Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anesthetic morbidity remains an important consideration in pediatric anesthesia practice, with risk increasing as the age of the patient decreases. This is despite the development of newer anesthetics and techniques. In addition, advances in medical and surgical care have led to increasing complexity of patients because of increased survival. Serious adverse events in pediatrics occur in 1.4 per 1000 anesthetics. Among these serious events, respiratory and cardiac events are most commonly seen ( ). In the Pediatric Perioperative Cardiac Arrest Registry from 1998 to 2004, cardiovascular and respiratory etiologies were the most common causes of anesthesia-related cardiac arrest in anesthetized children ( ). Using the same registry with data from 1994 to 2005, pediatric patients with complex heart disease had a higher mortality rate after arrest than patients without heart disease ( ). reviewed cardiac arrest data at the Mayo Clinic, finding that the respective incidences of cardiac arrest and mortality during noncardiac procedures were 2.9:10,000 and 1.6 :10,000, respectively, while the incidence of cardiac arrest in children undergoing cardiac surgeries was 127 :10,000. Cardiac arrests were highest in infants under 30 days of age, followed by children less than 1 year. In the National Anesthesia Clinical Outcomes Registry, the incidence of cardiac arrest in infants was more than seven times that of the incidence for children of all ages ( ). The National Anesthesia Clinical Outcomes Registry also shows morbidity and mortality to be higher in the OR than in non-OR settings, despite the growth in non-OR anesthesia practice ( ). Clearly pediatric patients undergoing anesthesia are a high-risk population, requiring careful attention, staff vigilance, and monitoring to minimize or prevent physiologic perturbations.
The American Society of Anesthesiologists ( ) has published guidelines for intraoperative monitoring of patients under anesthesia (see Box 17.1 in Chapter 17 : Equipment). These standards mandate the continuous presence of an anesthesiologist or other qualified anesthesia personnel throughout the conduct of anesthesia and require continuous monitoring of oxygenation and electrocardiography and adequacy of ventilation and circulation. The minimum standard for monitoring oxygenation includes an oxygen analyzer in the anesthesia breathing circuit, sufficient illumination to evaluate the patient’s color, and a quantitative method such as pulse oximetry, except under extenuating circumstances. Tracheal intubation must be verified by physical examination and qualitative detection of carbon dioxide in the exhaled gas. Regardless of whether endotracheal intubation has been performed, continuous capnography is required unless it becomes invalidated by the nature of the patient, procedure, or equipment. Furthermore, quantitative monitoring of the volume of expired gas is strongly encouraged. The ASA also recommends monitoring of ventilation using observation of chest excursion and the reservoir breathing bag, as well as auscultation of breath sounds. When ventilation is controlled by a mechanical ventilator, a device that is capable of detecting disconnection of components of the breathing system must be in continuous use, and the device must give an audible signal when its alarm threshold is exceeded.
ASA monitoring standards for circulation mandate that every patient receiving anesthesia will have continuous ECG and determination of arterial blood pressure and heart rate at least every 5 minutes. In addition, every patient will have circulatory function continually evaluated with use of at least one of the following methods: palpation of a pulse, auscultation of heart sounds, monitoring of an intraarterial pressure tracing, ultrasound peripheral pulse monitoring, or pulse plethysmography or oximetry. Finally, a method by which the temperature can be measured should be readily available during general anesthesia, and patients should have their temperature monitored when clinically significant changes in body temperature are intended, anticipated, or suspected.
As a reflection of the increasing role of anesthesiologists in the perioperative arena, many of these provisions have been extended to the PACU in standards adopted by the ASA in 1988 and updated in 2014 ( ). PACU monitoring should emphasize assessment of oxygenation, ventilation, circulation, and temperature, with specific capability for quantitative determination of systemic oxygenation by pulse oximetry or its equivalent. Consistent and timely use of patient monitors on arrival to PACU on a recently anesthetized patient can improve safety and reduce adverse events. Equipment should be readily available to enable the practitioner to meet these standards in all pediatric patients.
The anesthesiologist can gain a tremendous amount of information from observation and vigilance. In the spontaneously breathing patient, anesthetic depth can be inferred from the respiratory rate and pattern, and airway obstruction can be detected by chest wall retractions or “seesaw” paradoxical motion. Skin and mucous membranes should be continually evaluated to assess adequate oxygenation, as a pulse oximeter reading may significantly lag behind other indices of hypoxemia when placed on an extremity ( ; ), or it may not detect a pulse at all during intense vasoconstriction ( ). Physical exam with vigilance during observation of anesthetic care is paramount to patient safety even in the presence of monitors.
Capillary refill can provide valuable information about the intravascular volume and cardiac output of a euthermic patient. A child with cool, mottled, poorly perfused extremities should be examined closely for additional evidence of hypovolemia or reduced cardiac output, even if the systemic arterial pressure remains normal. Progression of this mottled appearance onto the trunk indicates the extreme vasoconstriction that may herald imminent cardiovascular collapse.
Continuous auscultation of heart and lung sounds by means of a precordial stethoscope is useful during all phases of general anesthesia and during transport of the child between hospital locations. A precordial stethoscope allows the anesthesiologist to immediately detect changes in the rate and character of heart and breath sounds and is often the first warning of a physiologic alteration ( ). Use of the precordial and esophageal stethoscope has decreased and currently can be hard to find in many clinical use settings because of the ubiquitous presence of capnography and pulse oximetry, even though experienced pediatric anesthesiologists have noted earlier detection of critical events when a precordial or esophageal stethoscope was used ( ). Breath sounds and heart tones are best heard when a precordial stethoscope is positioned near the left sternal border between the second and fourth interspaces (above the nipple line). An esophageal stethoscope is reserved for patients whose anesthetic management includes endotracheal intubation and in whom a precordial stethoscope either provides inadequate information or violates the surgical field. The proper method for accurate placement of the esophageal stethoscope is to listen while simultaneously advancing the device and placing it at the level where the heart and lung sounds are maximal. In small infants, unintentional placement of the esophageal stethoscope into the stomach can occur easily.
Esophageal stethoscopes are contraindicated in patients with esophageal atresia or in those who have a disease process involving the proximal portion of the esophagus. They confer a rigid feel to the esophagus, which might be mistaken for the trachea ( ). As a result, use of an esophageal stethoscope is relatively contraindicated in surgeries where the trachea is a critical landmark.
More advances in auscultation technology have been in the development of electronic stethoscopes. Electronic stethoscopes are designed to augment acoustic signals from the patient so that the clinician can better detect changes in patient respiratory or cardiac status. In addition, electronic stethoscopes have the ability to digitally record and save patient examinations for future review and research opportunities ( ). Furthermore, electronic stethoscopes have enabled telemedicine examinations to be more comprehensive and familiar. Compared with conventional stethoscopes, early studies demonstrate that electronic stethoscope use may lead to greater accuracy in identifying heart and pulmonary sounds. The ability, however, for electronic stethoscope use to improve clinical outcomes is still to be determined ( ).
In pediatric anesthesia, the electrocardiogram (ECG) is most useful for tracking the heart rate and diagnosing intraoperative arrhythmias, with bradycardia and supraventricular tachycardia (SVT) being the most commonly observed in otherwise healthy children ( ). In small infants, bradycardia secondary to hypoxemia may occur before the pulse oximeter reveals oxyhemoglobin desaturation. Premature ventricular contractions (PVCs) were formerly more commonly observed when halothane was used as the general anesthetic agent, especially during periods of hypercapnia and/or catecholamine release. Dysrhythmias are less common with sevoflurane, desflurane, and isoflurane. In children, the normal heart rate varies with age ( Table 18.1 ). Heart rates tend to decrease with age and in parallel with decreases in oxygen consumption. In addition, many children have a noticeable variation in heart rate with respiration (i.e., sinus arrhythmia).
| HEART RATE (BEATS/MIN) | ||
|---|---|---|
| Age | Mean | Range (±2 SD) |
| 0–24 hr | 119 | 94–145 |
| 1–7 days | 133 | 100–175 |
| 8–30 days | 163 | 115–190 |
| 1–3 months | 152 | 124–190 |
| 3–12 months | 140 | 111–179 |
| 1–3 years | 126 | 98–163 |
| 3–5 years | 98 | 65–132 |
| 5–8 years | 96 | 70–115 |
| 8–16 years | 77 | 55–105 |
Electrolyte abnormalities may be revealed through observation of the ECG. Hyperkalemia produces characteristically prominent T waves. Hypocalcemia, which can occur during rapid administration of citrated blood products, prolongs the QT interval. Because ischemic changes in pediatric patients are rare and lead II provides a good view of atrial activity for arrhythmia diagnosis, the latter is recommended for routine intraoperative electrocardiographic monitoring of pediatric patients.
The arterial pulse pressure profile changes from the proximal to the distal portions of the arterial tree. The systolic pressure is higher proximally and the diastolic blood pressure is lower distally. Mean blood pressure is the same throughout the arterial tree but the arterial pulse pressure increases distally. These changes can be accounted for by the different physical characteristics of the vascular tree and ejection velocity that result in changes of impedance and harmonic resonance.
Blood pressure is measured noninvasively in infants and children using oscillotonometry. In children, oscillometric measurements of systolic arterial pressure ( ; ) and mean arterial pressure ( ) usually correlate well with the Riva-Rocci mercury column method and with direct arterial pressure measurement, but they tend to underestimate the diastolic component. With oscillometric measurements the sensor inside the cuff detects the arterial oscillations. As cuff bladder declines, the oscillations increase in amplitude. The maximum amplitude represents the mean blood pressure. The systolic and diastolic blood pressures are calculated. The general formula is:
During routine uncomplicated cases, measurement of blood pressure should be performed every 3 to 5 minutes while the child is anesthetized. The most current ASA guidelines for intraoperative blood pressure management, last affirmed in 2015, state that arterial blood pressure should be determined and evaluated at least every 5 minutes ( ). The blood pressure cuff is most commonly placed on the upper arm but can be placed on the forearm, thigh, or calf. There is inconsistent correlation of measurements obtained between the upper and lower limbs. Importantly, depending on patient condition, patient intraoperative positioning, and surgical needs, the blood pressure cuff may have to be moved to a different extremity that may adversely impact accurate blood pressure management. Keidan and colleagues studied blood pressure measurements in the arm, forearm, and leg in anesthetized healthy children during a prospective randomized study and determined that forearm and ankle noninvasive blood pressure measures were less reliable and more inconsistent than those from the arm ( ).
Variation in patient size due to age and other factors requires the proper choice of blood pressure cuff size to be selected to optimize measurement accuracy. The width of the blood pressure cuff should cover approximately two-thirds the total length of the extremity portion to which it is applied. A cuff that is too small or too narrow incompletely occludes the artery, resulting in the premature return of detectable flow and thus falsely increasing the blood pressure measurement ( ). A cuff that is too wide may dampen the arterial wave and result in a falsely low pressure, but the magnitude of this error can be small ( ). Blood pressure increases gradually throughout childhood ( Tables 18.2 , 18.3, and 18.4 ) and is dependent on the height of the child such that taller children demonstrate a higher blood pressure. Blood pressure ranges in premature infants have been defined ( Table 18.5 ) and vary according to the health of both the infant and the mother.
| Age (Years) | 50th Percentile (mm hg) | 90th Percentile (mm hg) | 95th Percentile (mm hg) |
|---|---|---|---|
| 1 | 85–86 | 99–100 | 103–104 |
| 2 | 87–88 | 100–102 | 104–106 |
| 3 | 88–91 | 102–105 | 104–109 |
| 4 | 91–93 | 104–107 | 108–111 |
| 5 | 93–95 | 106–108 | 110–112 |
| 6 | 94–96 | 108–110 | 111–114 |
| 7 | 96–97 | 109–111 | 113–115 |
| 8 | 98–99 | 111–112 | 115–116 |
| 9 | 100 | 113–114 | 117–118 |
| 10 | 102 | 115 | 119 |
| 11 | 103–104 | 117 | 121 |
| 12 | 105–106 | 119–120 | 123 |
| Age (Years) | 50th Percentile (mm hg) | 90th Percentile (mm hg) | 95th Percentile (mm hg) |
|---|---|---|---|
| 1 | 37–40 | 52–54 | 56–58 |
| 2 | 42–45 | 57–59 | 61–63 |
| 3 | 46–49 | 61–63 | 65–67 |
| 4 | 50–52 | 65–66 | 69–70 |
| 5 | 53–54 | 68 | 72 |
| 6 | 55–56 | 70 | 74 |
| 7 | 57 | 71–72 | 75–76 |
| 8 | 58–59 | 72–73 | 76–78 |
| 9 | 59–60 | 73–75 | 77–79 |
| 10 | 60–61 | 74–75 | 78–80 |
| 11 | 61 | 75–76 | 79–80 |
| 12 | 62 | 76 | 80–81 |
| Mean Systolic (mm Hg) | Mean Diastolic (mm Hg) | |
|---|---|---|
| Premature | 55–75 | 35–45 |
| 0–3 months | 65–85 | 45–55 |
| 3–6 months | 70–90 | 50–65 |
| 6–12 months | 80–100 | 55–65 |
| Age (Days) | SYSTOLIC BLOOD PRESSURE (mm Hg) | DIASTOLIC BLOOD PRESSURE (mm Hg) | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| 1 | 48 ± 9 | 63 ± 12 | 25 ± 7 | 35 ± 10 |
| 2 | 54 ± 10 | 63 ± 10 | 30 ± 0 | 39 ± 8 |
| 3 | 53 ± 9 | 67 ± 10 | 31 ± 8 | 43 ± 8 |
| 4 | 57 ± 10 | 71 ± 11 | 32 ± 8 | 45 ± 10 |
| 5 | 56 ± 9 | 72 ± 14 | 33 ± 9 | 47 ± 12 |
| 6 | 57 ± 9 | 71 ± 11 | 32 ± 7 | 47 ± 10 |
Though the measurement of arterial blood pressure for patients is clearly a standard of care requirement and important for clinical anesthetic management, additional work has been conducted on determining more accurate reference ranges of blood pressure for children during anesthesia. Studies have been motivated by concerns that neurotoxicity could be mediated by hypotension and subsequent hypoperfusion ( ). Challenges in variation in pediatric patient ages and size, creates difficulty in defining normal reference valves for arterial blood pressure during anesthesia. For example, data from a survey of American and British pediatric anesthesiologists define intraoperative hypotension as a systolic blood pressure of 25 to 70 mm Hg in neonates and higher in patients 2 to 12 years of age at 40 to 100 mm Hg ( ). Using retrospective data from the Multicenter Perioperative Outcomes Group (MPOG), de Graaff and colleagues presented the first reference ranges for blood pressure in children during anesthesia and could be the basis for future clinical outcomes research using blood pressure measurements ( ). See Chapter 21 : Induction, Maintenance and Recovery.
The arterial waveform has distinct components, and these components change contour from the proximal to peripheral sites of the arterial tree (see Fig. 18.1 A–C). Owing to changes in impedance and harmonic resonance, the arterial upstroke becomes steeper and its slope increases. The systolic peak is higher and the dicrotic notch appears later in the cycle; the end-diastolic runoff is lower. There is an increase in systolic pressure and a decrease in diastolic pressure and a wider pulse pressure when one moves the sampling site further away from the thoracic aortic root into the medium-sized arteries ( Fig. 18.1 C). This is secondary to an increase in resistance of blood movement and a decrease in compliance of the smaller vessels.
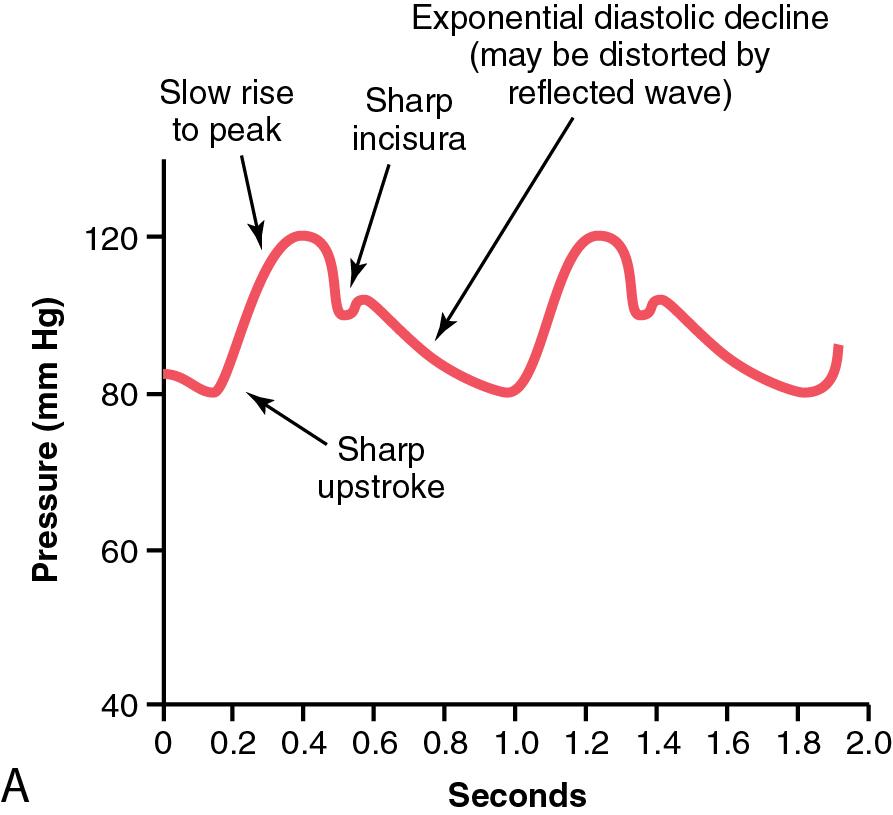
Direct measurement of blood pressure via an arterial catheter is indicated when there is a need for precise beat-to-beat blood pressure monitoring, difficulty obtaining noninvasive blood pressure measurements, cardiopulmonary bypass or pulseless flow, and/or frequent determination of arterial blood gas values. This patient population includes children who are at increased risk for developing unstable hemodynamics or those undergoing a surgical procedure that could result in profound hemodynamic alterations related to blood loss (i.e., total loss >50% estimated blood volume [EBV] or acute loss >10% EBV), fluid shifts (i.e., third space losses >50% EBV), deliberate hypotension, or nonpulsatile blood flow (e.g., cardiopulmonary bypass). Respiratory indications for direct arterial monitoring include significant abnormalities in gas exchange secondary to either preexisting disease or the surgical procedure (e.g., thoracotomy).
Rarely, direct arterial monitoring is necessary because of the inability to measure systemic arterial pressure by any indirect technique, or for beat-to-beat assurance of cardiac output in the pacemaker-dependent child. Although, it is important to note that direct blood pressure measurement can also be indicated in unique clinical scenarios in pediatric anesthesia, including the need for prolonged measurement in patients who are limited in extremities due to congenital disease or where repeated blood pressure cuff use can cause severe injury. While a small retrospective study demonstrated no association with the use of intraoperative blood pressure cuffs in patients with osteogenesis imperfecta, there continues to be a heightened sense of awareness of minimizing pressure and blood pressure measurements in patients at risk for fracture due to disease progression ( ).
There are no absolute contraindications to placing an arterial catheter, but a risk-benefit analysis should be performed in patients with a hypercoagulable state or bleeding disorder and in children who have previously required or will require frequent arterial access as a result of ongoing disease processes. The site of arterial catheter placement can be critically important in pediatric patients to maximize successful placement and minimize the risk of complications associated with cannulation. The radial artery is a favored site for arterial cannulation because it is superficial and easily accessible. However, ultrasound-guided arterial cannulation allows for increased access to arteries that were normally not as easy to access in the past using traditional palpation techniques ( ). Other anatomic sites frequently used are the ulnar, dorsalis pedis, posterior tibial, and femoral arteries. The axillary artery has gained favor because of increased collateral blood flow compared with the brachial or femoral arteries ( ; ; ; ). Common practice has been to avoid the brachial artery because of the concern of median nerve damage and poor collateral flow around the elbow. However, there is minimal scientific evidence indicating increased risk with this approach. Umbilical vessels provide an alternate site via which the aorta and inferior vena cava may be cannulated in neonates. In determining a site, one needs to consider whether the vessel has been cannulated before, sources of collateral flow, the experience of the person inserting the catheter, and special physiologic issues (e.g., whether it arises on an aortic root proximal to the ductus arteriosus) or surgical issues (e.g., whether it arises from a vessel likely to be clamped or sacrificed during the procedure). Cannulation of vessels with good collateral flow, such as the arch vessels of the wrist or foot, may reduce the risk of ischemic tissue damage distal to the catheter.
As the largest superficial vessel, the femoral artery can be cannulated most predictably in situations where intense peripheral vasoconstriction may accompany low cardiac output and blood pressure. Although, in one retrospective study, the risk of arterial thrombosis was higher in pediatric patients receiving a femoral arterial catheter versus other locations. In this study, younger age was also associated with a higher rate of thrombosis ( ). In less dire circumstances, the selection of a vessel may reflect a variety of anatomic and physiologic characteristics exhibited by certain vessels. The pedal vessels exhibit pressure wave amplification that results in pressure determinations exceeding aortic values by as much as 30% ( ).
After palpation and localization of the artery with the nondominant hand and/or ultrasound, one can cannulate the artery either by inserting the catheter directly using a catheter-over-needle device or by using the Seldinger technique. The Seldinger technique involves entering the vessel with a needle, placing a guidewire through the needle after the vessel is entered, removing the needle, and then placing the catheter over the wire into the vessel. A 22- or 24-gauge catheter is appropriate for peripheral artery cannulation in infants and children younger than 5 years, whereas a 20-gauge catheter may be substituted in older children. Aseptic technique should always be followed when placing an arterial catheter. When cannulating a peripheral artery, it is helpful, although not necessary, to immobilize the extremity with a board.
Ultrasound-guided arterial catheterization and a Doppler flow transducer can also be used to locate an artery that may be difficult to palpate (see Chapter 20 : Ultrasound). Ultrasound guided arterial catheterization is rapidly becoming widespread and evidence has emerged that suggests it can be clinically superior to more traditional techniques such as palpation or Doppler assistance. In a Cochrane meta-analysis that incorporated five different randomized control trials, the authors concluded that there was moderate-quality evidence to suggest that ultrasound guidance for radial arterial line placement was better for first and second attempt success rate and lower rates of complications compared with traditional cannulation techniques. This may particularly be true for smaller and younger patients ( ). Some studies have suggested that ultrasound guidance for arterial cannulation be considered best practice ( ). Ultrasound guidance allows for arteries that are typically not accessed, such as the posterior tibial artery, to become more reasonable alternatives to the radial artery in small children ( ).
Surgical cutdown may be the preferred option for patients in whom percutaneous placement is likely to be difficult or has failed. Indwelling arterial catheters are associated with several potential complications, including proximal emboli, distal ischemia, arterial thrombosis, and infection. Thrombosis of the radial artery is generally temporary, although it is more likely to persist after a cutdown ( ). Although small flush volumes (0.3 mL) in radial arterial catheters can be detected in the aortic arch vessels, cerebral infarcts have not been reported ( ).
In neonates, the umbilical artery is a potential site for cannulation. The tip of an umbilical artery catheter should be placed in either a high (above the diaphragm) or a low (below L-3) position to avoid direct flushing into the renal arteries. Despite these precautions, as many as 10% of neonates exhibit hypertension as a late complication attributed to umbilical artery catheterization ( ; ; ). Minor complications of umbilical artery monitoring include vasospasm of the lower extremity vessels, which are more common with low tip placement. Major complications (e.g., necrotizing enterocolitis, renal artery thrombosis) occur independent of location ( ; ). The rarity of clinical complications is remarkable given an incidence of aortic thrombosis on removal of umbilical artery catheters that approaches 95% in some series ( ), although most series define the incidence at 12% to 31% of neonates ( ; ; ). Gleich and colleagues demonstrated that the overall major complication rate of arterial cannulation for monitoring in pediatrics was 0.2% over the course of 5142 arterial cannulations in a single institution. Furthermore they concluded that all major complications occurred in femoral arterial catheters in children younger than 5 years of age and, importantly, no complications in the distal arterial cannulation sites were reported. Infants and neonates had the greatest complication rates, at 16 and 11 per 1000 arterial catheters, respectively ( ).
Systolic pressure variation, pulse pressure variation (PPV), and stroke volume variation (SVV) are noninvasive methods for evaluating volume status and fluid responsiveness ( ). It is defined as the difference between the maximal and minimal values of systolic blood pressure during a positive pressure breath. Initially during a positive pressure breath, there is a transient increase in systolic blood pressure (delta up) followed within 4 to 5 beats by a decrease in systolic blood pressure (delta down). Increases in intrathoracic pressure during positive pressure ventilation cause a decrease in systolic blood pressure because of decreased preload to the right ventricle, increased afterload to the right ventricle, and decreased afterload to the left ventricle. This decrease is greater during hypovolemia. Systolic pressure changes in response to respiratory variation have been used to determine hypovolemia ( ) ( Fig. 18.2 ).
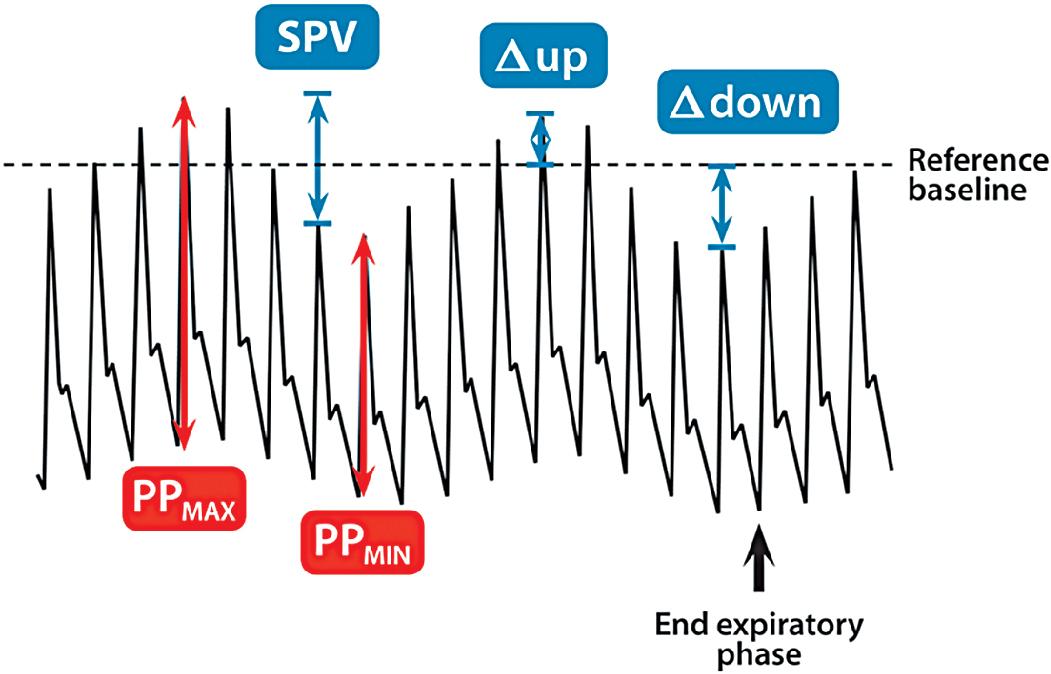
The difference between the maximal systolic blood pressure and minimal systolic blood pressure during a respiratory cycle may help predict volume status. In fact, when this value is divided by the mean of the two systolic pressure values, it provides a percentage of respiratory change in arterial pulse pressure. The equation for this calculation is as follows:
demonstrated a strong relationship in mechanically ventilated adult patients between pulse pressure changes and cardiac output. Patients with pulse pressure changes (delta PP) that are greater than 10% may be fluid responsive and benefit from the administration of intravenous fluids.
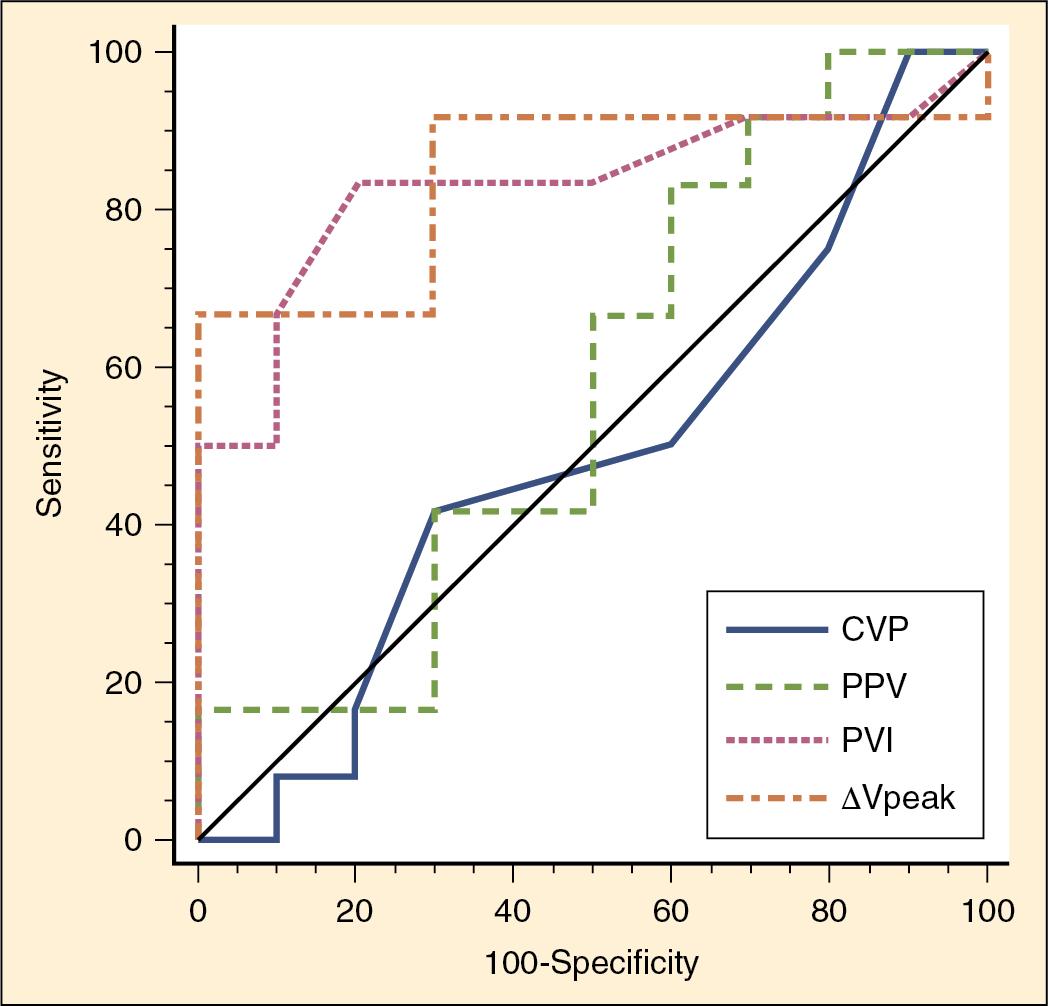
In children, pulse pressure changes may not be as prognostic of fluid responsiveness because of higher chest/lung compliance and higher vascular compliance, resulting in less predictable changes in blood pressure in the face of hypovolemia. Changes in the waveform of pulse oximetry plethysmographic waveform amplitude have been shown to predict fluid responsiveness and may be more predictive than CVP in mechanically ventilated children ( Fig. 18.3 ) ( ; ). Bedside use of this variable is challenging. The Pleth variability index (PVI) automatically calculates the waveform amplitude variation and may predict fluid responsiveness noninvasively ( ).
Four relative indications exist for central venous catheterization: inadequate peripheral venous access, central venous pressure monitoring, infusion of hyperosmolar or sclerosing substances, and a planned operative procedure with a high risk of hemodynamically significant venous air embolism. There is no absolute indication for central venous pressure monitoring in pediatrics. Unlike direct systemic arterial pressures, central venous pressure itself rarely provides the sole basis for therapeutic action. It does, however, provide useful information that, taken together with other data, helps to form a management plan. The procedures in which this monitoring deserves consideration include those involving large estimated blood loss or fluid shifts (>50% EBV), deliberate hypotension, cardiac surgery with cardiopulmonary bypass, situations in which the usual signs of hypovolemia are likely to be misleading (e.g., renal failure, congestive heart failure), and procedures with expected moderate blood loss or fluid shifts. The normal values for central venous pressure in children are similar to those in adults (mean, 2 to 6 mm Hg).
Every insertion site that has been used in adults can be used in children. Access to the central circulation can be achieved from the internal and external jugular, subclavian, basilar, umbilical, and femoral veins. Proper insertion depth and subsequent length of the central line catheter need to be taken into consideration. If the position of the tip is too shallow the catheter can be more easily displaced out of the vessel or lead to inaccurate central venous pressure measurement. On the contrary, if the position is too deep, the catheter may enter the right atrium and lead to erosion of the catheter tip through the wall of the right atrium ( ). Other risks include cardiac tamponade ( ; ) and increased risk of thrombosis ( ). The catheter should be advanced only until the orifice lies in the intrathoracic great vessels, where the junction of the RA and the superior vena cava meet, and its position should be confirmed radiographically. Because of the small size of the pediatric patient, proper length, depth, and positioning are critical as small changes in length can lead to inappropriate positioning.
Catheters of various sizes (2.5 to 10 Fr), lengths, and composition are available for pediatric applications (Cook Critical Care, Bloomington, IN, and other companies). Selection is based on the size, including the height and weight of the patient ( ) and the purpose of the catheter ( Table 18.6 ). Length of central line catheter is an important consideration because of the varying nature of pediatric patient sizes. While the determination of central line length in pediatric patients has been shown through the use of mathematical calculations, anatomic landmarks, transesophageal echocardiography, and others, radiographic confirmation is widely used for confirmation.
| Weight (kg) | Age | Size (French) | Insertion Length (cm) |
|---|---|---|---|
| <5 | 0–2 months | 3–4 | 4–5 |
| 5–10 | 2 months–2 years | 4 | 6–7 |
| 10–30 | 2–10 years | 4–5 | 8–10 |
| 30–50 | 10–15 years | 5 | 11–13 |
| >50 | >15 years (adult) | >5 | 13–15 |
The composition of the catheter depends on its intended use. Teflon is more resistant to thrombus formation, but concerns about catheter perforation have prompted the development of softer materials, especially for long-term use (e.g., Silastic and polyurethane). Catheters are generally inserted via a Seldinger technique using landmarks and techniques similar to those used in adults.
While there are no absolute contraindications to placement of a central venous catheter, each site has potential risks. All sites share the common complications of infection (site cellulitis, bacteremia), venous thrombosis with potential emboli, air embolism, catheter malfunction (occlusion, dislodgment, or fractures), dysrhythmias (when the catheter tip is in the heart), and bleeding. Universal precautions and sterile technique should be used when placing a central venous catheter. Risks involved in cannulating the internal jugular vein include carotid artery puncture, Horner’s syndrome, pneumothorax, and injury to the thoracic duct with cannulation of the left internal jugular vein. The high approach to the internal jugular vein, at the midpoint of the sternocleidomastoid muscle, results in comparable success, with fewer complications than lower approaches ( ).
Ultrasound guidance improves localization of the internal jugular vein and increases the success rate of central venous cannulation in adults and children, especially because there is significant variation in the relationship of the internal jugular vein and the carotid artery ( ; ; ) ( Fig. 18.4 ). Ultrasound guidance for central line placement was shown in a meta-analysis to reduce the incidence of failed line placement and inadvertent arterial punctures in pediatric patients compared with landmark-only technique ( ). Ultrasound allows for variation in anatomy to be visualized in real time during placement of central lines.
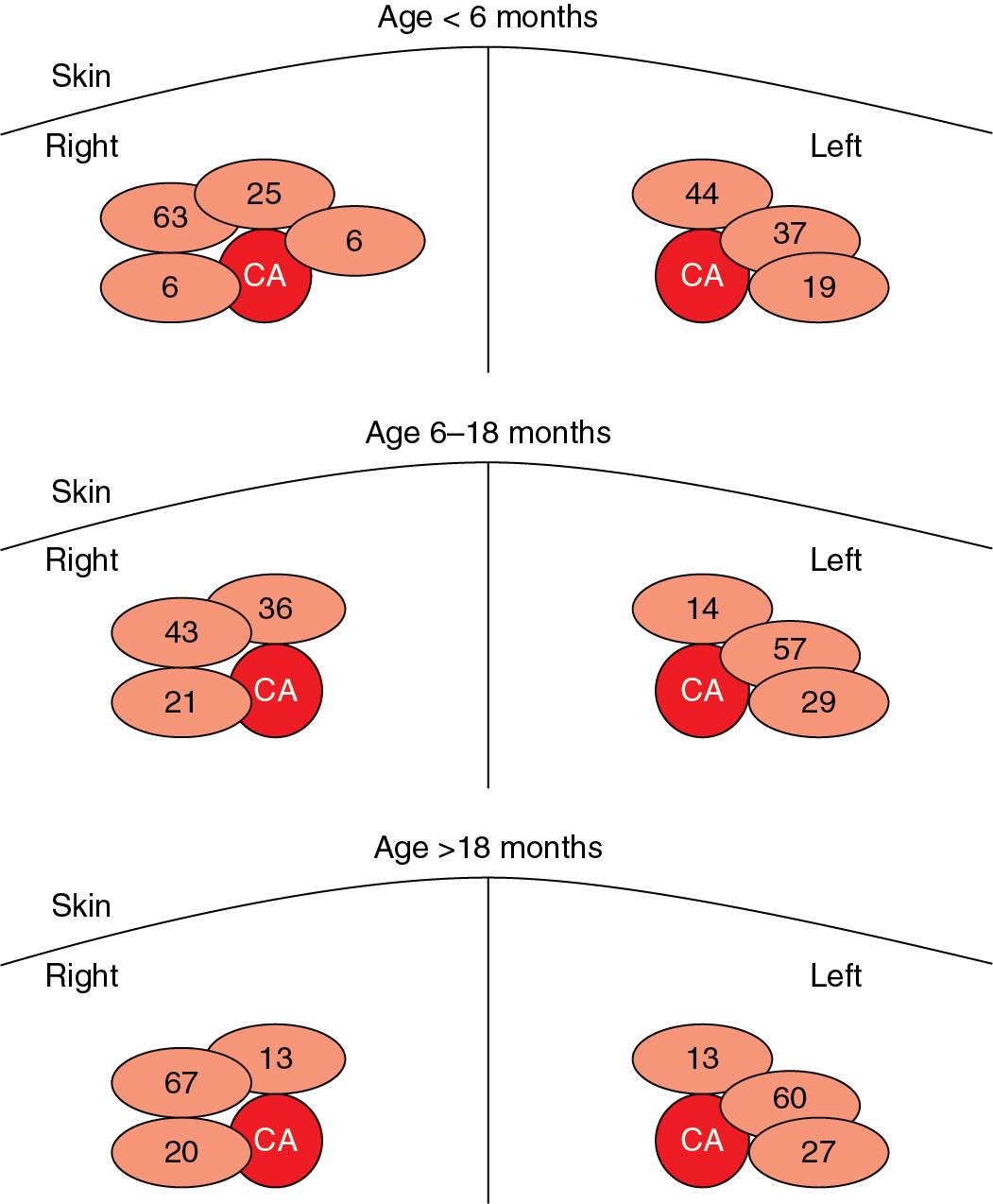
Variation in anatomy has also been shown to be influenced by the patient’s head position ( ; ), demonstrating the need to appropriately understand optimal positioning during line placement. Using ultrasound techniques, reported an 18% prevalence of anatomic variations in children younger than 6 years that would preclude or significantly hinder the successful cannulation of the internal jugular vein using anatomic landmarks alone. In addition, reported that rotating the head away from the neutral position increases the degree of carotid artery and internal jugular vein overlap and decreases the incidence of lateral positioning of the internal jugular vein to carotid artery ( ).
Mixed venous oxygenation is defined as the oxygenation saturation or content of venous blood from the pulmonary artery. When the Fick equation is solved for mixed venous saturation (SvO 2 ), four variables are necessary to determine mixed venous saturation: oxygen consumption (VO 2 ), cardiac output, hemoglobin, and arterial oxygen saturation.
Normal values for mixed venous saturation range from 65% to 75%. A decrease in SvO 2 occurs because of increased oxygen consumption (stress, pain, hyperthermia, shivering) or decreased oxygen delivery (anemia, decreased cardiac output, decreased Pa o 2 , decreased Sa o 2 ). An increase in SvO 2 occurs because of a decrease in oxygen consumption (hypothermia, anesthesia) or an increase in oxygen delivery (increased hemoglobin, increased cardiac output, increased Pa o 2 , increased Sa o 2 ). Mixed venous oxygenation is a global index of tissue oxygenation and is used to assess the balance between oxygen delivery and oxygen consumption for patients in the operating room and intensive care unit.
A significant limitation of mixed venous saturation, particularly in smaller patients, is the necessary collection of blood from the pulmonary artery. Blood from the superior vena cava (central venous ScvO 2 ) and right atrium (SraO 2 ) have been investigated as possible surrogate markers for mixed venous oxygen saturation (SvO 2 ) with mixed results. found a significant variation between measurements of central venous saturation (ScvO 2 ) and mixed venous saturation within the same patient. However, although absolute values do not correlate, there is a correlation between trends in ScvO 2 values and SvO 2 values. In a retrospective pediatric study, identified a correlation between venous oxygen saturation measured in the right atrium and ScvO 2 .
Since the introduction of the flow-directed balloon-tipped pulmonary artery (PA) catheter in 1970, indications for its use in pediatric patients have remained limited. Additionally, interpretation of the flow data generated can be hindered by several factors. First, the desired cardiac output varies according to age, disease state, and other elements of management that alter metabolic demand in complex ways, thereby introducing significant uncertainty in assigning a target value. Second, the existence of intracardiac communications that permit right-to-left or left-to-right shunting of blood can result in measurement discrepancies. In patients with congenital heart malformations, the risk of improper placement of the flow-directed PA catheter is also increased. Finally, despite several studies demonstrating reasonable accuracy when thermodilution is compared with other methods of flow determination, such as the Fick equation ( ) and dye dilution ( ), the precision of these determinations in small infants is low and has a 25% intersample variability. Alternatively, directly placed PA catheters can provide necessary information regarding pulmonary vascular resistance and residual left-to-right shunts, and left atrial catheters may be used to reflect filling and diastolic function of the left ventricle after cardiac surgery.
Clinical situations in which PA catheters can potentially provide useful information include children with pulmonary arterial hypertension, severe respiratory failure, refractory shock, and multiorgan dysfunction ( ). In children with severe coexisting pulmonary and circulatory failure, PA catheters can help quantify the hemodynamic effects of extreme respiratory support measures and guide complex fluid and pharmacologic regimens. They may also be useful in patients with underlying pulmonary hypertension or poorly compensated left ventricular dysfunction who undergo acute surgical stress (e.g., arteriovenous malformation clipping or aortic cross-clamping). Given the uncertainty regarding optimal systemic flow in a child, mixed venous oxygen saturation may serve as a better indication of oxygen delivery and global perfusion.
Pulmonary artery catheters can be technically difficult to insert, especially in infants or in children with low cardiac output. They may be placed in any vein used for access to the central venous system, but the most frequently used veins are the right internal jugular and the femoral. In infants and children smaller than 15 kg, it is technically difficult to place an introducer sheath in the neck vessels; the femoral veins are preferable. Multilumen catheters capable of thermodilution are available in two sizes, 5 and 7 Fr, with four options for the right atrium–pulmonary artery interluminal distance. Catheter recommendations are based on age ( Table 18.7 ). The proper placement of these catheters can be time consuming, and thus the assistance of fluoroscopy is recommended in infants and children less than 30 kg and in larger children who have a low cardiac output.
| Age (Years) | Size (F) | Distance (cm) |
|---|---|---|
| Newborn to 3 | 5 | 10 |
| 3–8 | 6 | 15 |
| 8–14 | 7 | 20 |
| >14 | 7 | 30 |
The risks of balloon-tipped PA catheters are numerous and include the risks of central venous catheter placement discussed previously and the complications seen in adult patients with PA catheters: infection, air emboli, thrombus, PA rupture, acute right bundle branch block, and intracardiac knots. Certain complications are more common in children: generation of misleading information, paradoxical systemic emboli, disruption of an intracardiac repair, and high-grade right ventricular outflow tract obstruction secondary to the relatively large balloon diameter. The presence of intracardiac and extracardiac malformations may result in an aberrant catheter course, leading to incorrect data as well as an increased risk of systemic emboli.
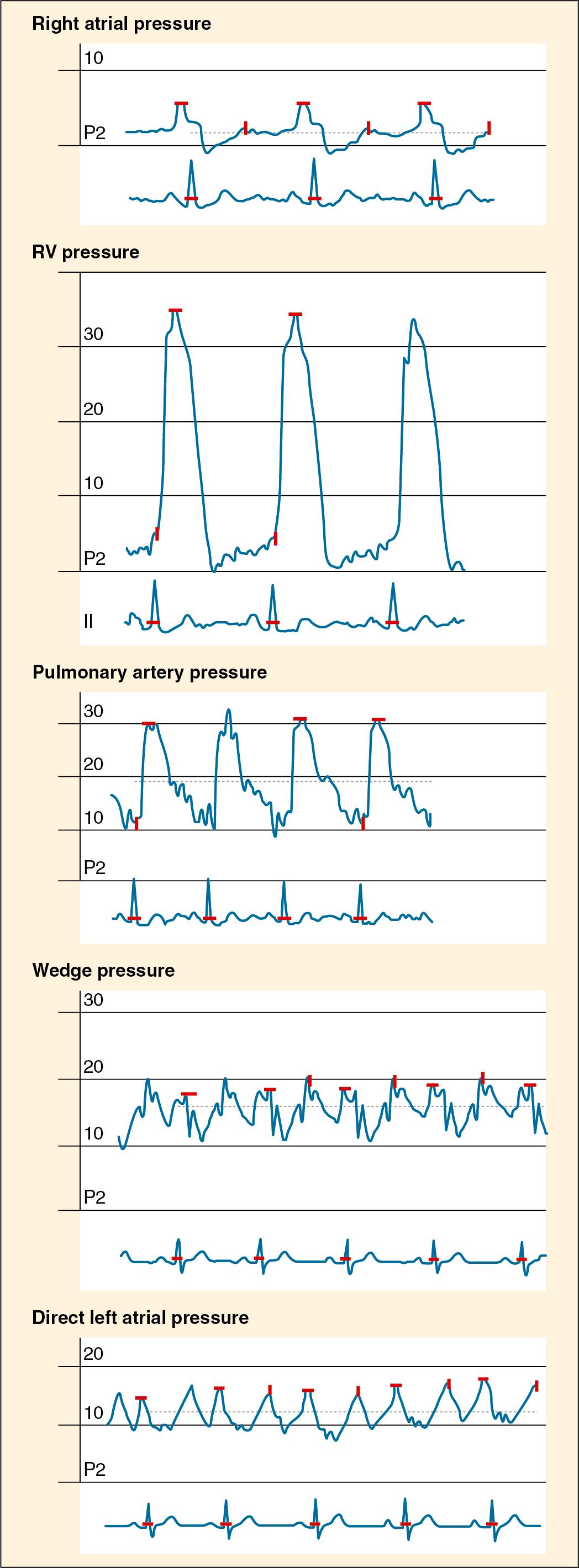
Characteristic waveforms seen as the catheter tip traverses the cardiac and pulmonary system are shown in Fig. 18.5 . Cardiac output can be estimated in children through indicator dilution (e.g., thermodilution or dye dilution) and noninvasive techniques. Transesophageal Doppler determinations of aortic blood velocity can be used to quantify system flow. These flow measurements have been validated by corresponding thermal dilution measurements. However, the probes are sensitive to positional changes, and optimal signal acquisition can be challenging, particularly for less experienced practitioners ( ; ). Thoracic bioimpedance, a method that estimates stroke volume on the basis of changes in thoracic impedance, has been applied to children as small as 3.6 kg. Although some correlation exists between bioimpedance and indicator dilution methods, reproducibility is poor ( ; ). The Electrical Cardiometry (ICON, Osypka Medical, San Diego, CA), which estimates changes in cardiac parameters by measuring changes in thoracic electric bioimpedance during the cardiac cycle, was shown to provide real-time information regarding developing hemodynamic events and successfully track responses to hemodynamic interventions in children of all ages ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here