Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bone is a dynamic living tissue composed of two major constituents, an organic and a mineral component. The organic components consist of (1) cells, including osteoblast, osteoclast, and osteocytes, and (2) proteins, mainly type 1 collagen (COL1) and other non-collagenous proteins. Maintenance of healthy bone is a complex process that involves the synchronization of multi-organ systems that controls calcium and phosphate metabolism, collagen formation as well as the cellular components of bone. Several organs such as parathyroid gland, kidney, liver, intestine, and bone itself regulate bone metabolism. Calcium, phosphate, vitamin D, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), estrogens, androgens, tissue nonspecific alkaline phosphatase (TNALP), and calcium-sensing receptor (CaSR) are among some of the factors known to affect bone metabolism. Furthermore, the bone cellular components are in constant signaling with each other through factors such as receptor activator of nuclear factor Kappa-B ligand (RANKL), phosphate regulating factor with homologies to endopeptidase on X chromosomes (PHEX), dentin matrixprotein-1 (DMP-1), , ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) guanine nucleotide-binding protein G(s) subunit alpha isoforms (GNAS1), FGF23, sodium inorganic phosphate cotransporters ( NaPi 2a and NaPi2c ), sclerostin, osteoprotegerin, and ephrin. Metabolic bone disease is caused by a dysfunction of one of these factors alone or in combination ( Table 38.1 ).
| Gene | Function | Type of Mutation | Effect |
|---|---|---|---|
| NPT2 a | Main renal phosphate transporter | Loss of function | Hypophosphatemia, renal lithiasis, bone demineralization |
| NPT2 b | Phosphate transporter in the lung and intestine | Loss of function | Lung microlithiasis |
| NPT2 c | Renal phosphate transporter | Loss of function | Hypophosphatemia, hypercalciuria, renal lithiasis, bone demineralization |
| PiT1 | Phosphate transporter | Not identified | Not known |
| PiT2 | Phosphate transporter | Not identified | Not known |
| PTH1R | TTH and PTH-related peptide receptor | Gain or loss of function | Gain of function: chondrodysplasia; loss of function: can be fatal in homozygotes |
| GCMB | Transcription factor mandatory for parathyroid gland development | Loss of function | Hypoparathyroidism |
| NHERF1 | Protein that binds to NPT1a and PTH1R, modulates PTH-induced cyclic AMP production | Loss of function | Hypophosphatemia, renal lithiasis, bone demineralization |
| FGF23 | Phosphaturic factor, inhibitor of calcitriol production | Gain of function | Hypophosphatemia, bone demineralization |
| Loss of function | Hyperphosphatemia, ectopic calcifications | ||
| KLOTHO | FGF23 coreceptor | Loss of function | Hyperphosphatemia, ectopic calcifications |
| GALNTE3 | Glycosylates FGF23, which increases its stability | Loss of function | Hyperphosphatemia, ectopic calcifications |
| PHEX | Unknown, putative endopeptidase | Unknown | Hypophosphatemia, bone demineralization |
| DMP1 | Transcription factor and secreted peptide | Loss of function | Hypophosphatemia, bone demineralization |
There are three types of cells in bone: osteoblasts, osteoclasts, and osteocytes. Osteoblasts are mesenchymal in origin and are responsible for COL1 production and bone mineralization. The cell membrane of the osteoblasts have matrix vesicles (MVs) budding from its cell membrane. MV is rich with TNALP required for liberation of inorganic phosphate necessary for bone mineralization, from inorganic pyrophosphate. Mutations in TNALP gene results in hypophosphatasia (HPP).
Some mature osteoblasts are destined to become embodied within the extracellular matrix to become osteocytes. Osteocytes are the most abundant cell type in bone. They have long slender processes extending through canaliculi forming connective network within bone. This network is important in the integrity of extracellular matrix. Recent evidence suggests that osteocytes regulate phosphate metabolism via PHEX, DMP 1, and FGF23. PHEX gene mutation results in X-linked hypophosphatemic rickets (XLH). A mutation in FGF23 can lead to autosomal dominant (AD) hypophosphatemic rickets. Osteocytes also modulate bone formation and resorption by producing sclerostin that inhibits osteoblastic activity and promotes osteoclast differentiation.
The macrophage is the progenitor of the osteoclast. Osteoclast formation is mediated via RANKL. In healthy bone osteoclasts are rare. Osteoclasts are responsible for resorbing bone matrix necessary for bone growth and remodeling. Defective osteoclasts can cause osteopetrosis, while pharmacologic inhibition of osteoclasts can be used to treat hypo-mineralized bone disease. Medications that block osteoclastic activity (anti-RANKL antibody and bisphosphonate [BP]) or those that stimulate osteoblastic cellular activity (Teriparatide) have been used for a variety of metabolic bone disease as discussed in detail later in the text.
Extracellular organic bone matrix constitutes up to 30% of bone mass. Organic bone matrix supports bone cell function and provides a structure in which cells reside. Bone matrix is a composite structure made of a proteinaceous interwoven scaffold on which mineral components deposit. Type 1 collagen (COL1) is the predominant collagen present in the bone. It is the major structural macromolecule of the bone matrix fiber network. A defect or deficiency of the quantity of COL1 chains can have a detrimental effect on the function and integrity of bone, as seen in osteogenesis imperfecta (OI). Mineralization of bone starts by TNALP within the cell membrane liberating inorganic phosphorus from inorganic pyrophosphate and combining with calcium forming hydroxyapatite micro crystals. As these microcrystals coalesce, further crystals deposit over COL1 leading to hardened and mineralized bone.
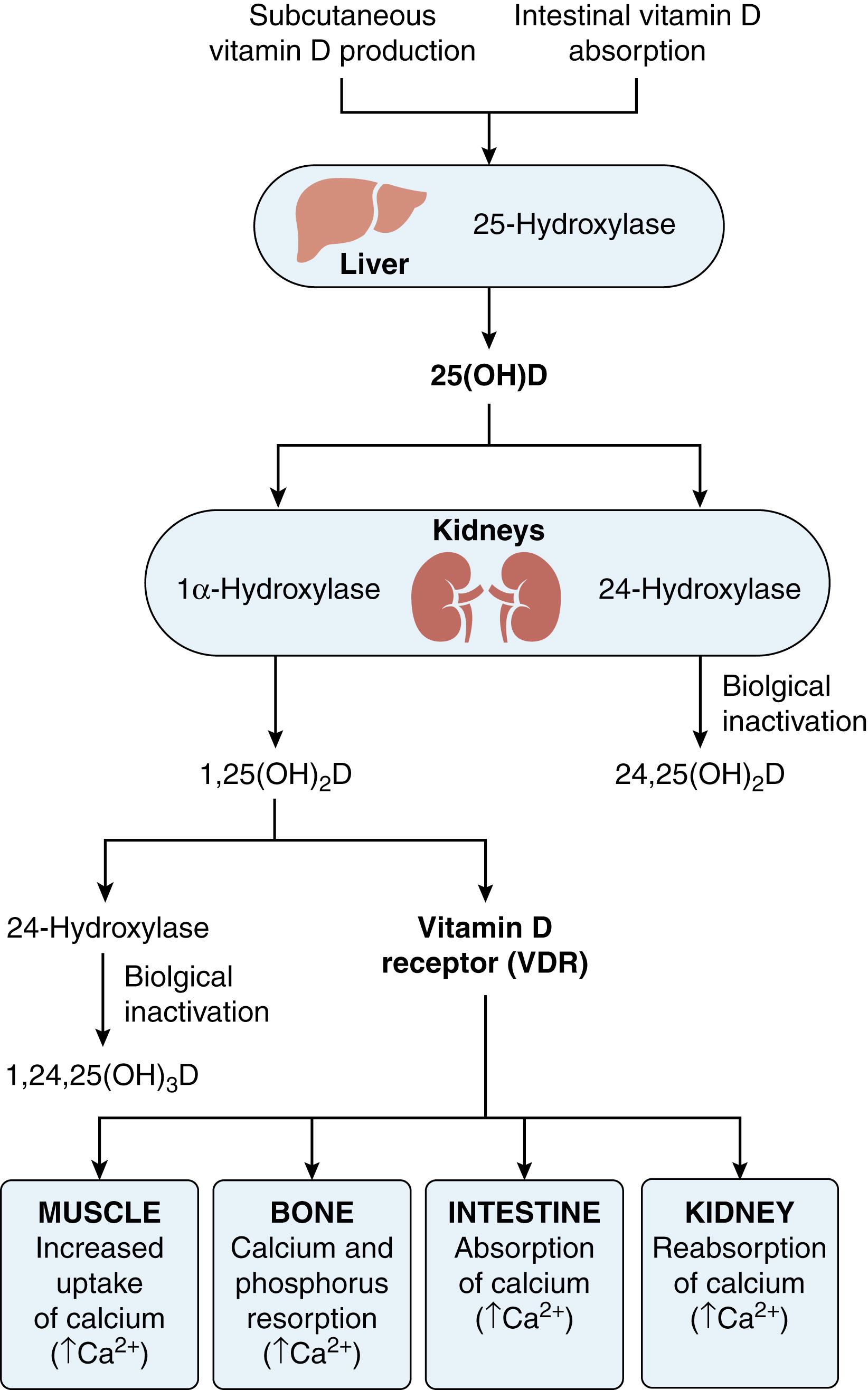
The human body is sensitive to changes in serum calcium concentration; a disturbance in extracellular versus intracellular calcium balance leads to cellular irritability, deranged conductivity, and contractility abnormalities. Almost 99% of the body’s calcium is stored in bone and teeth as hydroxyapatite, which is composed of calcium and phosphate compounds. If extra calcium is needed in the bloodstream to maintain cardiac or neurologic function, bone is the source of the required calcium, which is released via osteoclast-mediated bone resorption. Various factors, including PTH and calcitriol (1,25(OH) 2 vitamin D), regulate the activity of osteoclasts. Serum calcium is under the regulation of vitamin D and PTH ( Fig. 38.1 ). , , , , PTH functions to increase serum calcium concentration by increasing osteoclastic bone resorption and renal reabsorption of calcium in the proximal tubules, and stimulating the conversion of inactive 25-hydroxyvitamin D (calcidiol) to its active form, calcitriol. Hypocalcemia drives the conversion of the inactive form of vitamin D to its active form. The active form of vitamin D increases the intestinal absorption of calcium and increases renal reabsorption of phosphate. FGF23 is also an important negative regulator of vitamin D activation produced by bone cells. It inhibits 1α-hydroxylase activities, which is required for vitamin D activation. FGF23 also plays a key role in phosphate homeostasis by increasing renal phosphate excretion, thus decreasing serum phosphate level (hypophosphatemia). An increase in FGF23 activity is an important pathophysiologic component of vitamin D–resistant rickets (VDRR), which is associated with hypophosphatemia, hyperphosphaturia, and relatively low calcitriol activity.
Vital amines or what is known as vitamins are essential micronutrients that the body needs but cannot be synthesized by the body.
Vitamin D 3 (Cholecalciferol) is endogenously produced in keratinocytes of the skin within the epidermal layer, predominantly in the stratum basale and spinosum, upon exposure to ultraviolet light B; wavelength 290 to 315 nm. Vitamin D is then twice hydroxylated to its active metabolite, calcitriol (see Fig. 38.1 ). In that sense vitamin D is not actually a vitamin but rather a hormone producing endocrine, autocrine, and paracrine signaling effects. Endocrine signaling occurs when a hormone synthesized by a glandular cell is released into the circulation to be delivered to a receptor on a different target cell to express its biologic function. Autocrine signaling occurs when a cell secrets a hormone that binds to receptors on the same cell, and paracrine signaling occurs when the hormone released by a cell induces a response in an adjacent but different cell.
Vitamin D formation and activation is a complex process that involves the gut, skin, liver, and kidney. Ergosterol (provitamin D) is a sterol precursor found in plant tissue (see Fig. 38.1 ). Ergosterol is lipid soluble, ingested and absorbed from the small intestine, and is irradiated in the skin to vitamin D 2 (ergocalciferol). On the other hand, 7-dehydrocholesterol is a zoosterol that is converted also in the skin to cholecalciferol, as a result of exposure to ultraviolet light. Mal-absorption syndromes can result in vitamin D dependent rickets. Conditions resulting in decreased skin conversion to cholecalciferol, such as scarce sunshine, skin shielding, and dark-toned skin, can also result in vitamin D deficiency rickets.
Both cholecalciferol and ergocalciferol are biologically inert and must undergo a series of hydroxylation reactions in their transformation to the active form, calcitriol (1,25(OH) 2 vitamin D). The first hydroxylation takes place in the liver to produce 25(OH) vitamin D mediated by CYP2R1 enzyme. The liver also produces 7-dehydrocholesterol (provitamin). Deficiency in CYP2R1 result in VDDR type 1B ( Table 38.2 ). Liver disease or medications that increase the degradation of calcidiol can result in VDRR. Ninety percent of calcidiol is bound to vitamin D binding protein (DBP). Calcidiol is the major form of vitamin D in circulation with a half-life of 15 days whereas the half-life of the active form, calcitriol, is only 15 hours (see Fig. 38.1 ). Thus, serum calcidiol level better reflects vitamin D stores in the body and is a better indicator of vitamin D level than serum calcitriol level. Calcidiol is transported to the cite of the second hydroxylation via DBP. Megalin is involved in internalizing calcidiol-DBP complex from the luminal side into the proximal tubule of the kidney. Cubilin is a megalin-calcidiol co-receptor. It is proposed that cubilin binds the excreted DBP-calcidiol complex on the cell surface of the tubular cell thus facilitating endocytosis. The second hydroxylation occurs in the kidney via 1α-hydroxylase enzyme (CYP27B1) , which produces the active form, calcitriol. A mutation in CYP27B1 causes VDDR type 1A ( Table 38.3 ). Calcitriol binds to specific nuclear, vitamin D receptors (VDR) that regulate the transcription of target genes which control mineral homeostasis and cell differentiation. The VDR gene is located on the long (q) arm of chromosome 12 at position 13.1 (12q13.1). A mutation in the VDR gene causes VDDR type 2A. Post VDR defects result in VDDR type 2B.
| Calciopenic Rickets Calciopenic rickets is the consequence of the inability to maintain serum calcium concentrations in the normal range, leading to secondary hyperparathyroidism and hypophosphatemia. The hypocalcemia can be caused by vitamin D or dietary calcium deficiencies, and abnormalities of vitamin D metabolism and action. Vitamin D-Associated Causes of Rickets
Rickets as a consequence of dietary calcium deficiency due to insufficient dietary calcium intake or gastrointestinal disorders resulting in calcium malabsorption.
Rickets as a consequence of dietary phosphate deficiency or impaired bioavailability:
Rickets Caused by Increased Renal Phosphate Loss
FGF23-Independent Types of Rickets
Rickets Associated With a Direct Inhibition of Mineralization
|
| Type of Rickets | Clinical Features | Salient Biochemical Features | Genetic Mutations |
|---|---|---|---|
| Vitamin D deficiency rickets |
Classic x-ray findings, classic clinical features of rickets either nutrition-related or associated with systemic disease (see text for details) | ↓ 25 (OH) vitamin D, nl 1,25 (OH) 2 Vit D, ↑ PTH | None |
| VDRR | |||
| XLH | Classic x-ray findings, classic clinical features (see text for details), symptoms are more severe in males | ↓ Pi, ↓ 1,25(OH) 2 vitamin D, nl Ca, nl PTH ↑ urinary phosphate excretion, ↑ FGF23 | PHEX |
| AD VDRR | Classic x-ray findings, classic clinical features (see text for details) | ↓ Pi, ↓ 1,25(OH) 2 vitamin D, nl Ca, nl PTH ↑ urinary phosphate excretion ↑ FGF23 |
FGF23 |
| AR VDRR Type 1 Type 2 |
Classic x-ray findings, classic clinical features (see text for details) | DMP1 ENPP |
|
| AR VDRH | Classic x-ray findings, classic clinical features (see text for details) | ↓ Pi, ↓ 1,25(OH) 2 vitamin D, nl Ca, nl PTH ↑ urinary phosphate & calcium excretion | NaPi 2c |
| Fibrous dysplasia (McCune-Albright) | Classic x-ray findings, classic clinical features (see text for details), cafe au lait spots | ↓ Pi, ↓ 1,25(OH) 2 vitamin D, nl Ca, nl PTH ↑ urinary phosphate excretion ↑ FGF23 | GNAS |
| Fanconi | Variable depending on cause | Acidosis, ↓ Pi, ↑ urinary-reducing substances and amino acids | Variable |
| RTA | Classic x-ray findings, classic clinical features (see text for details), growth delay | Acidosis | |
| TIO | Classic x-ray findings, classic clinical features (see text for details) | ↓ Pi | Not Known |
| VDDR | |||
| Type 1A | Classic x-ray findings, classic clinical features (see text for details), seizure | ↓ 1,25(OH) 2 vitamin D | CYP27B1 |
| Type 1B | Classic x-ray findings, classic clinical features (see text for details), seizure | ↓ 25(OH) vitamin D | CYP2R1 |
| Type 2A | Classic x-ray findings, classic clinical features, seizure, ± alopecia dwarfism | ↑↑↑↑ 1,25(OH) 2 vitamin D | VDR |
| Type 2B | Similar to type IIA details), seizure, dwarfism normal receptors | ↑↑↑↑ 1,25(OH) 2 vitamin D | VDRE |
Calcitriol plays a critical role in the intestinal absorption of calcium and phosphorus. Several intestinal transport proteins found in the brush border of intestinal cells are involved in calcium absorption: Ca/Mg ATPase, alkaline phosphatase, intestinal membrane calcium binding proteins, and calcium channels TPRV5 and TRPV6. Calcitriol has additional roles independent of its action on the gut. This includes anti-proliferative effect on parathyroid cells, stimulation of osteoclast differentiation, and regulation of formation of several matrix proteins. Calcitriol in turn increases FGF23 production, which down-regulates 1α-hydroxylase activity as a negative feedback mechanism. Calcitriol and calcidiol are further catabolized into an inactive form 1,24,25 (OH) 3 vitamin D by CYP24A1 enzyme (see Fig. 38.1 ). Deficiency in CYP24A1 causes hypercalcemia.
Vitamin D deficiency rickets leads to decreased mineralization of bone or osteomalacia. When osteomalacia occurs in a growing skeleton the condition is termed rickets. Vitamin D deficiency rickets can result from various causes: decreased intake (e.g., malnutrition), decreased vitamin D stores (e.g., prematurity), malabsorption syndromes (e.g., due to biliary atresia, inflammatory bowel disease, or liver disease), medications that stimulate cytochrome P450 (e.g., antiepileptic), kidney disease, and conditions leading to DBP loss (e.g., nephrotic syndrome) (see Table 38.2 ). , , ,
In the early twentieth century, Hess used a trial of cod liver oil to treat 65 infants with rickets and found that the rickets resolved in 92% of the infants during a 6-month course of treatment. Mellanby and Park, in the mid-1920s, were the first to suggest that rickets could be prevented by adequate vitamin D intake. Since that time, milk and dairy products have been fortified with vitamin D. Although nutritional rickets is thought to be rare in developed countries because of vitamin D fortification, some studies show a reemerging burden of rickets in developed countries. , , , Certain populations are at risk, including premature infants, infants with prolonged breast feeding, infants with food allergies, children on a vegetarian diet, dark-skinned children, and immigrant populations from the Indian subcontinent, Africa, and the Middle East. a
a References , , , , , , .
In the United States, an increase in the frequency of nutritional rickets has been reported in children with dark skin pigmentation who are breast-fed past 6 months of age without vitamin D supplementation. , , , ,
In rickets, the primary disturbance is failure of mineralization of growth plate cartilage and osteoid tissue, which decreases longitudinal bone growth and weakens the mechanical properties of tubular bone. , , , , Thus the pathologic manifestations of the disease are most noticeable around growth plates and along long bones. Normal longitudinal growth of bone is the result of endochondral ossification. The key elements of the process are proliferation of chondrocytes in columns (zone of proliferation), cellular maturation to become hypertrophic chondrocytes, calcification of the cartilage matrix (zone of provisional calcification), vascular invasion of the terminal hypertrophic chondrocytes, and deposition of new bone. In rickets, failure of deposition of calcium along the matrix of the maturing chondrocyte columns is observed, along with a disorderly invasion of cartilage by blood vessels, lack of resorption at the zone of provisional calcification, and consequently increased thickness of the growth plate ( Fig. 38.2 ). The chondrocytes proliferate normally, but the normal process of endochondral ossification fails to take place.
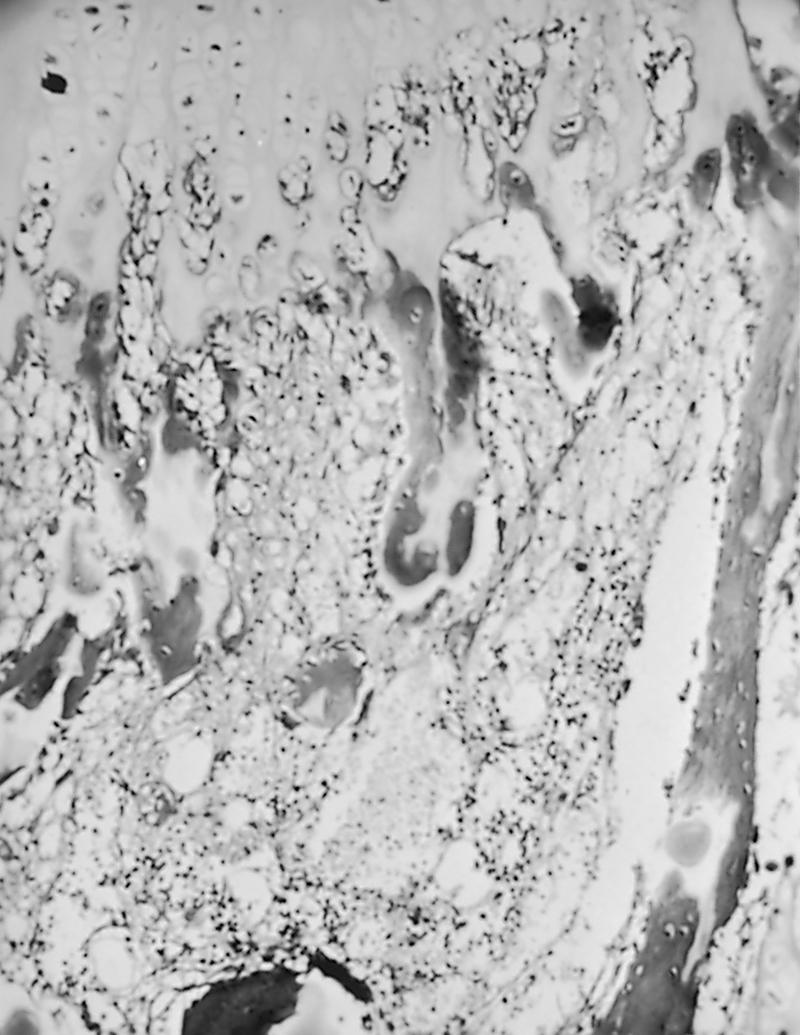
Osteoblastic activity on the endosteal and periosteal surfaces of bone is normal, and abundant osteoid (nonmineralized bone matrix) is formed. With defective mineralization of osteoid, osteoclastic resorption of the osteoid does not take place. However, increased resorption of the mineralized bone occurs from secondary hyperparathyroidism due to hypocalcemia. Hence, the overly abundant osteoid produced by normal osteoblasts generates widened osteoid seams. Because of a lack of resorption of osteoids, the osteoid islets may even persist down into the diaphysis.
In rickets, an abnormality in the arrangement of bundles of collagen fibers in compact bone also exists. Instead of running parallel to the haversian canals, they course perpendicularly and are biomechanically inferior. Grossly, the rachitic bone is soft and becomes misshapen under the force of weight bearing. If the disease remains untreated, angular deformities of the lower extremities and deformities of the thoracic cage and pelvis may develop.
After treatment of rickets with vitamin D, calcium absorption increases and calcification of the cartilage columns and osteoid occurs. Osteoclasts resorb the calcified cartilage, and normal remodeling and improvement of bone follows.
Vitamin D deficiency rickets mainly results in an inability to absorb calcium and phosphorus from the gut. Vitamin D status is best evaluated by measuring the level of serum 25(OH) vitamin D (calcidiol), which reflects the degree of deficiency as discussed above. In contrast, the serum level of 1,25(OH) 2 vitamin D (calcitriol) is not helpful because it may be normal in most patients with vitamin D deficiency rickets. The PTH level is elevated in response to hypocalcemia (secondary hyperparathyroidism), which attempts to ameliorate the serum calcium level. Serum calcium levels are normal to mildly decreased, but phosphate levels are low (hypophosphatemia). Bone turnover markers such as alkaline phosphatase, osteocalcin, pro-collagen I N-propeptide, and COL1 N-terminal and C-terminal telopeptide levels are elevated ( Table 38.4 ). , , , , Urinary excretion of calcium is low because of enhanced renal tubular reabsorption.
| Type of Rickets | Calcium | Phosphate | Alkaline Phosphatase | PTH | 25(OH) Vitamin D | 1,25(OH) 2 Vitamin D |
|---|---|---|---|---|---|---|
| Nutritional | Nl | Nl ↓ | ↑ | ↑ | ↓↓ | ↓ |
| Vitamin D–resistant (XLH, RTA, Fanconi, oncogenic) | Nl | ↓ | ↑ | Nl | Nl | Nl |
| Vitamin D–dependent type I (inability to hydroxylate) | ↓ | ↓ | ↑ | ↑ | ↑↑ | ↓↓ |
| Vitamin D–dependent type II (receptor insensitivity) | ↓ | ↓ | ↑ | ↑ | Nl ↑↑ | ↑↑↑↑ |
| Renal osteodystrophy | Nl ↓ | ↑ | ↑ | ↑↑ | Nl | ↓↓ |
Children with nutritional rickets are usually first seen between the ages of 6 months and 3 years, a period of rapid growth. The clinical features of nutritional rickets depend on the severity of the disease and may be subtle. Initial findings are listlessness, periarticular swelling, or angular deformities. Hypocalcemic seizure is also a common presentation during the first 2 years of life. Infants demonstrate generalized muscular weakness, lethargy, and irritability. Sitting, standing, and walking are delayed. The abdomen may appear protuberant.
Early bone manifestations include a slight thickening of the ankles, knees, and wrists. Beading of the ribs, referred to as the rachitic rosary, is caused by enlargement of the costochondral junctions. As the disease continues, the pull of the diaphragm on the ribs produces a horizontal depression known as Harrison’s groove. Short stature results from insufficient longitudinal growth. Pectus carinatum is caused by forward projection of the sternum. Closure of the fontanelles is delayed and the sutures are thickened, which leads to a skull appearance described as resembling hot cross buns. The dentition is affected, with delays in appearance of the teeth and defects in the enamel.
As the child begins standing and walking, the softened long bones bow, and it is at this time that the child is usually brought to the orthopaedic surgeon for a diagnosis. In toddlers, bowleg, or genu varum, is one of the most common initial signs. In older children, genu valgum and coxa vara may be initial features. Stress fractures of the long bones may be present. Children may be seen acutely with unexplained fractures suggesting child abuse, tetany, and hypocalcemic seizures. Later, kyphoscoliosis may develop.
Failure of the physeal cartilage to calcify and undergo normal endochondral ossification leads to an increased thickness of the physis and a hazy appearance of the provisional zone of calcification. , , The widened growth plate is particularly suspect for rickets, which differentiates this rare condition from the more common physiologic angular deformities of the lower extremities ( Fig. 38.3 ). The metaphysis abutting the physis is brush-like in appearance, with islands or columns of cartilage persisting well into the metaphysis ( Fig. 38.4 ). The metaphysis also appears cupped or flared. The bones have an osteopenic appearance overall, with thinning of the cortices. The bony trabeculae are indistinct. Looser lines, or radiolucent transverse bands (pseudofracture lines) that extend across the axes of the long bones, are evident on radiographs in 20% of patients with rickets.
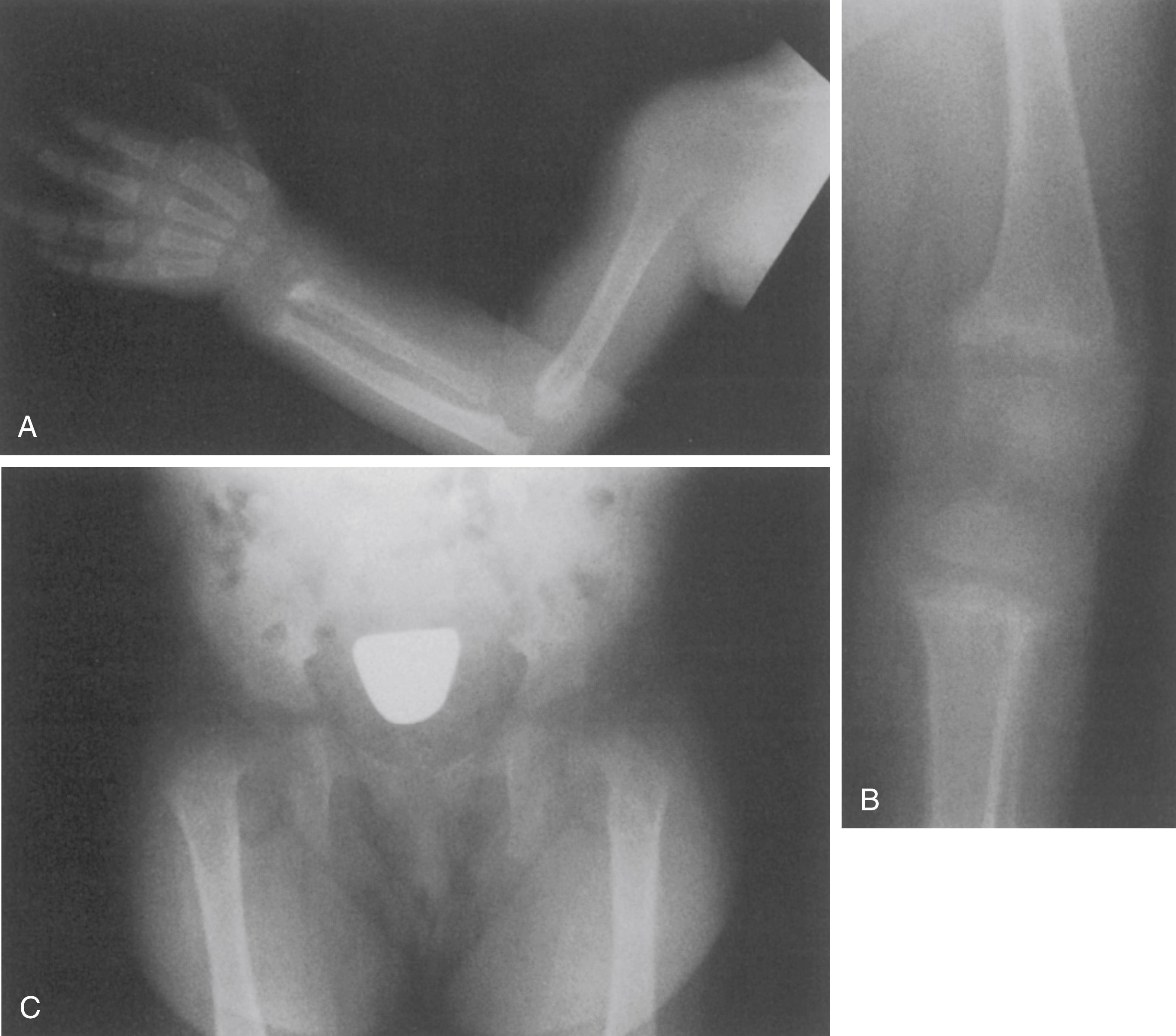
As the rickets continues, deformities of the long bones, ribs, pelvis, and spine develop. Thoracolumbar kyphosis—rachitic cat back—may be apparent on radiographs.
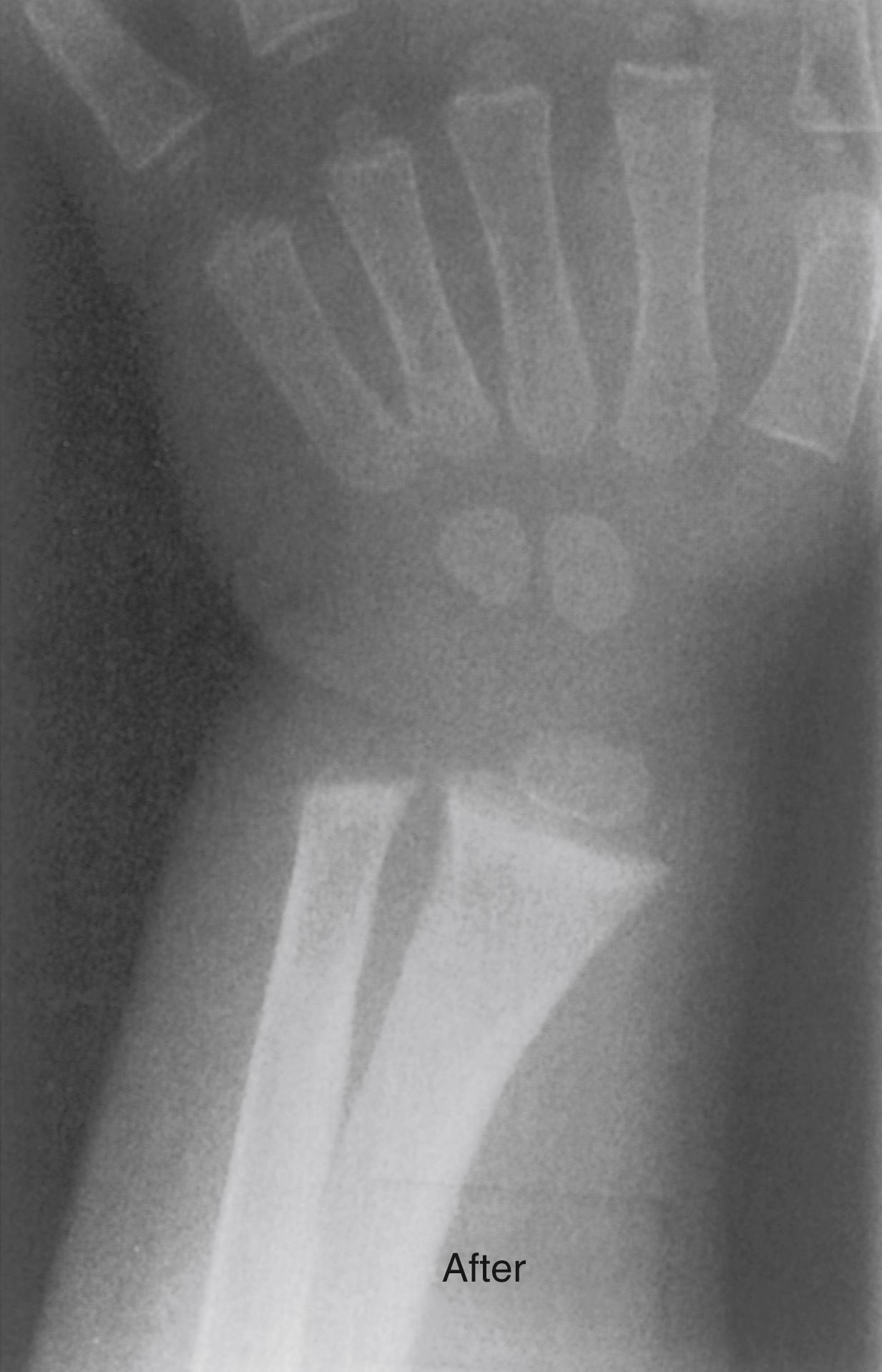
After diagnosis of vitamin D deficiency rickets is confirmed, therapy should be initiated. Medical treatment of rickets is multidisciplinary and involves primary care provider and a dietitian. Dietary counseling evaluating daily intake of calcium, phosphorus, and vitamin D is an essential part of managing vitamin D supplementation in the form of either cholecalciferol or ergocalciferol, which is the backbone of therapy. Vitamin D replacement could be daily or high doses involving biweekly or monthly regimen. 25(OH) vitamin D serum concentration should be checked 6 to 8 weeks after initiation of therapy. The usual duration of initial treatment is 6 to 10 weeks. After 2 to 4 weeks, radiographs show improvement in mineralization. The physis thins, and bone density increases ( Fig. 38.5 ). , Vitamin D stores are usually replenished after 8 weeks of therapy and vitamin D intake should be maintained afterward. Because residual deformity is rare after medical treatment of nutritional rickets, there is no specific orthopaedic treatment of nutritional rickets.
Other forms of vitamin D deficiency rickets need to be treated based on understanding of the pathogenesis. Malabsorptive causes of vitamin D deficiency rickets, such as biliary atresia, and urinary losses of DBP are treated with high doses of vitamin D. Vitamin D deficiency rickets secondary to liver disease should be treated with calcitriol or calcidiol when available. Vitamin D deficiency rickets secondary to kidney disease is treated with calcitriol.
VDRR is a term used when rickets is resistant to physiologic replacement doses of vitamin D. VDRR could be secondary to a variety of clinical conditions. These include XLH, AD and autosomal recessive hypophosphatemic rickets (ADHR, ARHR), Fanconi syndrome, McCune Albright syndrome, oncogenic rickets (OR), hypophosphatemic rickets with hypercalciuria (HRH), and renal tubular acidosis (see Table 38.2 )
Several disease conditions are associated with hypophosphatemic rickets (see Tables 38.2 and 38.3 ). XLH, also known as familial hypophosphatemic rickets, is the most common cause of VDRR. XLH disease is the most frequent form of hereditary rickets, with an incidence of 1 in 20,000, and is considered the prototypic disorder of renal phosphate wasting. In 1995, the gene regulating PHEX was identified as the cause of XLH disease. The PHEX mutation produces elevated levels of FGF23 by an as yet unidentified mechanism. FGF23 is a circulating hormone produced by osteoblasts and osteocytes that reduces sodium phosphate co-transporter (NaPi) 2c, with subsequent decrease of renal phosphate reabsorption (i.e., urinary phosphate wasting) and the conversion of 25(OH) vitamin D to its active form, 1,25(OH) 2 vitamin D, by suppressing renal 25-hydroxy-1α-hydroxylase activity. Inhibition of 1α-hydroxylase by FGF23 prevents the normal compensatory increase in active vitamin D formation associated with hypophosphatemia. Increased renal phosphate excretion and hypophosphatemia produce short stature, long bone bowing, and other radiographic features of rickets.
In the AD form of hereditary rickets, gain-of-function mutations in FGF23 have been identified that prevent the degradation of this hormone and produce renal phosphate wasting. In the autosomal recessive form of hereditary rickets, mutations in the DMP1 gene and ENPP1 gene have been reported. DMP1 is an important regulatory protein produced by osteoblasts and osteocytes that regulates the growth and development of dentin, bone, and cartilage and also plays a role in matrix mineralization. Thus, DMP1 mutations impair osteocyte maturation and bone mineralization. DMP1 mutations also produce elevated levels of FGF23 through an undefined mechanism, leading to phosphaturia and hypophosphatemia. Other rare forms of hereditary rickets include hereditary hypophosphatemic rickets with hypercalciuria (autosomal recessive inheritance with mutation in NaPi2a, also known as SLC34A3). AR Fanconi syndrome is caused by SLC34A1 mutation. Fanconi could also be secondary to cystinosis, tyrosinemia, or Wilson disease. McCune Albright syndrome is a rare genetic disorder associated with GNAS 1 gene mutation (20q13.2) characterized as the triad of polyostotic fibrous dysplasia of bone, precocious puberty, and café-au-lait skin pigmentation. Fibrous dysplasia in patients with McCune Albright produces FGF23, hyperphosphaturia, and subsequently rickets. OR is a form of endocrine paraneoplastic syndrome where cancer cells produce FGF23. , , , , , Mesenchymal tumors, neurofibromatosis, and fibrous dysplasia are among the tumors that produce FGF23. , , , Osteoblastoma, hemangiopericytoma of bone, and skin tumors can also produce rachitic changes in bone by disrupting the renal tubular resorption of phosphate. , Tumor-induced osteomalacia (TIO) should be suspected in older children with hypophosphatemic rickets because the true genetic form is generally apparent by 2 years of age. The rachitic changes resolve with excision of the tumor. , ,
In renal tubular acidosis, the kidney excretes fixed base and wastes bicarbonate, which leads to wasting of calcium and sodium. The alkaline urine results in the precipitation of calcium and severe renal calcinosis. ,
Laboratory studies in VDRR depend on the primary etiology (see Table 38.3 ). In XLH, normal or almost normal levels of calcium are found. The serum phosphate concentration is significantly decreased, whereas the 1,25(OH) 2 vitamin D level may be inappropriately low in response to the hypophosphatemia. The PTH level is normal. Urine assays for phosphate demonstrate an increased concentration of phosphate in the urine. The serum alkaline phosphatase concentration is elevated (see Table 38.5 ). The diagnosis is often confirmed by genetic testing/sequencing for PHEX, DMP1, FGF23, and SLC34A1 gene mutations or by FGF23 peptide assay.
| Type | Inheritance | Teeth | Bone Fragility | Deformity of Long Bones | Growth Retardation | Presenile Hearing Loss (%) | Prognosis | Sclerae | Spine | Skull | Other | Incidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | Autosomal dominant | Normal | Variable, less severe than other types | Moderate | Short stature, 2%–3% below mean | 40 | Fair | Distinctly blue throughout life | Scoliosis and kyphosis in 20% | Wormian bones on radiographs | Premature arcus senilis | 1/30,000 |
| IB | Autosomal dominant | Dentinogenesis imperfecta | Variable, less severe than other types | Moderate | Short, 2%–3% below mean | 40 | Fair | Distinctly blue throughout life | Scoliosis and kyphosis in 20% | Wormian bones on radiographs | Premature arcus senilis | 1/30,000 |
| II | Autosomal recessive | Unknown (because of perinatal death) | Very extreme | Crumbled bone (accordion femora) marked | Unknown (because of perinatal death) | Perinatal death | Blue | Marked absence of ossification | 1/62,000 live births | |||
| III | Autosomal recessive | Dentinogenesis imperfecta | Severe | Progressive bowing of the long bones and spine | Severe, smallest of all patients with osteogenesis imperfecta | Nonambulatory, wheelchair- bound; may die in third decade | Bluish at birth, become less blue with age, white in adult | Kyphoscoliosis | Hypoplastic, more ossified than in type II, wormian bones | Very rare | ||
| IVA | Autosomal dominant | Normal | Moderate | Moderate | Short stature | Low frequency | Fair | Normal | Kyphoscoliosis | Hypoplastic, wormian bones | Unknown | |
| IVB | Autosomal dominant | Dentinogenesis imperfecta | Moderate | Moderate | Short stature | Low frequency | Fair | Normal | Kyphoscoliosis | Hypoplastic, wormian bones | Unknown |
When suspected, XLH is often diagnosed within first 6 months of life. First signs include hypophosphatemia, increased alkaline phosphatase, and rachitic bone changes in the metaphysis of the ulna. With positive family history, the diagnosis is confirmed by clinical and biochemical changes on a genetic test for PHEX gene mutations. Most patients become symptomatic between 1 and 2 years of age. Because XLH is inherited as an X-linked dominant trait, the disease is more severe in males than females. Severe hypophosphatemic rickets can be recognized in early infancy. When the disease is suspected because of clinical presentation and the family history, laboratory determination of phosphorus concentrations can lead to the diagnosis in infants as young as 3 months. The usual initial complaints are delayed walking and angular deformities of the lower extremities. In contrast to what is seen in nutritional rickets, systemic manifestations such as irritability and apathy are minimal. TIO often presents in early childhood. AD VDRR has milder presentation than male patients with XLH.
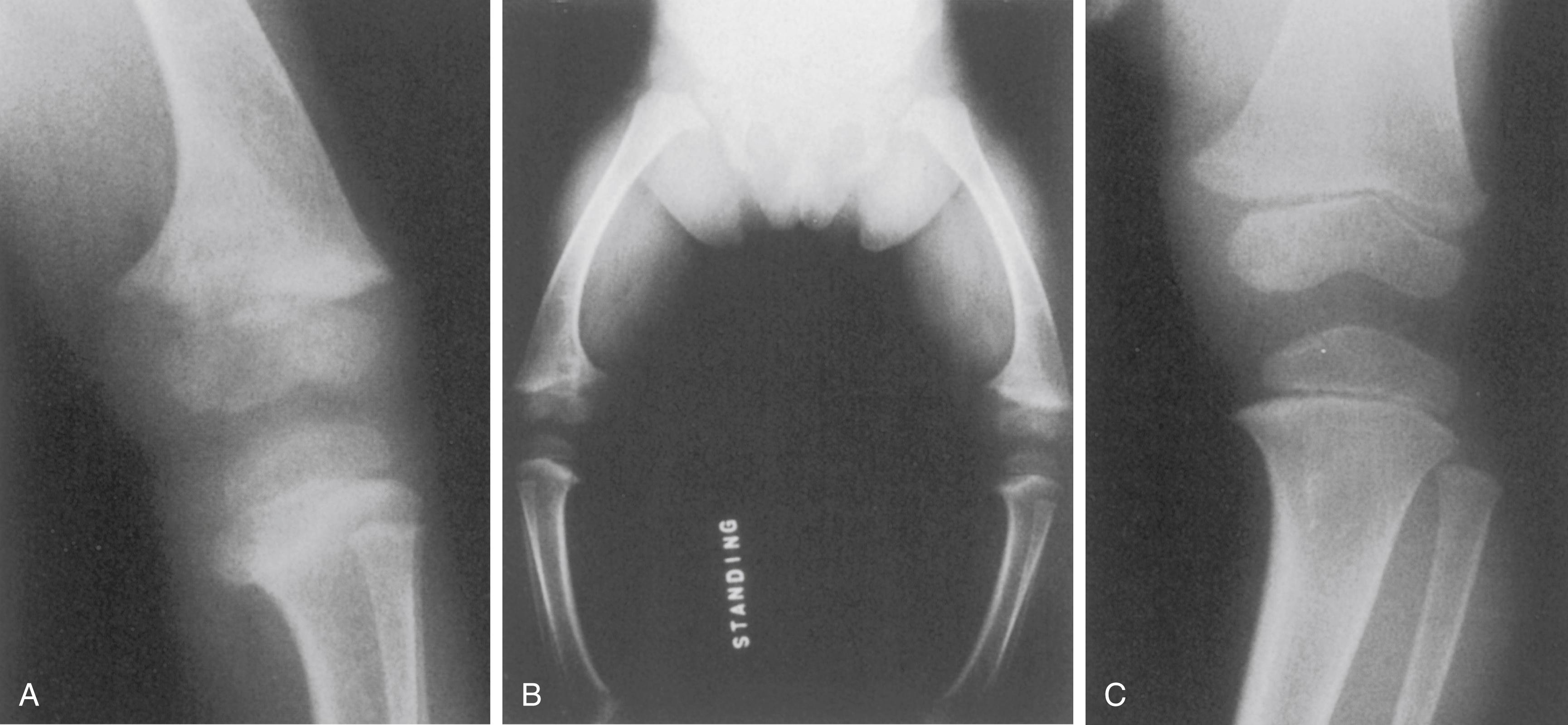
Physical findings in hypophosphatemic rickets include skeletal deformities, which resemble those seen in other forms of rickets. Once affected children begin to walk, genu varum develops, although genu valgum may occur in some children ( Fig. 38.6 ). Periarticular enlargement is present as a result of widening of the physes and metaphyses. The rachitic rosary may also occur.
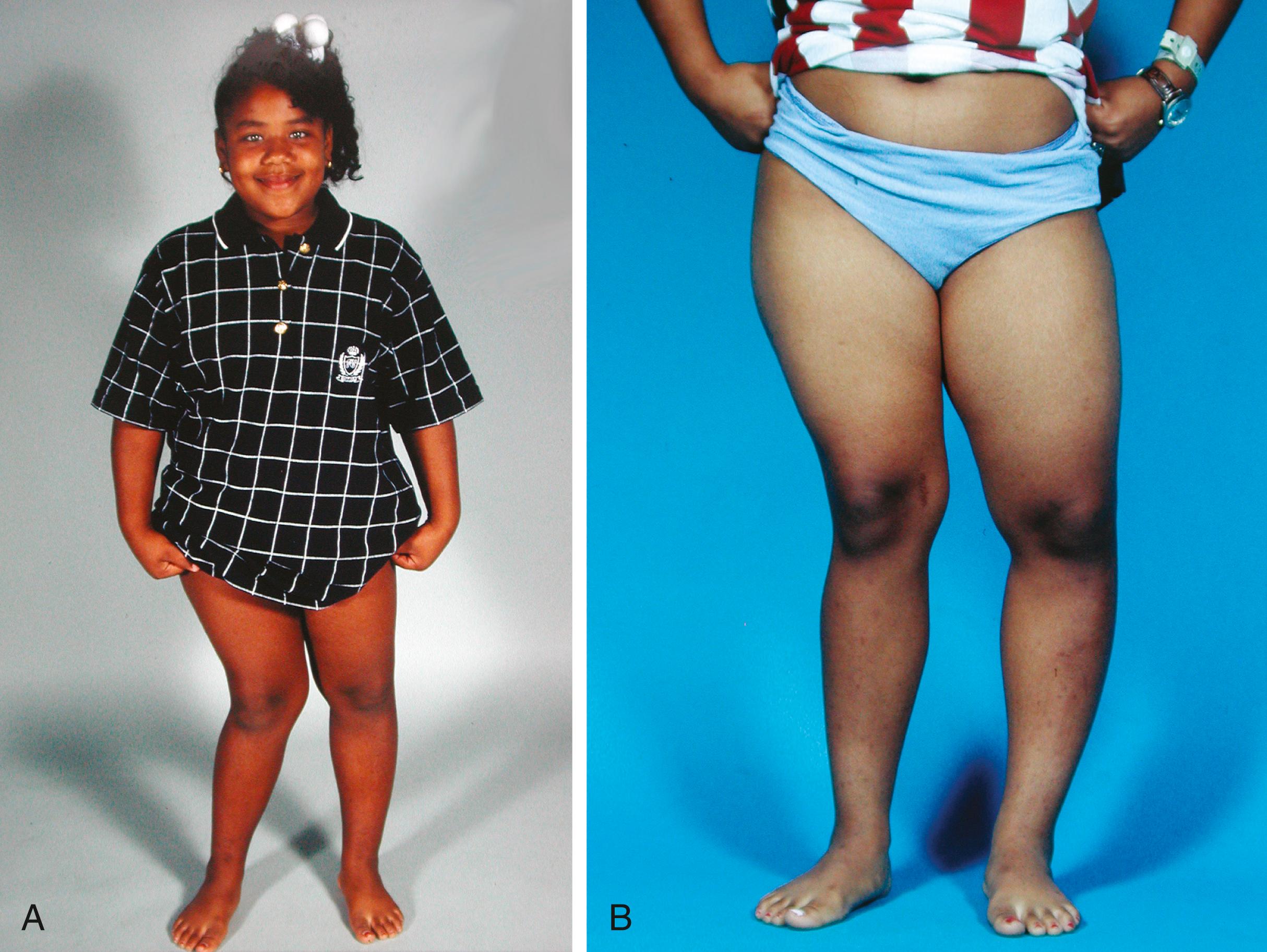
Short stature is a feature of hypophosphatemic rickets. Height is usually two standard deviations or less below the mean for age in these patients.
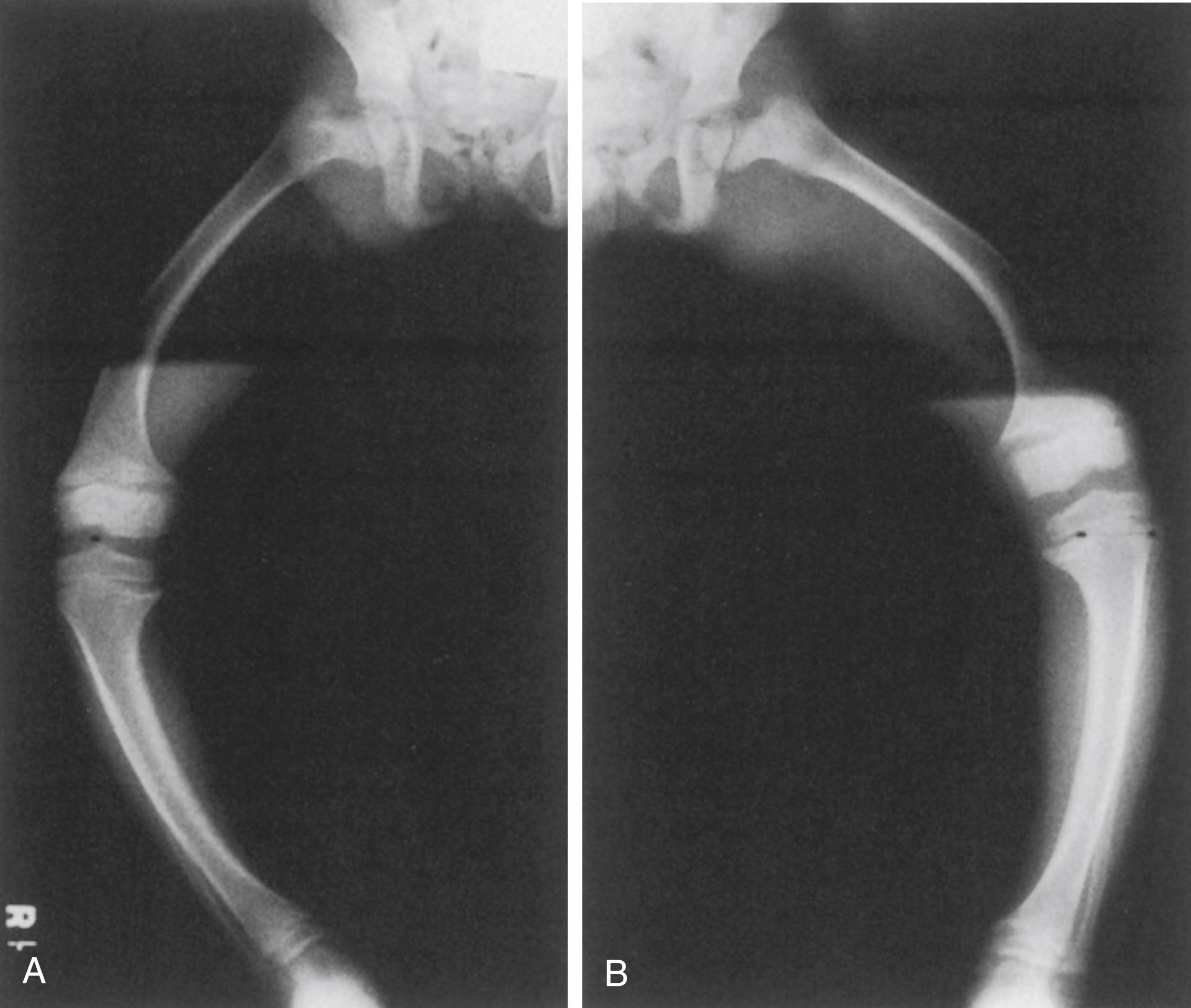
The radiographic changes are the same as those seen in nutritional rickets and include physeal widening and indistinct osteopenic metaphyses. In the lower extremities, genu varum is obvious, and the distal femoral and proximal tibial physes are particularly widened medially ( Fig. 38.7 ). Coxa vara is present, and there may be general anterior and lateral bowing of the entire femur. The varus of the tibia is also generalized, not only present proximally but also producing varus angulation of the ankle. Treatment can result in craniosynostosis and dolichocephaly.
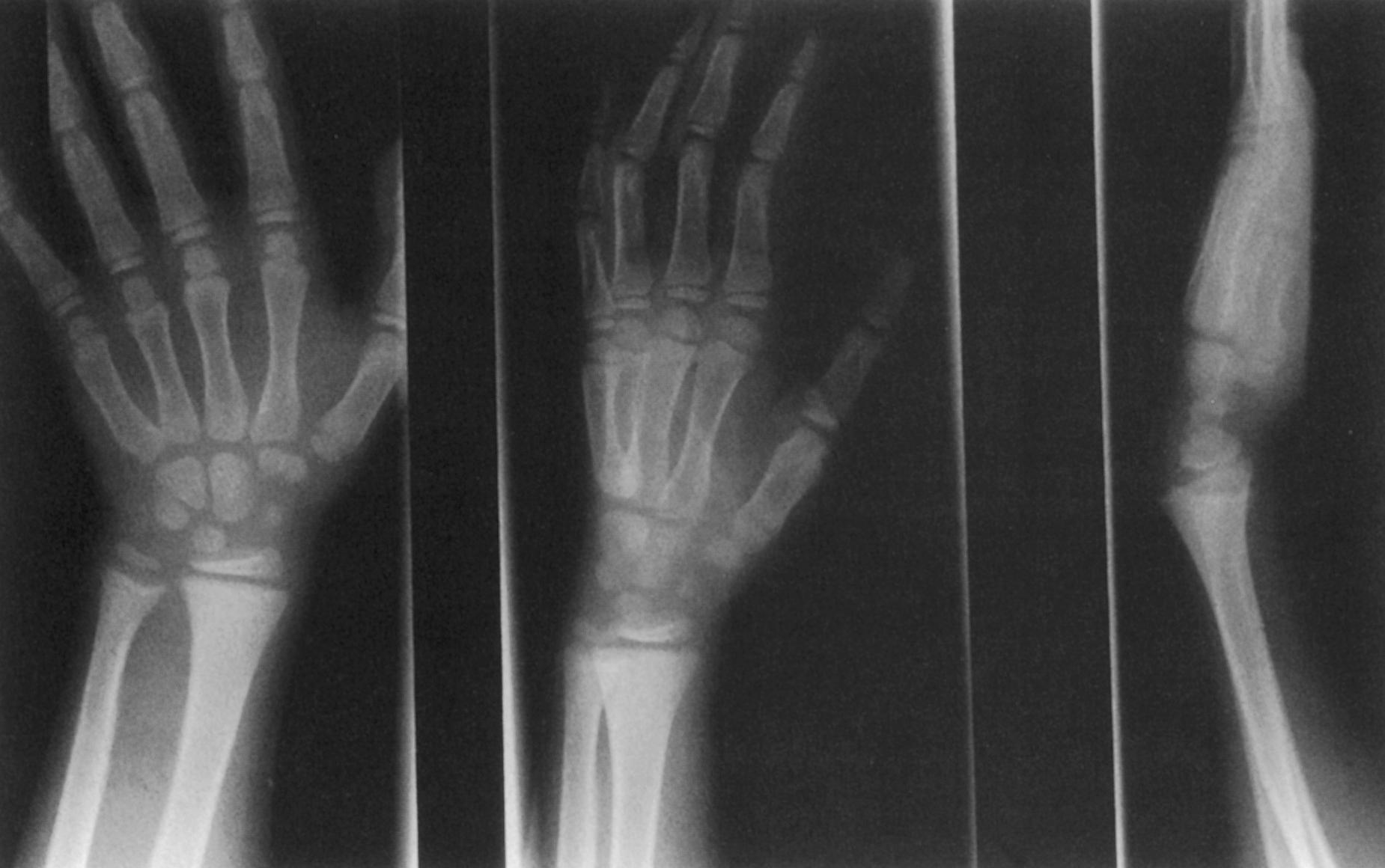
The upper extremities are involved as well, but to a lesser degree because of absence of the influence of weight bearing ( Fig. 38.8 ).
The medical treatment of hypophosphatemic rickets is best managed by a metabolic bone disease specialist. The usual treatment consists of oral replacement of phosphorus in large doses along with high doses of calcitriol or alfacalcidol. , , A large dose of phosphorus alone will result in hyperparathyroidism and gastrointestinal intolerance. Analogues of vitamin D 3 (1,25-dihydroxyvitamin D 3 ) are several hundred times more potent than the original form of vitamin D in treating hereditary rickets. , , The therapeutic target of medical therapy should not be to normalize the serum phosphate level because achieving normalization may not be a practical goal and may lead to overmedication and greater side effects. Focus should be on improving bone turnover markers such as alkaline phosphatase and osteocalcin. Other markers of response to therapy are improvement in skeletal deformity, height, and physeal function. In general, growth and skeletal deformities improve with medical therapy. Initiation of therapy in infancy has a greater impact on height but does not completely normalize skeletal development. In one study, 62% of patients with X-linked dominant rickets required surgical intervention for bowleg deformities despite being on optimal medical therapy.
Nephrocalcinosis is a significant complication of medical treatment. In one study, renal calcinosis was present in 79% of treated children with hypophosphatemic rickets, and the severity of the calcinosis correlated with the dose of phosphorus. Nephrocalcinosis could be treated with thiazide diuretics.
Treatment of children with hypophosphatemic rickets with growth hormone has been shown to increase height and have beneficial effects on bone density and phosphate retention. , Preliminary studies reported that the administration of growth hormone with vitamin D increases the serum phosphate concentration and may reduce the incidence of nephrocalcinosis. Cinacalcet is a calcimimetic agent used to treat both XLH and tumor-induced osteomalacia.
Burosumab is a recombinant humanized monoclonal (IgG 1 ) antibody against the FGF23. It increases serum levels of phosphorus and calcitriol by regulating phosphate excretion and increasing vitamin D production by the kidney. At the time of preparation of this chapter the US Food and Drug Administration (FDA) has accepted the Biologics License Application (BLA) for burosumab to treat pediatric and adult patients with XLH. Burosumab was developed to treat XLH and TIO, both diseases characterized by excess levels of FGF23 which produces phosphate wasting. By binding and blocking the biological activities of FGF23 in patients with XLH and TIO, burosumab is intended to increase renal tubular phosphate reabsorption and vitamin D production, which enhances intestinal absorption of phosphate and calcium. Serum phosphorus and calcitriol increase in response to subcutaneous dosing within 8 to 15 days. The mean half-life of burosumab was 13 to 19 days after subcutaneous injection. The effect on urinary phosphate excretion, serum Pi, and calcitriol levels correlated with serum burosumab concentration. Side effects such as increased hypercalcemia, hypercalciuria, nephrocalcinosis, anti-FGF23 antibodies, elevated serum PTH, or creatinine were not observed. The effect of anti-FGF23 antibodies on long-term outcome of children with XLH is awaiting further studies. TIO has also been treated with cinacalcet, a calcimimetic agent that lowers PTH level.
The deformity most commonly seen in patients with hypophosphatemic rickets is a gradual anterolateral bowing of the femur, combined with tibia vara. Genu valgum can also occur. The orthotic management of VDRR is not efficacious. Surgical intervention should be considered in patients having pain or difficulty walking due to persistent or progressive worsening of the deformity despite optimal medical therapy. In patients with growth remaining and on medical therapy, guided growth has been shown to successfully correct varus or valgus limb deformity ( Fig. 38.9 ). In a retrospective study of patients with X-linked dominant rickets, 15 out of 23 limbs (65%) treated with guided growth achieved neutral alignment while 7 limbs attained some improvement but not neutral alignment. The mean correction time was 16 months (range 9 to 27 months). Patients with ≥3years of growth remaining or valgus deformity responded better to guided growth than older patients or those with varus deformity, respectively. The rate of recurrence of deformity was low, and the authors did not recommend overcorrection as one patient had a persistent overcorrected deformity. Interestingly, improvements in knee alignment also improved femoral and tibial diaphyseal bowing and the alignment and radiographic appearance of proximal femoral and distal tibial physes in younger patients. These results are explained by the Hueter-Volkmann principle of normalization of mechanical load across the physis correcting the metaphyseal-diaphyseal deformity over time. Because of the potential benefit of restoring neutral mechanical axis to lower limb physeal function, early intervention using guided growth is advocated by some surgeons.
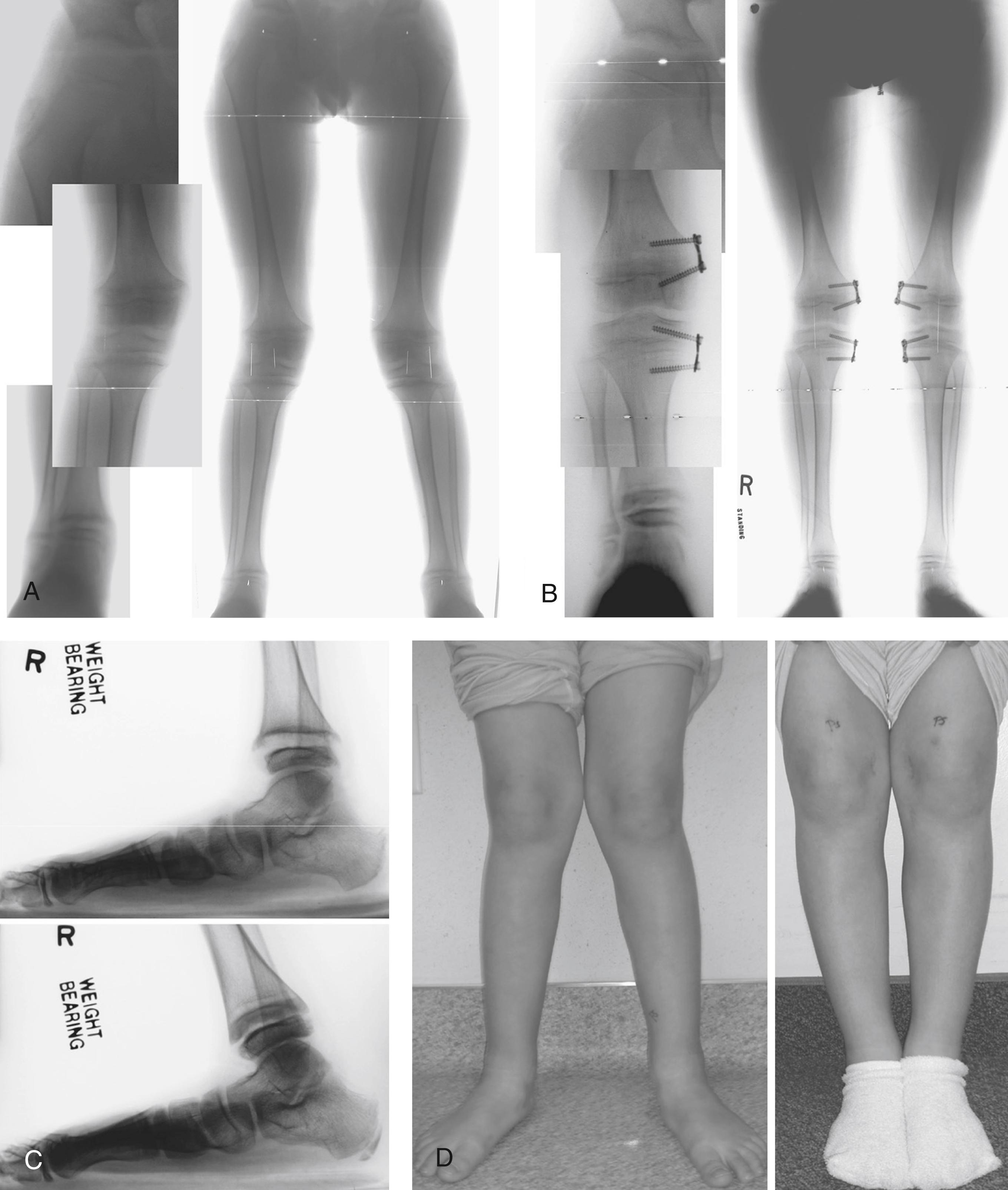
For symptomatic patients with significant deformity or near skeletal maturity, multilevel osteotomy is generally required to satisfactorily correct the mechanical axis of the limb ( Fig. 38.10 ). In those patients with growth remaining, mechanical axis should be mildly overcorrected at surgery as recurrence of deformity is a common sequela of osteotomies in patients with hypophosphatemic rickets. , Younger patients have a higher risk of recurrence. One study reported a recurrence rate of 90% after the first corrective procedure. For this reason, milder deformities should be corrected by guided growth in early childhood. Some children have severe varus at a very young age that leads to thrust during gait. When gait is compromised or patient is symptomatic, guided growth should be considered.
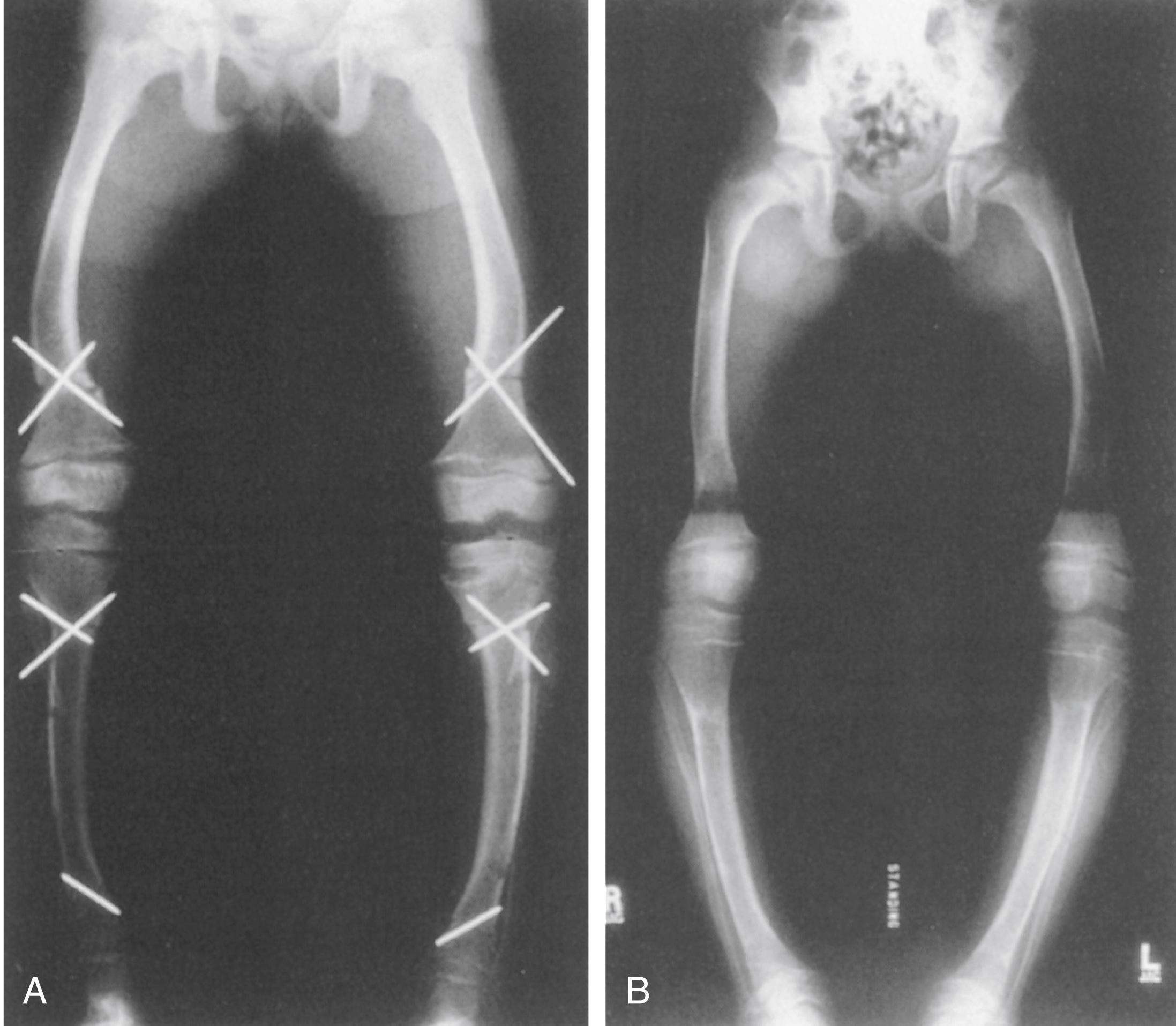
The suggested fixation for osteotomies varies among reports. External fixation allows fine tuning of the alignment postoperatively, when the patient is able to stand ( Fig. 38.11 ). Others advocate the use of intramedullary fixation or plating ( Fig. 38.12 ). , Regardless of the type of fixation used, careful preoperative planning of the surgical treatment of these multiplanar deformities is crucial to restoring alignment. It is important to work closely with the nephrologist or endocrinologist who is managing the medical therapy because calcium levels can suddenly increase in a patient who is immobilized after surgery. Discontinuation of vitamin D before surgery should be discussed.
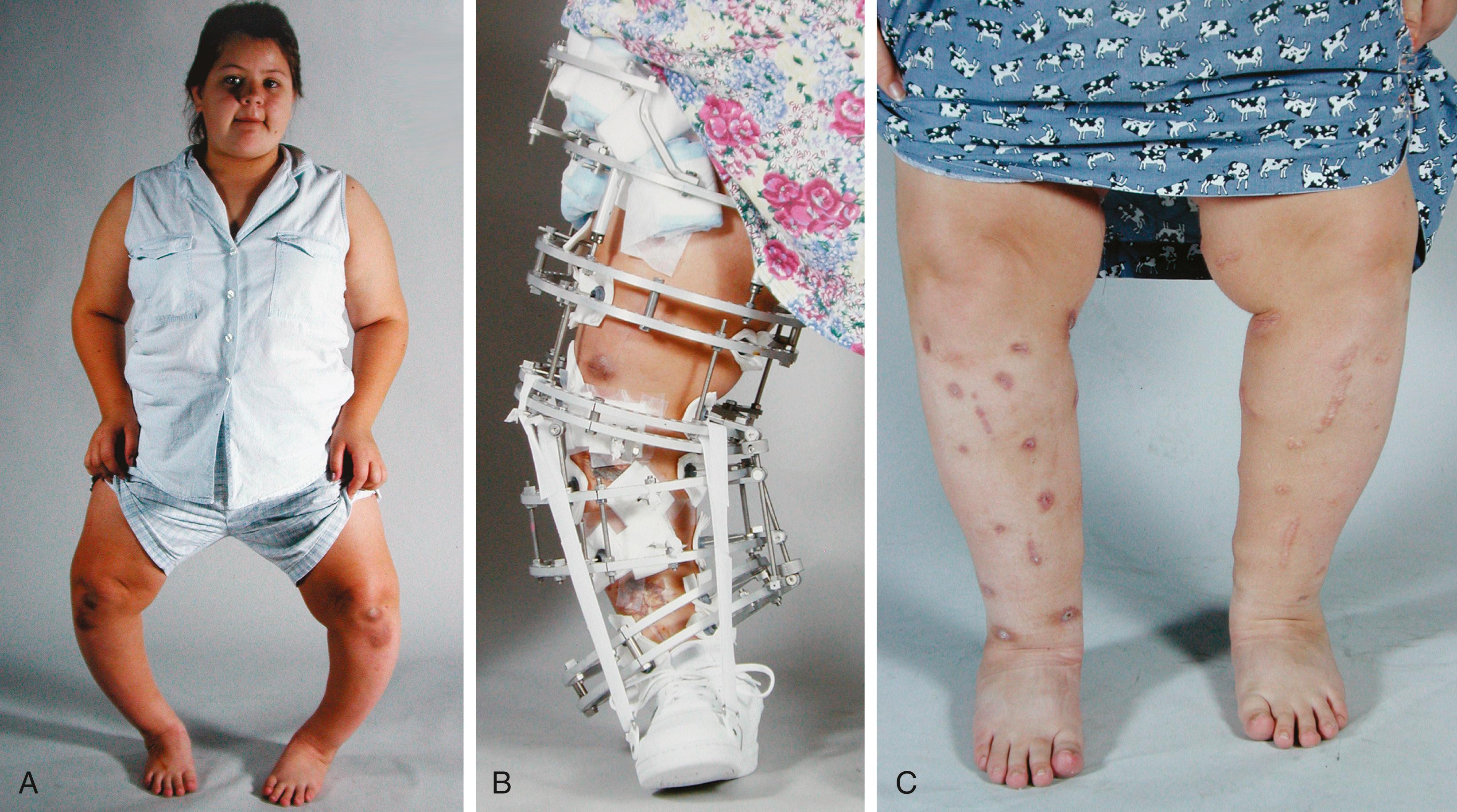
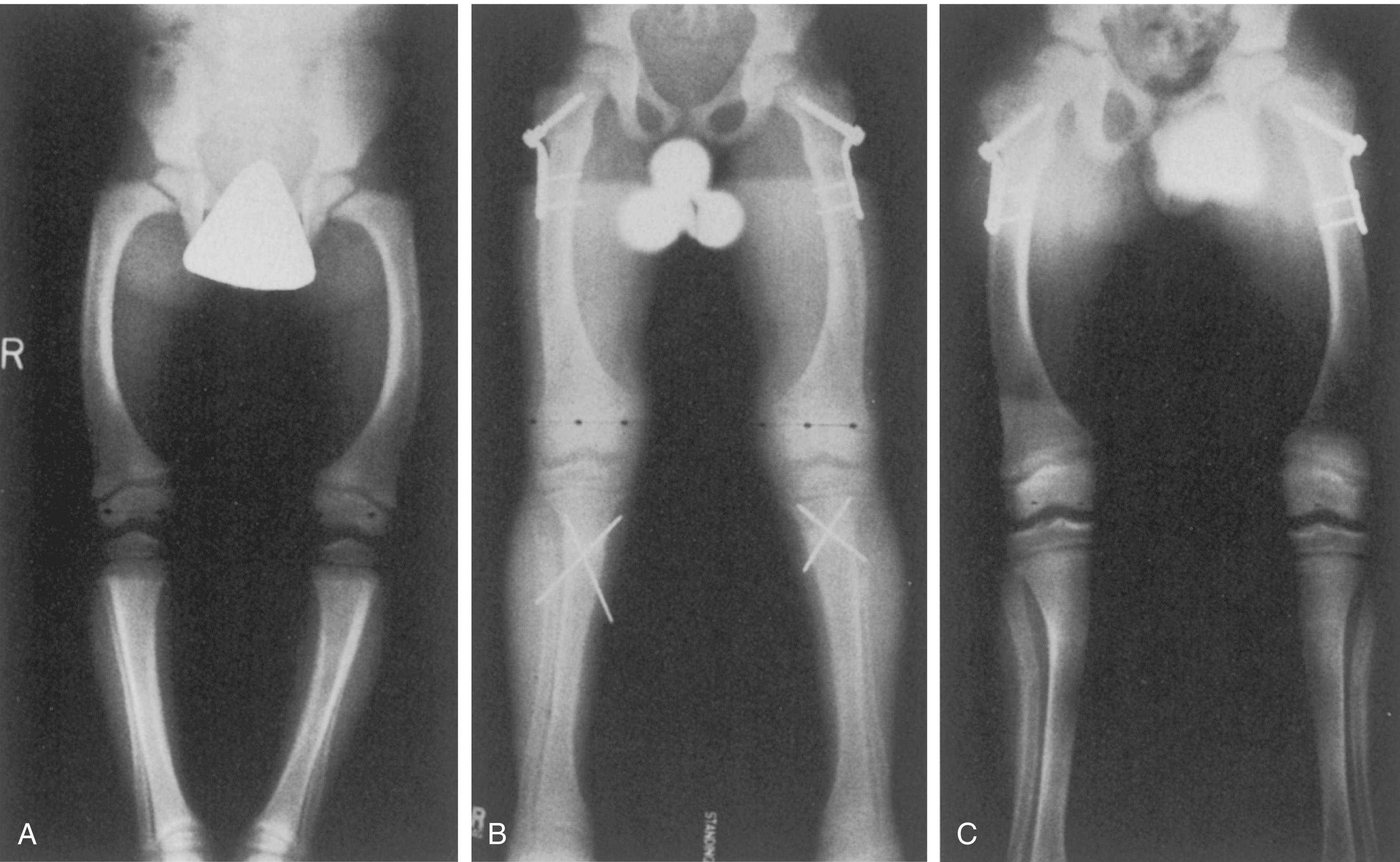
Spinal deformity may be seen in patients with hypophosphatemic rickets. Kyphoscoliosis, Arnold-Chiari malformations, and spinal stenosis have all been described in patients with VDRR. ,
Adults with hypophosphatemic rickets are prone to the development of arthritis. Degradation of articular cartilage resembling osteochondritis dissecans has been described. Joint stiffness and bone pain are common complaints.
VDDR has four types (see Table 38.3 ). Type 1A is related to 1α-hydroxylase deficiency due to mutations in CYP27B1 , gene or renal disease, resulting in low calcitriol levels. Type 1B is related to 25-hydroxylase deficiency owing to mutations in CYP2R1. Type 2A is related to mutations in VDR preventing it from interacting with calcitriol. As a result, VDR cannot regulate gene activity. Type 2B is related to an overexpression of a vitamin D response element (VDRE)-binding ribonucleoprotein. VDDR type 2B is a post receptor deficiency. Laboratory and x-ray findings: VDDR is very similar to other forms of rickets biochemically and radiographically.
25-Hydroxylase deficiency, also known as VDDR type 1B, is a rare autosomal recessive trait described in a handful of children from Africa and the Middle East. The clinical picture is similar to other forms of rickets. 1-α hydroxylase deficiency is another rare condition that often presents within the first 24 months of life with clinical features of rickets and seizure. A hallmark is normal 25(OH) vitamin D and very low 1,25(OH) 2 vitamin D and a mutation in CYP27B1 gene. , VDDR type 2A, also known as hereditary 1,25(OH) 2 vitamin D-resistant rickets, is characterized by hypocalcemia and maybe seizures, hyperparathyroidism, normal serum concentrations of 25(OH) vitamin D, and substantially increased (3-fold to 30-fold) serum 1,25(OH) 2 vitamin D levels. A hallmark is the finding of either sparse body hair or total alopecia in the majority of affected patients. Patients with alopecia, which can be present at birth or develop within the first year of life, seem to have an earlier age of onset of rickets and greater resistance to 1,25(OH) 2 vitamin D treatment than those with hereditary 1,25(OH) 2 vitamin D-resistant rickets with or without alopecia. Type 2B is a very rare condition, and is thought to be a post-receptor disorder that is clinically similar to type 2A with normal receptors.
Calcitriol and calcium supplement are used to treat all forms of VDDR. Very high doses of calcitriol are needed to treat type 2A and 2B VDDR.
Because residual deformity is rare after medical treatment, there is no specific orthopaedic treatment for VDDR.
Hypervitaminosis D results from ingestion of excessive amount of vitamin D. , , Patients at risk are those who are taking vitamin D for the treatment of metabolic bone diseases such as VDRR and hypoparathyroidism. The elevated vitamin D level promotes the intestinal absorption of calcium and thereby leads to hypercalcemia. Increased serum Ca can result in hypertension, acute kidney injury, and if persistent, nephrocalcinosis and calciphylaxis.
Histologically, wide osteoid seams are found around the trabeculae, similar to what is seen in rickets. The physis, however, is well calcified and normal in width and length. Metastatic calcification may be found in the kidneys, arteries, thyroid, pancreas, lungs, stomach, and brain. Deposition of calcium salts in the kidneys and degenerative changes in the arteries may produce significant morbidity.
The hypercalcemia can be severe. The serum phosphate concentration is normal, with a diminished alkaline phosphatase concentration. ,
Symptoms and signs of hypercalcemia are seen. Early manifestations include hypertension, acute kidney injury, anorexia, constipation, nausea and vomiting, polyuria, thirst, and symptoms of dehydration. The child feels very tired. With progression of the intoxication, mental depression and stupor develop. Renal failure and hypertension are common.
Dense metaphyseal bands are seen in the long bones and result from an increase in the proximal zone of calcification. The diaphyses show osteopenia as a result of demineralization. Osteosclerosis is visible at the base of the skull, and there may be premature closure of the sutures. The vertebral endplates are dense. Metastatic calcifications are seen in soft tissues and joints ( Fig. 38.13 ). ,
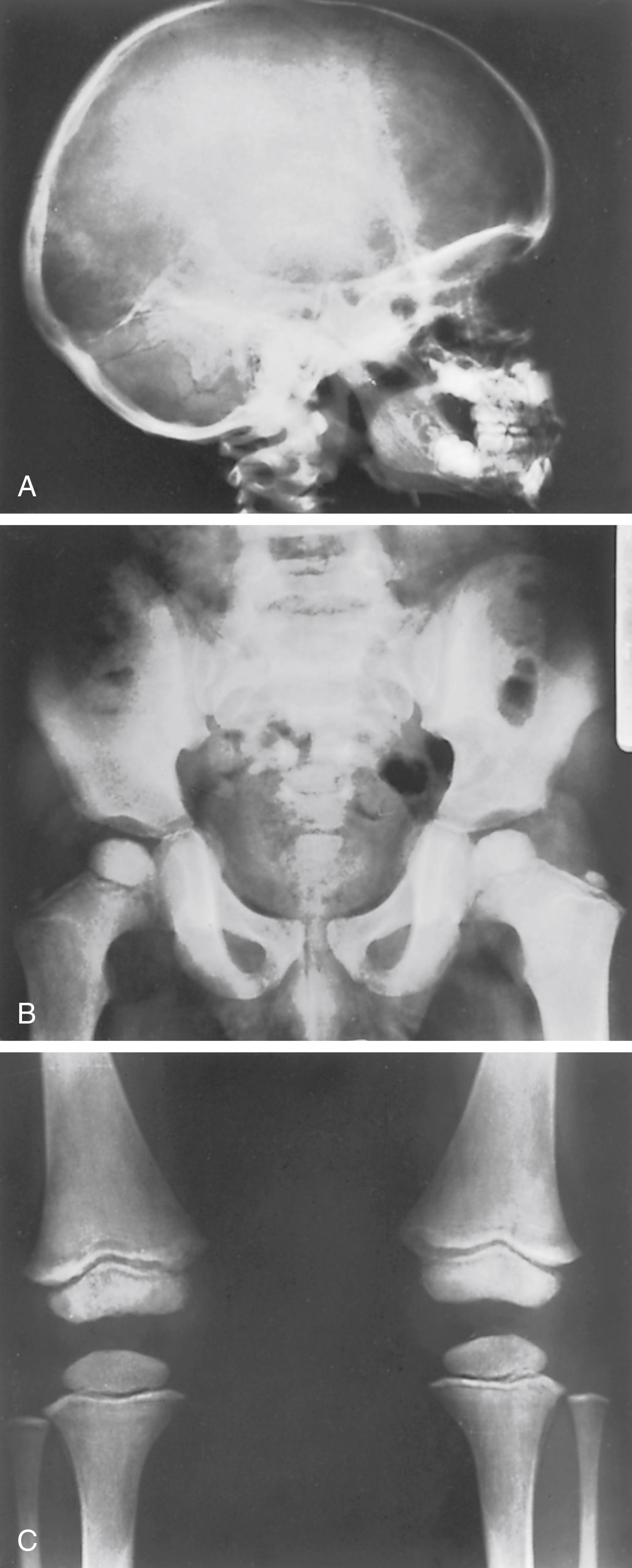
Treatment is medical and consists of the immediate cessation of vitamin D supplements. Due to the availability of different forms of medicinal vitamin D the duration of vitamin D toxicity varies with their half-life. Saline diuresis along with loop diuretics are used to treat hypercalcemia. Steroids inhibit calcium intestinal absorption in the kidney and gut and are helpful in correcting the calcium level. BPs inhibit bone release of calcium from bone and have been useful in treating vitamin D intoxication. Sodium phosphate intravenous infusion should be used sparingly because its administration leads to extra-skeletal calcification.
Vitamin A is a fat-soluble vitamin whose primary biologic functions are concerned with skeletal growth, maintenance and regeneration of epithelial tissues, and preservation of visual purple in the retina. It is also necessary for membrane stability. The normal plasma level of vitamin A is 80 to 100 IU/100 mL. Deficiency of vitamin A due to malabsorption syndromes of nutritional causes can produce hypocalcemia, resulting in rickets complex. Hypervitaminosis A is rare and usually results from inappropriate use of vitamin supplements or its accumulation in chronic kidney disease (CKD). , , Skeletal changes associated with hypervitaminosis A can mimic rickets and increase the intracranial pressure. Retinoids used for treatment of acne also contain vitamin A and can lead to toxicity. , , Children with advanced stages of CKD have problems metabolizing vitamin A and thus are prone to hypervitaminosis A.
Clinically, the soft tissues overlying the hyperostotic bones are swollen and tender. Proliferation of basal cells and hyperkeratinization cause dry, itchy skin. Anorexia, vomiting, and lethargy are caused by increased intracranial pressure and rachitic skeletal changes. The child fails to thrive. Hepatomegaly with cirrhosis-like liver damage or splenomegaly may be present.
Radiographs may appear normal initially as the development of bone changes in patients with hypervitaminosis A is slow. Once changes do occur, there is periosteal hyperostosis and thickening of the cortex of the long bones. The ulna, radius, metacarpals, and metatarsals are particularly affected. The mandible is spared, a fact that distinguishes hypervitaminosis A from Caffey disease. Subperiosteal new bone formation is seen (previous Fig. 38.14 ). Bone scintigraphy shows increased uptake. Premature partial or complete physeal closure may be present. , ,
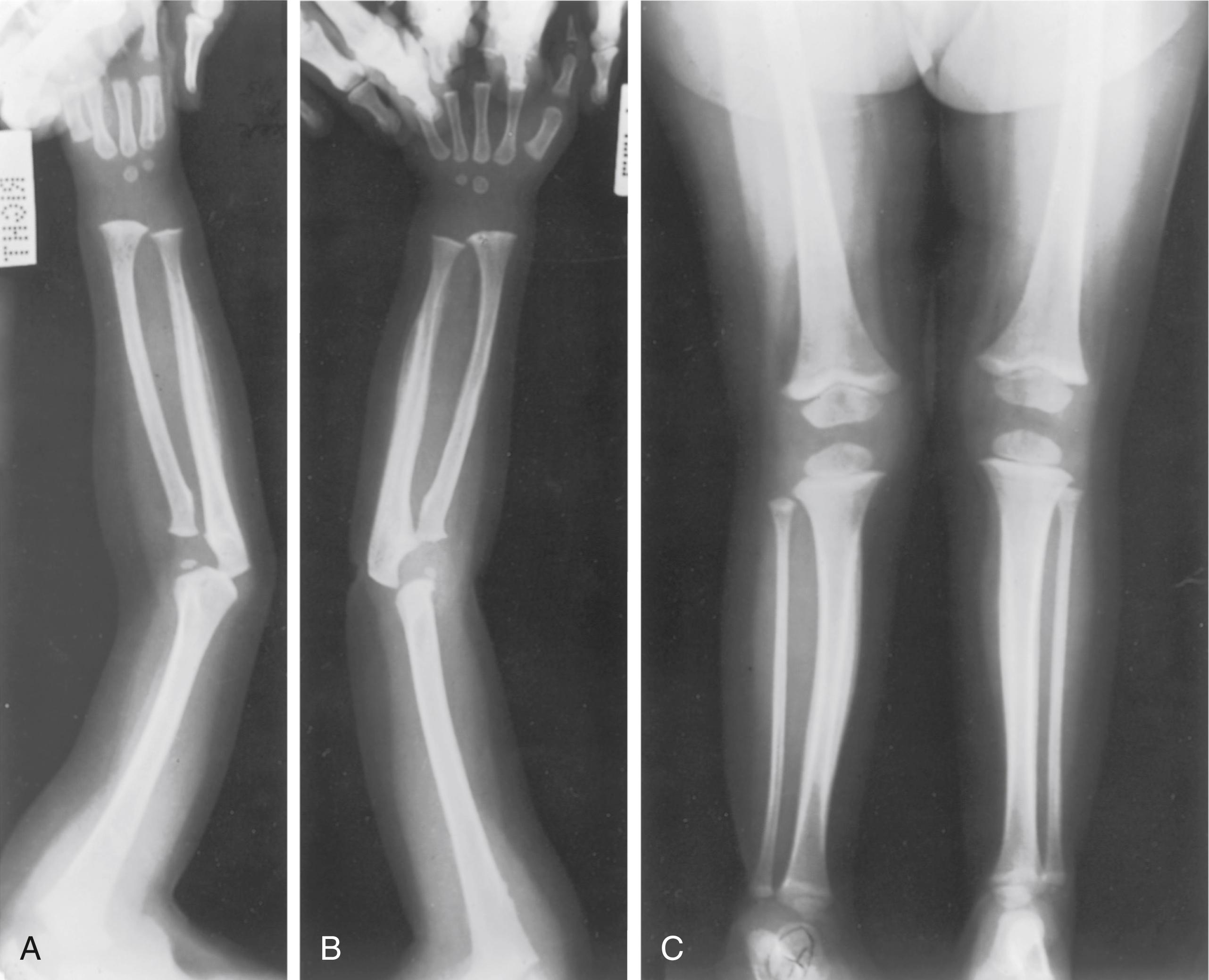
The diagnosis is made by determining the plasma concentration of vitamin A, which will be elevated 5 to 15 times the normal value. Hypercalcemia can be present. , Hypervitaminosis A must be differentiated from infantile cortical hyperostosis (Caffey disease), scurvy, and congenital syphilis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here