Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blood is the principal vehicle delivering oxygen and nutrients to the various tissues and organs of the body. Blood flow and the integrity of the vasculature are essential to life itself. The hemostatic process has evolved to provide damage recognition and protection from blood loss after perforation of the vasculature while at the same time preventing the systemic activation of the clotting system. However, pathologic occlusions are associated with dysregulation of the intravascular system, resulting in venous or arterial thrombosis. The fine line between vascular occlusion and hemostasis is defined by the complex interplay between pro- and anticoagulant materials provided by the blood, the vasculature, and subvascular elements. The appropriate functions occur as a consequence of intense focal development and regulation of enzymatic activity at sites of vascular injury.
The development of the inventory of components involved in plasma clotting were initially based on the most abundant procoagulant plasma proteins, notably prothrombin and fibrinogen, and extended during the past century with the identification of genetic abnormalities that led to bleeding and deviations in laboratory tests that evolved as the inventory of congenital defects expanded. In some instances, laboratory test results indicating a defect in the procoagulant system were not mirrored by hemostatic pathology. In a similar fashion, the congenital defects associated with thrombosis led to the discovery of anticoagulant proteins in blood and vascular counterparts associated with their presentation and activation.
The functional connections between procoagulant “factors” were developed by mixing and matching plasmas associated with different hemostatic disorders. This inventory and its connectivity were ratified and expanded by experiments performed with transgenically mutated mice.
The dynamics of the plasma coagulation process as expressed are a consequence of the molar concentrations of the pro- and anticoagulant components in blood and the vasculature, and the kinetic processes associated with the dynamics of both the activation and functions of the various proteins associated with the process.
The initial result of the activation of the procoagulant hemostatic process is the formation of a fibrin–platelet plug that forms the temporary seal of the vascular perforation in hemostasis. The generation of an occlusive fibrin–platelet plug blocking further flow through an element of the vasculature is thrombosis. In both instances, the fibrin–platelet scaffold is ultimately removed and substituted by vascular repair, new cells, and connective tissue. In thrombosis, the platelet–fibrin plug is removed mechanically or by biochemical intervention to restore flow to the flow-starved vascular bed.
The elements of a clotted fibrinogen–platelet plug are dissolved by the fibrinolytic system, a tightly regulated, dynamic system involving enzyme activation, feedback regulation, and blockade by a potent series of inhibitors. Just as there is an interplay between the pro- and anticoagulant components that brings about a blood clot in hemostasis, similar mechanisms occur when blood clots are dissolved via the fibrinolytic process, which is essential for tissue repair. This chapter describes the components of the pro- and anticoagulant system and the pro- and antifibrinolytic system, and the interplay between these systems. The common feature of both systems is the focal presentation of activity that is dependent on the presentation of surface-bound enzymatic complexes that can cleave their respective substrates.
Blood coagulation can best be understood if viewed as a choreographed system that starts from an inventory of the key players, the relationship or connectivity of these players, and the dynamic catalytic processes. These processes together keep blood in a fluid state but primed to react to vascular injury in an explosive manner. Therefore following sections describe the process of blood coagulation in terms of the inventory, the connectivity, and then the dynamics.
Putting together an inventory of the blood coagulation components is still ongoing, but what we currently know to date began from initial observations that were made in the fifth century and recorded in the Babylonian Talmud. It was noted that if two male children died of bleeding after circumcision, the third should not be circumcised. Over the centuries, many more hypotheses were made regarding what happens to blood when it escapes from the body. The realization that clots stem blood loss only occurred in the 18th century. The existence of thrombin, the key enzyme in blood coagulation, was recognized in the 19th century.
In 1905, Paul Morawitz proposed the classic theory of coagulation. He hypothesized that in the presence of calcium and thromboplastin, prothrombin was converted to thrombin, which in turn converted fibrinogen to the fibrin clot. These clotting factors were subsequently assigned Roman numerals, factor I (fibrinogen), factor II (prothrombin), factor III (thromboplastin; tissue factor membrane), and factor IV (calcium). As more coagulation factors were introduced, they were assigned consecutive Roman numerals. The activated forms are distinguished by a lower case “a” after their Roman numeral designation. Therefore, the activated form of factor V becomes factor Va.
More complex descriptions of the coagulation system were as a “cascade” or “waterfall.” Macfarlane and Davie and Ratnoff proposed that in an intrinsic pathway, involving only plasma, blood coagulated by a sequence of events in which the reactions occurred in a defined series leading to fibrin clot formation. Over the past six decades, the Morawitz/Davie and Macfarlane pathways have been significantly expanded ( Fig. 125.1 ). Each reaction shares a similar mechanism in which an inactive zymogen protein is converted to an active enzyme and each “enzyme” is a surface-bound multiprotein complex consisting of a surface, divalent calcium ions (Ca 2+ ), a protease, and a cofactor. Although some facets of these initial descriptions are still valid, the emerging concept of coagulation and fibrinolysis centers on a complex network of highly interwoven concurrent processes. Procoagulant and fibrinolytic events occur simultaneously with many positive- and negative-feedback loops regulating the processes.
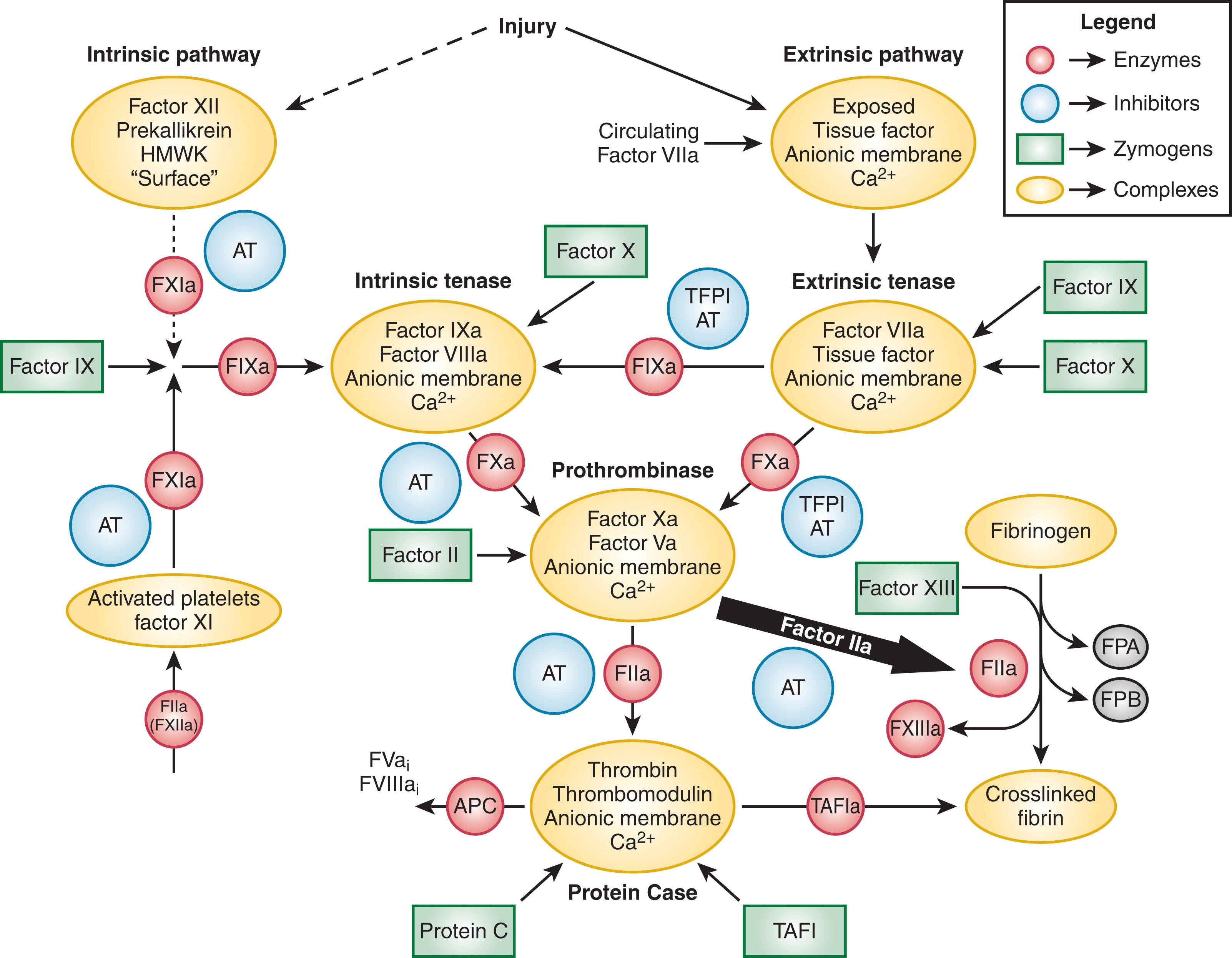
To fully understand the multiple simultaneous processes that occur to effect a hemostatic response, we will first inventory the key procoagulant, anticoagulant, and fibrinolytic participants. The inventory sections discuss the vitamin K–dependent protein family, cofactor proteins, the intrinsic accessory pathway proteins, endothelium, platelets, proteinase inhibitors, clot proteins, and fibrinolysis proteins.
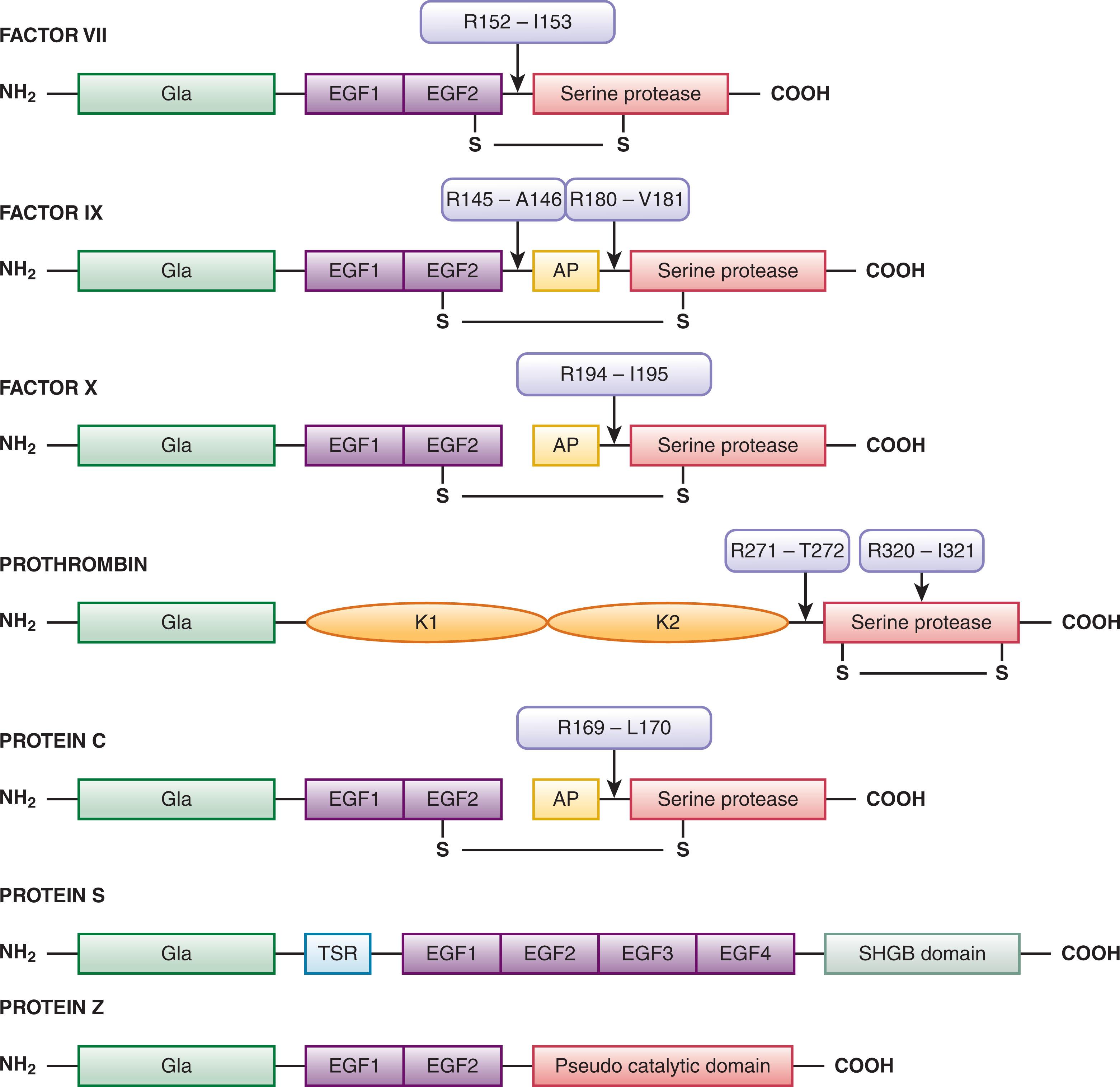
Vitamin K–dependent proteins, synthesized in the liver, play a central role in blood coagulation through either procoagulant or anticoagulant mechanisms. The vitamin K–dependent protein family includes the zymogen procoagulant factors VII, IX, X, and prothrombin, and the anticoagulants protein C, protein S, and protein Z ( Fig. 125.2 and Table 125.1 ). Except for protein S and protein Z, after cleavage to their active forms these proteins are serine proteases related to the trypsin and chymotrypsin superfamily. Peptide bond cleavage at specific sites converts the vitamin K–dependent zymogens to their active serine protease forms. All share noncatalytic domains, each of which is characterized by highly conserved regions that fold, independently from the rest of the molecule, into a characteristic three-dimensional shape. The domains of the vitamin K–dependent proteins are illustrated in Fig. 125.2 . Several reviews have been written on vitamin K–dependent proteins.
| Protein | Molecular Weight (kD) | Plasma Concentration | Plasma t 1/2 (Days) | Clinical Phenotype Associated with Deficiency | Functional Classification | |
|---|---|---|---|---|---|---|
| (nmol/L) | (μg/mL) | |||||
| Procoagulant Proteins and Receptors | ||||||
| Factor XII | 80 | 500 | 40 | 2–3 | None | Protease zymogen |
| HMW kininogen | 120 | 670 | 80 | None | Cofactor | |
| LMW kininogen | 66 | 1300 | 90 | Cofactor | ||
| Prekallikrein | 85/88 | 486 | 42 | Protease zymogen | ||
| Factor XI | 160 | 30 | 4.8 | 2.5–3.3 | Sometimes bleeding | Protease zymogen |
| Tissue factor | 44 | N/A | Cell-associated cofactor | |||
| Factor VII | 50 | 10 | 0.5 | 0.25 | Bleeding (occasionally thrombotic) | VKD protease zymogen |
| Factor X | 59 | 170 | 10 | 1.5 | Bleeding | VKD protease zymogen |
| Factor IX | 55 | 90 | 5 | 1 | Bleeding | VKD protease zymogen |
| Factor V | 330 | 20 | 6.6 | 0.5 | Bleeding a | Soluble procofactor |
| Factor VIII | 285 | 1.1–1.5 | 0.3–0.4 | 0.3–0.5 | Bleeding | Soluble procofactor |
| vWF | 255 | Varies | 10 | Bleeding | Carrier for factor VIII | |
| Factor II | 72 | 1400 | 100 | 2.5 | Bleeding b | VKD protease zymogen |
| Fibrinogen | 340 | 7400 | 2500 | 3–5 | Bleeding c | Structural clot protein |
| Factor XIII | 320 | 94 | 30 | 9–10 | Bleeding | Transglutaminase zymogen |
| Anticoagulant Proteins, Inhibitors, and Receptors | ||||||
| Protein C | 62 | 65 | 4 | 0.33 | Thrombotic | Proteinase zymogen |
| Protein S | 69 | 300 | 20 | 1.75 | Thrombotic | Inhibitory cofactor |
| Protein Z | 62 | 47 | 2.9 | 2.5 | Sometimes thrombotic | Inhibitory cofactor |
| Thrombomodulin | 100 | N/A | N/A | N/A | Cofactor/modulator | |
| Tissue factor pathway inhibitor | 40 | 1–4 | 0.1 | minutes | Proteinase inhibitor | |
| Antithrombin | 58 | 2400 | 140 | 2.5–3 | Thrombotic | Proteinase inhibitor |
| Heparin cofactor II | 66 | 500–1400 | 33–90 | 2.5 | Often thrombotic | Proteinase inhibitor |
| α 2 -Macroglobulin | 735 | 2700–4000 | 2–3000 | <1 h | Proteinase inhibitor | |
| α 1 -Proteinase inhibitor | 53 | 28,000–65,000 | 1500–3500 | 6 | Proteinase inhibitor | |
| Endothelial protein C receptor | Receptor | |||||
| Fibrinolytic Proteins, Inhibitors, and Receptors | ||||||
| Plasminogen | 88 | 2300 | 210 | 2.2 | Proteinase zymogen | |
| t-PA | 70 | 0.07 | 0.005 | <5 min | Proteinase zymogen | |
| u-PA | 54 | 0.04 | 0.002 | 5 min | Proteinase zymogen | |
| TAFI | 58 | 75 | 4.5 | 10 min | Thrombotic | Carboxypeptidase |
| FSAP | 64 | 190 | 12 | Fibrinolytic zymogen | ||
| PAI-1 | 52 | 0.2 | 0.01 | <10 min | Bleeding | Proteinase inhibitor |
| PAI-2 | 47/60 | <0.070 | <0.005 | — | Proteinase inhibitor | |
| α-Antiplasmin | 70 | 500 | 70 | 2.6 | Bleeding | Proteinase inhibitor |
a Factor V Leiden mutation associated with thrombosis.
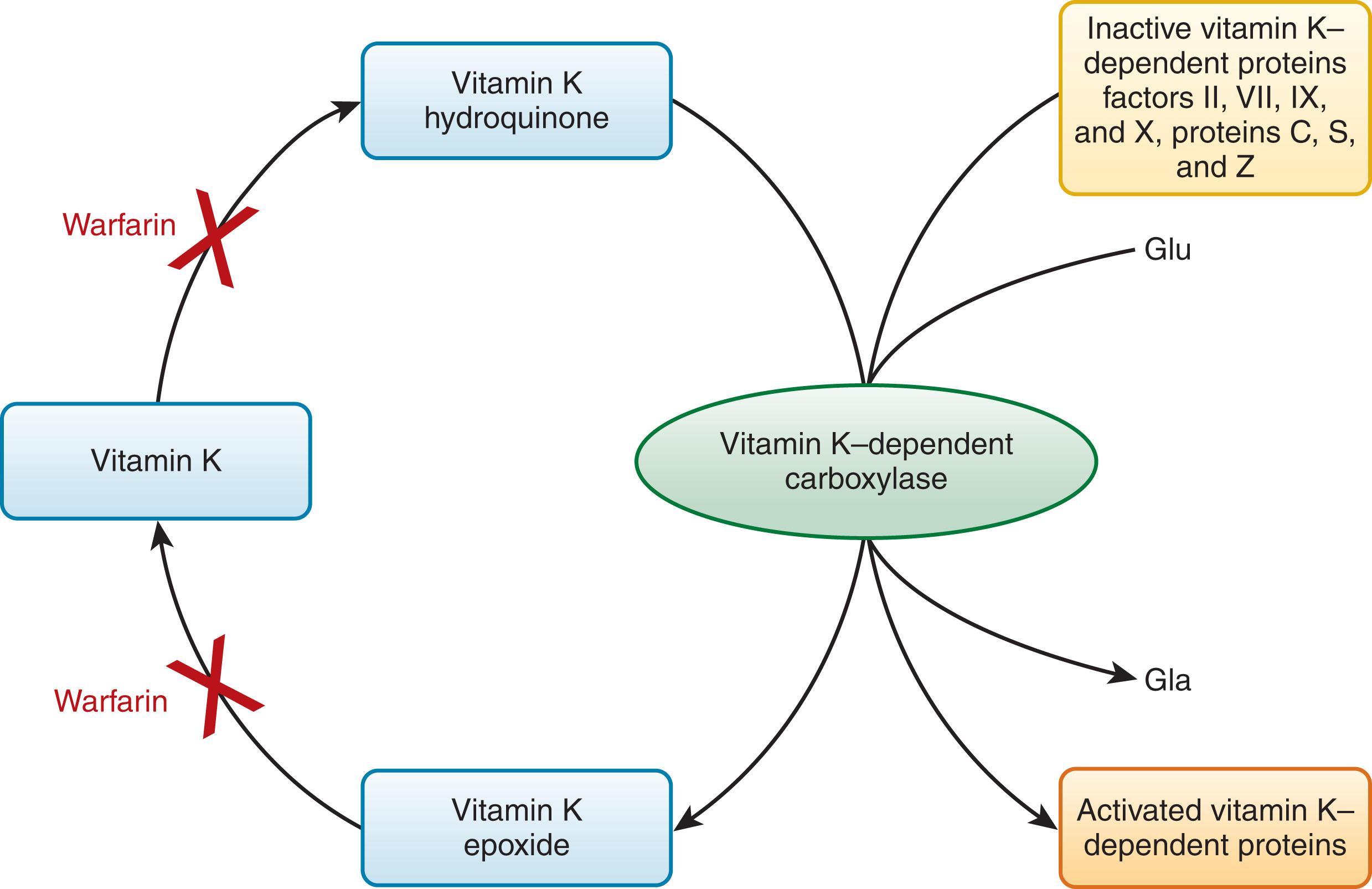
Vitamin K is essential for the biosynthesis of these clotting factors by participating in the cyclic oxidation and reduction of the enzyme that converts 9 to 13 N-terminal glutamate residues to γ-carboxy glutamate (Gla; Fig. 125.2 ; see reviews listed in the References ). This posttranslational modification to form Gla residues adds a net negative charge to the molecules that enables the vitamin K–dependent proteins to interact with Ca 2+ and a membrane surface. Blocking the formation of the Gla residues is the basis for anticoagulant therapy with coumarin (warfarin) derivatives, which are chemically similar in structure to vitamin K ( Fig. 125.3 ). The Ca 2+ binding association with this modification is also the basis for the anticoagulant activity of sodium citrate, a calcium chelator, found in the blue-top vacuum tubes used for clinical laboratory testing of clotting activity. The level of inhibition achieved with the same dose of warfarin varies among patients. Increased sensitivity to warfarin has been identified in patients when started after surgery. Factors affecting the level of anticoagulation include dietary intake of vitamin K; liver function; concomitant medications that either reduce or enhance the warfarin effect ; common polymorphisms in the vitamin K epoxide reductase complex subunit 1 (VKORC1), which is responsible for vitamin K reduction; and polymorphisms in CYP2C9, which affect warfarin metabolism (see reviews listed in the References ). Therefore, genotyping may be helpful to personalize starting doses of warfarin. Ultimately, proper monitoring of warfarin therapy is essential. This is done using the prothrombin time (PT) with the assay sensitivity corrected using the international normalized ratio (INR).
The NH 2 -terminal Gla domains are followed by either a kringle domain in factor II or an epidermal growth factor–like domain (EGF) in factor VII, factor IX, factor X, protein C, protein S, and protein Z (see Fig. 125.2 ). Protein S is not a serine protease precursor and instead contains a thrombin-sensitive region before the EGF domain and a sex hormone–binding globulin–like domain (SHBG) in the COOH-terminus. Protein Z contains a “pseudo catalytic domain” in the COOH-terminus and does not function as a serine protease enzyme.
Vitamin K–dependent protein complexes are essential for establishing hemostatic balance ( Fig. 125.4 ). Each complex is composed of a serine protease enzyme, a cofactor that functions as a surface receptor or enhancer for the enzyme Ca 2+ , and a negatively charged membrane surface provided by activated or damaged cells (e.g., endothelial cells, monocytes, and platelets). There are four vitamin K–dependent complexes: the extrinsic tenase complex (factor VIIa–tissue factor), the intrinsic tenase complex (factor IXa–factor VIIIa), the prothrombinase complex (factor Xa–factor Va), and the anticoagulant protein Case complex (thrombin–thrombomodulin).
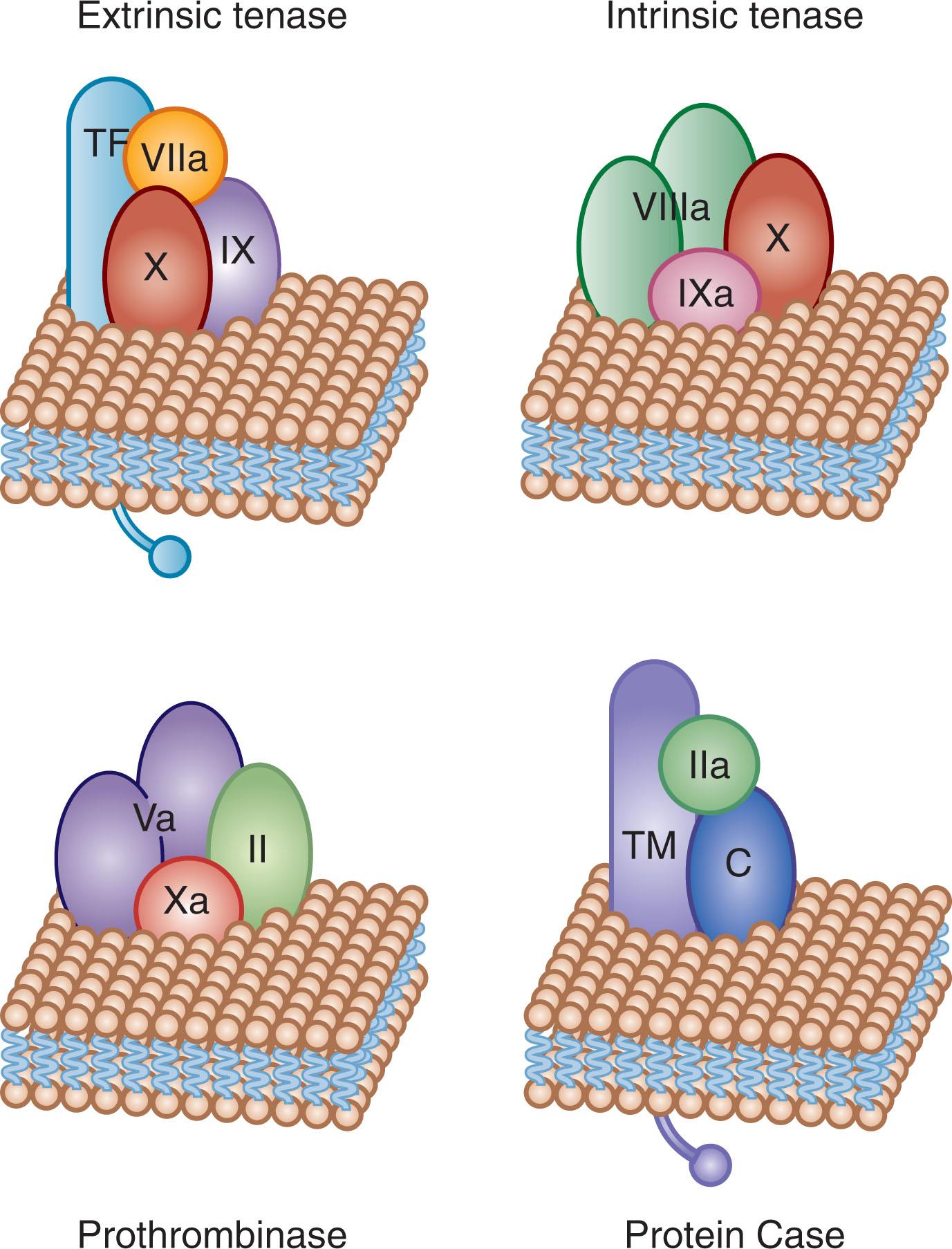
When the serine protease is associated with its respective cofactor on an appropriate membrane surface with Ca 2+ , the specific reactions occur at a rate that is 10 4 - to 10 9 -fold faster than that of protease–substrate combination alone. One way to visualize the importance of the assembly of these macromolecular complexes in the formation of the hemostatic plug is to note that if a healthy person takes 4 minutes for his or her blood to clot, then in the absence of membrane and cofactor, blood clot formation would take approximately 3.8 years.
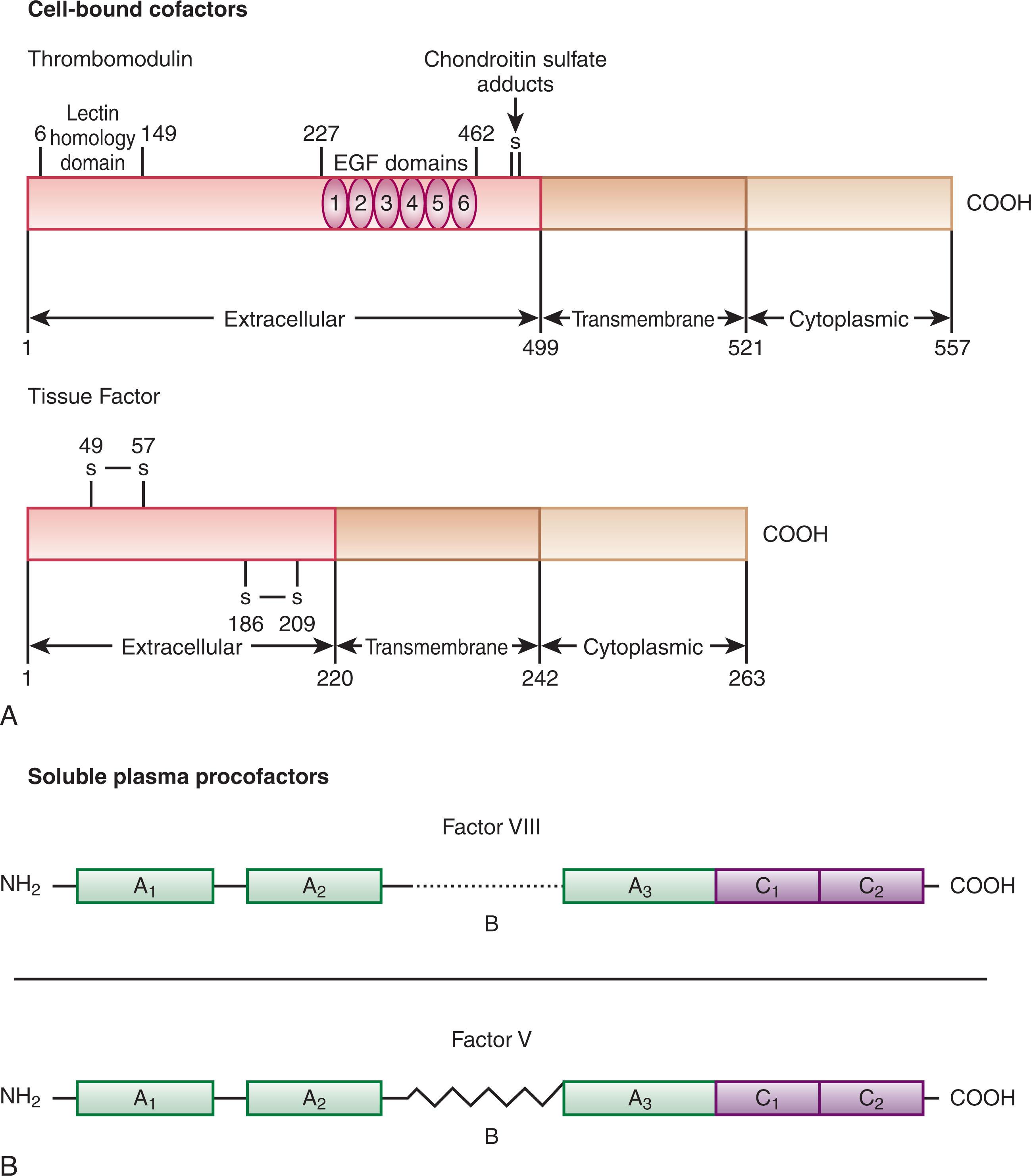
There are two categories of procoagulant cofactor proteins: the cell-bound cofactors (tissue factor and thrombomodulin) and the soluble plasma-derived procoagulant procofactors (factor V and factor VIII with its circulating carrier von Willebrand factor [vWF]).
Tissue factor is a transmembrane protein that functions as a nonenzymatic cofactor for factor VIIa in the extrinsic tenase complex (see reviews listed in the References ; Fig. 125.5A ). In the absence of injury or inflammatory stimuli, tissue factor is not expressed on cellular surfaces in direct contact with circulating blood (see the review listed in the References ). Presentation of tissue factor to the circulation is the event that triggers the primary procoagulant pathway of coagulation (see Fig. 125.1 ). There are no known mutations or deficiencies of human tissue factor, and tissue factor deletion in mice is lethal during embryonic development, leading to the speculation that tissue factor is essential for life.
Tissue factor activity is primarily regulated by controlling its presentation. The commonly accepted sources of functional tissue factor are the subendothelium exposed upon vascular damage or monocytes stimulated by cytokines. However, there is controversy regarding the source and presentation of active tissue factor and whether functional tissue factor circulates in blood in healthy and pathologic states. Microparticle sources of blood-borne tissue factor are generally defined as submicron-sized cell-derived membrane fragments produced in response to activation or apoptosis. Their role in hemostasis is still debated.
Thrombomodulin is a type 1 transmembrane protein constitutively expressed on the surface of vascular endothelial cells (see Fig. 125.5A ). Thrombomodulin is a high-affinity receptor for all thrombin forms and acts as a cofactor for the thrombin-dependent activation of protein C. The endothelial cell protein C receptor (EPCR) provides cell-specific binding sites for both protein C and activated protein C (APC). When bound to thrombomodulin, thrombin’s procoagulant activities (e.g., its capacity to generate fibrin and activate factor V, factor VIII, factor XI, and platelets) are neutralized, and the rate of inactivation of thrombin by antithrombin is increased. The generation of APC by the thrombin–thrombomodulin complex leads to inactivation of the procoagulant cofactors factor Va and factor VIIIa, thus suppressing thrombin formation. Thrombin–thrombomodulin, or the “protein Case” (see Fig. 125.4 ) complex, also has an antifibrinolytic role via activation of the fibrinolysis inhibitor, thrombin-activatable fibrinolysis inhibitor (TAFI; see reviews listed in the References ).
Thrombomodulin activity on the surface of endothelial cells is decreased by inflammatory cytokines, and this decrease may contribute to the hypercoagulation characteristic of inflammatory states.
Factor V is a large single-chain glycoprotein (GP) that circulates in human plasma (see Table 125.1 and Fig. 125.5B ) and is also contained in the α-granules of human platelets, with approximately 18% to 25% of the total factor V found in platelets. The procofactor factor V is proteolyzed by α-thrombin to the active cofactor factor Va. Factor Va functions both as a factor Xa receptor and positive modulator of factor Xa catalytic potential in the prothrombinase complex (see Fig. 125.4 ). Factor Va is proteolytically inactivated by APC. The importance of this regulatory mechanism is demonstrated by the “APC resistance” syndrome associated with factor V Leiden . Individuals with factor V Leiden have a G to A substitution at nucleotide 1691 in the factor V gene that results in an Arg 506 →Gln mutation at the protein level. Factor Va Leiden has normal cofactor activity as part of the prothrombinase complex. However, unlike normal factor Va, factor Va Leiden is more slowly inactivated by APC. The Arg 506 →Gln mutation hinders the first step in the series of inactivating cleavages by APC. Cleaved factor Va Leiden retains limited cofactor activity and continues to promote thrombin generation. The identification, role in coagulation, and overall importance of factor V in hemostasis have been described in numerous reviews.
The soluble procofactor factor VIII, or antihemophilic factor (see Fig. 125.5B ), circulates in plasma in complex with the large multimeric protein vWF. vWF acts to regulate the plasma concentration of factor VIII. Factor VIII interaction with vWF requires the NH 2 - and COOH-termini of the factor VIII light chain (A3, C1, and C2 domains), although a specific vWF binding site has been identified at residues 1673–1684 on the light chain. Factor VIII is activated by thrombin cleavage at three sites (Arg 372 , Arg 740 , Arg 1689 ) to generate the heterotrimeric cofactor factor VIIIa. The vWF binding site is removed from the factor VIII protein by thrombin cleavage at Arg 1689 . After forming, vWF–free factor VIIIa forms a complex with the serine protease factor IXa, Ca 2+ , and a membrane (provided by platelets), resulting in the intrinsic tenase complex (see Fig. 125.4 ). Factor VIIIa function is downregulated by the relatively rapid dissociation of the noncovalently associated A2 subunit, which is produced by the cleavages required for activation, and by cleavage by APC. Factor VIII is homologous (40% identity) to the procofactor factor V.
Deficiency of factor VIII, or hemophilia A, is a well-characterized bleeding disorder linked to the X chromosome (see Chapter 134 ). Hemophilia A therefore occurs almost exclusively in males at a frequency of 1 in 5000 to 1 in 10,000 individuals.
vWF has several key roles in coagulation. It is synthesized in endothelial cells and also contained in the α-granules of human platelets. vWF is a large adhesive GP that circulates in plasma as a heterogeneous mixture of disulfide-linked multimers that range in size from dimers ( M r =600,000) to extremely large multimers of more than 20 million kDa. vWF has binding sites for factor VIII, heparin, collagen, platelet GPIb, and platelet GPIIb–IIIa. vWF acts as the bridge between platelets to promote platelet aggregation. The primary platelet binding site for vWF is the GPIb–IX–V receptor complex (see Chapter 124 ). GPIb–IX–V is an active receptor on unstimulated platelets and serves to promote platelet aggregation and adhesion to vWF in the absence of platelet activation.
vWF is a structural protein and is part of the subendothelial matrix. Endothelial cells secrete vWF multimers, which are larger than those found circulating in plasma. The function of these large multimeric forms of vWF is to bind to and agglutinate blood platelets under high shear conditions. These large multimers of vWF are degraded by a specific metalloprotease called a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS)-13. In familial and acquired thrombotic thrombocytopenic purpura, ultra-large vWF multimers are correlated with defective ADAMTS-13 activity (see Chapter 133 ).
ABO blood type has a significant influence on vWF levels, with individuals of types A, B, or AB blood having much higher levels of vWF than those with type O blood. vWF is also known for its role in ristocetin-induced platelet agglutination, which is the basis of clinical assays for von Willebrand disease, a fairly common disorder that is estimated to occur in 1% to 2% of the general population (see Chapter 132 ). vWF is also an acute-phase reactant, and vWF levels are elevated as a result of stress, pregnancy, or surgical trauma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here