Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Writing of this chapter was supported by National Institutes of Health grants R01-DK-412174 (F.K.G.), R01-DK-33209 (F.K.G.), and R01-DK-73638 (F.K.G. and H. Xu).
The mammalian intestinal tract undergoes dramatic changes during the first few weeks of postnatal life. Genetic and neurohormonal regulators influence the changes in digestive and transport functions that mediate the development of the gut. In precocial species, such as rats and mice, gut maturation occurs predominantly after birth during the suckling and weaning periods. Conversely, in altricial species, such as humans and pigs, significant gut maturation occurs during the late fetal period, and they are born with limited absorptive and secretory function. In all mammals, the most significant changes occur during the suckling/weaning transition. At birth, the digestive and secretory function of the intestine is not fully developed, and thus nutrient absorption occurs throughout the small and large intestines and along the entire length of the crypt-villus axis. Contents of the mother’s milk signal intestinal maturation. These milk-derived factors (i.e., immunoglobulins, growth factors and peptide hormones, and antigens such as bacteria) affect intestinal function and regulate the expression of genes involved in nutrient transport and immune function. On weaning, the gut epithelium is under the influence of a different set of physiologic regulators and dietary factors; thus, alterations in function rapidly ensue. This chapter reviews the key discoveries that have led to the current understanding of gastrointestinal function during ontogeny. This chapter also discusses secretory, digestive, absorptive, and regulatory aspects of gut maturation and, whenever possible, provides insight into the molecular physiology of experimental findings.
Ontogeny is the development of a single individual, or a system within the individual, from the fertilized egg to maturation and death. As observed in several mammalian species, ontogenetic development of the intestine is an organized process driven by genetic and environmental factors that results in an adaptive, functional intestinal tract. Advances in cellular and molecular biology have allowed a better understanding of the mechanisms responsible for the ontogenetic changes of the mammalian intestine. The development of the gastrointestinal tract in the early stages of life is characterized by morphologic and functional changes of the intestinal epithelium. However, the timing and the rate of maturation depend on the gestational period of mammals. Species with short gestational periods, such as the mouse and the rat, show maturation of their gastrointestinal function at weaning. In contrast, species with long gestational periods, such as the sheep, pigs, and humans, show intestinal development even in utero.
Complete development of gastrointestinal function refers to fully developed secretory, digestive, and absorptive functions; this process is guided by genetic factors and influenced by neurohormonal regulation. The anatomic differentiation of the human fetal gastrointestinal tract occurs by 20 weeks of gestation, and the anatomic structure resembles that of a newborn. However, functional secretion and absorption develop later and at different stages. Intestinal absorptive function is partially developed by 26 weeks of gestation, whereas gastric and pancreatic secretory processes are minimal and can only be partially stimulated in the full-term newborn. The maturation of digestive and absorptive functions of nutrients (carbohydrates, proteins, fats, minerals, and vitamins) in the newborn is determined in relation to the availability of hydrolytic enzymes, such as amylase, glycosidases, lactase, protease, and lipase.
The gastrointestinal epithelium consists of multiple functional cell types. The stomach has parietal cells, chief cells, and mucous neck cells. The intestines have enterocytes, enteroendocrine cells, goblet cells, and Paneth cells. Enterocytes absorb water, solutes, vitamins, and other nutrients. Enteroendocrine cells secrete peptide hormones. Goblet cells produce a variety of mucins, and Paneth cells secrete digestive enzymes, growth factors, and antimicrobial cryptdins. In the crypt compartment of the intestines lies a small group of proliferating, undifferentiated stem cells that give rise to several cell types that migrate onto adjacent villi. All four epithelial cell types of the intestine are differentiated from the common stem cells in the crypt compartment. The enterocytes, goblet cells, and enteroendocrine cells differentiate as they migrate up the crypt-villus axis turning over every 5 days while Paneth cells migrate downward turning over more slowly. As mature enteroendocrine cells and enterocytes migrate to the tips of the villi, they presumably undergo apoptosis and are extruded into the lumen.
How stem cells are allocated to differentiate into different cell types at different stages of development is not fully understood. One proposed explanation is that “hardwired” genetic programming coupled with dietary influences as triggered by neurohormonal factors regulate the development of gastrointestinal function. In the past decade, molecular biology techniques have been widely applied to investigate the development of gastrointestinal function. This chapter summarizes the most recent updates in the ontogeny of gastrointestinal function, especially in the developmental regulation of overall gastrointestinal function.
Gastrointestinal secretion occurs throughout the gut from the salivary glands, the stomach, the pancreas, the small intestine, to the colon. Each segment has specialized secretory functions. In the stomach, acid secretion acidifies the gastric lumen and creates an environment that promotes protein and bacterial breakdown. Bicarbonate secreted by the pancreas is released into the duodenum to neutralizes the gastric acid to ensure the proper pH conditions for digestive enzyme function in the intestine. Chloride secretion drives active intestinal secretion. Thus, these secretory processes are critical for normal gastrointestinal functions, including digestion and absorption. As the digestive tract develops in the organism, developmental changes also occur in secretory functions of the different segments of the gastrointestinal tract. Genetic, environmental, and dietary factors guide these changes.
Gastric acid secretion . Gastric acid secretion is a critical step for food digestion, which activates enzymes that break protein bonds into smaller absorbable units and more available for intestinal enzymes. Gastric acid also functions as an antimicrobial agent by creating an environment inhospitable to bacteria. The production of acid by the stomach is a tightly controlled physiological process that involves neural and hormonal mechanisms and the input of several epithelial cell types. Studies have advanced our understanding regarding the molecular ontogenesis of the stomach and the factors controlling stomach innervation, as well as the differentiation of gastric epithelial cell lineages and their respective hormones/factors that influence acid production. All gastric epithelial cells are originated from a common stem cell in the isthmus/neck region of the gastric glands. In humans, a variety of mediators/hormones and cell types are involved in acid production and regulation. Although gastric acid production occurs at birth in the term infants as well as in preterm infants, the physiological management of gastric acid is age-dependent. Illnesses associated with altered gastric acid secretion are more common in neonatal patients compared to older children, and this condition affects more preterm infants than term infants among neonatal ICU patients. The primary player for gastric acid production in parietal cells of the gastric mucosa is the H + /K + /ATPase, which is situated on a secretory membrane. By the 13th week of gestation, parietal cells and gastric H + /K + /ATPase can be detected, and their presence increases significantly with gestational age. The H + /K + /ATPase is an α,β-heterodimeric enzyme that exchanges cytoplasmic H + with extracellular K + . The β-subunit of the gastric H + /K + /ATPase is required not only for the normal digestive function but also for normal development of the gastric mucosa and attainment of the normal structure and composition of secretory membranes. Although the gastric H + /K + /ATPase is strongly regulated by gastrin, the precise role of gastrin in regulating acid secretion during early postnatal life is unclear. In human newborns, high circulating levels of gastrin is detected, but these newborns have low acid secretion. On the other hand, exogenous gastrin is ineffective in stimulating acid secretion, despite the presence of anatomically developed parietal cells in newborns. These findings suggest that gastrin not only regulates acid secretion but also modifies the programmed differentiation of gastric epithelial linages.
Water and electrolytes secretion . The gastrointestinal tract is known for its absorptive and secretory roles, and interplay among the secretion of water and electrolytes is central to these physiologic functions. Secretion of bicarbonate, potassium, and chloride occur along the entire intestine. Potassium is passively secreted down the electrochemical gradient through apical K + channels. On the other hand, fluid secretion, which usually occurs in the colon, is driven by chloride secretion. Chloride is secreted into intestinal lumen via the coordinated action of the basolateral Na + /2C1 − /K + cotransporter (NKCC1) and the apical cystic fibrosis transmembrane conduction regulator (CFTR). Accompanying chloride secretion is the paracellular and transcellular movement of sodium. The resulting luminal accumulation of sodium chloride provides the osmotic basis for water movement. The movement of chloride also supports some bicarbonate secretion. Apical anion exchangers bring chloride into the cell in exchange for bicarbonate. The ductal epithelium-mediated
secretion in the pancreas has several functions. It neutralizes the gastric acid and protects the intestinal mucosa and also protects the pancreatic-biliary tract from ascending infection. The mechanism of regulating bicarbonate secretion is a complex process involving neural, hormonal, and intracellular signal transduction in ductal and acinar pancreatic cells. Interestingly, the intestinal secretion of K + , Cl − , and
changes with age. Colonic K + secretion is higher in suckling rats, and this process is regulated by aldosterone. Although the intestine is considered incompletely developed during the suckling stage, intestines of many mammalian species are capable of secreting chloride during this early stage of development. In addition, the activity of cyclic adenosine monophosphate (cAMP)-dependent chloride channels and the CFTR transcript have been detected even in the fetal intestine, and their expression levels were similar in prenatal and early postnatal development. Chloride secretion also varies segmentally during development. In pigs, Cl − secretion in the jejunum is higher in young piglets, while Cl − secretion in the colon is the same between young piglets and grown pigs.
Antidiarrheal function . The fact that adults are more resistant to bacterial enterotoxin-induced secretory diarrhea reflects on the age-dependent aspect of secretory function development. The jejunum of 2- and 3-week-old rats have higher sensitivity for heat-stable toxin (ST) of Escherichia coli than that of the adult. Infants aged 0–11 months exhibit a great risk of death after exposure to enteropathogenic E. coli (EPEC). Furthermore, the small intestine of 14-day-old pigs was more sensitive to cholera toxin (CT) than that of 14-week-old pigs.
CT and ST are members of a larger family of A–B toxins of bacterial and plant origin that are composed of structurally and functionally distinct, A and B subunits or domains. A is enzymatically active while B binds to receptors. The mechanism of CT- and ST-induced diarrhea involves the formation of a channel by a pentamer of the toxin’s B subunit and translocation of the enzymatic A1 domain of the A subunit into the target cell cytosol. Resulting covalent modification of intracellular targets leads to the activation of adenylate cyclase and the production of cAMP. cAMP opens Cl − channels and inhibits Na + /H + and
exchangers, which subsequently causes a sequence of events culminating in life-threatening diarrhea. It has been suggested that differential expression of G proteins and ion transporters (e.g., Na + /K + /ATPase and Cl − channels) during development alters the neonatal hosťs responsiveness to CT and ST. Therefore, the developmental control of microvillus membrane receptors, signal transduction mechanisms, and ion transport systems in the gastrointestinal tract may, in part, contribute to the altered host sensitivity in infant toxigenic diarrhea.
The developmental difference in responsiveness to enterotoxins could also be explained by the affinity and the avidity of the receptors on enterocyte membranes, receptor-effector transduction pathways, and neurohormonal regulation. The number of ST receptors increases in the brush-border membranes of porcine, murine, rat, and human intestine during development. . In the pig model, the increase of the susceptibility to ST-mediated diarrheal disease during the first week of life and at weaning is partially caused by an increase of ST receptor numbers. In addition, ST receptor mRNA expression was found to be greatest in the perinatal period in the jejunum and ileum, suggesting that the age-dependent changes in ST receptors are transcriptionally and/or posttranscriptionally controlled. However, the immature gut exhibited increased host sensitivity to CT stimulation that was not correlated with initial receptor binding but was related to decreased Na + /K + /ATPase activity, which suggests that an underexpressed sodium pump may be partially responsible for the high incidence of secretory diarrhea in neonates.
On the level of the receptor-effector transduction pathways, one study found that CT-induced interaction between adenylate cyclase and its receptors is significantly greater in preweaned rats than that in weaned rats. In addition, the CT-induced fluid secretion was closely correlated with increased adenylate cyclase activities. Thus, the increase in adenylate cyclase responsiveness to CT is, at least in part, responsible for the increased incidence of toxigenic diarrhea in neonates. Furthermore, the presence of an antisecretory factor, which naturally inhibits fluid losses in enterotoxin-induced diarrhea, is partially responsible for the age-related reduction in the response to CT. Study has showed that higher levels of antisecretory proteins were found in 28-week-old pigs compared with 3- and 5-week-old pigs. A recent study also showed that RegIIIγ, an antibacterial C-type lectin, produced by goblet cells in neonatal mice and by enterocytes and Paneth cells in adult mice is involved in EPEC infection. These observations suggest that multiple factors are involved in the developmental regulation of intestinal secretion.
Neuronal effect on secretory function . The enteric nervous system appears to modulate intestinal secretion. At least two major common neuronal pathways, cholinergic and noncholinergic, influence the secretory epithelium of the intestinal crypts. The stimulatory effect of CT binding to enterocytes is estimated to explain only one-third of the secretory response. The remainder is induced indirectly through activation of the enteric nervous system. In the small intestine, CT induces the goblet cells to release mucin in addition to the secretory effects. However, exposing cultured goblet cells in vitro to CT does not evoke mucous secretion. Furthermore, CT-induced secretion can be attenuated by 5-HT, or serotonin. These findings suggest that the release of toxins could influence enterocytes and/or activate the enteric nervous system and inflammatory mediators to evoke fluid secretion.
Chemosensory effect on secretory function . The gut chemosensory system also plays roles in intestinal secretion. Short-chain-fatty-acids (SCFAs) are the predominant anions in the content of the large intestine, mainly consisting of acetate, propionate, and butyrate. They are produced by bacterial fermentation of specific indigestible dietary fibers and oligosaccharides. Luminal SCFAs are not only absorbed as nutrients across the intestinal epithelia but also utilized as chemical signals that influence epithelial proliferation, mesenteric blood flow, colonic motility, and colonic ion transport. Recent studies demonstrate that SCFAs produced by gut bacteria stimulate colonic
secretion though SCFA receptors FFA2 (GPR43) and FFA3 (GPR41).
Carbohydrates digestion . Dietary adaptation from the neonatal period to adulthood dramatically changes the expression of digestive enzymes in the small intestine. Disaccharidases are located in the brush-border membrane of mature enterocytes of the small intestine, and their activities are the greatest in the proximal and mid-jejunum. The two main disaccharidases found in humans are β-galactosidase (lactase) and α-glucosidases (sucrase, isomaltase, and glucoamylase). Lactase hydrolyzes lactose to glucose and galactose. Sucrase hydrolyzes sucrose to fructose and glucose. Isomaltase hydrolyzes the αl-6 bonds of α-limit dextrins to two glucose units. Maltase hydrolyzes maltose to two glucose units.
Lactase activity is low before the 12th week of fetal development, but lactase expression increases to only 30% of perinatal expression level between the 26th to 34th week of gestation and peaks soon after birth. The peaking of lactase activity coincides with the fact that lactose is the major carbohydrate in the human milk. The rapid ontogenic change in intestinal lactase activity during the early stages of human life clearly demonstrates physiologically relevant dietary adaptation after birth. The lactase levels remain relatively high through 6 months of age, and decline at 5 years of age. In adults, two phenotypes exist with regard to lactose digestion. Most of the world population is characterized by a loss of lactase activity before adulthood, resulting in adult-type hypolactasia. For this group, the ingestion of lactose-containing products will result in osmotic-type diarrhea. The other group is lactase-persistent phenotype, which is characterized by continued high lactase activity throughout adulthood. The adult-type hypolactasia is inherited in a recessive manner, whereas the persistence of lactase activity is inherited in a dominant manner. Several nucleotide polymorphisms have been described in the promoter region of the lactase gene. Two particular single-nucleotide polymorphisms (SNPs) are tightly associated with adult-type hypolactasia. LCT-13910C/T and LCT-22018G/A are associated with lactase nonpersistence in the Finnish and other populations, and LCT-13910C > T is associated with high expression of lactase in the intestine in adults. The functional role of these SNPs has been confirmed in two studies demonstrating that the genomic region containing these two polymorphisms possesses enhancer activity. Currently, it is believed that lactase expression in both humans and animals is regulated by a complex spatial and development pattern in the small intestine. A conserved 150-basepair (bp) proximal promoter is important for regulation of lactase expression. This promoter region binds transcription factors, such as CDX-2, hepatocyte nuclear factor 1α (HNF-1α), and GATA, that are important for the regulation of the lactase expression.
In humans, all glucosidases are present by the third intrauterine month. An increase in maltase, glucoamylase, and sucrase-isomaltase occurs at the 12th week of gestation, but their expression levels reach 70% of adult levels between 26th and 34th weeks of gestation Although these developmental changes have been well documented over the past several decades, the molecular mechanisms of how those genes are precisely regulated remain largely unknown. Transcriptional mechanisms have been suggested in regulating these developmental changes. Indeed, the expression of sucrase-isomaltase is increased by weaning in the mouse and the rat intestine. A combinatory role of HNF-1α, Cdx-2, and GATA-4 is involved in the timing and position of the sucrase-isomaltase expression along the small intestine. In addition, glucocorticoids and microbiota also regulate the ontogeny of the sucrase-isomaltase expression in the intestine.
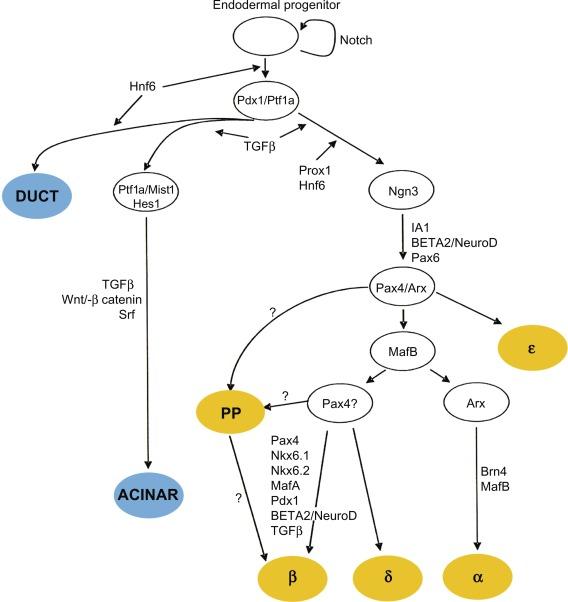
Pancreas development . Pancreas is a glandular organ in the digestive system and endocrine system of vertebrates. As a digestive organ, pancreas secrets pancreatic juice containing digestive enzymes that assist digestion and absorption of nutrients in the small intestine. As an endocrine organ, pancreas produces several important hormones, including insulin, glucagon, somatostatin, and pancreatic peptide. Remarkable advances have been made in recent years in understanding the events of the ontogeny of islets of Langerhans in the mammalian pancreas. The previously held concept that one cell type secretes one hormone during ontogeny has been changed by the new idea that there is a single precursor cell for all of the four types of principal endocrine cell types that produces all of hormones associated with the pancreas (i.e., insulin, glucagon, somatostatin, and the pancreatic polypeptide). Ontogenic changes of the endocrine pancreas involve series of stages in the differentiation of islet cells, and in one of these stages, the precursor islet cells can secrete more than one hormone. Early in development, glucagon and insulin secretion are usually mediated by the same cell. Somatostatin and the pancreatic polypeptide are secreted together by cells much later in development, but at one point in mouse development, polypeptide YY is present in all four principal islet cell types.
Pancreatic development starts from two separate and independent endodermal primordial. The molecular events supporting the early morphological changes that give rise to the formation of the dorsal and ventral pancreatic buds result from coordinated responses to extrinsic and intrinsic signals ( Fig. 10.1 ). The roles of extrinsic and intrinsic signals during pancreas development are variable, depending on the particular competence of each progenitor cell. This requires complex combinations of transcription factors to control organogenesis and cell differentiation ( Fig. 10.2 ). Interruption of genes for Pdx1, Hlb9, Isl1, or Hex results in an arrest of pancreas development at a very early stage (embryonic d8-9). Disruption of genes encoding for the Notch signaling pathway, e.g., Hes1 or neurogenein-3, abrogates development of the endocrine pancreas (isolates of Langerhans). Inactivation of transcription factor genes expressed more downstream in the development cascade (Beta2/NeuroD, Pax4, NKx2.2, and Nkx6.1) curtails the formation of insulin-production β-cells. In particular, the Pax family of transcription factors is involved in the formation of the pancreas and many other organs including the eyes, brain, kidney, thyroid gland, and immune system. A number of murine and human genetic disorders are linked to mutations in specific Pax genes. Two of its members, Pax4 and Pax6, are important in islet differentiation. Pax4 null mice lack pancreatic β- and δ-cells, and Pax6 null mice eliminate pancreatic α-cells. In addition, Pax4 null mice also lack serotonin and somatostatin-secreting cells in the gastric antrum, as well as most endocrine cell types in the proximal small intestine. Pax6 null mice lack glucagon-like peptide-1 (GLP-1)- and GLP-2-expressing cells in the distal intestine, gastrin and somatostatin cells in the antrum, and gastric-inhibitory-peptide (GIP) cells in the duodenum. These observations indicate that Pax4 and Pax6 play crucial roles in normal pancreatic development. The Nkx6 transcription factor family is also important in controlling pancreas differentiation. Nkx6.1 and Nkx6.2 are expressed in the central nervous system and pancreas. Loss of Nkx6.1 function results in a failure in β-cell precursor expansion, and deficiency in both Nkx6.1 and Nkx6.2 displays more severe defect in pancreas as indicated by fewer pancreatic β-cells and α-cells. Thus, Nkx6 subfamily is important in controlling endocrine cell differentiation in pancreas.
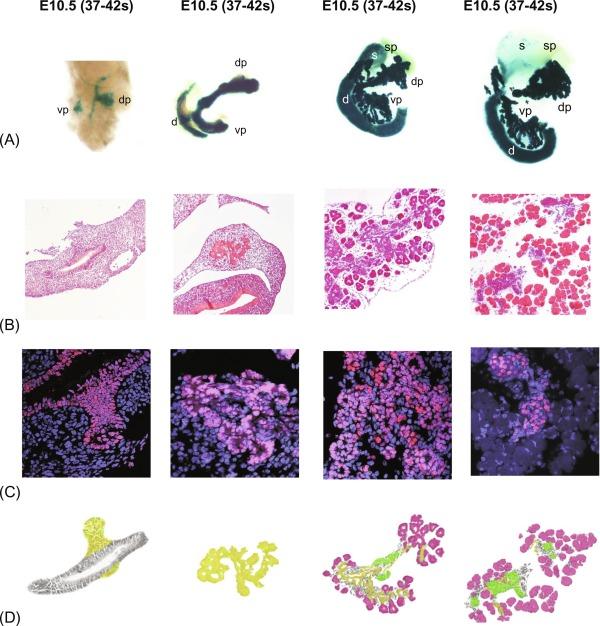
Two other proteins are also important for normal islet development. One is a new member of the protein tyrosine phosphatase (PTP) family, the PTP-NP (for neural and pancreatic). PTP-NP is a receptor-type transmembrane molecule and is an early marker of pancreatic development, which is detected in all pancreatic cells at stage E5.5. Another regulatory peptide, adrenomedullin, has been found to play a role in insulin secretion. Adrenomedullin is an almost ubiquitously expressed peptide that is involved in growth, development, and endocrine signaling. It is also expressed in islet cells during development. This hormone appears first within cells containing glucagon, then eventually within all principal islet cell types, and ultimately only within those cells expressing pancreatic polypeptide. Thus, early expression of adrenomedullin is critical for normal islet development.
The pancreatic duodenal homeobox gene-1 (Pdx-1) is a master regulator of both pancreatic development and the differentiation of progenitor cells into the β-cell phenotype. Pdx-l regulates insulin and somatostatin gene expression in the endocrine pancreas and has particularly important role in the survival of the β-cell. Stable expression of Pdx-1 in a glucagon-secreting cell line induces the expression of the β-cell-specific genes insulin, glucokinase, and islet amyloid polypeptide. The expression of Pdx-1 in the developing pancreas is maintained throughout development and provides both spatial and temporal contributions to the commitment of endoderm to a pancreatic phenotype. Targeted disruption of the Pdx-1 gene in mice results in agenesis of the pancreas. A case where a child with a homozygous inactivating mutation in Pdx-1 gene is born without a pancreas highlights the importance of the Pdx-1 transcription factor in pancreatic development.
The neonatal/suckling and the weaning/postweaning stages are important periods in the development of intestinal transport function. Milk-derived factors, hormones, and macromolecules during the suckling period, distinct hormonal changes associated with weaning, and new dietary factors after weaning all influence gut physiology. The degree of intestinal maturation also plays a major role in affecting absorptive function during ontogeny. Thus, the mechanisms and levels of intestinal transport of nutrients and electrolytes vary throughout early postnatal development. Nutrient transport in the neonatal intestine occurs along the entire crypt-villus axis, whereas only enterocytes along the upper villus have an absorptive phenotype after weaning. Changes in transport function during postnatal development can be related to alterations in transporter density and turnover rate in the plasma membrane of intestinal epithelial cells, or changes in affinity constants and/or expression levels of different transporter isoforms. Changes in transport activity can also be related to alterations in mucosal surface area, proliferation and differentiation of epithelial cells, membrane fluidity, and paracellular permeability. During ontogeny, altered cholesterol-to-phospholipid and protein-to-phospholipid ratios and also altered lipid composition all change plasma membrane fluidity of intestinal cells. These changes in the biophysical properties of the membrane can modify the activity of various transporters in addition to modifying the physical characteristics. During intestinal development in the postnatal period, there is a transient appearance of villus-like structures in the proximal colon. Enterocyte amplification also occurs to increase the absorptive surface area of microvilli.
In general, the neonatal intestinal epithelium is more permeable than the adult intestine. During early postnatal life, growth factors, immunoglobulins, food antigens, other macromolecules, and bacteria may be absorbed from colostrum and milk by adhering to the gut epithelium. This transport occurs through enterocytes and specialized M cells that appear early in fetal development. Milk-derived factors are transported via interaction with specific receptors on epithelial cells. Whole proteins from dietary sources are transported by M cells. The high permeability of the neonatal intestine for macromolecules decreases after birth, a process labeled “gut closure,” but the precise timing of this event is species dependent. In some rodent species and in pigs, transport capacity decreases a few days after birth, whereas transport ceases completely around weaning in rats and rabbits. The process of gut closure is dependent on epithelial-derived factors and increased pancreatic secretions into the gut lumen.
Transport of macromolecules during the neonatal period can also occur nonspecifically, whereby adherent or soluble molecules are moved across the epithelium by vesicular transport. Only molecules that adhere to the microvillus membrane are transported across the cells, whereas soluble molecules in the fluid phase are mostly degraded. This ability of the neonatal gut is due to an apical, tubular system and numerous supranuclear vacuoles, of which distribution and developmental pattern vary dramatically between mammalian species. What makes the macromolecules are more likely to adhere to intestinal epithelial cells during the neonatal period compared to the mature gut is not very clear, although the differences in membrane composition is one possible factor. The relative contribution of enterocytes versus M cells in nonspecific transport of macromolecules is unknown, but selective adherence of milk IgGs to M cells has been reported.
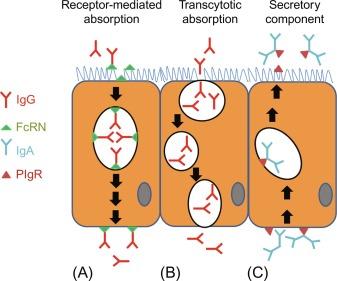
Immunoglobulin transport in the neonatal period is enhanced by protease inhibitors found in colostrum and milk. These inhibitors ensure that IgGs escape degradation and bind to their cognate receptors in the intestinal epithelium. This process is important for the development of passive immunity, which is involved in regulating the expression of development-specific genes in the neonatal intestine. Rodents express a specific IgG receptor that selectively binds to the Fc portion of IgG (the Fc receptor of the neonate, FcRN) and mediates endocytosis and transport across epithelial cells ( Fig. 10.3 ). Fc receptor of the neonate is maximally expressed in the proximal small bowel, with decreasing expression levels toward the distal gut segments. The low pH of the duodenum facilitates FcRN/IgG interaction and endocytosis mediated uptake. The enclosed IgGs are then transported within vesicles that are protected from fusion with lysosomes to the basolateral membrane for extrusion into the interstitial space. IgG may also be transferred by the placenta during late fetal development in humans, but humans and other precocious mammals are born in a hypoglobulinemic state, and thus absorption of IgGs from the maternal milk is critical for development of passive immunity. The expression of IgG receptors in the intestinal epithelium (and macromolecular transport) during the fetal and neonatal periods gradually diminishes after birth. Studies suggest that FcRN expression may be transcriptionally mediated by the Sp transcription factor family members, which are known to regulate the expression of many genes during postnatal development. In addition to IgG absorption, the neonatal intestine also actively secretes IgAs via the activity of the polymeric IgA receptor (PIgR) ( Fig. 10.3 ), and the expression of this PIgR may be modulated at the transcriptional level by glucocorticoid hormones.
Several lines of experimental evidence suggest that milk-based hormones and growth factors can survive digestion and are absorbed by epithelial cells of the neonatal, mammalian intestine ( Table 10.1 ). In suckling animals, the rate of protein degradation is low, but it increases dramatically upon weaning. This low degradative capacity facilitates the transport of biologically active substances by the neonatal intestine, as intestinal epithelial cells express receptors that mediate transepithelial transport of these molecules across intestinal mucosa ( Table 10.2 ). Because gastric acid secretion and pancreatic protease secretion are low during the neonatal and suckling periods, peptide hormones, and other proteins are able to reach the absorptive enterocytes of the proximal small bowel. The principal target of these milk-borne substances seems to be the intestinal epithelium, where they influence proliferation and differentiation of enterocytes. A small fraction of these absorbed molecules may survive intracellular proteolytic processes and become absorbed into the neonatal circulation; however, the physiological importance of this observation is currently unknown. In rat ileum, it has been demonstrated that epidermal growth factor (EGF) and nerve growth factor may be transported by fluid-phase and receptor-mediated mechanisms, but only small fraction of both peptides actually reach the basolateral membrane for extrusion into the circulation.
| Milk-Derived Factor | Physiologic Effects |
|---|---|
| Corticosterone | Hormone shown to be absorbed from formula-fed, glucocorticoid-deficient rats |
| Epidermal growth factor (EGF) | Transport occurs in suckling and weanling rats. Some EGF is degraded during transport, whereas only a small amount is actually transported into the circulation. |
| Insulin | Orally ingested insulin decreases blood glucose levels in suckling rats. Neonatal calves receiving greater amounts of dietary insulin have higher circulating levels of insulin (colostrum vs milk) |
| Insulin-like growth factor (IGF) | Insulin-like growth factor may be absorbed in an undegraded form in pigs. Plasma concentration of IGF is greater in pigs fed a diet containing higher IGF levels (colostrum vs milk). Orally ingested IGF-I modulates intestinal growth in rats and is absorbed intact. Insulin-like growth factor type l is absorbed by a nonsaturable, nonreceptor-dependent mechanism. |
| Prolactin | Prolactin is transferred from mother to rat pups and can be detected in the serum of the offspring; it can also be detected in the serum of suckling rats given an oral dose of prolactin. |
| Prostaglandins | Some prostaglandins are absorbed intact and can be detected in the liver of neonatal rats. |
| Somatostatin | Oral administration to rats results in a small amount being absorbed in an undegraded form. |
| Thyroid-releasing hormone | Thyroid-releasing hormone treatment of maternal rats leads to an increase of serum levels in the pups. |
| Thyroid-stimulating hormone (TSH) | TSH was shown to be absorbed in a biologically active form. Injection (subcutaneous) of neonatal rats with TSH increased serum T4 in both sucklings and weanlings, whereas oral administration had the same effect only in sucklings. |
| Transforming growth factor β (TGF-β) | Mouse pups born to TGF-β knockout mouse heterozygotes have detectable tissue levels of TGF-β, whereas those born to null females do not. Transforming growth factor-β given orally can cross the intestinal epithelium and can be detected in various tissues. |
| Receptor | Developmental Stage | Experimental Observations |
|---|---|---|
| Epidermal growth factor (EGF) | F, S, W, Ad | Epidermal growth factor binding capacity decreases in the perinatal period, and then increases to adult levels by postnatal day 14. A factor present in rat milk decreases EGF binding. Epidermal growth factor receptor expression is greater during the gestational period than in suckling and adult rats. A higher binding capacity and lower receptor affinity is present after weaning in newborn pigs. Virtually no receptors were observed in suckling pigs. |
| Glucocorticoid | N, S, W, PW | Binding capacity increases from fetal life through the suckling period, and then decreases until adulthood in rats. Binding capacity reaches a maximum during gestation, followed by a decrease to adult levels within 3 days of parturition of rabbits. |
| Insulin | S, PW | Decreased binding capacity occurs during development in high- and low-affinity receptors, with no changes in binding affinity. Binding capacity is much greater in crypt cells than in villus cells in rats. |
| Insulin-like growth factor type I | N, S, W | Binding capacity rapidly declines after birth and remains low during the suckling period, and then increases by postnatal day 21 in pigs. Receptor affinity remains unchanged during ontogenic development. |
| (IGF-I) | S, W, PW, Ad | Age-dependent increase of binding capacity during suckling and weaning periods in rats, with decreased capacity noted thereafter. |
| IGF-II | F, W | Receptor messenger RNA expression is significantly higher during fetal development of the rat intestine. |
| S, W, PW, Ad | Age-dependent decrease of binding capacity from early suckling period until adulthood in rats. | |
| Prostaglandin E1 | W, PW, Ad | An age-dependent increase in binding capacity has been noted in rats. |
| Somatostatin | N, S, W, PW, Ad | Binding capacity decreases immediately after birth, and then increases within 2 weeks to adult levels, in the absence of alterations in receptor affinity. In the perinatal period, only high-affinity receptors are expressed, whereas later in postnatal development, high- and low-affinity receptors are expressed in rats. |
| Thyroid hormone | N, S, W, PW | Receptor messenger RNA expression is not altered during postnatal development in rats. |
| Vasoactive intestinal peptide | S, W, PW | An age-dependent increase in binding capacity and expression of both high- and low-affinity receptors has been noted in the absence of changes in affinity in rats. |
| F, N, W | High- and low-affinity receptors have been described; the binding capacity of both receptors has been noted during ontogeny. | |
| Vitamin D | S, W, PW, Ad | Receptor binding capacity dramatically increases on weaning in rats, although receptor affinity does not change during postnatal development. |
Most nutrient transporters are first expressed during gestation and are particularly important during the third trimester when the fetus actively absorbs nutrients from the amniotic fluid. The postnatal ontogeny of nutrient transporters reflects the need to absorb increasing quantities of nutrients for rapid growth and high metabolic activity. The developmental changes in transport capacities are due to several factors: changes in intestinal mass, physicochemical properties of the cell membranes, and types of transporters being expressed and their kinetic properties. In sucklings, even the colon participates in nutrient absorption, which might be a compensatory mechanism for decreased transport capacity in the small bowel and less developed colonic bacterial fermentation. Total intestinal transport capacity increases with age predominantly because of increased intestinal mass, but surprisingly, the absorptive capacity for many carbohydrates and amino acids decreases in proportion to intestinal mass. In some mammalian species, this decline maybe up to 50% of initial values and is not attributable to decrease of mucosal surface area. The phenomenon is not fully understood, but three possible mechanisms may explain the decline: changes in transporter expression levels, induction of other isoforms or related transport systems, and alterations in transporter turnover rates. Decreased absorptive capacity could also be related to the smaller proportion of enterocytes that express various active transporter processes during early ontogeny.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here