Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mohs micrographic surgery is a specialized surgical procedure indicated for basal cell carcinoma, squamous cell carcinoma, melanoma, and other malignancies of the skin and mucous membranes.
Mohs micrographic surgery provides complete microscopic margin control through the use of horizontal frozen histologic sections of the entire periphery of the excised tumor.
Mohs surgery is cost-effective and provides the highest cure rate for tumors that spread by direct extension.
Mohs surgery requires a physician trained in surgery and pathology to accurately excise and map tumor extensions.
Mohs surgery entails a series of precise steps that lead to tumor extirpation and allow for immediate wound repair.
Mohs surgery has few complications with proper preoperative planning, but can be a time-intensive procedure.
Mohs micrographic surgery (MMS) is a method of excision that provides complete microscopic control of tumor margins and offers cure rates superior to those of other treatment options. It is most commonly used for cutaneous malignancies, and can be used for benign neoplasms in special situations. It is a meticulous technique performed by a physician skilled in cutaneous surgery and pathology, in which horizontal frozen histologic sections of the surgical margins of the excised tumor are generated for the most complete microscopic examination possible. Residual malignant extensions of the margins are mapped and excised selectively until the entire tumor is removed. This selective removal of malignant tissue allows preservation of healthy tissue and thus smaller defects. Defects may be repaired immediately, allowed to heal by second intention, or undergo delayed reconstruction. MMS is usually performed in an outpatient setting over several hours using local anesthesia. Training programs for performing Mohs micrographic surgery are available throughout the USA via a 1- or 2-year fellowship after completion of a dermatology residency. In 2003, the US Accreditation Council for Graduate Medical Education (ACGME) approved procedural dermatology fellowships in which Mohs surgery is the backbone.
Cutaneous tumors have been successfully treated in a number of ways. In addition to Mohs surgery, the most common methods include standard fusiform excision, cryotherapy, radiation therapy, curettage, and electrodesiccation. Other alternative treatments are intralesional interferon, topical and intralesional 5-fluorouracil (5-FU), intralesional methotrexate, topical ingenol mebutate gel, photodynamic therapy (PDT), and ablative laser treatment. Vismodegib is an FDA-approved oral drug for the treatment of recurrent, locally advanced, or metastatic BCC. Topical imiquimod, an immune response modifier which stimulates interferon-α, is also used for cutaneous tumors. These treatments can provide excellent results in many circumstances. However, unlike Mohs surgery, none of them examine 100% of the margins or offer the satisfaction of immediately knowing that the tumor has been completely removed at the time of the procedure. Because of this, MMS is often the method preferred by both patient and physician. In both the original form designed by Dr Frederic Mohs using a zinc chloride paste fixative on the skin prior to excision ( Fig. 45.1 ) and as now practiced without the fixative, it consistently shows the lowest 5-year recurrence rates compared with all other modalities.
1930s: Frederic E. Mohs, a general surgical trainee, developed a technique of excisional surgery in which a complete margin of skin cancer was plotted in three dimensions and he named it “chemosurgery” because it involved chemical fixation in situ before excision as follows:
Zinc chloride (as fixative), combined with stibnite (as permeant) and bloodroot powder ( Sanguinaria canadensis ) (as agglutinant) ( Fig. 45.1 ) was applied to achieve in-vivo tissue fixation while maintaining cytological detail for microscopic evaluation of the excised specimen.
One day later, the tumor and saucer-shaped specimen containing a narrow margin of normal-appearing tissue were excised painlessly in a bloodless surgical field; frozen sections were generated from the specimen with the deep portion and peripheral margin of the excised tissue in a single horizontal plane, and residual tumor was precisely mapped based on the microscopic findings.
Overnight, zinc chloride was reapplied (if indicated) and the procedure was repeated to remove another saucer-shaped tissue specimen in the precise location of any residual tumor.
The process was repeated until all residual tumor was removed.
The wounds were usually left to heal by second intention. Mohs demonstrated significantly higher cure rates than conventional excision, but this technique had limitations, including the time taken (1 day for each surgical layer) and the delay in wound reconstruction as a result of the postoperative fixed tissue slough.
1953: Mohs and others demonstrated that the zinc chloride fixation could be replaced by local anesthesia and frozen section processing, while still maintaining high cure rates, making the technique simpler and quicker.
1985: The official name of this procedure was changed to Mohs micrographic surgery to reflect the shift away from chemical fixation toward a fresh tissue fixation technique, while maintaining reliance on microscopic evaluation of a layer-by-layer pictorial representation of tumor. This allows most tumors to be removed in 1 day, permits immediate wound repair, eliminates preoperative fixative discomfort, and has a cure rate comparable with that of the chemosurgical technique. It is now used almost exclusively by most Mohs micrographic surgeons.
1986: American College of Chemosurgery, founded by Mohs, renamed American College of Mohs Micrographic Surgery.
Subsequently, the College renamed American College of Mohs Micrographic Surgery and Cutaneous Oncology to reflect changes in the clinical scope of the practice as the procedure enjoyed increasingly widespread application.
It was recently renamed again as the American College of Mohs Surgery.

MMS is predicated on the observation that skin cancers generally grow through direct extension without “skip” areas. Tumor cells can be found a significant distance from the clinically visible and palpable neoplasm, yet are in continuity with the tumor itself as a result of the tumor's microscopic spread. Thus, the tumor can be traced to its termination by excising it layer by layer and examining the entire margin of each layer under the microscope. Critical in this process is that the skin edge and deep surface are visualized in one plane on the glass slide, and thus the entire margin can be analyzed. A map is constructed for each layer to accurately locate residual tumor and selectively excise it with the next layer ( Fig. 45.2 ).
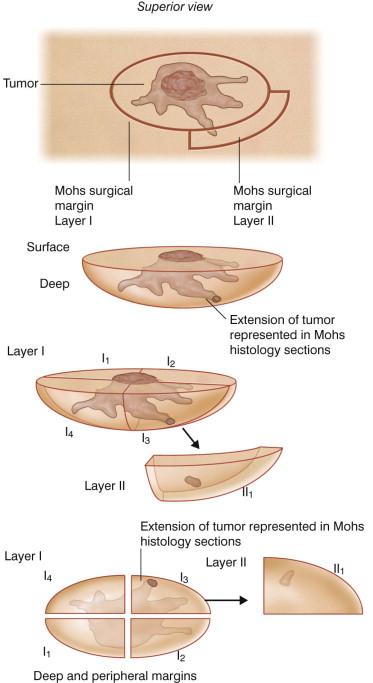
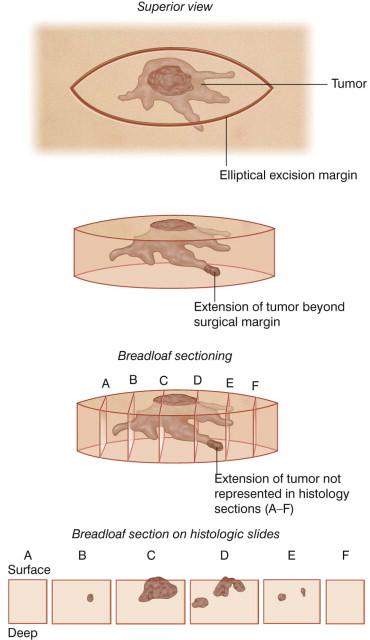
MMS was conceptualized to address the inadequacy of conventional means of surgical excision with margins. Vertically cut serial sections of an entire excisional specimen require an excessive number of sections to be examined. Step sections, representative vertical sections taken at 2–4 mm intervals as in the “breadloaf” method ( Fig. 45.3 ), are customarily obtained when excisional specimens are sent to the pathology laboratory. However, this method leaves marginal areas between the sections that are not microscopically visualized, and allows examination of less than 1% of the tumor's margin. The quadrant method similarly fails to identify residual tumor between sampled areas. Failure to detect subclinical microscopic tumor foci is often responsible for recurrence of the tumor despite “clear” margins on the pathology report.
There are many advantages of using the Mohs technique:
First, with its improved method of evaluating the margin, the Mohs technique has significantly improved cure rates. The 5-year cure rate for primary cancers treated by Mohs surgery is 99%, compared with 93% for conventional excision. For recurrent cancers, the 5-year cure rate is 95%, compared with 80% for excision or any other treatment modality.
Second, Mohs surgery compares favorably in cost-effectiveness to other modalities of treatment of non-melanoma skin cancer.
Mohs surgery is indicated in situations where other treatment modalities have failed. The Mohs technique is used for recurrent tumors or those at high risk for recurrence, those with clinically indistinct margins, and for those tumors that are anatomically located where tissue conservation is important ( Table 45.1 ). It can be used for virtually any neoplasm that spreads by contiguous growth along cutaneous or mucosal surfaces.
| Type of tumor | Examples |
|---|---|
| Recurrent | |
| Positive margin after simple excision | |
| At high risk for local recurrence and/or metastasis |
|
| In an area where tissue conservation is important |
|
| Facial – in area of embryonic fusion planes |
|
| Poorly-defined clinical margins | |
| Perineural spread | |
| Larger than 2 cm in diameter | |
| Immunosuppressed patient | |
| Skin previously treated with ionizing radiation | |
| Demonstrated biological aggressiveness |
One additional advantage of the Mohs technique is that it is tissue sparing. In specific cosmetic or functional areas, this may allow a more cosmetically acceptable or functional result after surgery. Frequently, it also can simplify the reconstruction both by tissue conservation within critical cosmetic units, as well as by allowing the reconstructing surgeon to be as confident as possible that the entire tumor has been removed.
Mohs surgery is not limited to the skin – treatment of mucosal cancers of the head and neck can benefit from its many advantages. The face (especially the eyelids, ears, lips, and nose), the digits, the genitalia, and the scalp, are specific anatomic locations in which Mohs micrographic surgery can be most helpful in obtaining clear tumor margins while sparing as much uninvolved tissue as possible. These are locations in which tumors can spread along nerves, cartilage, periosteum, bone, and embryonic fusion planes, making them high risk for recurrence.
MMS should be considered for virtually all cutaneous malignancies of the face. The face has multiple unique anatomic structures which must be preserved in reconstruction, making tissue conservation critical.
Lesions on the eyelids present special challenges. It was with eyelid tumors that Dr Mohs had his earliest successes with extremely high cure rates. Some 90% of malignant tumors of the periorbital area are basal cell carcinomas (BCCs). If the tumor involves the tarsal plate, levator muscle, or lacrimal apparatus, especially the canaliculus, oculoplastic repair is frequently required. Intraocular mucosal spread of basal cell carcinoma has been documented. When operating on the free eyelid margin, layers may be taken in succession, so that the canaliculus is sacrificed only if involved. The postoperative size of medial canthal lesions can be minimized with the Mohs technique, allowing for second intention healing in select cases. Regardless of the size and extent of the defect, repair of the Mohs defect can commence immediately, saving the patient great anxiety and days of non-productive time while awaiting repair.
Lesions on the ears may also present significant challenges to the cutaneous surgeon. Making up only 6% of all skin cancers, those that occur on the ear account for a disproportionate number of recurrences. The difficulty in eradication likely stems from the many embryonic fusion planes in the periauricular area. Tumors of the ear may have a predictable pattern of spread. Tumors that arise in the postauricular area tend to spread to the ear itself. Tumors that arise in the preauricular area spread toward the tragus and the medial and superior aspects of the helix. Once at the tragus, the tumor can spread down the external part of the tragus between tragal cartilage and the parotid gland to involve deeper structures such as the facial nerve. Neuropathic pain in this area is frequently an indicator for such involvement. Treatment of tumors involving deep structures, the mastoid, or extending from the concha into the external auditory meatus may require sedation or general anesthesia and consultation with other specialists. Tumors extending beyond the outer third of the ear canal may be difficult to access, requiring specialized surgical expertise and skills. Tumor extirpation can involve significant morbidity to the patient.
The nose is the most common site for recurrence of BCC on the entire integument. Like the periauricular area, embryonic fusion planes may offer the path of least resistance and facilitate tumor spread along contiguous anatomic areas. In particular, the alar rim and nasal tip are two areas where tissue conservation is critical and size can affect reconstruction possibilities dramatically. Lesions involving the melolabial folds may be particularly deep and extensive. The Mohs technique should be considered for virtually any malignant neoplasm of the nose.
Tumors of the skin of the upper lip are usually BCCs, while those of the cutaneous lower lip are most commonly squamous cell carcinomas (SCCs). The latter type is highly capable of regional and metastatic spread, especially if over 2 cm in diameter. Larger and more aggressive tumors may require wider excision with lymph node evaluation (i.e., sentinel lymph node mapping), and involvement of a head and neck surgeon may be helpful.
Tumors of the digits may be advanced at the time of presentation. Mohs surgery is useful and often avoids the amputation of a digit, thus maintaining function. Treatment of subungual or periungual tumors is particularly challenging to maintain function, avoid infection, and allow for adequate cosmesis. The nail matrix in particular is extremely vulnerable to tissue loss, resulting in visible deformity of the nail plate with loss of function.
The treatment of tumors of the genitalia may also benefit from MMS. Patients may require penectomy for advanced stages of malignancy; Mohs surgery may provide the necessary tissue conservation to avoid this outcome. Larger and more aggressive tumors may require wider margins of excision and regional lymph node examination. The precise location and size of the tumor can greatly influence prognosis. Higher cure rates can be obtained when tumors involve only the glans or prepuce of the penis, and when lesions are less than 1 cm in diameter.
Patient consultation is provided before the procedure. Critical in this process is the accurate localization of the tumor, which can be obtained using photographs, diagrams, and descriptions, often from the referring physician. Physical examination should include contiguous areas and assessment for pre-existing cosmetic and functional deficits. Draining regional lymphatics should be palpated and documented for all patients. Histologic proof of diagnosis is generally required, and the histologic slide can be reviewed to confirm the tumor type and pattern. The patient's health history and review of systems should be focused, but comprehensive, to minimize complications ( Box 45.1 ). Any history of previous cutaneous procedures (especially those involving the operative site), ultraviolet exposure, and radiation therapy should be elicited. A detailed medication history should also include dietary supplement use. In one study, 49% of patients undergoing Mohs surgery reported taking at least one dietary supplement. Patients are instructed to avoid aspirin-containing products, antiplatelet agents, and other supplements such as vitamin E, ginkgo biloba, and garlic, which have demonstrated anticoagulation properties. Cessation of warfarin is controversial because of the well-documented risks of abruptly discontinuing therapy perioperatively. There are several newer agents being used in clinical practice that dermatologic surgeons should be aware of; these include the oral activated factor X inhibitors rivaroxaban and apixaban and the oral direct thrombin inhibitor dabigatran. It is good practice to consult with the treating physician, if possible, when the patient is on a prescribed aspirin-containing product, antiplatelet agent, or anticoagulant. Improving hypertensive control and optimizing other medical conditions are also important to ensure operative success. Preoperative anxiolytic agents, usually administered sublingually or orally, can be considered in patients with a high level of apprehension. It may be advisable for such patients to come for the procedure after having a light meal and being well rested and accompanied by a family member or friend. Warm layered clothing is advisable in most operating room environments.
Hepatitis
AIDS
Herpes simplex virus
Medications
Vitamins
Nutritional supplements
Allergies to medicines, anesthetics, topical creams, adhesive tape
Immunosuppression
Concurrent malignancies
Concurrent cutaneous disorders
Prosthetic joints and valves
Pacemaker/defibrillator
Bleeding diathesis
Smoking history
Other medical illnesses (e.g., gout)
The cutaneous surgeon must successfully alleviate the patient's anxieties about their surgery. Many patients present with closely held perceptions about their aesthetic ideal, and some have unrealistic expectations. It may be useful to obtain preoperative digital photographs to document both tumor location and specific anatomic features. A number of patients have researched their cutaneous neoplasm on the internet or by other sources of information, but the cutaneous surgeon should still take this opportunity to educate the patient to ensure the transmission of accurate information. Patient education can take many forms, including websites, brochures, pictures, and models. However, none is a total replacement for the direct consultation between the physician and patient.
A thorough discussion of risks, benefits, alternatives, and complications of the proposed surgical procedure must take place. Specific alternatives to surgery such as radiation therapy and other modalities of treatment (perhaps even observation) should be discussed. An explanation of the Mohs technique with respect to anesthesia, stages, waiting periods, and repair options should be tailored to the patient's specific case. The possibility of tumor spread to infiltrate contiguous soft tissue and cartilaginous and bony structures should be anticipated and discussed with the patient before or during the operation before proceeding further. In some cases of large or deep tumors, the extent of tumor involvement may be assessed preoperatively by computed tomography (CT), magnetic resonance imaging (MRI), or other diagnostic aids, although a negative test result may be misleading.
The reconstruction of large tumors that infiltrate widely may benefit from the expertise of plastic, oculoplastic, and head and neck surgeons, or other specialists, and their evaluation should be incorporated into the preoperative evaluation of the patient. Medical oncologists may provide useful input and therapeutic options in certain cases. In essence, a team approach may serve the patient best in challenging cases.
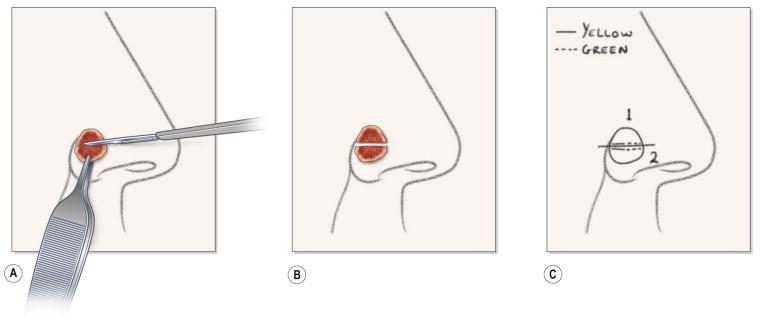
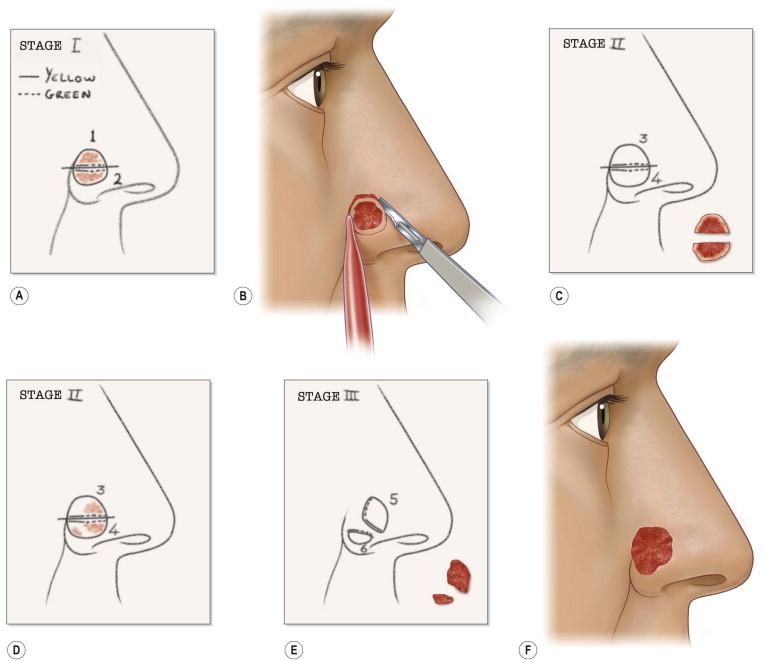
The execution of MMS requires several precise steps. Before surgery can commence, vital signs, including blood pressure and pulse are recorded, the patient medication history is updated, and informed consent is obtained in writing. The exact location of the lesion must also be confirmed by the patient and physician prior to surgery. Patients sometimes have difficulty with this step. The site of the tumor may be identified with the aid of adequate lighting, magnification, and/or Wood's light. Dermoscopy may also be helpful in this process. The surgeon may also reference photographs, diagrams, or descriptions of the lesions during initial presentation or time of biopsy. The tumor size is recorded on an anatomic diagram, which functions as a surgical map. A 1–5 mm surgical margin, depending on tumor type, is drawn around the clinically apparent tumor with a surgical marking pen ( Fig. 45.4A ). Because local anesthesia can distort the anatomy, landmarks and borders of cosmetic subunits may also be outlined at this time. For lesions of the lip, it may be helpful preoperatively to mark the white roll of the vermilion border with small, superficial nicks using the scalpel after injecting a small amount of local anesthetic because marking ink may be wiped away by skin cleansers or blood. The lesion may be photographed preoperatively before or after marking (and at subsequent stages of the procedure as deemed necessary for documentation).
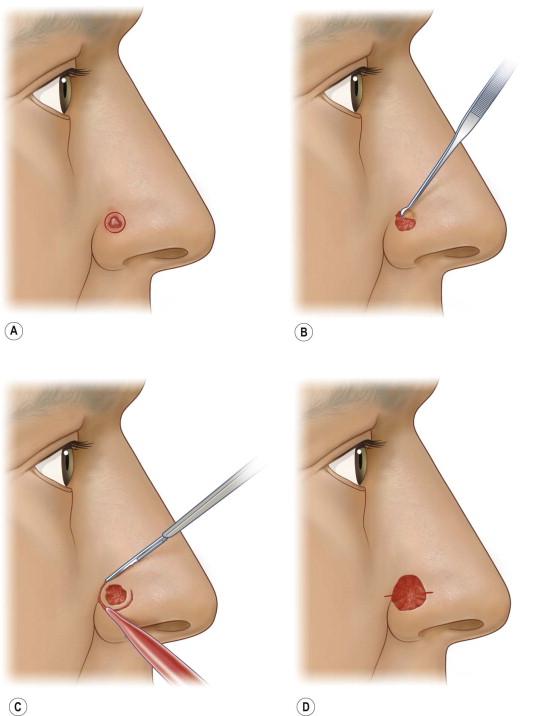
Local anesthesia is obtained with 1% or 2% lidocaine with epinephrine 1 : 100 000 or 1 : 200 000 in most situations. Factors to make injections more comfortable are buffering anesthetic with sodium bicarbonate, injecting slowly, and using distraction techniques such as pressure, vibration, or cold. Topical 20% benzocaine or lidocaine preparations may alleviate the discomfort of the injection. It is advisable to wait several minutes to allow for complete anesthesia and vasoconstriction to occur before proceeding. Occasionally, regional nerve blocks may be helpful for large tumors. This may be supplemented with a longer-acting anesthetic agent such as bupivacaine to enhance patient comfort between layers.
The tumor and surrounding area is then prepped with povidone-iodine or chlorhexidine gluconate and draped. If the tumor is large or thick, it may be debulked using a curette or surgical razor blade with or without the use of electrodesiccation ( Fig. 45.4B ). This may further delineate margins and reduce the number of layers necessary to obtain clear margins. A small specimen of this material may be obtained for a vertical transverse section for documentation and to identify specific tumor growth pattern within the tissue, especially if the biopsy was not definitive. Immediately thereafter, the incision is made along the outlined margin with a scalpel, usually with a no.15 or no.15c blade, and the tumor is sharply excised to an appropriate depth. A no.10 blade can be useful for larger tumors overlying thick skin. A saucer-shaped specimen with 30° beveled edges is ideal so that the specimen may be flattened, but the degree of beveling varies among Mohs surgeons ( Fig. 45.5 ). Flattening means that the bottom and margins can be sectioned by the Mohs technician in the same plane. Surgical reference marks are made on the specimen and adjacent tissue with gentian violet or the scalpel for precise orientation of the specimen before its removal. Once removed, maintaining proper orientation of the specimen is critical to the integrity of the entire procedure ( Figs 45.4C,D, 45.6 ). Hemostasis may be achieved with direct pressure, chemical cautery, electrocoagulation, or a hand-held battery-operated thermal electrocautery unit if avoidance of electrical interference with implanted devices is required.
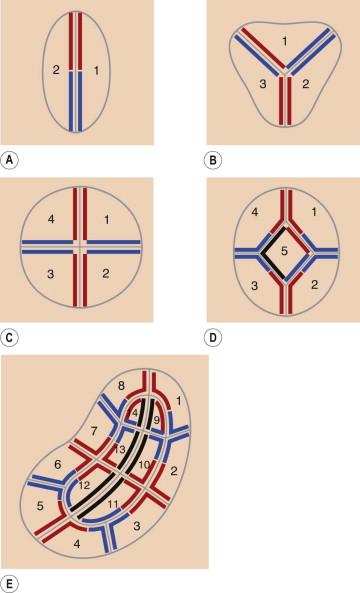
A two-dimensional map is then created while the patient is still in the operating suite to document the exact tumor location. It may be helpful to transpose the map onto a digital photograph of the lesion. The specimen is then divided into sections small enough to fit on a microscope slide (0.5–1.5 cm), and its non-epidermal edges are stained to allow proper orientation ( Figs 45.6C, 45.7A,B ). Two to three colors are used for each specimen to allow for adequate orientation. The number of specimens depends on the size of the tumor, but the numbering sequence of the tissue specimens should be consistent from patient to patient. The clockwise numbering pattern is the one historically and most commonly used, starting at the 1 o'clock position. When large specimens have central portions, the sequence is initiated at the skin edges and then continued into the central portions ( Fig. 45.8 ). Ideally, the layer of tissue should be cut into as few blocks as possible to minimize the risk of false-positive and false-negative margins that increase with each additional subdivision.

When specimens contain cartilage or bone, or subcutaneous tissue without any skin edge, this should be indicated on the map. At this point, the tissue is handed to the Mohs technician for further processing. Patients are advised that each layer may require 1–2 h, and they may proceed to the waiting area after the placement of a pressure dressing. Occasionally, patients remain in the Mohs operating suite during tissue processing if their mobility is limited or if they require continuous monitoring.
Once in the laboratory, rather than being cut vertically, each specimen is placed bottom-side up toward the microtome stage so that horizontal sections can be taken from the deep surface. This process requires many precise steps with attention to detail to avoid mapping errors. First the specimen is embedded in optimal cutting temperature (OCT) or similar agents compound (Tissue-Tek; Miles Inc, Diagnostics Division, Elkhart, IN) and a forceps or flat portion of a scalpel handle is used to ensure the specimen is completely flat. If the specimen contains cartilage or fat, this can be more difficult. A variety of devices and maneuvers is available to assist in tissue flattening if the technician finds them of value.
The tissue may be immediately frozen with tetrafluoroethylchloride or liquid nitrogen and then transferred to the cryostat for thorough freezing (see Fig. 45.7C ). Obtaining a complete section is critical to prevent errors in tissue interpretation through missing sections. It requires great skill and practice. Training and certification information is available through several organizations, including the American Society for Mohs Histotechnology (Milwaukee, WI). Flattening the specimen, raising its peripheral margins, and cutting through the tissue block with the microtome blade before representative sections are obtained are all helpful techniques to avoid missing skin edges on portions of the microscope slide. Slides are identified and prepared with the patient's name, date, and layer/specimen sequence number, with attention to detail to avoid mapping errors.
The first section obtained is the truest margin. It may be placed closest to the frosted edge of the slide or furthest from the frosted edge, depending on the preference of the technician or surgeon, but should be established by the surgical team in advance and performed consistently. Often one to three additional sections are obtained at 4–8 µm intervals and placed on the slide sequentially ( Fig. 45.9A ). The process is repeated for each tissue specimen. Once all tissue has been processed, the slides are stained, usually with hematoxylin and eosin (H&E), though toluidine blue is also popular in many practices. In high-volume laboratories using H&E, they are placed in an automatic slide stainer such as the Shandon Linistat automated system (Thermo Electron Corporation, Waltham, MA) to decrease processing time by about one-third ( Fig. 45.9B ). Finally, the slides are rinsed, a clear mounting medium such as Cytoseal-60 (Richard Allen Scientific, Kalamazoo, MI) or similar medium is applied, and coverslips are placed to facilitate microscopic review ( Fig. 45.9C ).
Most MMS laboratories use routine H&E staining for all specimens. Although there are many immunostains that can be used for certain challenging or special tumors, the most commonly used immunostains in dermatologic surgery include MART-1 for melanocytic neoplasms, cytokeratin stains for keratinocytic neoplasms, and CD34 stains for dermatofibrosarcoma protuberans (DFSP). In a comparison study with a cytokeratin marker, Smeets and colleagues found there was a greater than 99% sensitivity in the identification of BCC with H&E on Mohs sections. However, adjuvant cytokeratin staining may be useful in selected cases of BCCs exhibiting aggressive or poorly circumscribed growth characteristics. Ber-EP4 is one such widely available cytokeratin stain used for this purpose. Toluidine blue can also be used for BCCs, favored by some for its optical clarity. However, due to reagent cost, unavailability of automated staining equipment, additional processing time, and additional technician and physician time, the process of immunostains is impractical for routine use. For melanoma, several stains have been used to increase the accuracy of interpretation of frozen sections. These include human melanoma black-45 (HMB-45), melanoma antigen recognized by T cells (MART-1, also known as Melan-A,) S-100, microphthalmia transcription factor (MITF), and mel-5, with MART-1 being the most useful stain for most melanomas. S-100 is superior for desmoplastic melanomas, and MITF may be chosen because of its nuclear staining and more clearly delineates melanocytes from background staining. Menaker and co-workers found that frozen sections using HMB-45 stained comparably with paraffin-embedded permanent sections at each stage of resection in a study of 20 cases of melanoma treated with Mohs micrographic surgery. Albertini and associates found MART-1 to be superior in sensitivity to both HMB-45 and S-100 for frozen sections.
Immunostains may also be used for many other tumors. Smith and co-workers found S-100 staining useful for tracking perineural extension of granular cell tumor not evident with H&E. Harris and colleagues described the use of carcinoembryonic antigen (CEA) rapid staining in the treatment of extramammary Paget's disease treated by Mohs surgery. Similarly, the CD-34 immunostain has been studied in DFSP. Whether or not immunostains are used, permanent sections may be obtained for additional confirmation of clear margins in more challenging cases.
At this point, the Mohs technician hands the stained slides to the Mohs surgeon for histologic examination. Each microscope slide is examined under light microscopy, carefully noting the slide sequence numbers. Specimens should be superimposed on the Mohs map if there is any uncertainty about the origin or orientation of the specimen. There is a predictable 10–20% tissue shrinkage that occurs during frozen section processing, but any larger size discrepancy (or large holes) should alert the Mohs surgeon that the specimen (and thus the surgical margin) could be incomplete as a result of technical error. Technical errors are the most common cause of local recurrences after Mohs surgery.
The vertical specimen of the debulked tumor is first examined because it may provide more complete information than the biopsy, and may be the only view of the tumor if the first Mohs layer is clear. By examining the slides in numerical order corresponding to the map, residual tumor can be identified and its exact location determined based on the colors of the margins on the slide. It is noted on the map with red marking ink ( Fig. 45.10 ). Additionally, areas of calcification, perineural spread, inflammation, and other incidental skin neoplasms such as actinic and seborrheic keratoses can be identified. Sections need to be carefully examined because rete ridges, hair follicles, and other adnexal structures can mimic tumor. Dense inflammation may mask small collections of tumor cells. Patients with chronic lymphocytic leukemia and solid organ transplant recipients have a 36% and 13% incidence, respectively, of prominent inflammatory foci on Mohs sections compared with controls (1%), making slide interpretation more difficult. Macdonald and associates evaluated 22 419 Mohs cases in which 6233 (27.8%) had additional layers taken because of inflammation or fibrosis, resulting in 121 (1.9%) cases showing tumor. Interestingly, 14 of these were collision tumors which can be found in patients with severe actinic field damage. However, a study by Katz and colleagues obtained additional Mohs layers to remove dense inflammation in 25 cases of BCC and performed H&E as well as Ber-EP4. The investigators concluded that the presence of dense inflammation on Mohs sections of BCCs may be associated with close proximity to tumor (52%) but does not necessarily obscure the presence of any residual tumor and should be carefully evaluated before taking an additional layer. Similarly, although an intense infiltrate may surround SCCs, any residual tumor usually can be visualized without the use of special stains. In our practice, each case is approached uniquely because we have seen tumor masked by inflammation.
If the pathology on frozen sections is not clear, permanent tissue specimens can be obtained for improved resolution or increased depth of tissue evaluation through levels to trace specific anatomic structures of concern. If any of the specimens examined are missing epidermis, dermis, or subcutaneous tissue, a recut can be obtained after the specimen has been reoriented in the OCT medium. In cases with an extremely large number of specimens, the surgeon may have every other one processed first to give an initial view of the tumor extent.
If residual tumor is identified or a segment of the margin cannot be fully visualized even after recuts have been obtained, the patient is brought back to the operating room for removal of another layer. In this way, the Mohs practitioner functions as both surgeon and pathologist. In a manner similar to the first layer, but avoiding complete re-excision unless all margins are positive, a specimen containing the area of residual tumor and a small amount (2–5 mm) of surrounding tissue is removed after careful marking with gentian violet ( Fig. 45.10 ). These layers are successively numbered on the Mohs map using Roman numerals. This cycle is repeated until no residual tumor is identified on any slides of a particular layer. Proper interpretation of Mohs micrographic frozen sections is thus critical in the training and practice of the Mohs micrographic surgeon.
Once the tumor has been completely removed, the patient is evaluated for reconstructive options. Cosmetic and functional issues must be considered with each wound before a decision is made to allow second intention healing, primary closure, or cover the wound with a graft or flap. If tumor recurrence is possible, second intention healing or split-thickness skin grafting should be considered. In these situations, flaps are to be avoided because they may mask recurrence. Extensive undermining and tissue advancement, rotation, or transposition may facilitate tumor growth along the path of least resistance. Second intention healing may allow recurrent tumor to be visualized more easily. Similarly, split-thickness skin grafts may allow increased visualization of the defect to detect recurrent tumor. Definitive repair may be delayed to allow for histologic reconfirmation of clear margins at a future time, or to increase the success of a full-thickness skin graft. If tumor recurrence is unlikely, repair can proceed with linear closures, flaps, or grafts.
The use of MMS provides the maximal assurance that residual tumor cells do not remain in adjacent normal-appearing skin and tissue.
Ensure appropriate instrumentation and equipment are available (e.g., special instruments for infiltrating tumors that involve deeper structures; curved ophthalmic blades for perioral and perinasal areas).
Ensure all staff, including nurses and technicians, have special Mohs surgery training.
Recognize potential complications and plan for the unanticipated (e.g., perioperative bleeding or emergencies).
Proper instrumentation, equipment, organization, and set up in the Mohs surgery area are essential to a successful operative outcome ( Figs 45.11 , 45.12 ). Mohs surgery requires a qualified practitioner functioning both as a surgeon and a pathologist, as well as well-trained nursing staff and skilled Mohs technician. It also requires patient cooperation and patience while the time- and labor-intensive layers are performed.
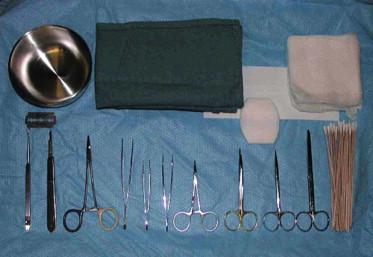
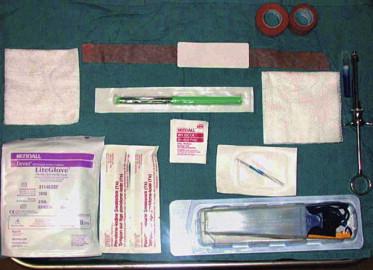
Recognizing potential areas of complication allows the surgeon to be proactive in primary prevention and risk management. Whenever surgery is being performed in the periorbital region, caution must be observed to prevent inadvertent injury to the eye. The eyes can be protected by taping cotton gauze over the eyelids, which also limits the intensity of the operating room lights. If the tumor involves the eyelid margin, a lubricated plastic eye shield may be inserted onto the cornea after topical anesthesia with tetracaine ophthalmic drops or a similar fast-acting anesthetic solution. The external auditory canal can be protected from undesirable blood by placing gauze into the meatus. Appropriate restraining belts on the operating room table are helpful if the patient is at risk of falling for any reason. A smoke evacuator should be readily available to remove malodorous electrosurgical plume. Suction is helpful to minimize brisk perioperative bleeding that obscures the surgical field. A plan for handling emergencies or unanticipated events is necessary. An efficient outpatient surgical area and laboratory are critical in ensuring overall patient satisfaction.
The removal of infiltrating tumors involving deeper structures requires special instrumentation to harvest the specimen without injuring adjacent structures. A spring-loaded Castro–Viejo needle driver and Westcott scissors are frequently used for periorbital excisions, allowing the cutaneous surgeon superior fine control ( Fig. 45.13 ). An operating room microscope may be used to further enhance precision. Ophthalmic mini-blades on cylindrical handles may be useful around the eye as well as for the ear and nose, to facilitate making the incision with the desired bevel. Tumors involving perichondrium, periosteum, and the nail can be dissected more easily using a periosteal elevator. For aggressive tumors that involve the skull, facial bones, or sinuses, the deep margin can be cleared by chiseling with a mallet. Bone specimens require decalcification in ethylenediaminetetra-acetic acid (EDTA) or similar softening reagent before they are sectioned the next day, necessitating a surgical delay to confirm tumor-free margins. For large tumors, a double-bladed scalpel can be used to allow for maximum tissue conservation and enhance mapping accuracy in peripheral margin control. Confocal mosaicing microscopy is a technique which may provide more rapid surgical observation of large areas of excised tissue with magnification. Mosaics are produced more quickly than frozen histology sections and could potentially expedite and assist surgery.

Immediately after the procedure, the patient's skin is cleaned of all blood, antiseptic, and markings, and a pressure dressing is secured with skin adhesive, to be left undisturbed for 24–48 h in most practices. For difficult facial concavities, such as the melolabial fold, dental rolls may be helpful to secure pressure. Wound care generally consists of cleansing with 3% hydrogen peroxide solution or a mild cleanser, followed by the application of petrolatum or antibiotic ointment, and then the wound is covered with a non-adherent dressing. There is a trend toward using plain petrolatum over topical antibiotics for wounds from clean dermatologic procedures in order to reduce the risk of antibiotic resistance and allergic contact dermatitis. Dressing changes are usually recommended twice daily. Patients should receive explicit instructions on when and how to call the Mohs surgeon in the event of unexpected problems.
Postoperative pain is usually easily managed after MMS. Patients should be instructed to take non-aspirin-containing analgesics for their discomfort. Anatomic locations subject to movement or tension such as the mid-chest, shoulders, and lips, may be more likely to be uncomfortable postoperatively. Procedures involving large tumors or patients with low pain thresholds may require narcotic analgesics. Patients experiencing pain several days postoperatively may have a wound infection or hematoma and should be evaluated.
Mohs surgery patients should be seen at least once in the 6-week postoperative period after suture removal to ensure proper wound healing and contraction. Patients may have an unexpected outcome such as a spitting suture, flap tip necrosis, partial graft slough, hypertrophic scar, or other event requiring the surgeon's intervention to optimize the ultimate outcome of the surgical procedure. An additional visit 3 months postoperatively may be used to monitor for recurrence and for patient education. Patients should receive regular skin examination, and be aware of their increased risk for additional cutaneous malignancies.
Careful preoperative planning minimizes complications.
Take an anesthetic history to identify allergy to lidocaine, which is rare but can cause anaphylaxis.
Monitor patients for procedural or needle anxiety, using sedation if necessary, and take appropriate precautions for fall risk.
Meticulous surgical technique and patient education help to reduce postoperative infection.
Assess whether cutaneous and sensory nerves are at risk, take precautionary measures (e.g., injection to tumesce tissue before removal, upward traction, and use of a nerve stimulator with an alarm), and provide appropriate informed consent explaining risk of nerve injury.
In the periorbital region, take care to avoid direct eye injury – where the eyelid is not directly involved, protect the eyes with taped gauze pads or drapes.
Advise patients on wound care and give explicit instructions on when to contact the surgeon if unexpected problems occur.
Complications of MMS are relatively infrequent. They may be minimized with careful preoperative planning. Cook and Perone, in a prospective study of 1358 cases, had a complication rate of 1.64%, most involving difficulties with hemostasis. Office-based Mohs surgical units provide a safe environment for the patient. A logbook should be maintained to detail complications, to establish trends, and to prevent recurrences as far as possible. A complete set of vital signs, including blood pressure and pulse, should be taken before the procedure.
Of the more serious complications, anaphylaxis to anesthetic agents or other administered medications is the most grave. Use of supplementary oxygen and administration of emergency medical care may be required. Training in Advanced Cardiac Life Support (ACLS) is a required component of fellowship training in MMS. Allergy to lidocaine, the most commonly used of the amide local anesthetics, is rare, but can result in anaphylaxis. This agent does not cross-react with the ester class of local anesthetics which are more commonly responsible for untoward effects. When a patient gives a history of “allergy” to local anesthetics, an episode of epinephrine sensitivity is most commonly elicited. In these patients and in the elderly with labile hypertension, the concentration of epinephrine used may be reduced while still maintaining the hemostatic effect. Some patients may need an allergy work-up to better characterize their precise sensitivity. Diluting the anesthetic agent with plain 1% lidocaine to achieve an epinephrine concentration of 1 : 300 000 can be used to provide sufficient hemostasis.
Patients may faint because of anxiety about the procedure or a fear of needles. Precautions must be undertaken to avoid the risk of a fall, including patient monitoring and observation, maintaining the surgical table as low as possible to the floor during non-operative periods, and light patient restraints. Patients with vasovagal symptoms should be placed in the Trendelenburg position. Patients should be encouraged to eat a light meal before surgery to prevent hypoglycemic episodes, except when a “nil per os” (NPO) status is indicated.
Procedural anxiety may be managed first by a caring staff attitude and interactions with comfortable, soothing surroundings, and soft, relaxing music and lighting. A surgeon's expression of confidence in a favorable outcome is also helpful in alleviating anxiety. If anxiety is excessive, the patient may be sedated with an oral or sublingual benzodiazepine, e.g., diazepam at a dose of 5–10 mg 1 h preoperatively. Benzodiazepines can reduce blood pressure, so careful monitoring is necessary before further administration. Important causes of patient anxiety are a fear of needles and pain with injection. The patient may recall biopsy-related pain, and fear a commensurate increase in pain with the excision. Slow injections of anesthesia through a small-bore needle (30 gauge) are critical. Advancing the needle through previously anesthetized sites and injecting upon withdrawal can greatly minimize discomfort.
Bleeding is the most common complication to be avoided. Despite the use of epinephrine and meticulous electrocoagulation to aid hemostasis, other measures may be required to limit blood loss. Suture ligation of large transected blood vessels may be necessary, especially of the superficial temporal artery, which is not infrequently encountered in excisions on the temple. If the field is large or bleeding persists despite appropriate hemostatic measures, other hemostatic agents such as bovine thrombin or hemostatic gauze (e.g., SeaSorb soft alginate dressing; Coloplast Corp, Marietta, GA) may be helpful. Pressure dressings between layers are important to minimize bleeding. Upon repair of the Mohs defect, closure of underlying dead space in layers is critical to prevent hematomas from small arterioles or capillary beds. Postoperatively, pressure dressings for 24–48 h are helpful. Dental rolls and other bolster materials placed on the skin and secured with tape augmented with skin adhesive (e.g., Mastisol, Ferndale Laboratories Inc, Ferndale, MI) are particularly helpful in the mid-face and periauricular areas.
Infection may develop in the setting of any cutaneous surgery, however the infection rate after Mohs surgery is very low. In a study by Rogers and colleagues, involving 1000 Mohs surgery cases in which both the Mohs layers and reconstruction were performed using clean surgical technique, the infection rate was only 0.91% and occurred more often with flap closures or as a complication of hematomas. Tumors treated with Mohs surgery that have required multiple layers to clear or prolonged exposed periods of time may have an increased risk of infection. The risk of infection is also increased if there is abraded or crusted skin and in immunocompromised patients. Patients who allow their wounds to desiccate or lack diligence in their postoperative care are also more likely to develop an infection. Risk can be minimized by meticulous surgical technique, including the removal of hair from the wound, preferably by clipping preoperatively, and ensuring appropriate eversion of skin edges and minimizing dead space.
According to the 2008 advisory statement on antibiotic prophylaxis in dermatologic surgery, prophylactic antibiotics are not routinely recommended unless there is high risk of infective endocarditis (IE), hematogenous total joint infection, or surgical site infection. Prophylactic antibiotics are indicated in high-risk patients in whom there is a breach in mucosa or infected surgical site or any patient with a surgical site in a location at high risk of infection. Patients at high risk of IE are those with a prosthetic cardiac valve, previous infective endocarditis, unrepaired congenital heart disease (CHD), repaired CHD with prosthetic device within 6 months of procedure, repaired CHD with residual defects, or cardiac transplant recipients with valvulopathy. Patients at high risk of joint infection are those having joint replacements within 2 years of surgery, previous prosthetic joint infections, immunocompromised patients, or those patients with type 1 diabetes, HIV infection, malignancy, malnourishment, or hemophilia. Finally, sites and locations at high risk of surgical infection include lower extremities, groin, wedge resection of lip or ear, skin flaps on the nose, skin grafts, or areas of extensive inflammatory skin disease.
Wound necrosis may occur when the repair places the wound under significant tension. Poor flap design resulting in vascular compromise may result in wound edge necrosis, requiring a portion to heal by second intention. Full-thickness or split-thickness grafts may be lost, again resulting in second intention healing. Sharp wound debridement may be required in some situations where eschar covers the wound.
Large or recurrent tumors in high-risk locations may be so deeply invasive that they may involve cutaneous and sensory nerves. Knowledge of the anatomy will enable the cutaneous surgeon to advise the patient pre- and perioperatively of the risk of injury and assess for any neurological deficits present. Nerve injury may be avoided in several ways:
First, an injection of dilute local anesthetic or 0.9% saline can be used to tumesce the tissues immediately before removal of the layer and thus makes it unlikely to inadvertently encounter vital structures including nerves.
Second, gentle traction upward on the tissue as it is dissected minimizes the risk to underlying nerves. A nerve stimulator with auditory alarm may be used in conjunction with the dissection.
Third, electrocoagulation-induced nerve injury can be minimized in critical locations such as the periorbital area by the use of Teflon-coated bipolar forceps.
Unrealistic expectations, poor surgical planning, intervening infection or hematoma, and other factors interfering with healing may result in wound cosmesis that is unacceptable to the patient. Although the main goal of MMS is complete extirpation of the tumor, the cosmetic and functional results are frequently of paramount importance to the patient.
Smokers have a higher rate of graft failure and delayed wound healing. Wounds closed under sustained tension frequently develop superficial necrosis, which can adversely affect the cosmetic result. Occasionally, a delayed repair is necessary, and the ultimate result may not be realized for a year or longer, requiring patience on the part of the physician and patient. Surgical repairs may need refinement in a series of steps for optimal cosmesis and functionality. However, most post-Mohs surgical wounds regain their texture, coloration, and sensation eventually.
Tumors that have extended to a free anatomic margin, such as the eyelid or lip, require additional surgical planning to prevent functional and cosmetic impairment. Ectropion may develop if tension is placed on the lower eyelid free margin. Xerophthalmia, keratitis, and impaired vision may result when the lacrimal apparatus of the eye is compromised. Chondritis may occur when the integrity of the cartilage is disturbed, particularly if perichondrium is lost and second intention healing is used. Air flow through the naris can be compromised when the lateral ala is unsupported after loss of cartilage, requiring cartilage grafts or composite flap reconstruction. Consultation with other specialists may be helpful pre-, peri-, and postoperatively in these situations.
In some situations, complete clearance of the tumor is not achievable, even with the Mohs technique. Occasionally, involvement of deep structures means that there is a great risk of functional injury. Sometimes the layers reveal perineural tumor or invasion into bone that cannot be cleared without sacrificing vital structures. In recurrent tumors, unpredictable growth patterns may occur as a result of fibrosis from previous radiation or excision. Tumor may spread far beyond the original site of recurrence along areas of previous surgery that were undermined for previously used flaps or grafts, and on rare occasions may be too extensive to clear completely. In these situations, postoperative radiation therapy is indicated to enhance local cure rates. On occasion, a combined approach is chosen after discussion with the patient midway during the procedure (i.e., between layers). A multidisciplinary approach is helpful in the management of these advanced malignancies.
Radiation therapy can have a primary or adjunctive role in cutaneous malignancy. The Mohs surgeon should be familiar with its capabilities and limitations. Radiation therapy has advanced greatly because of the wide availability of linear accelerators that generate electrons with energy that can be varied so that the physician can treat to different depths. Customization of the beam delivered to the patient through the use of Cerrobend cutouts, blocks, contoured Aquaplast masks, and lead shielding, and refined dose fractionation schemes that optimize treatment and limit side effects have also advanced the field. Electron beam radiation penetrates deeply into the tissue, in contrast to superficial X-ray treatments with diffuse energy scattering and superficial tissue penetration. Cognetta and colleagues evaluated 1715 BCCs and SCCs treated with superficial X-ray over a 10-year period and found the recurrence rate at 5 years to be as low as 5%, making it an acceptable alternative treatment for patients who are not surgical candidates, who decline surgery, or who would otherwise be faced with serious adverse cosmetic or functional effects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here