) | |
| Inhibition by cycloheximide | Many saprophytic fungi |
| Optimal growth temperature | 25°–30°C |
| 35°–37°C | |
| 40°–42°C | |
| 50°–58°C | |
| Biochemical tests | Assimilation |
| Fermentation | |
| Enzymatic degradation (e.g., urease) | |
| Growth enhancement | |
| Conversion from mold to yeast phase | Supplanted by DNA probe tests in most laboratories |
| Immunologic detection of antigens or antibodies | Cryptococcus, Histoplasma, Coccidioides, Blastomyces, Aspergillus |
| DNA probe | Histoplasma, Coccidioides, Blastomyces |
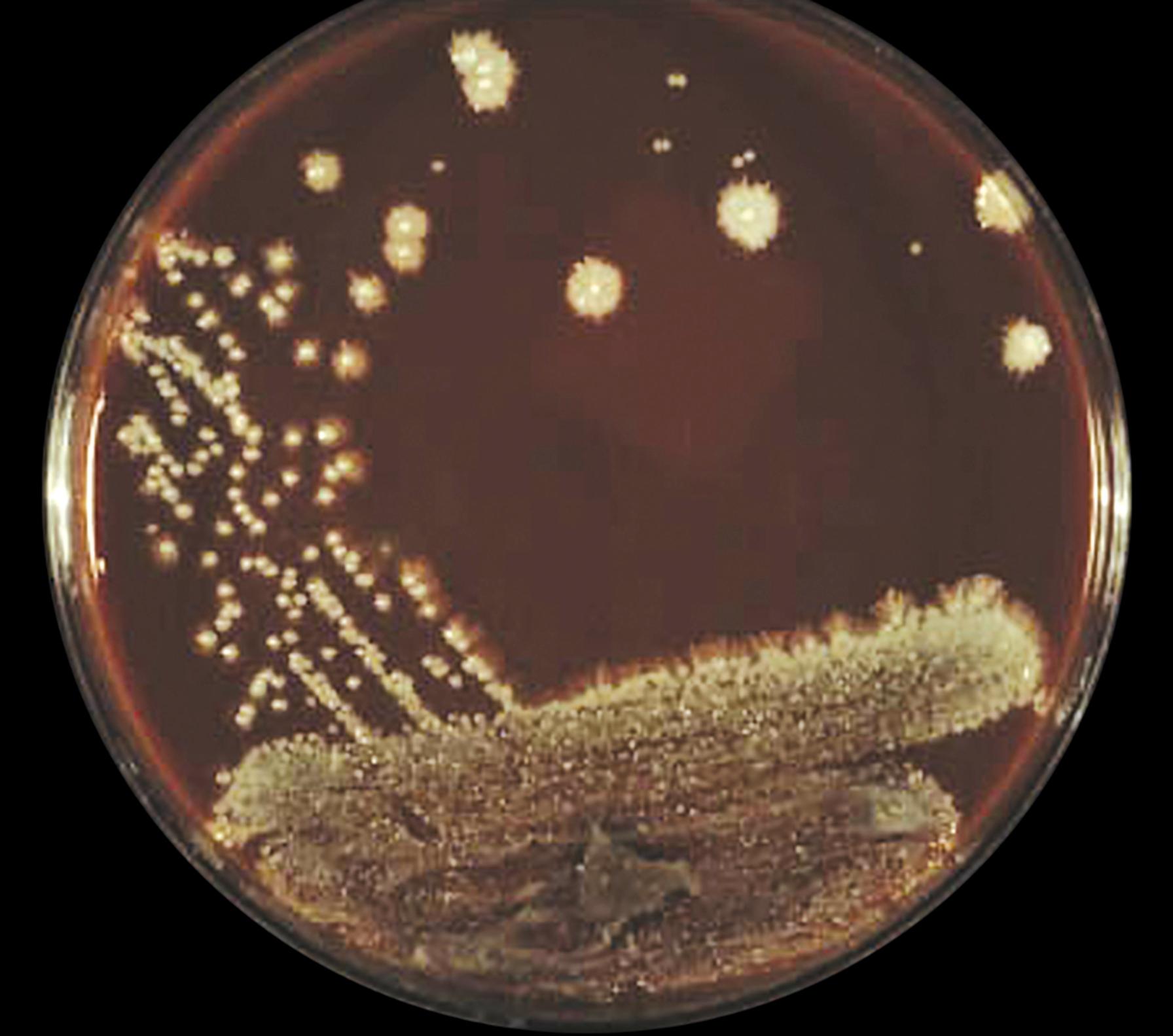
Two morphologic forms of fungi are recognized and include yeasts and molds. Yeasts are unicellular organisms that usually reproduce asexually by blastoconidia (also called budding ) to produce a daughter cell. The colonial mass of yeast is a collection of distinct individual organisms that macroscopically may resemble bacterial colonies on the surface of the agar. Many yeast species have colonies that are usually smooth with a regular edge, although some species may be rough with irregular edges. When the colonies become heaped and dull, they may resemble those of staphylococci. Yeast produces catalase, so the noncautious microbiologist who does not perform a Gram stain or a wet preparation may mistake a yeast colony for a bacterium and provide a misleading report. The microscopic presence of “budding yeast” with or without pseudohyphae would be adequate to identify the colony as yeast. Furthermore, the pseudohyphae of yeast are often visible macroscopically as filamentous extensions from the edges of the colony, known colloquially as feet ( Fig. 60.1 ).
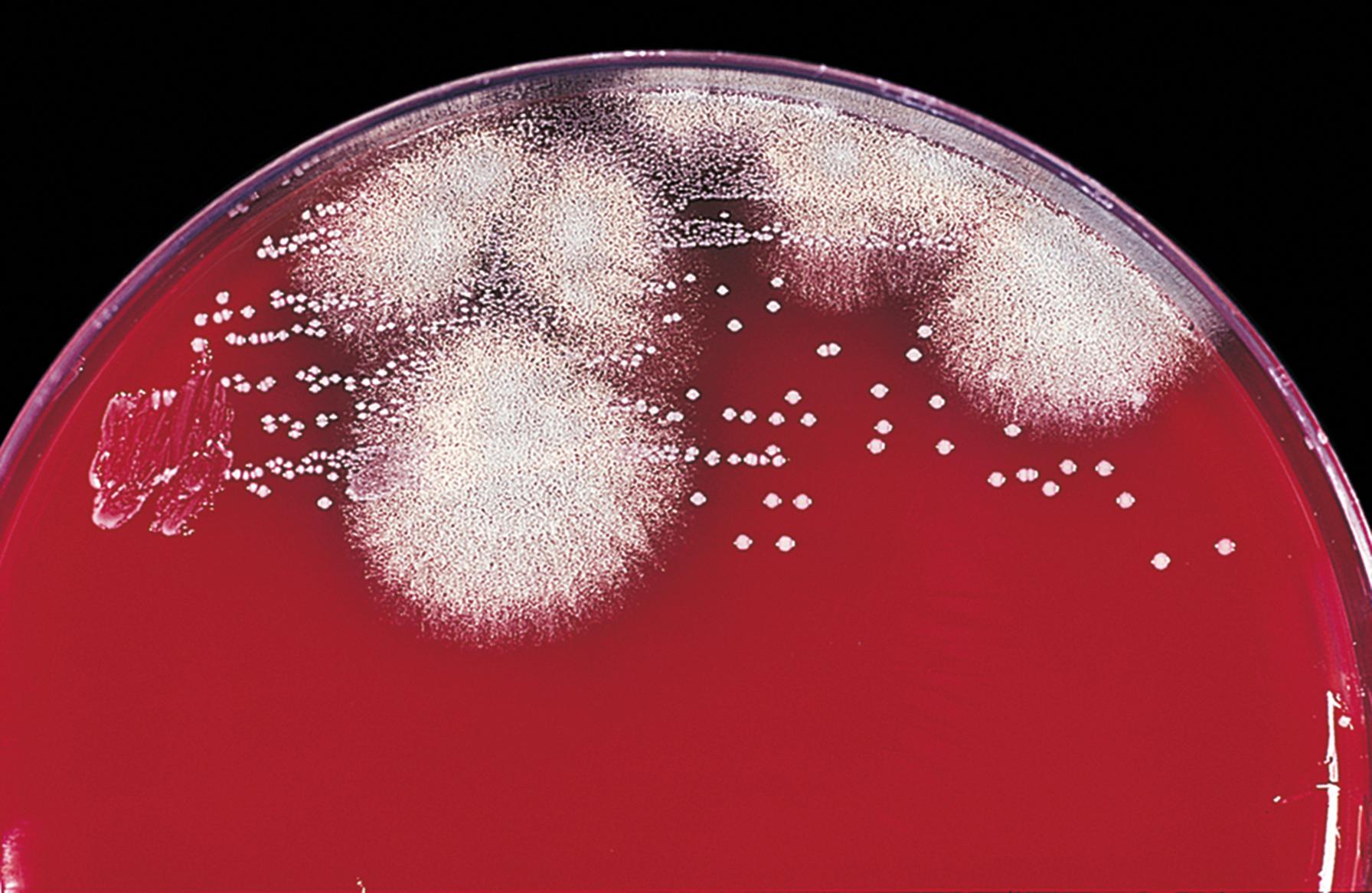
In contrast to yeasts, molds are described as filamentous fungi that are multicellular in structure. Although the term mold is used to describe this morphologic type, the term mould is historically recognized and more commonly used by mycologists to describe this morphologic form. The filamentous nature of the mold gives colonies a woolly, fluffy, or velvety appearance, sometimes punctuated with a granular or powdery aspect that is produced by the formation of asexual reproductive structures ( Fig. 60.2 ). At other times, the colony may have a glabrous (smooth) appearance.
The distinction between molds and yeasts is not firmly established. Some yeasts also develop a visually macroscopic filamentous component. For instance, Trichosporon spp. are yeasts that develop such filamentous extensions, whereas Exophiala jeanselmei is a dematiaceous yeast that develops a mycelium as it matures. Furthermore, dimorphic fungi can exhibit a mold form under some conditions and a yeast or yeastlike form in other circumstances. Clinically important dimorphic fungi are systemic pathogens that behave as molds in the environment and on agar media at 25° to 30°C. In contrast, the tissue form may be yeast (such as Blastomyces, Histoplasma, and Sporothrix ) or, in the cases of Coccidioides immitis and Coccidioides posadasii, a structure called a spherule is observed. When cultivated in vitro at 37°C on appropriate media, the tissue form is reproduced by these fungi. Other agents also exhibit dimorphism but are less common in the clinical laboratory. For instance, black molds such as Fonsecaea pedrosoi and Phialophora verrucosa produce nonhyphal cells in tissue called sclerotic bodies but appear as molds when grown on solid media at room temperature. Reverse dimorphism is exemplified by Malassezia furfur, which produces both yeast cells and hyphae in the cutaneous lesions of patients with pityriasis versicolor.
The morphology of the fungus is an important characteristic for the identification of both yeasts and molds. The filamentous structure of a mold is referred to as hyphae (singular, hypha ), and a mass of hyphae is known as a mycelium . The mycelium growing on the surface of or within the agar is known as the vegetative mycelium , whereas filamentous extensions above the colony are called aerial mycelium . True hyphae may have cross-walls that contain pores for communication through the hyphae or cross-walls that are complete, dividing the hyphae into multiple cells. Hyphae that have cross-walls are called septate , whereas those without cross-walls are referred to as aseptate . Some fungi that have septate hyphae also have aseptate or septate specialized hyphae (conidiophores) that bear asexual reproductive structures. Conversely, some so-called nonseptate fungi have occasional cross-walls and perhaps would be better designated as sparsely septate.
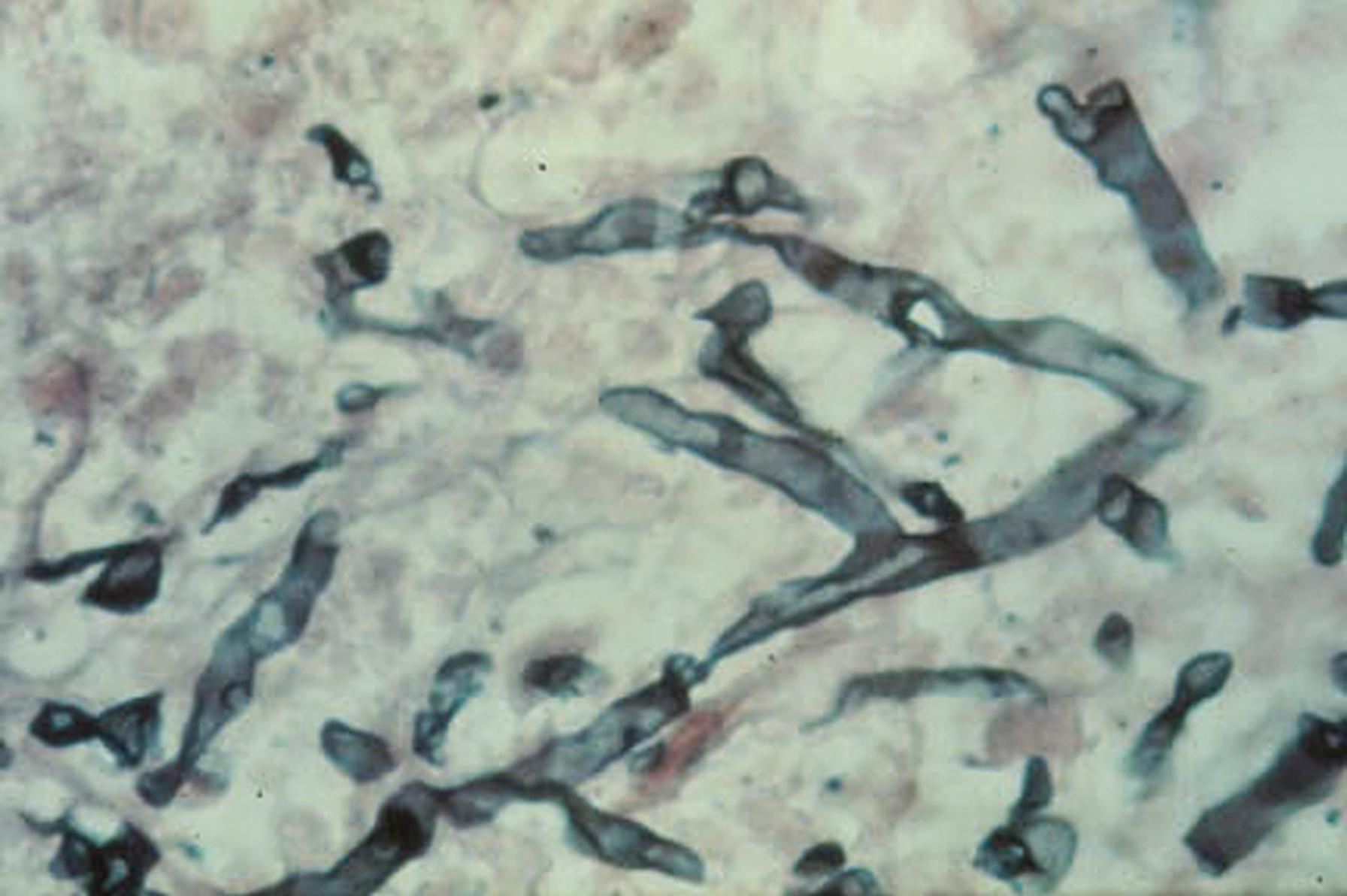
The width of the hyphae and the angle of branching are important clues to fungal identity. Mucormycetes (also called zygomycetes and members of the order Mucorales), such as Rhizopus spp., have broad, ribbonlike hyphae that have haphazard branching ( Fig. 60.3 ), whereas hyaline molds such as Aspergillus spp. have narrow hyphae that branch at acute angles (dichotomous branching) ( Fig. 60.4 ). The designation of a fungus as Aspergillus on the basis of hyphal morphology in a smear or tissue section is, in truth, only a statement of the a priori odds for this pathogen group. Describing the characteristics of the hyphae is a better approach to reporting this observation.
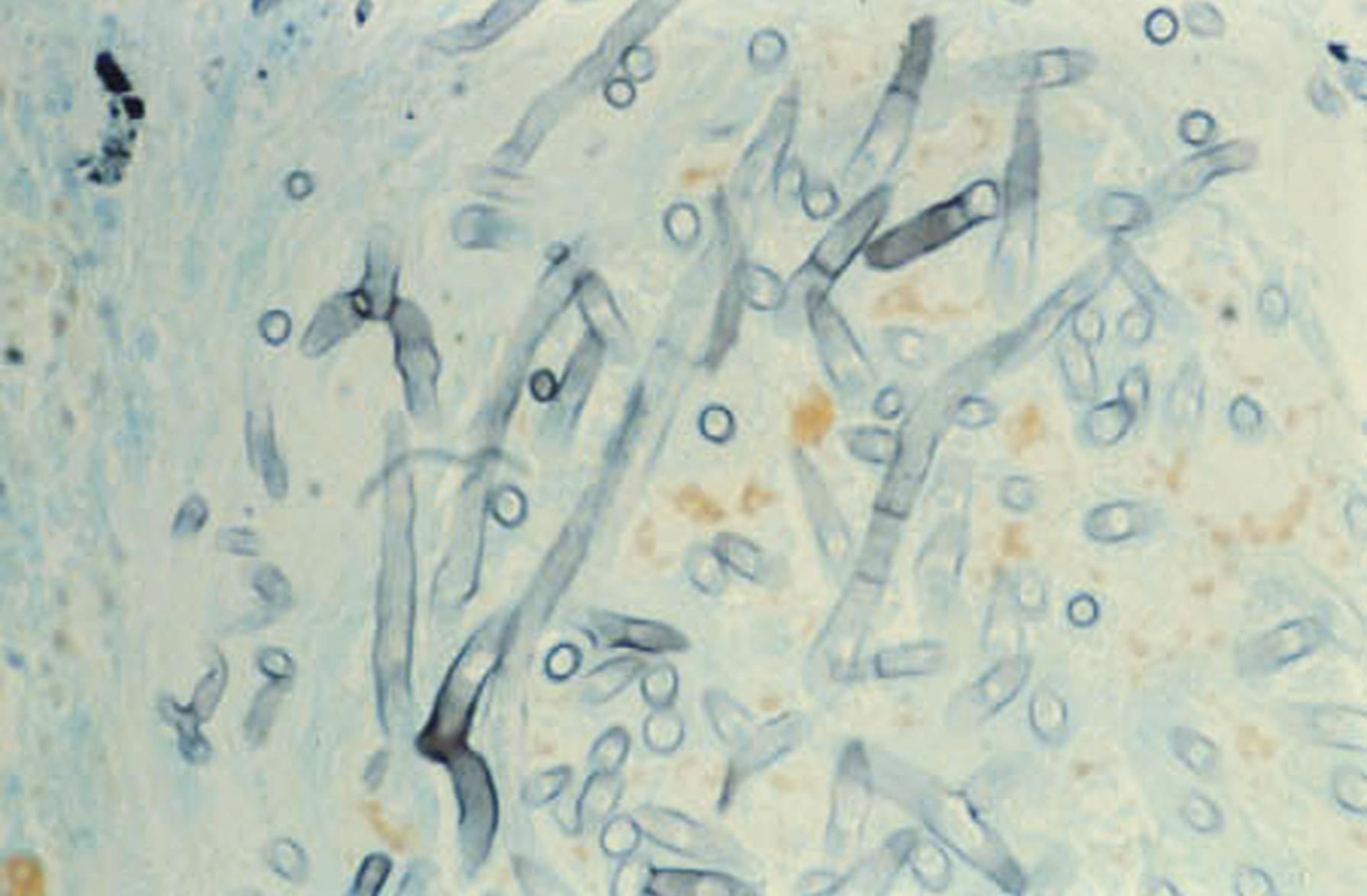
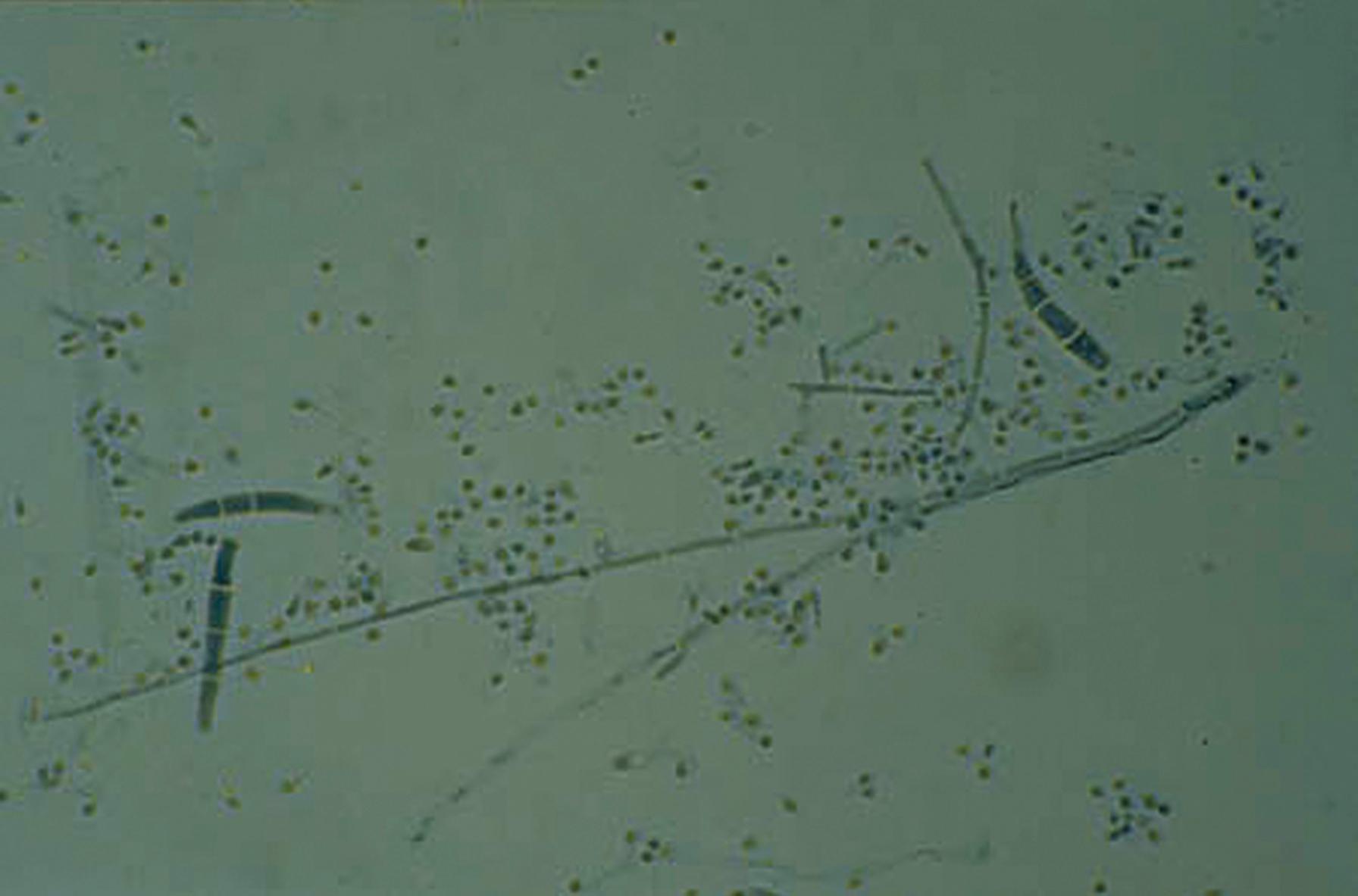
Yeasts reproduce asexually by the formation of blastoconidia, where the daughter cell usually appears at one end of a yeast cell and eventually enlarges to form a new yeast cell. This process is also referred to in a more generic sense as budding. If a series of daughter cells do not detach fully from the originating cells, a pseudohypha (plural, pseudohyphae) is produced. Pseudohyphae are distinguished from true hyphae by the presence of a constriction at the junction of adjacent cells and by the restriction in size of the daughter cell to that of the parent ( Fig. 60.5 ). In contrast, the walls of true hyphae are parallel without constrictions. Candida albicans and some strains of C. tropicalis may produce pseudohyphae that have the appearance of true hyphae, depending on growth conditions, and differentiation of Candida from hyaline molds such as Aspergillus in tissue sections on occasion may be problematic. In contradistinction, Cryptococcus spp. produces only budding yeast and rarely rudimentary or primitive pseudohyphae, but true hyphae are not formed.
Some mold hyphae and yeast can be characterized by the production of a dark pigment. These fungi contain melanin, which imparts a brown coloration to the fungal element in microscopic preparations and causes colonies of the fungi to appear dark green, brown, or black. The terms dematiaceous , melanized , dark , and phaeoid have been used interchangeably to denote those fungal elements that contain melanin ( ). Superimposed dyes in stained smears, wet preps, or histologic sections may partially obscure the color, which can be more obvious in unstained preparations. Those fungi that lack dark hyphal pigmentation are referred to as hyaline (clear or colorless). This term may not fully reflect the fungal species appearance, because in some cases a light pigmentation may produce colored colonies, and asexual reproductive structures of some hyaline molds may have green, brown, or black pigments that impart a color to the surface of the colony once the reproductive structures have formed. The true appearance of these molds usually can be discerned by observing the back of the colony, which maintains a light coloration. In contrast, both the front (obverse) and the back (reverse) of dematiaceous or highly melanized colonies usually demonstrate the dark pigment.
The primary means for identification of mold fungi is by characterization of asexual reproductive structures. For yeast, phenotypic studies are the mainstay in identification, with asexual reproductive structures serving as ancillary clues in the identification process. The two principal asexual structures are spores (which may also be present as sexual structures) and conidia. Asexual spores (called sporangiospores ) are produced by cleavage within an encompassing structure called a sporangium ( Fig. 60.6 ). Conidia (singular, conidium ) are much more diverse and form by differentiation from the tip or side of a fertile hypha, such as a conidiophore, or by hyphal differentiation. Unfortunately, interchangeable use of the terms conidium and spore in the literature has led to confusion. Sporulation and spores often are used as general terms for asexual reproduction, and the term spore is sometimes used when conidium would have been more accurate.
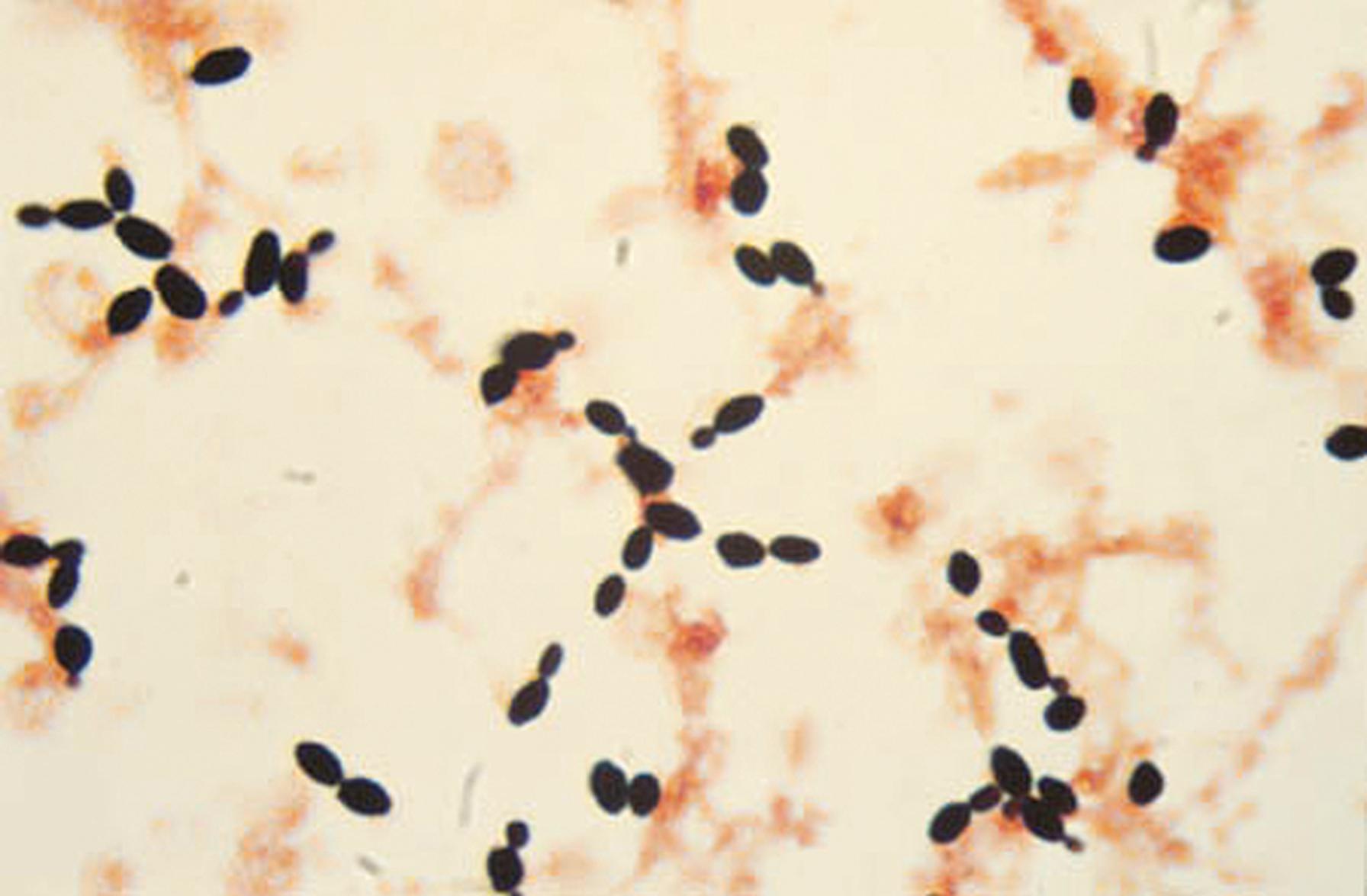
The principal means of asexual reproduction in yeast is by the formation of blastoconidia (i.e., budding). A bud starts as a softening of the cell wall of the mother cell, followed by expansion of the cell wall (blown out) and migration of nucleus and cytoplasm to the swollen area. A septum seals the boundary between daughter and parent cells ( Fig. 60.7 ). If separation does not occur, a pseudohypha results.
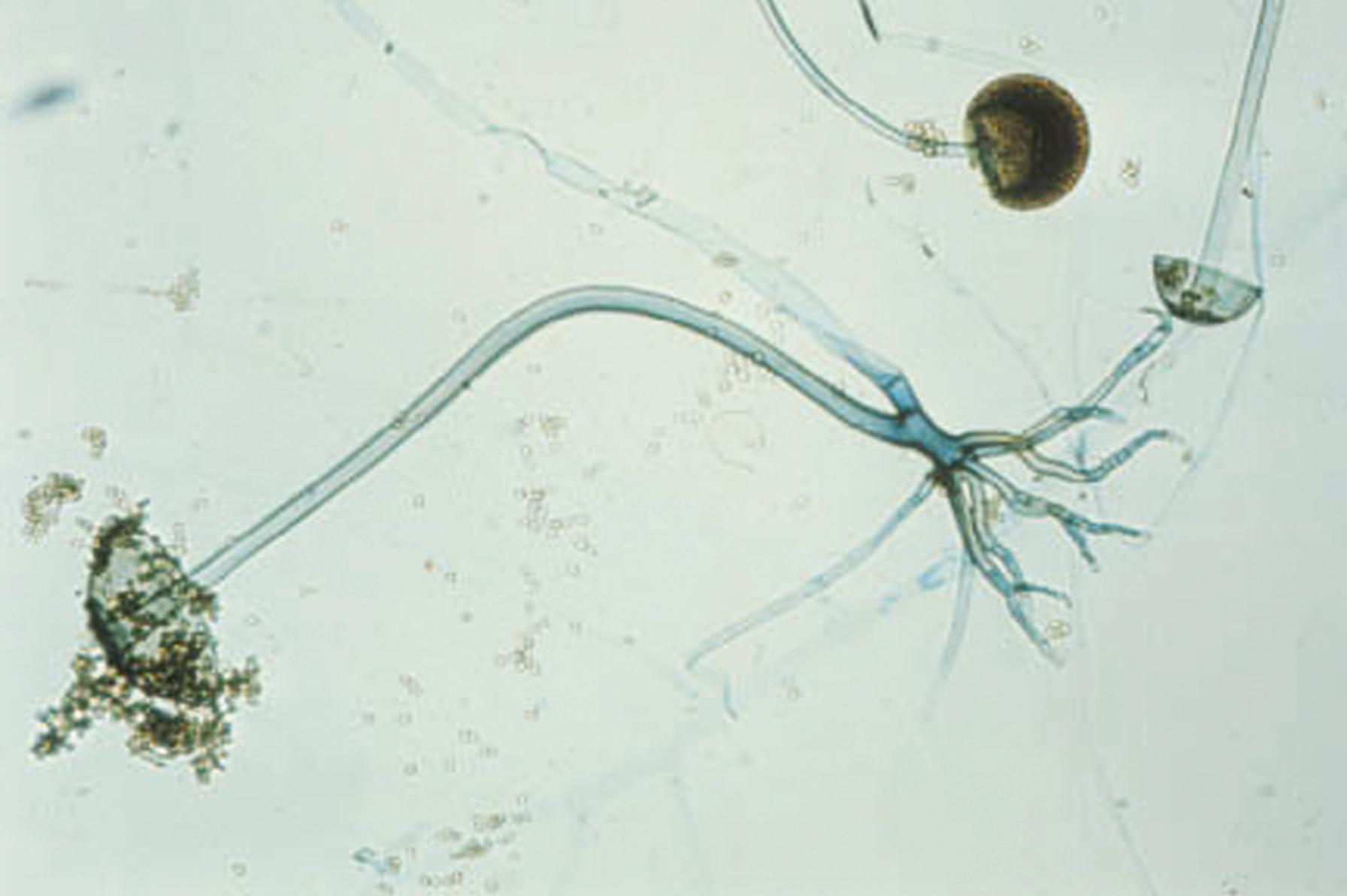
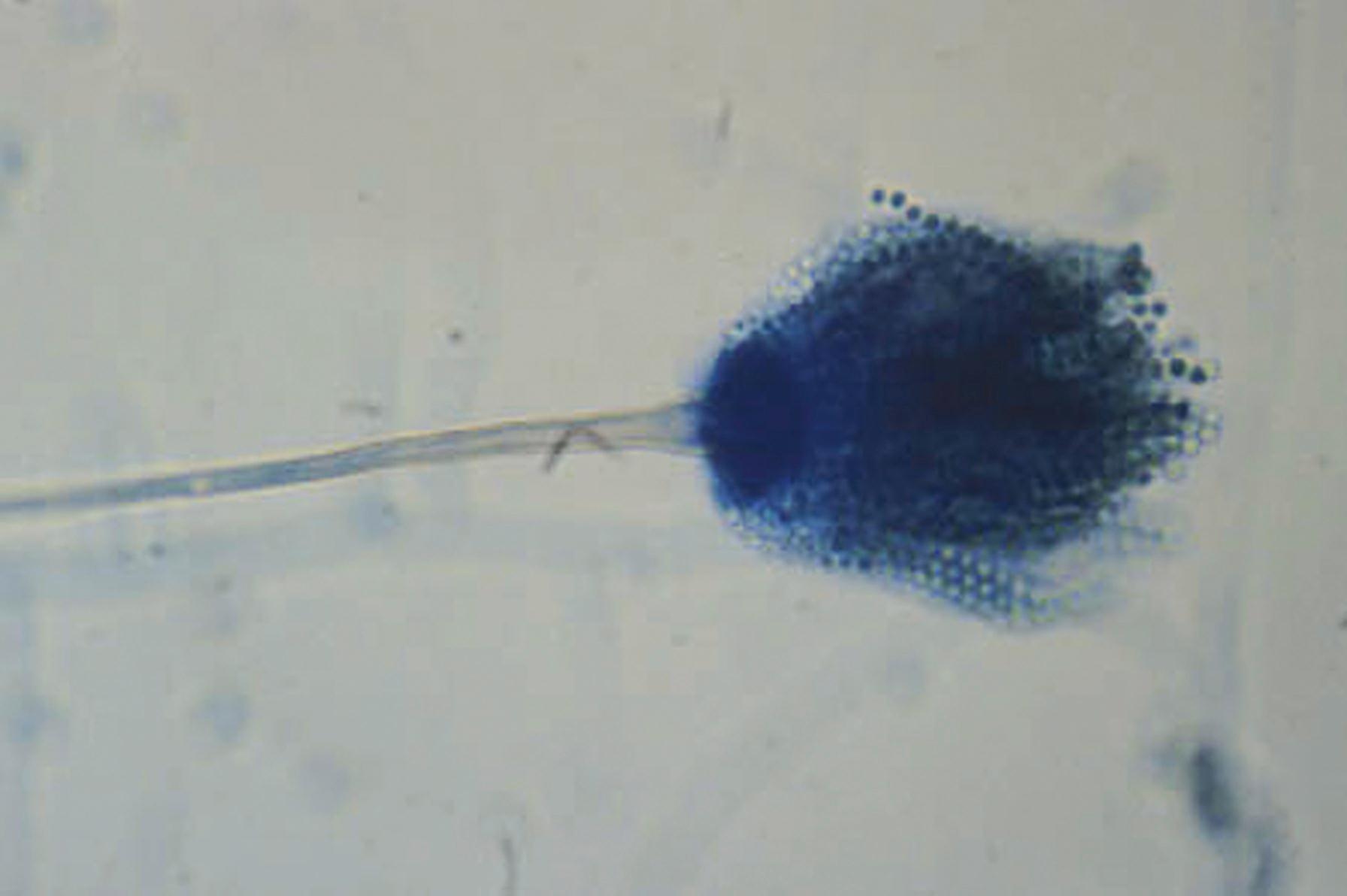
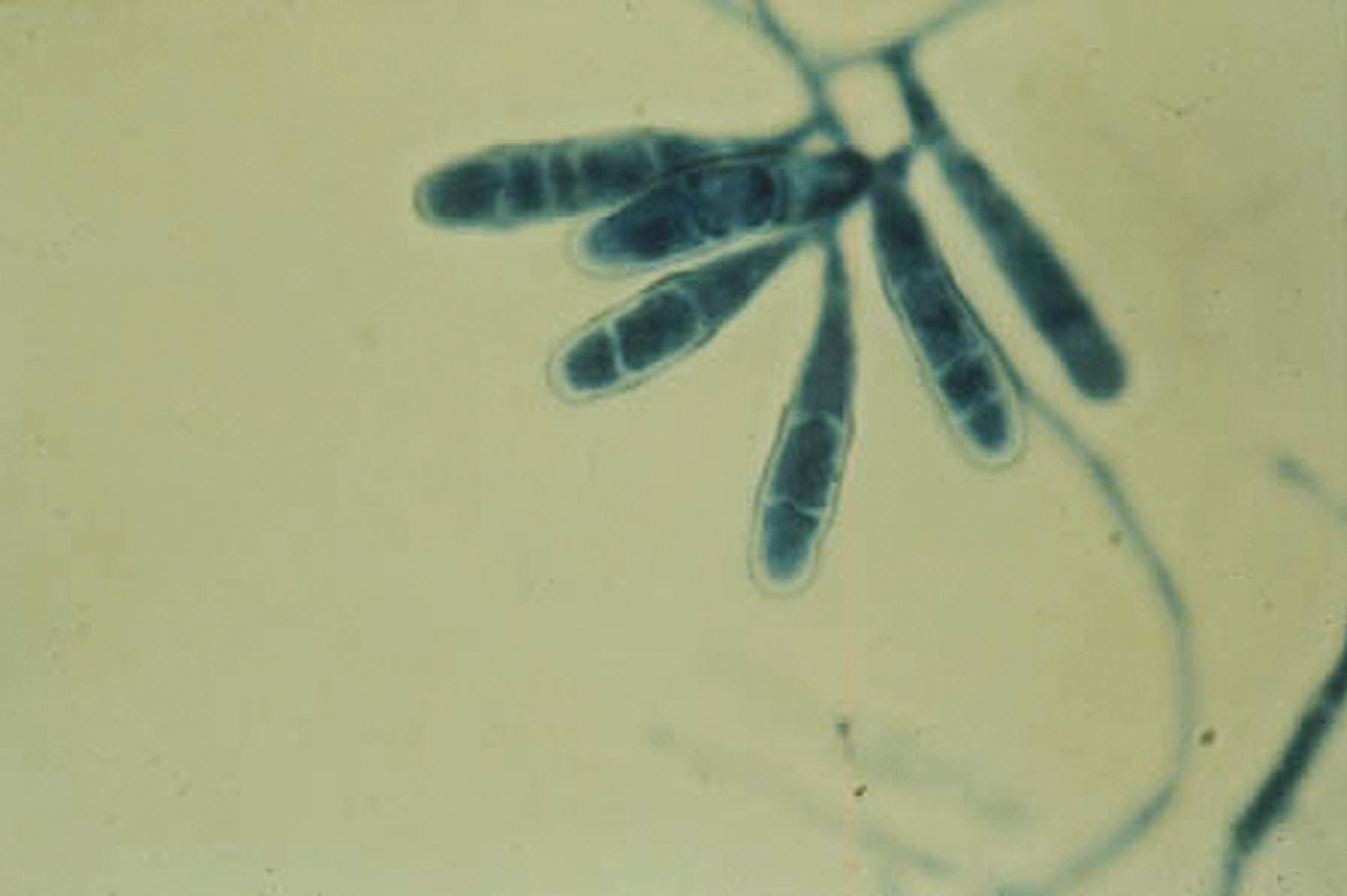
The portions of the vegetative mycelium that differentiate into conidia are referred to as conidiogenous cells . Specialized hyphae that support the conidia are termed conidiophores , which may be the conidiogenous cell itself arising from the vegetative mycelium or may be a supporting hypha. In Aspergillus spp., the conidiophore, which is aseptate, enlarges at the tip to form a swollen vesicle ( Fig. 60.8 ). Conidiogenous cells, now termed phialides , arise from the vesicle to support chains of conidia. Some species of Aspergillus produce a row of phialides, which occur on a row of sterile cells called metulae , with the conidia arising from the distal phialides. The mucormycetes produce structures called sporangiophores, which support the sporangium with enclosed sporangiospores (see Fig. 60.6 ). An extension of the apex of the sporangiophore into the sporangium is termed the columella .
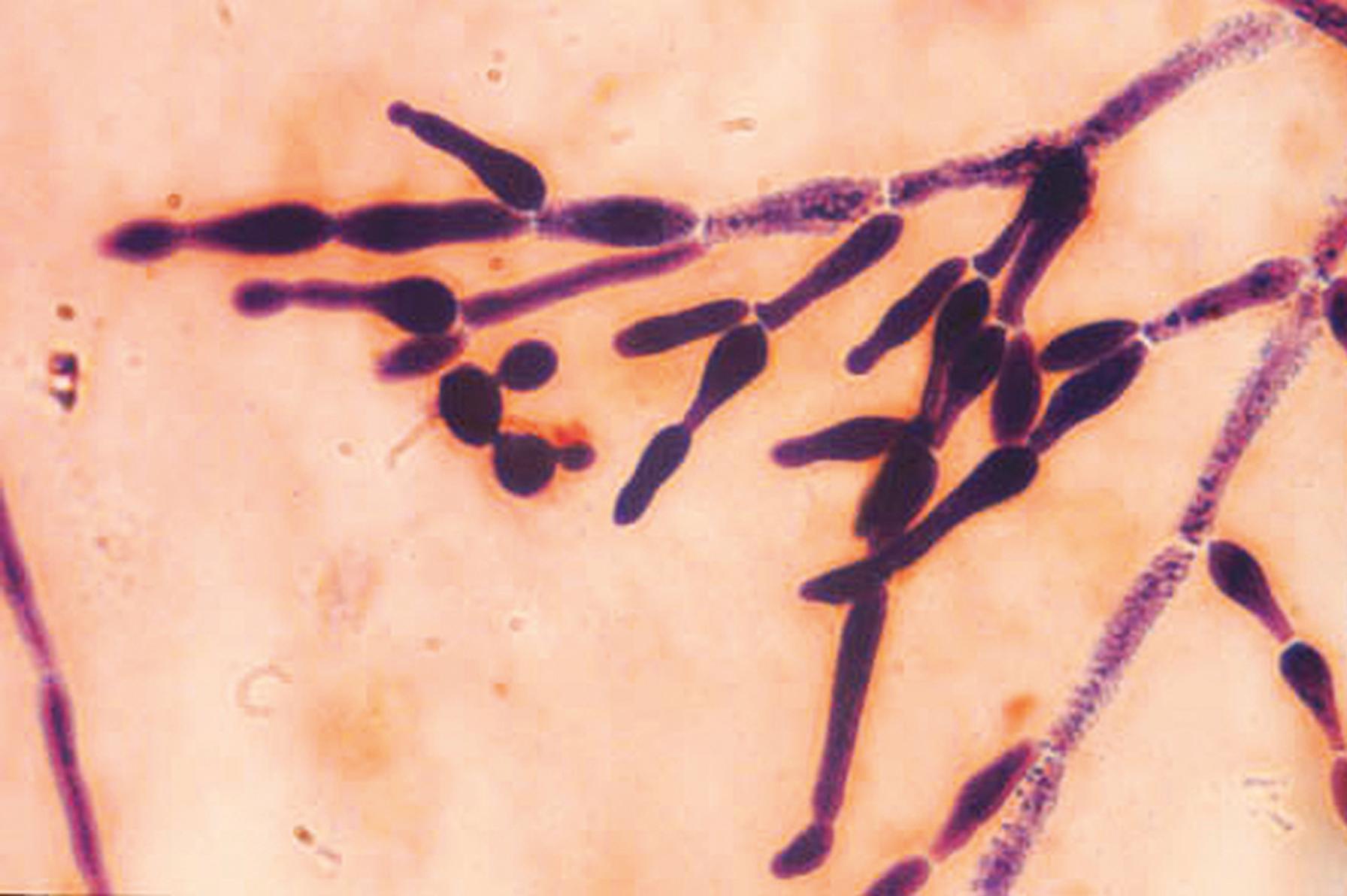
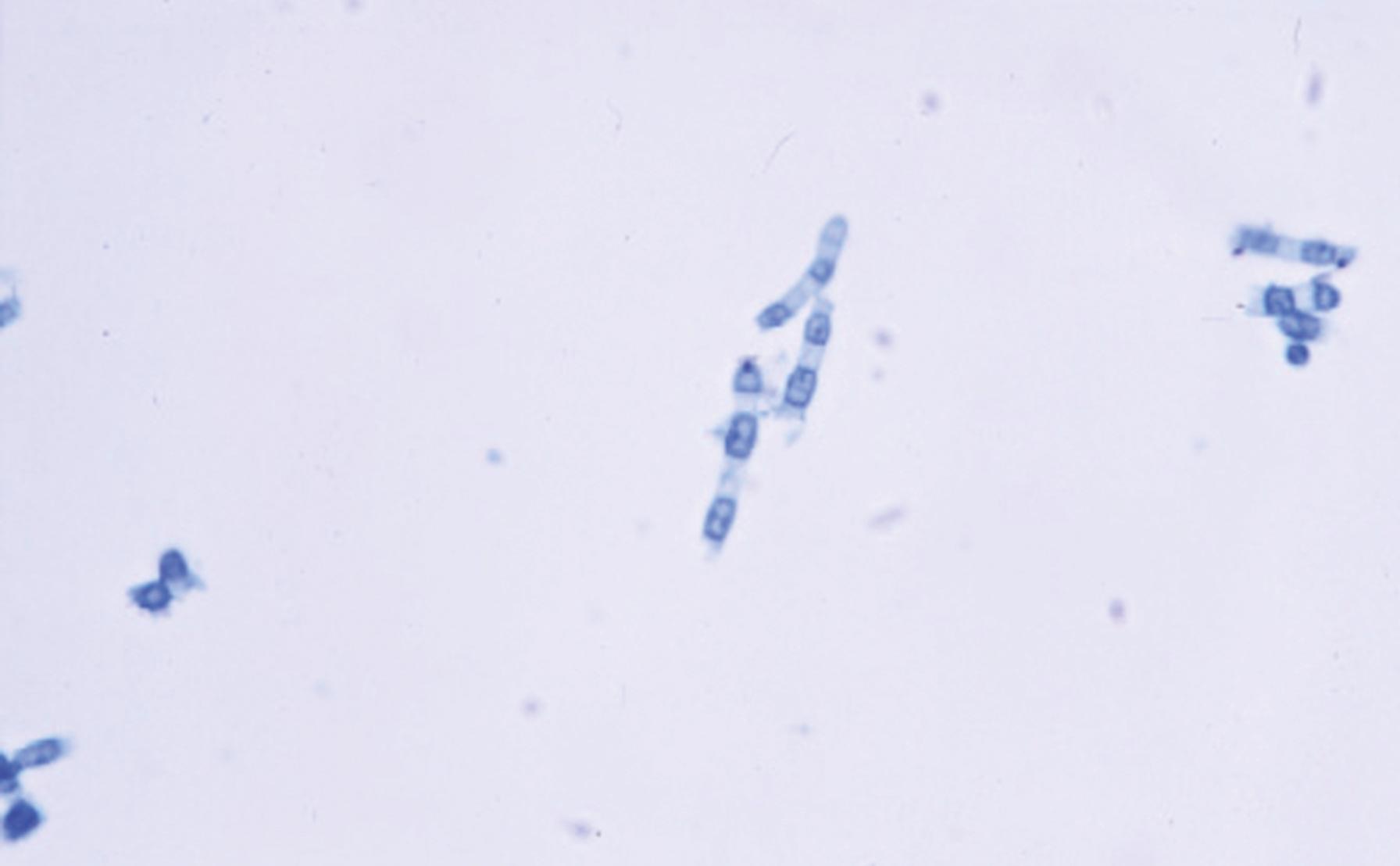
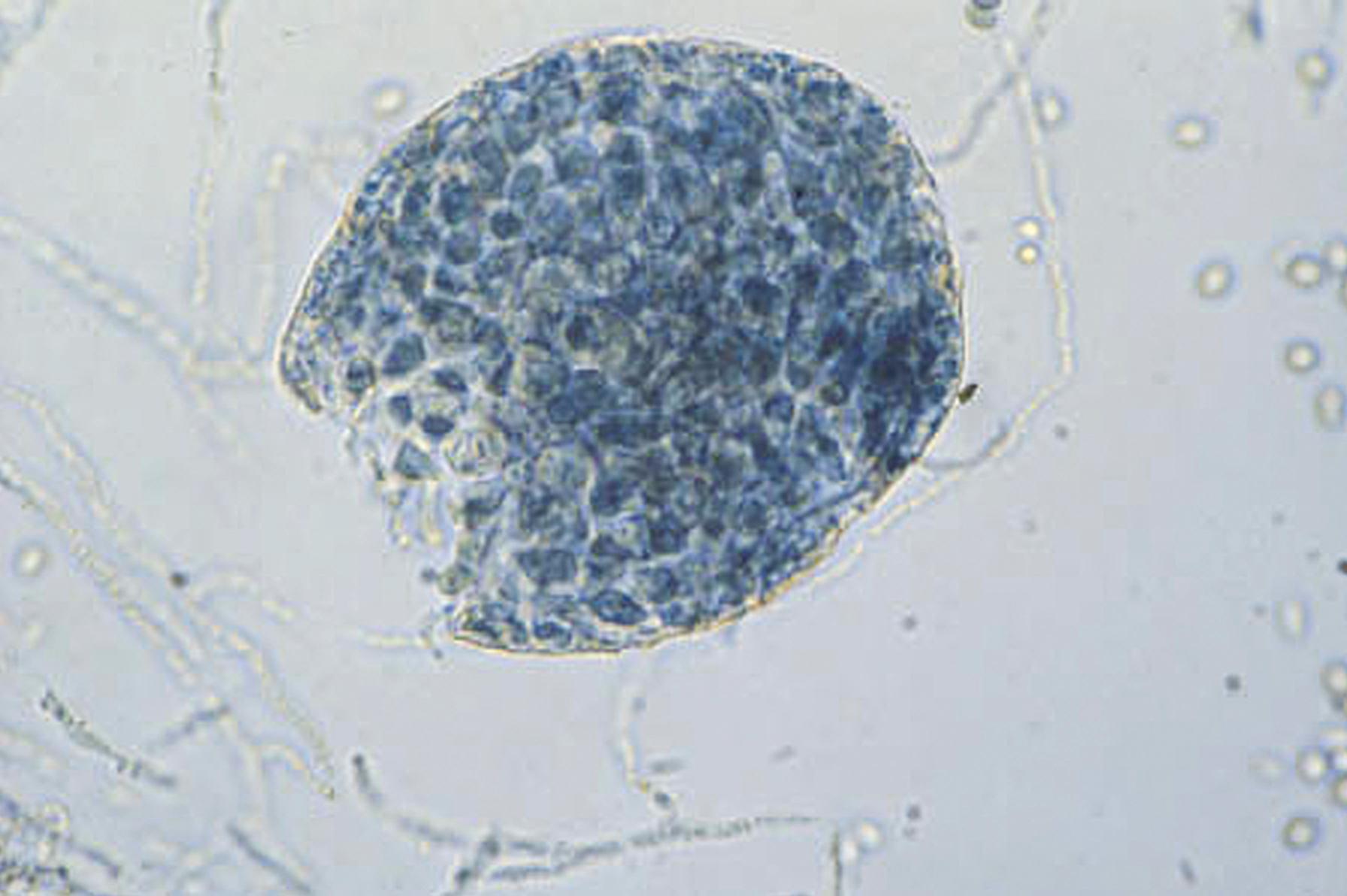
Thallic conidiogenesis is a process in which the conidium does not develop until a septum is formed between the conidium and the parent cell. The conidium originates from the whole of the parent cell. The most important human pathogens that exhibit thallic conidiogenesis are the dermatophytes and the dimorphic fungi in the genus Coccidioides . As conidiogenesis progresses in these species, barrel-shaped conidia called arthroconidia are produced; these fragment easily and are disseminated with little difficulty, resulting in a high degree of infectivity demonstrated by these important human pathogens ( Fig. 60.9 ). The thallic conidia of the dermatophytes are separated by size into two types: large septate macroconidia ( Fig. 60.10 ) and small, one-celled microconidia that are simpler structures ( Fig. 60.11 ).
The other type of conidiogenesis is called blastic conidiogenesis , whereby the protoplasm of the conidiogenous cell is blown out or blasted to form the conidium. The simplest form of blastic conidiogenesis is the budding process by which many types of yeast, including Candida spp., develop. As with thallic conidia, blastoconidia are divided into two types: one where the cell wall is involved in the process and the other where it is not. Further division of blastic conidiogenesis is made among those species in which the outer cell wall does not participate in the process (called enteroblastic conidiogenesis ). In enteroblastic fungi, phialidic and annellidic conidiogenous cells have been recognized. Phialides are conidiogenous cells that often have a collarette at the apices, produced when the tip releases the first conidium. The collarette may be a conspicuous flask-shaped structure, as observed in Phialophora spp., or an inconspicuous structure, as seen in Aspergillus spp. In contrast, annellides are conidiogenous cells that rupture to leave a distinct ring of cellular material at the base of the conidium when it separates from the annellide. Formation of sequential conidia at the base pushes the oldest cell to the tip of the chain and leaves a series of rings or annellations at the apex of the annellide, providing a record of past events, like rings on a tree. Although important for the microscopic identification of some mold fungi, the fine details of these structures may be difficult to visualize using the light microscope.
Differentiation of the various conidial structures is important for the correct identification of many fungal isolates. In some cases, the pertinent morphologic features are easily discerned; however, as in surgical pathology, pattern recognition is an essential tool for identification of the most common pathogens and saprobes. The pathologist should always appreciate the salient features of the entire morphologic preparation without primarily focusing on outliers or aberrant structures. Developing conidial structures of Aspergillus or Paecilomyces, for instance, may resemble the structures of Penicillium, and the observer must not be misled into thinking that two molds are present. At the same time, the possibility of a mixed culture must be remembered. Mycologists must use a low-powered objective for initial observation from fungal culture; however, the details of cellular subsets in that low-powered picture must be appreciated for study at a higher magnification.
The fine art of mycologic diagnosis rests in the appreciation and differentiation of cellular details, and in some instances, the task requires great subtlety and considerable experience. Sometimes, the isolate must be coaxed to produce the necessary diagnostic structures by selection of appropriate media ( Table 60.3 ). In general, enriched media favor the propagation of vegetative mycelium, whereas asexual reproductive structures are encouraged by basal (starving) media. Subculture onto water agar or potato flake agar is a general technique for encouraging the development of conidial structures. The colors of Aspergillus colonies are best studied on Czapek-Dox agar, whereas Trichophyton rubrum is encouraged to produce red pigment on potato dextrose or potato flake agar or on cornmeal agar with 1% glucose.
| Medium | Main Function |
|---|---|
| Ascospore medium, V-8 agar | Ascospore development |
| Birdseed (niger) agar | Demonstration of melanin in Cryptococcus neoformans |
| Brain-heart infusion agar with blood | Enhanced recovery of dimorphic fungi |
| Christensen urea agar | Differentiation of Trichophyton rubrum and T. mentagrophytes |
| CHROMagar Candida | Chromogenic mixture to identify Candida species |
| Cornmeal agar with Tween 80 | Visualization of yeast morphology |
| Czapek-Dox agar | Identification of Aspergillus species |
| Inhibitory mold agar | Selective medium that contains chloramphenicol and gentamicin |
| Potato dextrose agar or Potato flake agar | Pigment development of Trichophyton rubrum and mold morphology |
| Sabouraud dextrose (SAB) agar | General purpose fungal growth medium (traditional formulation or Emmon’s modification) |
| SAB agar with antibiotics | Selective medium to inhibit saprophytic fungi (antibacterials such as chloramphenicol or gentamicin also included) |
| Slide culture (various agars) | Study of microscopic structure |
| Trichophyton agars | Differentiation of Trichophyton species |
Experience also is important in determining whether a subculture has been incubated sufficiently long for diagnostic structures to form. Rootlike structures (rhizoids) that develop from the hyphae of some mucormycetes are important differentiating features, but they may not be detected if observation is terminated prematurely. Some of the techniques necessary for complete differentiation are difficult or even beyond the reach of most clinical laboratories. For instance, the melanized pathogen Exophiala dermatitidis produces blastic conidia with phialides and annellides. The presence of annellides was not recognized when the mold was studied with the light microscope, however, and this organism was once classified as Phialophora dermatitidis. Recognition that annellides were formed by the isolate led to a reassignment to a new genus, Exophiala, which contained both phialides and annellides.
Sexual reproductive structures are of occasional value for fungal identification in the general mycology laboratory. Although they are infrequently encountered in vitro, recognition of sexual structures for heterothallic fungi requires that a compatible mating strain be available for testing. Homothallic fungi, on the other hand, can produce sexual structures in culture without this mating process. The two structures most likely to be demonstrated in the laboratory from homothallic species are the naked asci of Saccharomyces cerevisiae and the cleistothecium of Scedosporium boydii (previously known as Pseudallescheria boydii and formerly considered to be the sexual phase of Scedosporium apiospermum but now recognized as a separate species) ( ), Aspergillus nidulans, or the Aspergillus glaucus group of fungi. The naked asci of Saccharomyces resemble oval yeast cells in which one to four individual haploid ascospores are enclosed ( Fig. 60.12 ). The ascospores in this species may be visualized in wet preparations but are detected more reliably using a Gram stain or an acid-fast stain. A cleistothecium is a sexual fruiting body (ascocarp) in which the ascospores are entirely enclosed and can be released only by rupture of the cleistothecial wall ( Fig. 60.13 ). The wall is composed of single or multiple layers of specialized hyphae. A perithecium is a sexual structure similar to a cleistothecium, but it contains an opening at the apex of the pear-shaped structure.
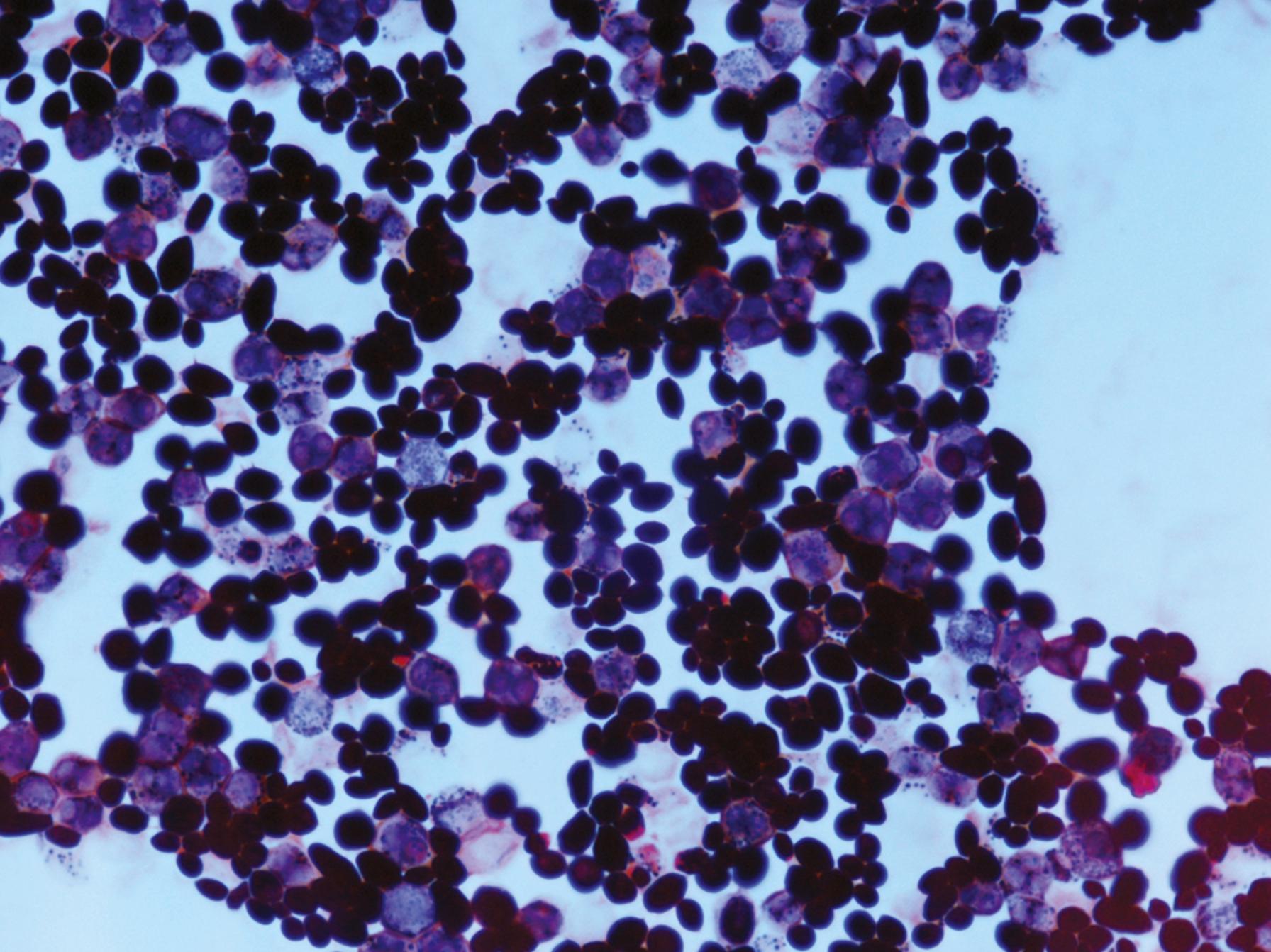
Although asexual and rarely sexual reproductive structures are necessary for the identification of fungi, especially molds, some isolates refuse to produce either of these structures for diagnosis. The colonies consist only of vegetative and aerial hyphae morphologically and subsequently are described as sterile hyphae or mycelia sterilia . Identification under this circumstance requires molecular or proteomic methods that may not be readily available in most routine diagnostic laboratories ( ; ; ).
Demonstration of dimorphism is the traditional approach to definitive identification of endemic fungal pathogens. In most laboratories, the initial isolate is the mold phase because culture plates usually are incubated at 25° to 30°C. Conversion to the tissue phase (yeast or yeastlike structure) is accomplished by incubating a subculture at 37°C. The transition from mold to yeast morphology may occur grudgingly, and hyphal forms are often intermixed with yeast cells. Some isolates may never be successfully converted. A rich medium, such as brain-heart infusion agar with a blood supplement, should be included with the primary isolation medium, particularly when H. capsulatum is suspected . Cysteine hemoglobin agar is a common medium used for the conversion of B. dermatitidis. Media for conversion of Coccidioides spp. have been described but are not often used. Similarly, inoculation of animals is infrequently employed in clinical laboratories, although this method is effective when used for conversion to the in vivo phase. The introduction of molecular probes has considerably simplified the task of definitive identification for these endemic fungi and has eliminated the need to demonstrate both phases of the dimorphic fungi in the laboratory ( ). Rapid deoxyribonucleic acid (DNA) probe tests, which utilize the technique of nucleic acid hybridization, are available for the identification of the three major dimorphic pathogens from culture (e.g., GenProbe AccuProbe, Hologic, Inc., San Diego, CA). Although demonstration of the characteristic yeast or yeastlike phase in tissue strongly supports the diagnosis of a dimorphic fungus, identification of the pathogen in culture is still necessary for disease confirmation ( ).
With the increased number of patients undergoing transplantation procedures or receiving aggressive immunosuppression and chemotherapy, the growing population of immunosuppressed hosts has dramatically increased, leading to a subsequent rise in the prevalence of invasive fungal infection ( ). Early recognition of a fungal infection to optimize patient management is becoming more realistic with advances in diagnostic applications for the identification of fungal etiologic agents.
Biosafety considerations from specimen collection through culture conformation in the mycology laboratory are critical (Clinical and Laboratory Standards Institute [ ). Inoculation of specimens and manipulation of mold colonies should always be performed in a biologic safety cabinet to prevent dissemination of the highly mobile fungal conidia. Scrupulous attention to cleaning the surfaces of both the biologic safety cabinet and the incubator should be maintained. Separate safety cabinets for the study of isolated molds may minimize contamination of other clinical samples.
The greatest hazard for laboratory personnel comes from handling mold cultures of the dimorphic pathogens, Coccidioides immitis/posadasii and H. capsulatum . These isolates are classified as risk group 3 (RG3) pathogens, and biosafety level 3 practices, containment equipment, and facilities are highly recommended for propagating and manipulating cultures, as well as for processing soil or other environmental materials known or likely to contain infectious conidia from these agents ( , , ). Screw-capped tubes of culture media should be used for culture if these pathogens are suspected. Unfortunately, the diagnosis may not be appreciated before the pathogen is isolated in some cases. All work with molds, however, should be performed in a certified biologic safety cabinet.
Of special note is that the tissue phase of dimorphic pathogens is not infectious by the airborne route, thus little or no biohazard is involved in handling tissue specimens from patients with blastomycosis, histoplasmosis, or coccidioidomycosis. A possible exception to this rule might occur if a Coccidioides spp. grew as a mold within a lung cavity that connected with the bronchial tree, which would allow for in vivo sporulation.
The correct specimen for submission to the mycology laboratory depends on the clinical presentation and the organ system affected ( ). A summary of the major categories of diseases produced by fungi along with suggested specimens is presented in Table 60.4 .
| Clinical Presentation/Organ System | Patient Group | Likely Pathogens | Specimen(s) |
|---|---|---|---|
| Skin, hair, or nail infection | All | Dermatophytes Candida Malassezia |
Skin scraping Hair Nail clipping |
| Subcutaneous tissue infection | All | Coccidioides Blastomyces dermatitidis Sporothrix schenckii |
Lesion biopsy |
| Patient with mycetoma | Scedosporium apiospermum Madurella Actinomadura |
||
| Primary pneumonia | All | Histoplasma capsulatum Blastomyces dermatitidis Cryptococcus neoformans Coccidioides |
Sputum Bronchoalveolar lavage Transbronchial biopsy Surgical lung biopsy Pleural fluid Thoracentesis fluid |
| Immunodeficient | Aspergillus Fusarium Mucormycetes/Zygomycetes (Mucorales) Scedosporium Pneumocystis jirovecii |
||
| Gastrointestinal infection | Immunodeficient | Candida Aspergillus Mucormycetes/Zygomycetes (Mucorales) |
Scrapings Curettings Biopsy |
| Urinary tract infection | All | Candida | Urine |
| Endocarditis | All | Candida Fusarium |
Blood |
| Meningitis | Immunodeficient | Cryptococcus neoformans/C. gattii Candida Coccidioides |
Cerebrospinal fluid |
| Encephalitis and brain abscess | Immunodeficient | Mucormycetes/Zygomycetes (Mucorales) Aspergillus Cladophialophora bantiana |
Brain biopsy |
| Osteomyelitis | All | Coccidioides Blastomyces dermatitidis Cryptococcus |
Biopsy |
| Keratitis | All | Aspergillus Fusarium |
Scraping |
| External otitis | All | Aspergillus | Scraping Swab |
| Sinusitis | All | Aspergillus Fusarium Dematiaceous molds |
Biopsy Curettings |
| Burn wounds | All | Aspergillus Mucormycetes/Zygomycetes (Mucorales) Fusarium Scedosporium |
Biopsy |
| Vaginitis | All | Candida | Vaginal secretions |
| Intravenous catheter infection | Indwelling catheter | Candida Malassezia (newborns) |
Catheter tip Blood |
| Disseminated infection | All | Histoplasma capsulatum Blastomyces Coccidioides |
Blood, bone marrow Tissue biopsy |
| Immunodeficient | Candida Mucormycetes/Zygomycetes (Mucorales) Aspergillus Fusarium Scedosporium Cryptococcus Potentially any fungus |
Blood Tissue biopsy |
Yeast, particularly Candida spp., may be recovered on occasion from swab specimens, especially from purulent lesions. Flocked swabs may be used for sampling oral or vaginal lesions suggestive of candidiasis and may be adequate for collecting specimens from patients with chronic external otitis, in which large numbers of Aspergillus conidia are usually present. However, swabs are decidedly inferior for collecting specimens in most areas of the infectious process. Particularly problematic is that swab fibers may be mistaken for hyphal elements upon direct examination of clinical specimens. A policy of rejecting swabs for fungal culture should be accompanied in most cases by assiduous and persistent educational efforts.
The best specimens for mycologic diagnosis are scrapings, curettings, aspirates, and lesion biopsies. Hairs infected with a dermatophytic fungus (e.g., Microsporum canis ) fluoresce under a long-wavelength ultraviolet light (Wood’s lamp) and can be specifically selected for examination. Cutaneous lesions of dermatophytosis (ringworm) are characterized by an active advancing edge with central healing, so that scrapings can be collected from the edge of the process. Hair, nails, scalp, and skin scrapings should be sent to the laboratory in a clean, dry container. Tissue samples should be sent in a sterile container with a small amount of sterile, preservative-free saline or a transport medium to prevent drying of the sample. Most samples can be handled as one would any other samples submitted for bacterial observation and culture.
Most clinical specimens can be examined directly for the presence of a fungal pathogen, although negative results are not sensitive enough to rule out the presence of a fungus. Multiple techniques are employed for the direct examination of clinical material. As a reminder, multiple manipulations of specimen material in the laboratory may render the material not acceptable for culture, so in cases in which culture is warranted, specimens must be separated before this type of examination is performed.
The simplest method for direct examination of a specimen is to observe a suspension placed on a slide and coverslip under reduced light. For those specimens that are dry or viscous, the addition of a wetting agent such as saline is necessary prior to observation. For specimens that contain distracting tissue debris and cells, such as vaginal secretions, nails, and skin scrapings, a solution of potassium hydroxide (KOH) may be used as a means to dissolve the tissue material, so that fungal elements are more visible ( Box 60.1 ). Some preparations also contain dimethyl sulfoxide (DMSO), which facilitates more rapid breakdown of keratinized tissue, and chlorazole, a lipophilic dye that is attracted to and stains fungal elements an iridescent green-black (i.e., Chlorazole Black E) ( ).
Place 1 drop of 10% potassium hydroxide (KOH) on a clean glass slide.
Immerse an aliquot of the specimen in the drop of KOH on the slide.
Gently heat the mixture by passing the slide through a Bunsen burner flame several times. Do not allow the mixture to boil.
Coverslip the slide and examine at 100× and 400× magnification using reduced light. Excessively tenacious specimens may require additional heating.
KOH may also be used as a simple mounting medium without heating (the addition of Lactophenol [aniline] blue or Calcofluor white stain with the KOH will enhance the visibility of fungal elements).
This commonly used microbiologic stain is particularly useful for the detection of yeast. Most yeasts stain partially or completely gram positive and are generally differentiated from bacteria by their larger size and by the presence of budding cells (see Fig. 60.5 ). The hyphae of molds may appear gram positive or gram negative by this stain but may stain less reliably than yeast cells and are easily missed in clinical specimens using this method.
In cases in which histoplasmosis is suspected, these hematologic stains are useful for demonstrating yeast cells within macrophages.
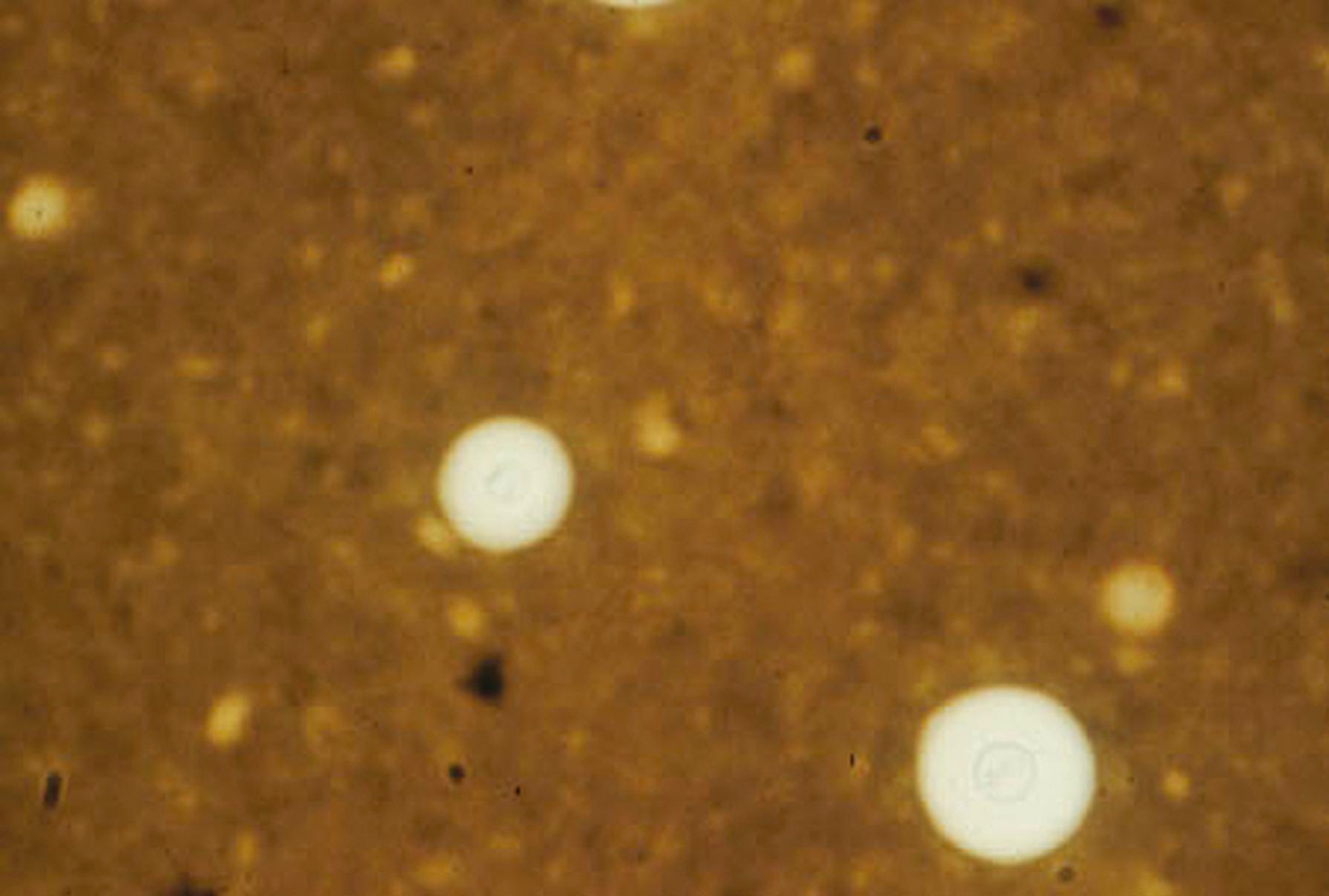
The polysaccharide capsule of species within the Cryptococcus neoformans/C. gattii species complex can be demonstrated by negative staining using India ink particles; it is especially evident when infected cerebrospinal fluid (CSF) is examined. When examined with the light microscope, the capsule stands out as a clear space around the fungal cell, with ink particles bouncing off the edge as brownian motion occurs ( Fig. 60.14 ). When budding yeast cells are demonstrated along with the capsule in the CSF of an infected patient, the specificity of the diagnosis of cryptococcal meningitis is ensured. Other potentially encapsulated fungi, such as Rhodotorula spp., Exophiala spinifera , or other cryptococcal species are infrequent pathogens and can be eliminated from practical consideration under this circumstance. Although the capsules usually are large in direct examination of specimens, isolation of the pathogen on agar media is usually characterized by the production of a much-diminished capsule, which is not as obvious. Unfortunately, some C. neoformans strains are poorly encapsulated; thus the sensitivity of the India ink test is generally less than 50%, and the organism is especially evident in patients who are not infected with the human immunodeficiency virus (HIV). However, direct detection of antigen in CSF or serum using a latex agglutination or enzyme immunoassay technique has increased the sensitivity to close to 100%. These techniques basically have replaced the use of India ink as a direct diagnostic technique, although the India ink technique is still useful when the antigen test is not immediately available and for examination of capsules in cells from isolated colonies that suggest C. neoformans/C. gattii species complex.
Surgical pathologic examination of biopsy material is a common practice for the identification of suspected fungal infection ( ). A number of stains have proved useful in recognizing fungal elements in tissue and in showing the immunologic response to infection. The periodic acid–Schiff (PAS) method is useful for demonstrating internal details. Gomori’s methenamine silver (GMS) technique is considered one of the better stains for demonstrating fungi because it provides high contrast with minimal background staining, thus allowing for the demonstration of sparsely present fungal elements in the sample. The hematoxylin and eosin (H&E) stain is best used for studying the host reaction and for determining whether a fungus is hyaline (colorless) or dematiaceous (naturally pigmented). Other more specialized stains include Mayer’s mucicarmine stain for demonstration of the mucoid capsule of C. neoformans and the Fontana-Masson stain for demonstration of melanin or melanin-like substances in the lightly pigmented agents of phaeohyphomycosis as well as the staining of minute amounts of melanin pigment in the cell wall of C. neoformans/C. gattii species complex. A book by provides greater detail on the utilization of stains to detect fungi in tissue.
This fluorochrome compound, a whitener used in the textile and paper industries, binds to the chitin in the walls of fungal cells and fluoresces white ( Fig. 60.15 ) or apple green (depending on the filter combination used) when exposed to short-wavelength ultraviolet light from a fluorescence microscope ( Box 60.2 ) ( ). Calcofluor white can also be mixed with potassium hydroxide to clear the specimen for easier observation of fungi. As is true with all fluorescent staining, care must be used in interpreting the results because nonspecific staining of substances that resemble fungal elements may also occur.
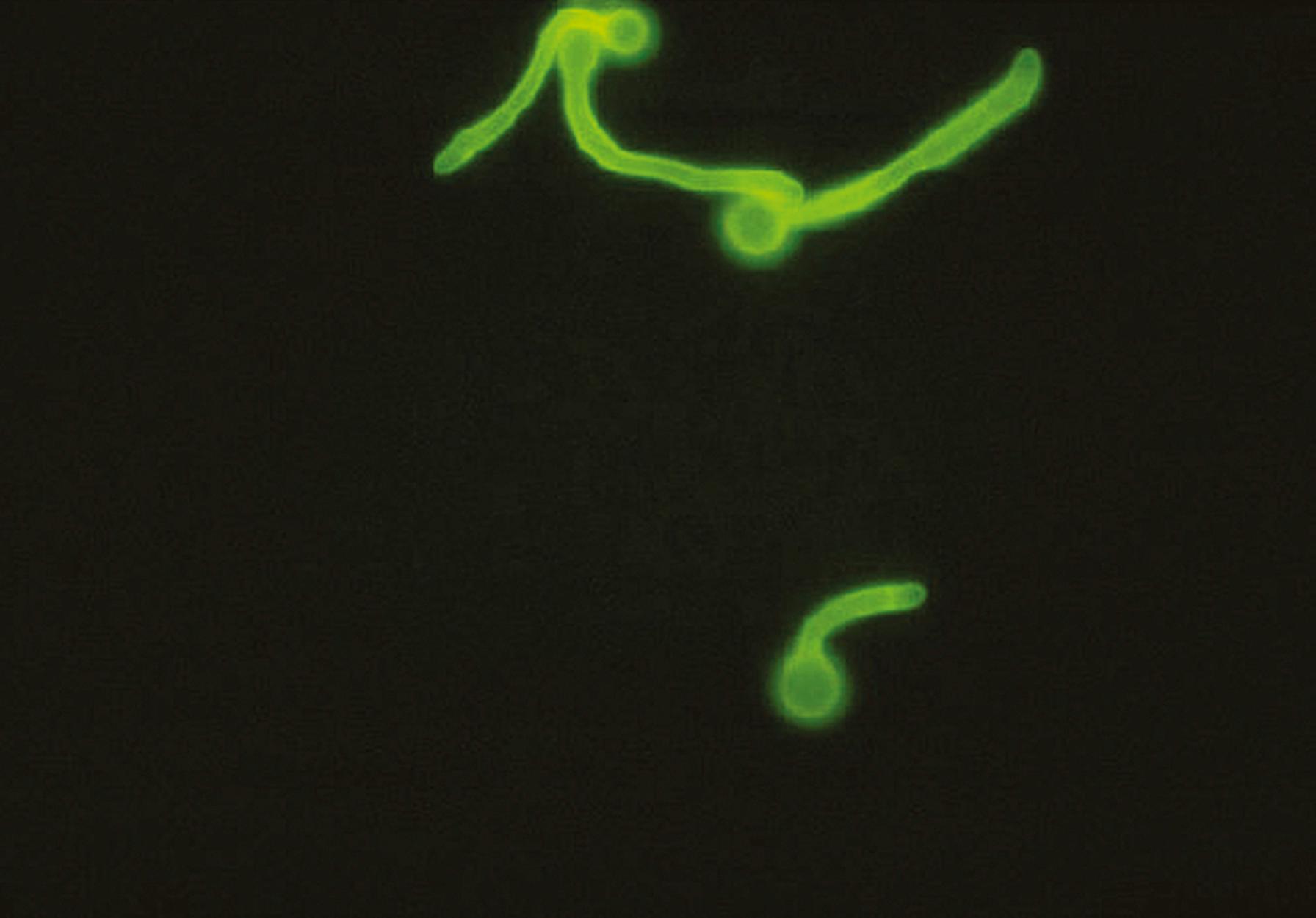
Place specimen on a clean glass slide.
Mix with Calcofluor white reagent.
Using a fluorescence microscope, examine the preparation for fungal elements at 100× to 400× magnification (will typically appear bluish white against a dark background). (Calcofluor white may be substituted for Lactophenol [aniline] blue stain when microscopic morphology of culture isolates is studied.)
Immunohistochemistry has been used to identify a wide variety of infectious agents, including the fungi, in fresh, frozen, or paraffin-embedded tissues ( ). Although many fungi can be readily identified by standard histologic staining, some fungal elements appear atypical in tissue sections and in some cases cannot be distinguished morphologically. Monoclonal and polyclonal antibodies for Aspergillus spp. and Candida albicans have been previously available (AbD Serotec, Bio-Rad Laboratories, Inc, Hercules, CA); however, these are not US Food and Drug Administration (FDA) approved and would require in-house validation testing prior to patient use. Cross-reactivity with Fusarium spp. has been found using immunohistochemicals to detect Aspergillus spp. ( ).
All specimens should be inoculated onto a general-purpose fungal growth medium (see Table 60.3 ). The medium used traditionally is Sabouraud dextrose agar, which has a pH of 5.5 to 5.6 and was designed for the isolation of dermatophytic fungi. Emmons modification of Sabouraud dextrose agar, which contains less glucose with a pH of 6.8 to 7.0, is more widely used in the clinical mycology laboratory as a general growth medium because of its wide application for the culture of fungi. Inhibitory mold agar and potato dextrose agar or potato flake agar are also utilized as general isolation media in the laboratory.
For specimens from nonsterile sites or from sites that are likely to be contaminated with other microbial flora, inoculation of a nonselective agar and a selective agar in tandem is recommended. The most commonly used selective agar employs cycloheximide (400 to 500 mg/L) to inhibit saprobic fungi and an antibacterial agent (usually chloramphenicol [50 mg/L] or gentamicin [50 mg/L]) to inhibit bacteria. For tissue specimens, especially when dimorphic fungi might be the etiologic agent, an enriched agar, such as brain-heart infusion agar with 5% to 10% sheep blood and supplemented with chloramphenicol and gentamicin or SABHI agar with antibiotics, may be added.
For the cultivation of yeast from clinical specimens the selective and differential medium called CHROMagar Candida (CHROMagar Company, Paris, France) may be used for the isolation and differentiation of major clinically significant Candida spp. This medium uses a chromogenic mixture to differentiate major Candida spp. by colony color: C. albicans , green; Candida krusei , pink; Candida tropicalis , metallic blue; and other species, white to mauve. Numerous reports have shown this medium to be useful for the isolation and presumptive identification of yeasts and to optimize detection of mixed cultures in clinical specimens ( ; ; ).
Processing of clinical specimens for fungal detection requires careful handling because some processes commonly used to prepare specimens for bacterial culture may be too disruptive for the fungal organism (e.g., grinding in a homogenizer). Inoculation of scrapings or curettings onto and into the agar at multiple points on the agar surface is a reliable technique for culture. Tissue should be teased or minced, after which the fragments are similarly inoculated.
Plates or tubes should be incubated in ambient air at 25° to 30°C. A controlled temperature incubator is essential. Although it is not necessary to incubate parallel plates or tubes at 37°C for recovery of the yeast phase of dimorphic fungi, in cases where strong suspicion of endemic fungi occurs, incubation at this temperature should be considered. Most fungi grow within a 2-week incubation period, and among commonly isolated fungi, only the dimorphic fungi such as H. capsulatum and B. dermatitidis are frequently detected after 14 days of incubation. General recommendations for incubation times are as follows: 7 days to detect the presence of yeast in mouth, throat, or vaginal specimens; 21 days to detect fungal pathogens in tissues and sterile body fluids other than blood; and up to 28 days for respiratory, bone marrow, and blood pathogens, and for specimens in which a dimorphic fungus is suspected. In general, all other specimens should be incubated for up to 14 days. Plates should be examined at least twice during the first week, when rapidly growing isolates may appear, and at least weekly thereafter.
Recovery of fungi from blood requires special attention and can be accomplished by inoculating broth media by using a biphasic broth-agar system or by using the lysis centrifugation technique (Isolator System; Wampole, Cranbury, NJ). The lysis centrifugation method consists of a tube containing a solution that lyses erythrocytes and leukocytes ( ; ). After the blood is drawn into the vacutainer tube, the lysed contents, including any microbes, are centrifuged into a pellet, which is removed and plated onto the surface of agar plates. Although the Isolator System is considered a sensitive method for detection of fungemia, this system is also more susceptible to contamination through handling of the tube or by airborne spores that settle onto the agar plates during processing. Because a high percentage of fungemias are caused by yeast, continuous-read automated-culture methods have become the mainstay for fungal detection. Two systems commonly used are the BACTEC System (Becton Dickinson Diagnostic Systems, Sparks, MD) and the BacT-Alert System (bioMerieux, Inc., Durham, NC). Because yeasts are aerobic organisms, aerobic culture bottles are used for detection. Two systems in combination (i.e., BACTEC MYCO/F Lytic blood culture bottles and the Isolator System) have been shown to optimize the recovery of fungi from blood ( ).
The rate of growth of fungal isolates provides a useful clue to diagnostic possibilities. In general, the pathogenic dimorphic and dematiaceous fungi grow slower, requiring 1 week or longer for colonies to appear. Rapidly growing mold colonies that appear after prolonged incubation on mycologic media should be questioned as possible contaminants. However, many exceptions to this generalization have been noted, and laboratorians need to be aware that fungi such as the Coccidioides spp . may grow quickly on nonfungal medium. Protocols on how to handle fungal growth on bacterial cultures are needed to prevent unnecessary exposure of laboratory personnel to highly infectious fungal pathogens that may be encountered.
Most molds grow best at 25° to 30°C. Most yeasts, however, grow well at 35° to 37°C and are often recovered first on enriched blood agar plates in the bacteriology laboratory. In some cases, growth of a fungus at elevated temperatures is useful for differentiation of species.
Some of the morphologic studies necessary for the identification of fungal isolates can be accomplished with colonies from the original isolation plates. However, it is often necessary to transfer portions of colonies to new or different media (subculture) to demonstrate diagnostic structures. Table 60.3 describes some of the media commonly used for the identification of fungal species. One of the media more commonly used is cornmeal agar supplemented with Tween 80 (CM-T80) for the visualization of yeast morphology. Recognition of pseudohyphae with chlamydoconidia on this medium is considered confirmatory for C. albicans and Candida dubliniensis . Other morphologic features can help to categorize yeasts into groups to facilitate separation into species. Although biochemical tests are important for identifying yeast isolates, complementation of these with a morphologic examination provides an important check on the biochemical information, especially if a commercial identification system has been used.
The germ tube test is an important initial step in the identification of yeast isolates. Germ tubes, which are elongated, fingerlike extensions from a yeast cell, represent the beginnings of a true hypha ( Fig. 60.16 ). This structure can be differentiated from pseudohyphae by the lack of a constriction at the junction of germ tube and yeast cell and by the parallel cell walls in the germ tube. True germ tubes are formed by both C. albicans and C. dubliniensis after growth in serum at 37°C for up to 4 hours. In many laboratories with well-trained staff, the germ tube in combination with results of morphology on CM-T80 agar is considered confirmatory for the identification of C. albicans/C. dubliniensis .
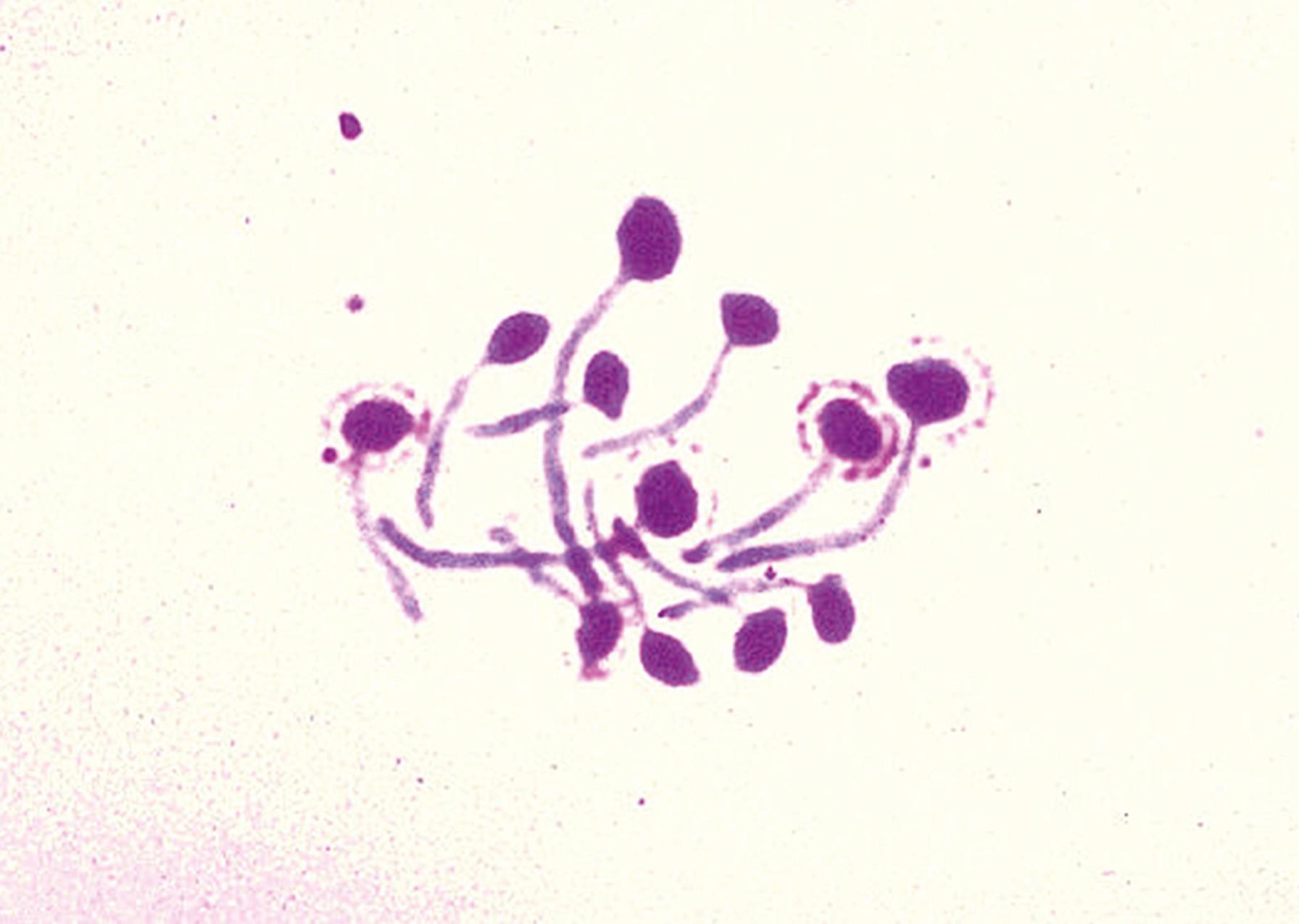
The traditional germ tube test involves inoculation of a tube of serum ( Box 60.3 ). After observation of the isolate for germ tubes at 37°C, incubation is continued for subsequent study of hyphal morphology and chlamydoconidia formation at 25° to 30°C. Thus all the information necessary for rapid identification can be collected in one procedure. The CM-T80 agar may be substituted, but regardless of the medium used, incubation conditions must be carefully controlled and the test monitored with controls to achieve good results. The traditional Dalmau technique for demonstration of chlamydoconidia on cornmeal agar is detailed in Box 60.4 .
Aseptically transfer several colonies of yeast to a 12-mm × 75-mm test tube containing approximately 0.5 mL of serum (human, fetal calf, bovine, or rabbit).
Incubate the tube at 35°C for up to 3 hours.
Place 1 drop of the mixture on a clean glass slide and coverslip.
Examine under high dry (400×) magnification and reduced light for the presence of germ tubes.
Streak a light inoculum of yeast onto a section of a cornmeal agar containing Tween 80.
Coverslip the area inoculated.
Incubate at 25° to 30°C for 24 to 72 hours.
Observe under 100× and 400× magnification using reduced light for morphologic structures.
A simple method for examination of molds is the cellophane tape mount, using clear tape and staining with lactophenol cotton (aniline) blue (LPCB) stain. If diagnostic structures are not observed, incubation can be continued and the process repeated. The established method used in observing mold morphology is to tease the mycelium apart with inoculating needles and examine the teased hyphae with LPCB stain.
Occasionally, a slide culture may be necessary to preserve easily disrupted conidial structures in their original relationships. The classic approach involves cutting a square of an appropriate agar medium (usually Sabouraud dextrose, potato dextrose, or potato flake agar), which is suspended on a glass slide and overlaid with a coverslip. The slide is supported by glass rods in a Petri dish, to which sterile water is added for maintenance of humidity. The coverslip subsequently can be removed after a few days of incubation, placed in a drop of LPCB, and observed for undisturbed reproductive structures.
When a mold isolate is suspected of being a dimorphic fungus (e.g., growth on cycloheximide-containing medium), a slide culture should not be performed, and a cellophane tape test or teased preparation should be examined only after the preparation has been sealed in a biosafety cabinet certified for use. Lactophenol cotton blue is fungicidal, but sealing the coverslip with nail polish before observation provides additional protection. Alternatively, the culture may be flooded with 10% formalin (4% formaldehyde solution) and incubated at room temperature overnight before the mold is manipulated.
Biochemical tests are at the core of identification schemes for yeast and occasionally are useful for identification of molds. A number of rapid tests for the presumptive identification of yeasts are described in Table 60.5 .
| Urease production | Cryptococcus gattii/C. neoformans |
| Germ tube production | Candida albicans / C . dubliniensis |
| Pseudohyphae present | Candida species |
| Chlamydoconidia present | Candida albicans/C. dubliniensis |
| Lipid growth requirement | Malassezia furfur species complex |
| Red colonial pigmentation | Rhodotorula species |
| Ascospore formation | Saccharomyces cerevisiae |
| Trehalose assimilation | Candida glabrata |
Biochemical characterization of yeasts may be accomplished by study of fermentation or assimilation patterns. Assimilation testing, which is used more extensively in the laboratory, assesses the ability of an isolate to use a carbohydrate as the sole source of carbon needed for growth, or of nitrate as the sole source of nitrogen. Numerous commercial identification systems with varying incubation times from 4 to 72 hours are available and have become the mainstay for yeast identification. These systems include the API 20C AUX System and the VITEK 2 System (both from bioMérieux, Hazelwood, MO), the MicroScan System (Siemens Healthcare Diagnostics, West Sacramento, CA), the RapID Yeast Plus System (Remel, Thermo Fisher Scientific, Lenexa, KS), and the Phoenix Yeast ID Panel (Becton, Dickinson and Co., Franklin Lakes, NJ). All of these systems perform well, as judged by reports in the literature and by proficiency testing surveys of the College of American Pathologists ( ; ; ; ; ; ; ; ). However, because no one system is known to be 100% accurate for identification of yeast species, a combination of methods should be considered, especially when rare species are recognized ( ).
In addition to fermentation and assimilation studies, stimulation of growth by biochemical compounds is a secondary test used in the differentiation of certain Trichophyton spp. ( ). Inclusion of inositol and thiamine in various combinations into agar media ( Trichophyton agars) allows assessment of growth-stimulating properties. The endpoint of the test, relative growth in comparison with a basal medium, is subjective, and both positive and negative controls should be included.
The test for urease production in cryptococci is of general utility in the clinical laboratory to differentiate these species from Candida spp., particularly in respiratory specimens ( ). C. neoformans is a pulmonary and systemic pathogen, whereas Candida spp. are frequent inhabitants of the upper airways but uncommon causes of primary pneumonia. Urease, however, may be produced by other nonpathogenic species of Cryptococcus, by Rhodotorula spp., and by some isolates of Trichosporon spp . and C. krusei . Urease production can be tested by inoculation of the yeast isolate onto a slant of Christensen urea agar or into urea broth. Alkalinization of the medium after production of NH 3 by urea-splitting organisms is detected by inclusion of a pH indicator in the system. Colonies that have macroscopically visible pseudohyphae (feet) or that grow on cycloheximide-containing agar need not be tested, because Cryptococcus does not produce pseudohyphae in vitro and does not grow in the presence of cycloheximide.
Production of urease also is useful to differentiate the dermatophytes Trichophyton mentagrophytes (urease positive) from T. rubrum (urease negative). The isolate should be subcultured to a slant of Christensen urea agar and incubated at 25° to 30°C for at least 3 days.
Although commercial biochemical tests are used for the identification of yeast, confirmation of the identification by examination of yeast morphology on agar media should also be considered, as should molecular sequencing or proteomic analysis. Trusting the results of automated or packaged systems implicitly is problematic, as highlighted by the emerging pathogen Candida auris. Studies have demonstrated that biochemical assays and even early versions of MALDI-TOF MS clinical databases were unable to correctly identify yeast of increasing clinical importance ( ; ).
Immunologic tests are available for detection of both antigens and antibodies to selected fungal pathogens; these assays have been shown to be of value for diagnosing infection and for monitoring therapeutic response ( ; , ; ). Although antibody testing for fungal pathogens has historically been used in some instances for diagnostics, these tests are no longer widely accepted owing to low sensitivity and specificity. In addition, the benefit of antibody detection remains unclear if patients are under immune suppression or are heavily colonized but not infected ( ).
Tests to detect fungal antigens or metabolic byproducts in serum or other body fluids, on the other hand, have been shown to be more useful for the diagnosis and management of fungal disease. Multiple antigen-detection methods are used for the specific diagnosis of a variety of diseases, including aspergillosis, cryptococcosis, histoplasmosis, blastomycosis, paracoccidioidomycosis, and talaromycosis (formerly penicilliosis) caused by Talaromyces marneffei (formerly Penicillium marneffei ) . One antigen-detection test is the cryptococcal antigen test for the diagnosis of cryptococcal infection ( ). The polysaccharide capsule of C. neoformans becomes dissolved in the serum and CSF and can be detected by the latex-based agglutination test or the enzyme immunoassay. Although these tests have limitations, they have been shown to be helpful in monitoring therapy over time through quantitative measurement of the titer of Cryptococcus polysaccharide in CSF ( ). A rapid, point-of-care lateral-flow device is now available for the diagnosis of cryptococcosis and can be performed on CSF or serum. This dipstick test (IMMY cryptococcal LFA, Immuno-Mycologics, Inc., Norman, OK) contains immobilized monoclonal antibodies to cryptococcal antigen and can provide results within 10 to 20 minutes without the use of specialized equipment ( ; ).
Antigen detection for the diagnosis of histoplasmosis has also proved useful when serum, urine, or bronchoalveolar lavage (BAL) fluid is tested ( ; ). As a screening test, detection of Histoplasma antigen plays a special role in the diagnosis of disseminated and diffuse acute pulmonary histoplasmosis ( ). Studies have shown that H. capsulatum –specific antigen can be detected in the urine of 90% of patients with disseminated infection and 80% of patients with acute histoplasmosis. Detection of antigen in BAL has also proved useful for the diagnosis of histoplasmosis, especially when the results of testing are combined with those obtained from cytopathology ( ).
The Platelia Aspergillus Ag (Bio-Rad, Marnes-la-Coquette, France) is an immunoenzymatic sandwich microplate assay for the detection of the galactomannan antigen, a reliable marker for invasive aspergillosis (IA). This assay is available to screen serum and bronchoalveolar fluid samples of high-risk patients for IA. Although this test has been shown to only have moderate sensitivity for the diagnosis of IA, the test is highly specific and considered a reliable screening for the detection of IA when used with other clinical parameters ( ; ; ). False-positive results have been reported in patients receiving piperacillin/tazobactam due to the presence of the galactomannan antigen in the preparation of this antibiotic, although some reports now suggest that the newer preparations no longer have this residual galactomannan present ( ). The FDA, however, still recommends that when reporting a positive Platelia Aspergillus galactomannan test result, laboratories inform physicians of the limitations of the test with regard to potential interaction with piperacillin/tazobactam.
A commercial test to detect (1-3)-beta- d -glucan (BG) is available for the presumptive diagnosis of invasive fungal infection (Fungitell Assay; Associates of Cape Cod, Inc., East Falmouth, MA) ( ). This antigen is present in the cell walls of most pathogenic fungi (including P. jirovecii ), with some notable exceptions such as Cryptococcus spp . and the Mucorales. Published studies have shown that BG may be useful as a screening tool for surveillance of invasive aspergillosis, as well as other invasive fungal diseases in populations at risk ( ; ). Although false-positive results appear to limit the use of this test, additional studies are needed to further define its diagnostic utility ( ).
Skin tests have been used for the epidemiologic study of some infections, but they have limited utility for diagnostic purposes.
Recent advances in molecular testing for the identification of fungal pathogens have had a significant impact on the diagnosis of fungal infections ( ; ; ; ). Numerous reviews have addressed these changes, and the general consensus is that approaches to species identification of many fungi today should include both morphologic and molecular testing methods ( ).
One of the greatest needs for clinical medicine is the development of a rapid and reliable detection method that can identify fungal pathogens directly from clinical specimens. This need reflects not only the inability of current laboratory tests to provide a timely result, but also the fact that fungal infections in immunocompromised patients usually appear suddenly, progress rapidly, and are often fatal unless treatment is started earlier in the course of infection.
Numerous molecular assays using both probe-based and amplification-based technologies have been developed for the identification of fungal pathogens. With any of these approaches, an initial goal has been to define the genetic loci within the genome for use as the molecular target. Although multiple areas within the fungal genome have been evaluated, the targeted areas most commonly used are those found within the ribosomal DNA (rDNA) gene complex ( Table 60.6 ) ( ). This section of the genome includes variable nucleotide sequence areas within the 18S gene and the 28S gene, as well as DNA sequence areas of the intervening internal transcribed spacer (ITS) regions called ITS1 and ITS2. Sequence homology within the rDNA genes and differences within the spacer regions are used as the genetic basis for the organization of fungi into taxonomic groups. The ITS regions of the nuclear rRNA gene had been proposed as the fungal barcode locus; however, more recent studies have now shown that other genetic markers are needed for the separation of species within many of the genera of fungi ( ; ; )
| Gene Target | Fungal Species |
|---|---|
| Actin | Candida albicans |
| β-Tubulin | Aspergillus species, Scedosporium species |
| Calmodulin | Aspergillus species, Fusarium species, Scedosporium species |
| Chitin synthetase | Dermatophyte species |
| Cytochrome b | Candida albicans |
| Dihydrofolate reductase | Pneumocystis jirovecii (carinii) |
| Elongation factor (EF-1α gene) | Fusarium species |
| Mitochondrial rRNA | Pneumocystis jirovecii |
| rDNA complex (5S gene) | Candida albicans, Pneumocystis jirovecii |
| rDNA complex (18S gene) | Multiple |
| rDNA complex (28S gene) | Aspergillus species , Candida species |
| rDNA complex (IGS regions) | Aspergillus species |
| rDNA complex (ITS regions) | Multiple |
| RNA polymerase (RPB1 and RPB2) | Fusarium species |
Probe-based assays are commercially available and are commonly used in the mycology laboratory for the identification of Histoplasma, Blastomyces, and Coccidioides spp. from culture (GenProbe AccuProbe, Hologic, Inc., Bedford, MA). Another probe-based method called the peptide nucleic acid fluorescence in situ hybridization (PNA-FISH) assay was developed and has become commercially available for the qualitative identification of certain Candida spp. in blood culture bottles ( ; ). This assay uses a fluorescent-labeled peptide nucleic acid probe that targets the species-specific ribosomal RNA of Candida spp. Two assays are available, and include the Yeast Traffic Light and QuickFISH diagnostic tests (OpGen, Inc., Gaithersburg, MD). The Yeast Traffic Light assay groups Candida spp. by color based on probable susceptibility to fluconazole. Candida albicans and C. parapsilosis fluoresce green since they are likely susceptible to this antifungal, C. tropicalis fluoresces yellow for variable susceptibility, and C. glabrata and C. krusei fluoresce red due to reduced susceptibility or resistance to fluconazole. The QuickFISH assay can detect C. albicans (green), C. parapsilosis (yellow/orange), and C. glabrata (red). The sensitivity and specificity of these tests have been shown to be greater than 97%, and the impact of rapid identification of Candida in the blood can be seen in the significant reduction in the empirical use of antifungals ( ; ; ; ). Incorporating the Candida PNA-FISH test as part of the initial identification algorithm for yeast recovered from blood can result in substantial savings for hospitals. Major drawbacks to this assay include the high costs of reagents, the need for fluorescent microscopy, and the changes in workflow required to incorporate testing in the laboratory.
Numerous amplification methods using both non–sequence-based and sequence-based approaches for analysis have been applied in identification of fungi. Most of the sequence-based analysis methods have used conventional polymerase chain reaction (PCR) with universal primers to target sequences within the 28S rDNA gene, the 18S rDNA gene, or the ITS1-5.8S-ITS2 target areas ( ; ).
The availability of a nucleotide-based sequence database that has the breadth of phylogenetics and the taxonomic accuracy needed to identify fungal species is desirable for sequence analysis alignment. The largest database currently used for sequence-based identification is the publicly available GenBank database (National Center for Biotechnology Information, Washington, DC). Unfortunately, because this is an open-source database, previous reports suggested that up to 20% of the sequenced entries listed for fungi may be associated with erroneously identified species ( ). Other publicly available databases include the International Society of Human and Animal Mycology (ISHAM) Barcoding database, which offers pairwise sequence alignments against reference ITS and translation elongation factor 1 alpha (TEF1α) sequences ( ), and the Westerdijk Fungal Biodiversity Institute (formerly the Centraalbureau voor Schimmelcultures Fungal Biodiversity Centre). Additionally, there is a need for sequences from a variety of genomic targets in the database that is phylogenetically represented with accurate species identification. Although multiple locus sequencing and alignment comparisons have been done using a variety of fungal species, additional studies using sequence comparison analysis are needed to determine the optimal target or targets for fungal identification. Multiple target evaluations for the identification of aspergilli and fusaria have been done and show this approach to be valid for identification purposes ( ; ).
High-order multiplex PCR assays have been developed for the direct detection of microbial pathogens in positive blood cultures. The FilmArray Blood Culture ID (BCID) Panel (BioFire Diagnostics, Salt Lake City, UT) tests positive blood culture broth for a complex panel of 24 pathogens to include five Candida spp. ( C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis ). This assay includes the ability to automatically carry out all steps of the identification process in a closed system from DNA extraction to the interpretation of the PCR data. Limited prospective studies have shown that the FilmArray panel was a reliable alternative to identify the most common yeast from positive blood cultures ( ; ). A similar meningitis/encephalitis (ME) panel is available that tests for 15 pathogens, including C. neoformans/C. gattii directly on CSF . However, false negatives with this assay in patients with positive cryptococcal antigen tests have been reported in the literature ( ). Additional studies are needed to determine how they will improve the management of patients by a reduction in the time of diagnosis and consequent length of hospitalization and mortality rates.
A fully automated assay is now available for the detection of Candida spp. directly from whole blood, which eliminates the time needed for blood culture bottles to become positive. In the T2 Candida assay (T2Biosystems), red blood cells and pathogen cells are first lysed, and Candida DNA is amplified using primers targeted for the ITS2 region ( ). The amplified DNA is then hybridized to supraparamagnetic nanoparticles coated with complimentary DNA, which agglomerate into microclusters and produce a signature T2 magnetic resonance signature. This signature is read and compared to a database for organism identification. This assay is able to detect between one and three colony-forming units/mL of whole blood of Candida , which is similar to the commercially available PNA-FISH assays, and groups species based on typical fungal susceptibility patterns ( C. albicans grouped with C. tropicalis ; C. glabrata grouped with C. krusei ; and C. parapsilosis by itself) ( ). In a clinical study, the specificity of the assay was 99.4%, and the time to negativity was 4.2 hours (vs >120 hours for traditional blood cultures) ( ). By rapidly ruling out candidemia, the inappropriate use of antifungals may be reduced.
Proteomics using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) has emerged as a reliable method for the rapid identification of microbial pathogens ( ; ). This technology involves combining of the pathogen with a suitable energy-absorbent chemical matrix (e.g., α-cyano-4-hydroxycinnamic acid dissolved in acetonitrile and trifluoroacetic acid), pulsing the sample with a laser for ablation and desorption, followed by ionization using time-of-flight technology to create a mass spectrum fingerprint of the ribosomal proteins of the organisms that is detected by mass spectrometry. This mass spectrum is then compared with a database of known spectra to allow for organism identification. Prior to performing the assay, fungal cells must be lysed, which may occur either “on plate” or “off plate.” Yeast typically undergo on plate extraction where cells are transferred to the target plate or slide from a growing culture, and this is followed by the addition of formic acid and drying, after which the matrix is applied. The preparation of filamentous fungi takes longer and may occur off plate. Success has been demonstrated with treating an appropriate amount of biomass with formic acid and acetonitrile prior to application to the target plate or slide ( ). Although the complexity of the composition of the fungal cell wall and the different protein compositions seen (e.g., between vegetative cells and spores and between young and mature colonies) has made the creation of reliable databases for analysis challenging, significant progress has been made, and two commercial systems are now FDA cleared for the identification of fungi. These include the Biotyper (Bruker Daltonics, Inc., Billerica, MA) and the VITEK MS (bioMerieux, Inc., Durham, NC).
Overall, both systems appear to have similar success rates in the identification of yeast species. One large study that included 1192 yeast isolates reported 97.6% correct species identification with the Bruker Biotyper and 96.1% with the bioMerieux MS ( ). Similar rates of correct species identification of various yeasts can be found in the literature using this technology (range 92–99%) ( ; ; , ; ; ; ; ; ). Studies have also demonstrated that this technology can discriminate between closely related species where biochemical assays have had difficulty ( ; ; ). However, the success of MALDI-TOF MS is dependent upon the availability of a well-curated database that contains reference spectra for the organisms being identified ( ). Neither of the FDA-cleared commercial systems was able to correctly identify C. auris when this species first began to be recognized as an emerging pathogen of clinical importance ( ). MALDI-TOF MS has also been evaluated for the identification of filamentous fungi with varying success. Both the Bruker Biotyper and bioMerieux VITEK MS FDA-cleared systems now contain molds within their databases, but the number is limited compared to yeast species, although more mold species are available within their research use only (RUO) databases ( ).
One of the main advantages of MALDI-TOF MS is the rapidity by which results are available. One study estimated that the turnaround time for species identification of bacteria and yeasts was on average 1.45 days faster than conventional methods ( ). However, access to available spectral databases is limited and those databases that are FDA cleared for use in clinical microbiology laboratories are maintained by the companies rather than publicly available like sequence databases (i.e., GenBank). Another limitation of MALDI-TOF MS is the need for a pure culture. Although MALDI-TOF MS has also been evaluated for the direct identification of yeast from positive blood culture bottles with accuracy in identification of greater than 90% in one study ( ), additional studies are needed. Studies have also been conducted to evaluate this technology for antifungal susceptibility testing ( ; ; ; ; , ). Some studies have reported good categoric agreement between MALDI-TOF MS methods and those obtained by broth microdilution ( ; ), although such agreement has not been universally reported ( ).
For many years, few antifungal chemotherapeutic agents were available, with amphotericin B virtually the only agent effective against most systemic fungal pathogens. Although this potent antifungal agent was frequently toxic, resistance to therapy was rare. With the recent introduction of new antifungal agents and with the widespread use of antifungal prophylaxis, the epidemiology of invasive fungal infections has changed, with non- albicans Candida, non- fumigatus Aspergillus, and molds other than Aspergillus becoming more common as causes of invasive disease ( ; ; ; ; ; ; ; ). These emerging fungi are resistant to or less susceptible than other fungi to standard antifungal agents and thus become more difficult to treat. Furthermore, although the predictability of innate resistance frequently means that species identification is often sufficient to alert the clinician to the likelihood of in vitro and often associated in vivo resistance, the emergence of resistance in a previously susceptible strain during the course of treatment has become more problematic ( ; ).
The Clinical and Laboratory Standards Institute has developed reference broth dilution methods for antifungal susceptibility testing of yeasts ( ) and molds ( ). Methods for antifungal disk diffusion susceptibility testing of yeasts ( ) and a method for antifungal disk diffusion susceptibility testing of nondermatophyte filamentous fungi ( ) have also been developed.
The CLSI M27 document provides the reference methods for the testing of yeasts ( ), and a new document ( ) has now been published that includes quality control and reference performance standards for 24-hour and 48-hour reading times for broth microdilution testing of the antifungals against yeast species and the species- and drug-specific breakpoints for the echinocandins (anidulafungin, caspofungin, and micafungin), fluconazole, and voriconazole against common species of Candida ( C. albicans, C. glabrata, C. guilliermondii, C. krusei, C. parapsilosis, and C. tropicalis ) ( ).
The CLSI M38 document provides the reference method for in vitro susceptibility testing of filamentous fungi that can cause invasive disease ( ). Organisms included in this document for evaluation are Aspergillus spp., Fusarium spp., Rhizopus oryzae (R. arrhizus), Scedosporium apiospermum/S. boydii, Lomentospora ( Scedosporium ) prolificans, and the mycelial form of Sporothrix spp. , as well as the dermatophytes ( Epidermophyton, Microsporum, and Trichophyton spp.). The CLSI M27 and M38 documents are reference standards developed through a consensus process to facilitate agreement among laboratorians in measuring the susceptibility of yeasts and molds to antifungal agents. Although the clinical relevance of testing yeasts is established and now included in treatment guidelines ( ), the need for testing filamentous fungi remains uncertain, and a breakpoint has been set for only one antifungal (voriconazole) against one species ( Aspergillus fumigatus ) (CLSI, 2020). The European Union Committee on Antimicrobial Susceptibility Testing (EUCAST), an organization similar in function to CLSI, has also published reference standards for susceptibility testing of fungi and have set breakpoints for antifungals against Candida spp. and some Aspergillus spp. ( ; ).
Since both reference broth methods are time consuming to perform, simplified alternatives to these methods have been assessed to include the disk-diffusion method, the Etest agar-gradient MIC method (bioMérieux, Inc., Durham, NC) ( ), the Sensititre YeastOne method (Thermo Scientific, TREK Diagnostic Systems, Inc., Cleveland, OH) ( ; ), and the VITEK 2 method (bioMérieux, Durham, NC) ( ). The disk-diffusion method has also been approved for the testing of yeast, and studies have shown that results obtained from testing using this method are comparable with results obtained with the CLSI microdilution broth method ( ; ; ).
The Etest uses a plastic strip that contains a predefined stable gradient of antifungal concentrations to determine the on-scale minimum inhibitory concentration (MIC) of the antifungal agent tested. These strips are now commercially available for several antifungals. Studies have shown the Etest strips to be similar to the results obtained by the CLSI broth microdilution methods for in vitro susceptibility testing of both molds and yeasts ( ; ; ).
The Sensititre YeastOne method uses a dried colorimetric microdilution panel in a microtiter format for antifungal testing. This method is the only FDA-cleared assay for in vitro diagnostic use in testing yeast susceptibility to fluconazole, itraconazole, 5-flucytosine, and voriconazole. Instead of reading the presence or absence of growth of the organism being tested, a change in color indicative of a change in the metabolism of the pathogen is used as the endpoint. Panels are also available for RUO in testing antifungal susceptibility to amphotericin B, anidulafungin, caspofungin, micafungin, and posaconazole. This testing platform has been shown to be dependable for the testing of yeasts ( ; ).
Although the key elements involved in selecting an appropriate antifungal agent include the type of patient (e.g., solid organ or stem cell transplant), the severity of immunosuppression, a history of prolonged exposure to antifungal drugs, and knowledge of the genera and species of the infecting pathogen, susceptibility testing may be warranted in some circumstances, including the following: (1) as part of periodic surveys that establish antibiograms for isolates in an institution; (2) to help in the management of refractory oropharyngeal candidiasis in patients with apparent therapeutic failure; and (3) to aid in the management of invasive candidiasis when the use of azole antifungal agents is uncertain ( ). Also, scant data are available regarding the clinical implications of in vitro susceptibility testing in many cases, and the refractile nature of many fungal pathogens to antifungal therapy makes susceptibility testing an option that needs to be considered. For instance, reports now show that resistance has emerged during treatment of Aspergillus infections, thus highlighting the potential need for in vitro susceptibility testing under this circumstance ( ).
Candida species are the most common recognized yeast pathogens and with increased use of immunosuppressive therapies, use of broad-spectrum antibiotics, and aging of the population, they have assumed a prominent place among nosocomial pathogens ( ; ; ). These species are currently the fourth most common cause of hospital-acquired bloodstream infection in the United States and rank third among causes of bloodstream infection in the intensive care unit ( ; ). Although C. albicans accounts for about 50% of invasive infections caused by Candida spp., the non- albicans Candida spp. have emerged as more common causes of disease ( ; ; ). In a multicenter evaluation of patients with candidemia (Prospective Antifungal Therapy Alliance registry), C. albicans accounted for 42.1% of cases, followed by Candida glabrata (26.7%), Candida parapsilosis (15.9%), Candida tropicalis (8.7%), and Candida krusei (3.4%) ( ).
Candida infections are limited in extent and severity, depending on the immune status of the host. Since Candida spp. are a part of the normal flora in the gastrointestinal (GI) tract, mucous membranes, and skin, infections generally occur as the result of an opportunity. For instance, the use of broad-spectrum antibacterial therapy upsets the balance of colonizing flora on the mucous membranes of the oral cavity, the skin, and the GI tract by eliminating the predominant competing bacterial flora, thus allowing overgrowth of yeast ( ; ).
Invasive disease by Candida spp. develops when host defenses are compromised. Diabetes, immunosuppressive disease or therapy, and neutropenia as a result of disease (e.g., AIDS) or treatment with high-dose chemotherapy are common risk factors. Bloodstream infections, including fungal endocarditis, are fostered by the use of indwelling vascular lines, which is common practice in the management of hospitalized patients ( ; ).
Although C. albicans is the most common pathogen within the genus Candida, the frequency of the non- albicans species associated with infections has increased over the last few decades ( ; ). Numerous reports describe invasive infections caused by C. tropicalis ( ; ), C. parapsilosis ( ), C. glabrata ( ), C. krusei ( ), and C. lusitaniae ( ). C. auris has recently emerged and represents a challenge to both infection control and treating clinicians alike ( ).
Resistance to antifungal agents has emerged as a problem with the introduction of newer agents, which is especially problematic within the non- albicans Candida species ( ). Resistance has been most notable within C. glabrata (resistance to the imidazoles [fluconazole, itraconazole, posaconazole, and voriconazole] and to the echinocandins) and within C. krusei (resistance to itraconazole), but also the recently recognized pathogen C. auris (resistance to triazoles, echinocandins, and polyenes in some cases) ( ). Antifungal intrinsic resistance is also present in C. krusei and in C. lusitaniae to fluconazole and amphotericin B, respectively. The Infectious Diseases Society of America has updated its clinical practice guidelines for the management of patients with invasive candidiasis and mucosal candidiasis to reflect changes noted with the addition of the new antifungals ( ).
Cutaneous disease is the most frequent infection caused by Candida spp. This disease presents topically as an erythematous lesion of the skin, sometimes accompanied by a creamy white exudate or scaling. Moist conditions, such as diaper rash in infants and infection of skin folds (intertrigo) in adults, are precursors to infection. Common sites are those in the groin, between fingers and toes, under the female breast, and in the axilla. Workers who immerse their hands in water for long periods of time are also at risk for infection of the skin of the hands, the nails (onychomycosis), or the nail bed (paronychia) ( ). Moreover, chronic cutaneous disease (referred to as chronic cutaneous candidiasis) is an uncommon manifestation of Candida infection in patients with defective cellular immunity, and the underlying genetic polymorphisms responsible are under active investigation ( ).
Oral candidiasis usually manifests as the appearance of creamy white patches overlying erythematous buccal mucosa (thrush) although can also present with an erythematous form, particularly under dentures ( ). Although symptoms are usually minimal, dysphagia may result from heavy infection or with esophageal candidiasis. Fissuring at the corners of the mouth is common and may be the primary complaint. Oral candidiasis is a common initial infection in patients with HIV and frequently is a marker of immune failure in these patients ( ). Especially notable is the prevalence of C. dubliniensis and the association with oral carriage and oropharyngeal infections in these patients with HIV ( ; ).
Gastrointestinal candidiasis occurs most frequently as esophagitis and uncommonly as gastritis ( ). Erosive lesions of the distal esophagus and stomach result in substernal pain, which is aggravated by swallowing. White plaques overlie the lesions when viewed by endoscopy. The differential diagnosis includes infection with herpes simplex virus, although these two processes may coexist. Esophageal candidiasis was previously described as a frequent opportunistic infection in persons infected with HIV; however, the use of highly active antiretroviral therapy has led to a striking decline in the prevalence of this disease ( ). Even though Candida spp. are seen frequently as flora of the lower GI tract, the significance of finding yeast in stool is uncertain. True invasive infection of the lower GI tract is less common than disease of the upper tract, although overgrowth of Candida spp. in the stool may accompany antimicrobial therapy.
Vaginal candidiasis afflicts postpubertal women; diabetes mellitus, utilization of antimicrobial therapy, pregnancy, and sexual activity are predisposing conditions ( ). Vaginal burning and itching, dyspareunia, and a discharge that is classically curdlike are associated with an infection that may be acute or chronic and difficult to eradicate.
Urinary tract infection caused by Candida spp. is difficult to diagnose because these yeasts are frequently recovered from the urine as a result of vaginal contamination or colonization of the bladder in patients with indwelling catheters, especially when systemic antibiotics have been administered ( ; ). Severe infection of the upper urinary tract, including necrosis of the renal papillae, is a serious complication that occurs particularly in patients who have obstructive uropathy. Quantitative cultures do not appear useful for assessing the significance of Candida spp. in the urinary tract.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here