Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Movement disorders, such as Parkinson disease (PD), are currently treated pharmacologically by systemic administration of drugs that replace, mimic, or potentiate lost neurotransmitters, and more recently with neurosurgical procedures such as deep brain stimulation (DBS) of the subthalamic nucleus (STN) or internal segment of the globus pallidus (GPi) , to replace older, more destructive procedures such as lesioning of the basal ganglia. However, advances in understanding of the pathophysiology underlying movement disorders, coupled with technical improvements in and increasing experience with stereotactic surgery for these diseases, have paved the way for application of molecular therapies to treat human movement disorders. Molecular therapies delivered with stereotactic surgical techniques offer the potential to combine the benefits of focal therapies that would not adversely influence other brain regions with the specificity of intervening in biological processes that may more effectively and efficiently alter or improve abnormal neuronal functions in movement disorders.
Molecular therapies can be subdivided into three fundamental categories, including infusions of chemicals, drugs, and neurotransmitters; infusion of growth factors or recombinant proteins; and transfer of genetic material using viral and nonviral vectors. While there have been an extremely large number of preclinical studies in each of these categories using animal models of movement disorders over the past 20 years, the clinical experience in human patients in each of these areas has been impressive. To date, none of these therapies has reached the point of regulatory approval for general sale and use. Nonetheless, there have been several promising studies that have supported ongoing late-stage clinical trials. The experience gained with all of the human studies, regardless of outcome, has helped advance the field of molecular therapies for functional neurosurgical disorders to the edge of a major breakthrough that will hopefully provide new options for neurosurgeons in the near future.
Drug infusion therapy directly into the central nervous system (CNS) is an attractive treatment modality in movement disorder patients with advanced disease. In PD, for example, the effectiveness of oral L-dopa therapy is limited due to its relatively short half-life, and the pulsatile stimulation of dopaminergic neurons can cause significant motor fluctuations and dyskinesias. Focal infusion of therapy to the area of dysfunction, however, could help achieve more constant striatal dopaminergic stimulation, and more closely mimic the body’s normal physiologic state.
The first demonstration of the effectiveness of focal infusion of medications within the brain parenchyma was conducted in PD patients, who were tested prior to stereotactic lesioning or electrode placement. These patients were infused with small-volume, low-dose muscimol, a GABA-A agonist, into the GPi just prior to pallidotomy, and they displayed a rapid onset of improvement in finger motor speed, reduced extremity tone, and decreased muscle fatigue, which all returned to baseline slowly after approximately 30 minutes. Following subsequent pallidotomy, the patients displayed a similar improvement in motor symptoms. In addition, studies in MPTP-treated nonhuman primates have demonstrated that local inactivation of neurons in the STN results in an improvement in parkinsonian symptoms. These observations prompted intraoperative exploration of microinjections of either lidocaine, an anesthetic that selectively blocks axonal sodium channels, or muscimol into the STN of six PD patients to study the effects of local STN inactivation. This resulted in transient improvement in akinesia, rigidity, and limb tremor in patients with PD. Electrophysiologic recordings were also performed, which confirmed inhibition of electrical activity in the nucleus following drug infusion as it spread from the injection site to more distant tissue.
Although muscimol microinjection and electrical stimulation by STN DBS have the same effects on PD motor symptoms, their mechanisms of action are quite different. The precise mechanism of action of electrical stimulation on symptomatic improvement in movement disorders is still unclear, and there is support for one or more functions, including excitation of neighboring axons, activation of recurrent inhibitory circuits, and direct inhibition of neuronal firing. As opposed to these more indirect mechanisms, muscimol acts by specifically activating chloride-dependent GABA-A receptors and thereby inhibiting neurons presynaptically. It is still unknown whether continuous infusion of muscimol is superior to stimulation, since the specificity of action provides opportunities for more direct conclusions regarding the relationship between neurotransmitter receptor action and symptomatic improvements, but there may as yet be unknown benefits of some more nonspecific or broader actions of electrical stimulation. In addition, a crucial question that must still be answered is whether patients will develop tolerance to the drug with time. In animals, one unpublished study has demonstrated that parkinsonian rats with unilateral 6-OHDA lesions show sustained effects when muscimol is administered by an implanted osmotic pump for 2 weeks, but longer-term consequences remain to be seen. However, it is clear to every practitioner of DBS that some patients can have adverse effects due to spread of electrical stimulation outside the STN or to nonmotor areas of the STN despite proper electrode location, and this could be less problematic with a biologically specific therapy such as muscimol infusion. The development of appropriate technology for chronic infusions into brain parenchyma, which have yet to be fully developed, would help facilitate development and ultimate determination of the value of continuous muscimol infusion into basal ganglia targets as a therapy.
In addition, patients with essential tremor (ET) have also been studied through a local drug infusion modality, and one study demonstrated the cessation of tremor in patients microinjected with muscimol into the ventralis intermedius thalamus (Vim). In this study, six ET patients undergoing unilateral stereotactic thalamic procedures for relief of tremor refractory to medications were given either saline and/or muscimol intracerebral microinjections prior to thalamotomy or thalamic DBS electrode placement. The patients displayed suppression of tremor for an average of 9 minutes, following a mean latency of 9 minutes to allow for sufficient drug diffusion to influence enough neurons for a therapeutic effect. This not only provides further support for the belief that inhibition of Vim and STN activity can improve symptoms of ET and PD, respectively, but this presents another opportunity for application of direct intraparenchymal drug infusion for a second movement disorder, should technology permit further development of this therapy.
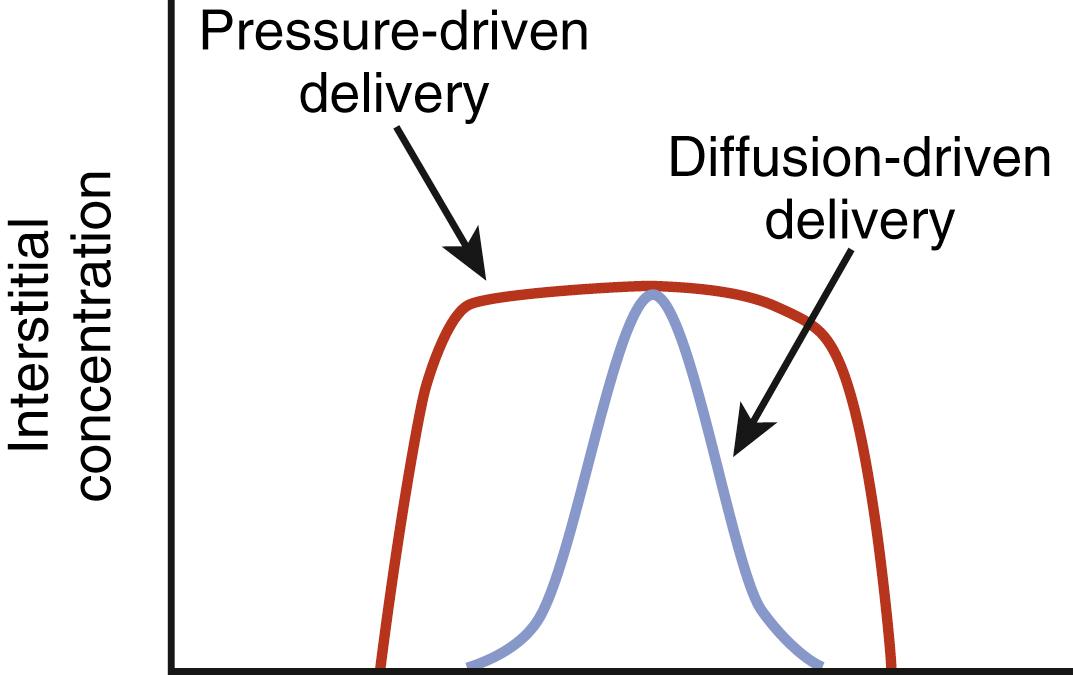
In addition to the therapeutic benefit of local microinjection of drugs such as lidocaine or muscimol, selective infusion of dopamine or dopaminergic medications into the basal ganglia has also been considered, in order to bypass the systemic side effects seen with conventional oral intake of these medications. Chronic intraventricular or intrastriatal dopamine infusions were investigated in 6-OHDA lesioned rats, which demonstrated improvement in motor symptoms following 7 days of continuous dopamine infusion compared to controls. However, local examination of the striatum adjacent to the point of intrastriatal dopamine infusion revealed a barrier, which limited diffusion of medication to only 1 to 2 mm from the catheter. In an effort to increase the size of the perfused region, convection techniques have been attempted that move both fluid and drug into the extracellular space by bulk flow driven by a pressure gradient ( Fig. 108.1 ). The data are still rather limited, and ideal flow rates, catheter diameter, and infusion volume have not yet been determined.
Other techniques that may allow for efficient delivery and monitoring of drug infusions locally within the brain include microdialysis and voltammetry ( Box 108.1 ). Cerebral microdialysis is a modality that allows measurement of the concentration of extracellular neurochemicals within cerebral structures, locally and in vivo. Initially conducted in 1966 by using a dialysis membrane filled with dextran solution into canine cortex, with measurement of local amino acid concentrations, this method has also been applied to PD patients. Currently, cerebral microdialysis consists of a double-lumen probe containing an inlet and an outlet port, surrounded by a semipermeable membrane, and sealed above and below a point at which the two tubes are inserted into the membrane lumen. This dialysis probe can be inserted through a burr hole to a preset depth through the brain parenchyma, allowing for perfusion of solutions through the inlet port as well as analysis of the returning solution for extracellular molecule concentrations with techniques such as high-performance liquid chromatography with electrochemical detection (HPLC EC). HPLC EC provides high sensitivity and specificity for the measurement of molecules, such as biogenic amine, including noradrenaline, dopamine, and serotonin, as well as for amino acids, including glutamate, aspartate, and GABA.
Muscimol (GABA-A agonist). Lidocaine (Na + -channel antagonist). Dopamine (both intrastriatal and intraventricular infusions).
Extended monitoring levels of a particular neurotransmitter in probe region; may allow for pump that would administer a drug or chemical in response to a certain level, allowing for achievement of steady-state levels of that neurotransmitter.
Detects changes in the concentration of certain neurotransmitters (transmitter oxidation), and allows for excellent temporal and spatial resolution of their release in real time.
Some limitations of microdialysis, however, include invasiveness, reports of complications in the literature, dependence of accuracy on factors such as flow rate and probe size, and a local fibrotic reaction to the probe tip seen during chronic microdialysis experiments. , In addition, the neurochemicals sampled during microdialysis are not only those released synaptically, but also include neurochemicals of local metabolism, capillary delivery, and neuronal release and uptake from groups of cells. , Despite these limitations, microdialysis has been used successfully in human patients, ranging from traumatic brain injury and subarachnoid hemorrhage to movement disorders and epilepsy. , In movement disorders, particularly PD, there have been published studies such as one demonstrating the changes in concentrations of GABA, cGMP, and glutamate before, during, and after 1 hour in the internal GPi, anteroventral thalamus, and putamen of six patients with refractory PD undergoing DBS in the STN. , In addition, an in vivo microdialysis method was developed permitting the continuous, chronic sampling of local neurotransmitter concentrations in the STN and substantia nigra pars reticulata (SNr) of patients for several days after placement of STN DBS electrodes. All six patients in this study had no complications, and the microdialysis probes were successfully removed without disrupting the DBS electrode location. In theory, microdialysis use may be extended to monitoring levels of a particular neurotransmitter in the region where the probe is implanted, which could then be attached to a pump that would administer a drug or chemical in response to a certain level, allowing for achievement of steady-state levels of that neurotransmitter. Microdialysis has in fact been used to monitor systemic drug levels and to infuse agents into the brain in epilepsy patients, and this could easily be extended to movement disorders if improved technology permitted chronic use of these devices.
In addition to microdialysis, voltammetry is another method that can detect changes in the concentration of certain neurotransmitters, and allows for excellent temporal and spatial resolution of their release in real-time. , Voltammetry is based on the principle that oxidation of certain chemicals leads to the release of electrons, and the resultant current can be measured with an electrode. Furthermore, a dialysis electrode was developed that combined microdialysis with voltammetry to measure glutamate, which specifically contained a dialysis probe with an electrochemical detection system utilizing the enzyme glutamate oxidase to detect glutamate levels. Other systems have been designed that measure the concentrations of molecules such as glucose and oxygen. , Most applications have currently been in animal models, including the construction of a micromanipulator in rats that allowed for both infusion of drugs and voltammetry in freely moving rats and mostly studied in tumors, cerebral ischemia, and traumatic brain injury. However, the application of voltammetry has also been extended to the measurement of dopamine in both rats and nonhuman primates. Voltammetry has been used to measure extracellular dopamine concentration changes in rats, such as during certain behaviors or in response to cocaine administration in the nucleus accumbens. In regard to movement disorders, there was a recent study using carbon fiber voltammetry to measure the release of dopamine in MPTP-induced dopaminergic lesions in nonhuman primates. In addition to its utilization in probing drug effects on dopamine release and uptake, there may also be future applications of voltammetry to measuring dopamine release in patients, with tailored therapeutic release of medication by external or internal means in response to certain changes in levels. Although microdialysis can sample any neurotransmitter that can diffuse across the dialysis membrane, voltammetry is currently limited to monitoring a select population of neurotransmitters due to technical constraints of the voltammetry methodology to measure transmitter oxidation. However, because it does not involve a membrane or a tube, physical blockade of fluid diffusion due to luminal obstruction is not a concern and oxidation could be more easily measured by an implanted electrical device compared with chemical analysis of dialysate fluids. Therefore voltammetry could represent an attractive prospect for long-term monitoring of brain neurotransmission as a component of a dynamic feedback mechanism to tailor intraparenchymal drug delivery to specific patient needs.
As an alternative to infusion of drugs into the CNS for the treatment of movement disorders, another potential strategy employs the infusion of growth factors or recombinant proteins. Since the central feature of patients with PD is the loss of dopaminergic neurons in the substantia nigra, researcher proposed the idea of administrating neurotrophic factors to degenerating neurons in order to promote their survival. Specifically, the leading candidate neurotrophic factor was glial cell–line-derived neurotrophic factor (GDNF), discovered in 1993 and shown to enhance the survival of dopaminergic neurons in vitro. In animal models, the goal of GDNF was to prevent further neuronal loss while encouraging reinnervation of the striatum from any surviving, intact terminal within the striatum. Since GDNF is a 134–amino acid peptide, it does not efficiently cross the blood brain barrier, and thus it must be delivered locally into the CNS either by administration into the ventricular system or directly into the brain parenchyma. In preclinical studies with both 6-OHDA- and MPTP-lesioned animal models, GDNF was delivered using both approaches into the CNS, and it indeed did show some benefit.
Eventually, infusion of a recombinant form of GDNF was administered in human clinical trials ( Box 108.2 ). Initially, 50 patients with moderately advanced PD were treated with intraventricular GDNF; however, the infusion trial was stopped early due to excessive complications from toxicity to the periventricular structures. Furthermore, there was a lack of therapeutic efficacy, potentially due to failure of GDNF diffusion across the greater distance between the ventricular system and substantia nigra when compared to nonhuman animal models. Subsequently, this led to direct intraparenchymal infusion of GDNF into the striatum (postcommissural putamen) in an open-label phase I study that demonstrated both encouraging safety and efficacy results. Five PD patients were treated, with a resulting significant decrease in total Unified Parkinson’s Disease Rating Scale (UPDRS) score in the “off” state at 3 months, as well as mean reduction in UPDRS motors score at 12 months of 48%. There were also correlational 18 F-dopa PET scans showing an increased uptake in the area of the GDNF infusion. Since 18 F-dopa PET measures dopamine synthetic capacity and by extension dopaminergic innervation of the striatum, these results did support potential enhancement of dopaminergic function in the remaining viable nigrostriatal neurons, and a postmortem case from the study later did demonstrate sprouting of dopaminergic fibers around the GDNF infusion site.
Loss of dopaminergic neurons in substantia nigra
Promote survival of degenerating dopaminergic neurons by neurotrophic factors. Encourage reinnervation of the striatum by remaining, intact intrastriatal terminals.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here