Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A significant number of soft tissue tumors either show no clear line of differentiation or appear to differentiate along the lines of a cell type with no normal counterpart. Some of these tumors, for example myxoma, appear to show principally fibroblastic differentiation, but have not traditionally been included among the fibroblastic tumors of soft tissue. Others, such as extraskeletal myxoid chondrosarcoma, have historically been considered to differentiate along the lines of a particular lineage (i.e., cartilage), but are now understood to represent translocation-associated tumors without a normal counterpart. Indeed, the number of soft tissue tumors of “uncertain lineage” appears to increase yearly, and there are likely tumors included in other chapters of this book which might easily be included here. As with all other soft tissue tumors, the tumors of uncertain lineage span a spectrum from benign to intermediate malignancy to fully malignant sarcomas.
The myxomas are a group of relatively common, probably unrelated lesions, which most commonly involve the large muscles (intramuscular myxoma), but may also occur around the large joints (juxta-articular myxoma) or in the skin (cutaneous myxoma). There are also poorly characterized myxomas occurring in subcutaneous locations but exhibiting morphologic features identical to their intramuscular counterparts. All are characterized by abundant myxoid matrix, bland stellate to spindled cells, and hypovascularity.
Intramuscular myxomas occur almost exclusively in middle-aged to older adults, more often in women than in men. There is a very strong association between the presence of multiple intramuscular myxomas and fibrous dysplasia, typically of the monostotic type (Mazabraud Syndrome). The large muscles of the buttocks, thigh, shoulder, and upper arm are most commonly involved, although rare cases have been reported in almost any location. These tumors usually present as a nonspecific soft tissue mass, without characteristic clinical or radiographic features. Most are 5–10 cm, although occasional cases can be considerably larger.
Intramuscular myxomas are almost always well-delineated, gelatinous-appearing masses, which may display cystic change ( Fig. 15.1A ).
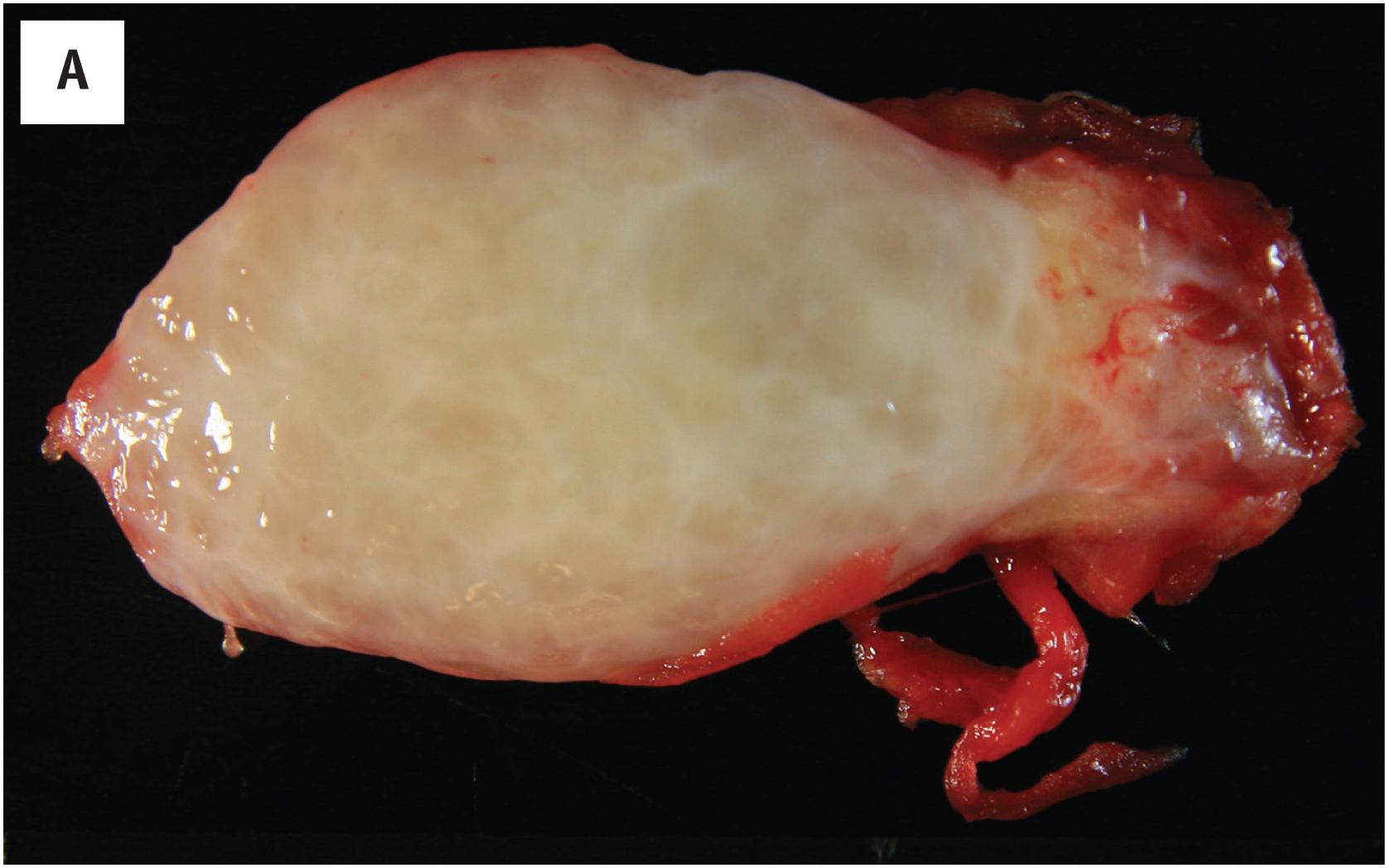
Despite their gross appearance of circumscription, intramuscular myxomas typically extend into the muscle to a limited extent, splaying the individual skeletal muscle fibers; this finding is a helpful clue to their diagnosis ( Fig. 15.1B ). Microscopically, they contain abundant myxoid matrix, which typically shows multifocal microcystic change ( Fig. 15.1C ). The tumor cells are spindled to stellate in appearance, with darkly staining, pyknotic appearing nuclei and a small amount of eosinophilic cytoplasm ( Fig. 15.1D ). Myxomas lack the well-developed, arborizing vasculature observed in myxoid sarcomas. Occasionally a myxoma may show greater cellularity and focal areas of fascicular growth, sometimes accompanied by a slightly greater number of blood vessels (“cellular myxoma”). The identification of areas of more typical myxoma is the key to recognizing such cellular myxomas.
The cells of intramuscular myxoma usually express only vimentin but may rarely express smooth muscle actin in a myofibroblastic, “tram-track” pattern.
Activating point mutations in GNAS are present in >90% of myxomas, although molecular testing is almost never needed for diagnosis.
Intramuscular myxomas are commonly confused with a wide variety of both benign and malignant myxoid tumors. Myxoid nodular fasciitis is usually much smaller, more often superficially located, and shows at least focal areas of increased cellularity, with short, randomly arranged fascicles of bland myofibroblasts. Myxoid variants of desmoid fibromatosis more often occur within the abdomen, contain a well-developed, thin-walled, nonarborizing vasculature and at least focal areas of typical fibromatosis. Myxoid liposarcoma contains an extremely well-developed, arborizing, capillary-sized vasculature and easily identifiable lipoblasts. Myxofibrosarcoma has a well-developed, thick-walled vasculature and contains hyperchromatic, pleomorphic tumor cells. Extraskeletal myxoid chondrosarcoma is composed of cords and chains of small eosinophilic cells, embedded in a “dense”-appearing myxoid matrix, often with abundant hemorrhage, but with relatively few blood vessels.
Benign mesenchymal tumor composed of bland spindled cells embedded in hypovascular, frequently cystic, myxoid matrix
Relatively common
Intramuscular myxoma: large muscles of thigh and buttock
Juxta-articular myxoma: near knees, shoulders, hips
None
Intramuscular myxoma is more common in women; may be associated with fibrous dysplasia (Mazabraud syndrome)
Juxta-articular myxoma is most common in men with degenerative joint disease
Painless soft tissue mass
Cured with complete excision
Well-circumscribed mass, myxoid-appearing, cystic
Abundant Alcian-blue positive, hyaluronidase-sensitive myxoid matrix
Splaying apart of skeletal muscle
Hypovascular, without well-developed arborizing vessels
Slender spindled cells with pyknotic nuclei
Juxta-articular myxomas often exhibit areas of increased cellularity, with partial fascicular growth pattern
Typically express only vimentin
Occasionally show limited smooth muscle actin expression
Intramuscular myxomas are entirely benign lesions that require only simple excision.
As indicated by their name, these myxomas occur in direct association with the large joints. By far the most common location is adjacent to the knee joint, followed by the shoulder, hip, and ankle. Most patients are older men, frequently with a history of osteoarthritis. The tumors are usually 2–6 cm at the time of diagnosis, although some may be significantly larger.
Juxta-articular myxomas are grossly identical to intramuscular myxomas.
In general, juxta-articular myxomas closely recapitulate the histologic features of intramuscular myxoma. However, a significant subset of these lesions may show features of cellular myxoma, with fascicular growth, areas of well-developed vasculature, and mild pleomorphism ( Fig. 15.2A and B ). As with cellular intramuscular myxomas, the identification of areas of typical myxoma is the key to the correct diagnosis ( Fig. 15.2C ).
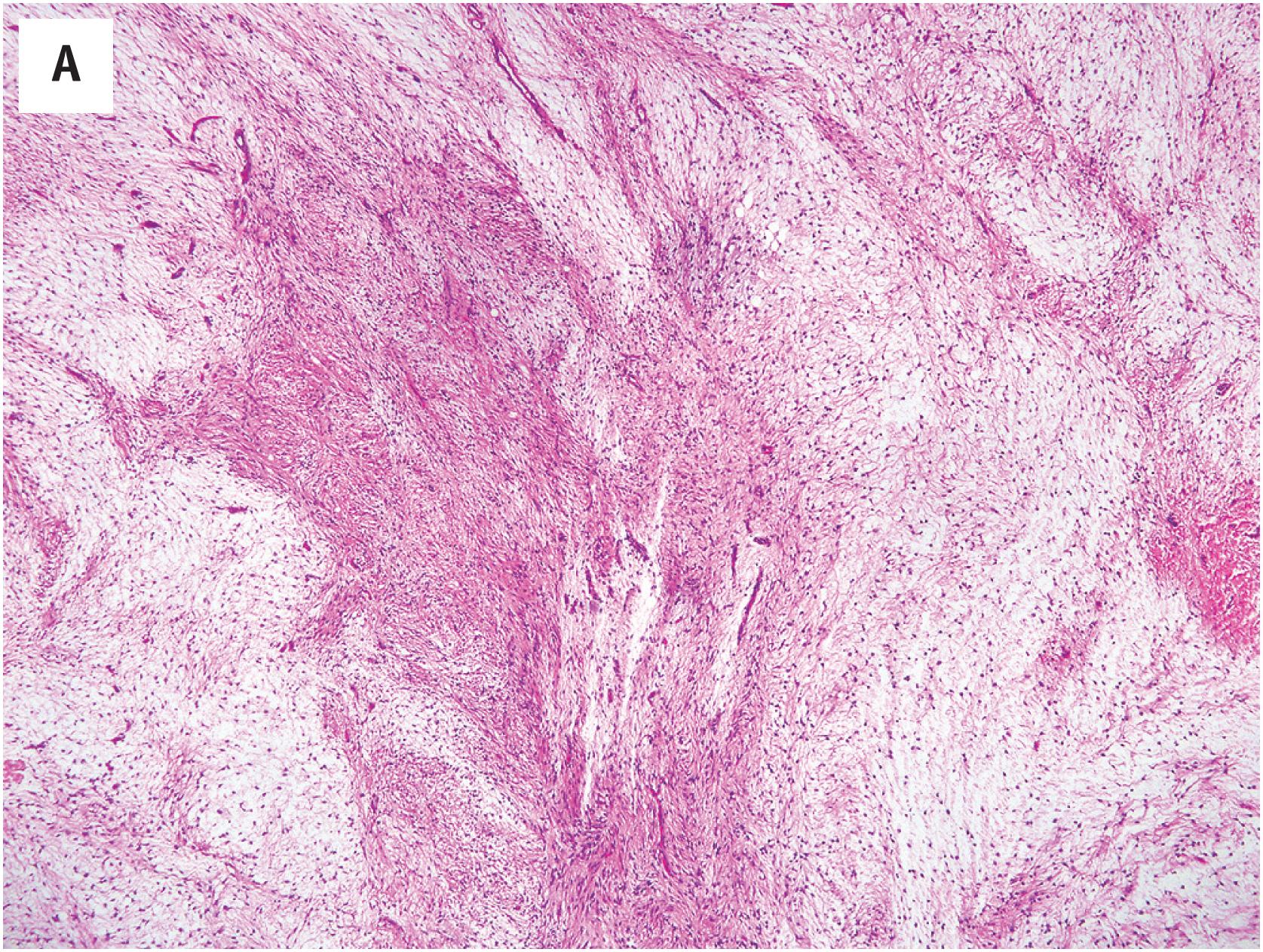
These are identical to those seen in intramuscular myxoma.
Juxta-articular myxomas display the same immunophenotype as do intramuscular myxomas, with the expression of vimentin and occasionally smooth muscle actin.
GNAS mutations are not a feature of juxta-articular myxomas.
The differential diagnosis of juxta-articular myxoma is essentially the same as that of intramuscular myxoma. Although cellular juxta-articular myxomas in particular may mimic myxofibrosarcoma, among others, the characteristic location around the knee joint and the frequent history of osteoarthritis should point one in the correct direction. In this location adjacent to the knee, a myxoid monophasic fibrous synovial sarcoma may also be a diagnostic consideration. Myxoid change in synovial sarcomas is almost always focal, rather than diffuse, and the identification of areas of more typical synovial sarcoma, characterized by hyperchromatic nuclei, alternating hypo- and hypercellular zones, wiry collagen, and focal keratin immunoreactivity is diagnostic.
Juxta-articular myxomas are entirely benign but may recur locally in up to 30% of cases, particularly if incompletely excised.
GNAS point mutations occur in intramuscular, but not juxta-articular myxomas
Myxofibrosarcoma, myxoid liposarcoma, myxoid chondrosarcoma, myxoid nodular fasciitis, myxoid desmoid-type fibromatosis.
Cutaneous myxoma, also known as “superficial myxoma” or “superficial angiomyxoma,” is a rare myxoid tumor of the skin and subcutis. Cutaneous myxomas are histologically identical to the cutaneous myxoid tumors seen in patients with Carney complex, although most occur sporadically. Men are affected slightly more often than are women, and the typical patient is a middle-aged adult. The most common locations for sporadic cutaneous myxomas are the trunk, lower extremities, and head and neck; Carney complex-associated myxomas are more often multiple and involve the eyelids and ear.
Cutaneous myxomas are typically small, sometimes polypoid tumors which have a gelatinous appearance. These lesions are often poorly circumscribed, and may involve the subcutis and occasionally deeper muscle.
Cutaneous myxomas are considerably more vascular than are other myxomas, as reflected in the alternative term, “superficial angiomyxoma” ( Fig. 15.3A ). This vasculature may be very well-developed and arborizing, reminiscent of myxoid liposarcoma. However, the overall cellularity of the lesion is quite low, and the lesional cells are small, cytologically bland, and spindled to stellate in shape. A prominent neutrophilic infiltrate is often present and is a helpful clue to this diagnosis ( Fig. 15.3B ). Epithelioid structures, probably representing the entrapped adnexal glands, are seen in approximately one-third of cases. A vaguely lobular growth pattern is often present.
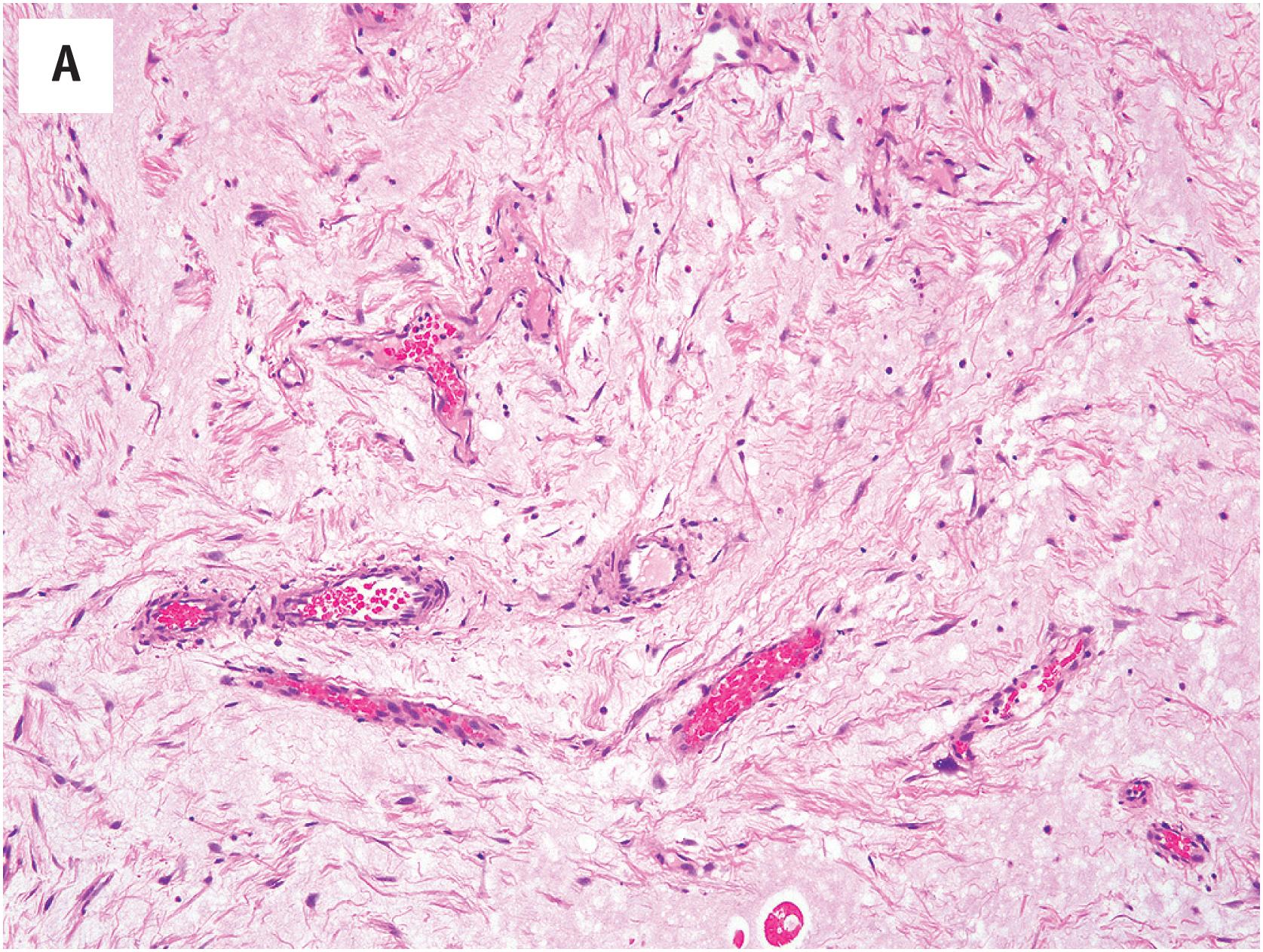
In general, cutaneous myxomas lack the expression of markers other than vimentin. The loss of PRKAR1A expression may be seen in cutaneous myxomas, even in the absence of other stigmata of Carney complex ( Fig. 15.3C ).
Focal cutaneous mucinosis lacks the neutrophils, lobular growth pattern, and elaborate vasculature seen in cutaneous myxoma. Digital mucinous cyst occurs exclusively in the digits and lacks a well-developed vasculature. Deep (aggressive) angiomyxoma is a deeply seated tumor of the genital region, which only focally involves the skin (if at all), lacks neutrophils, and contains ectatic, nonarborizing vessels. Nerve sheath myxoma is more distinctly lobular and is composed of plumper, occasional pleomorphic, S-100 protein-positive cells. Myxoid neurofibromas lack the well-developed vasculature of myxoma and contain wavy, “comma-shaped” cells that express S-100 protein. Myxoid dermatofibrosarcoma protuberans are much more infiltrative, CD34-positive lesions which typically show at least small areas of more typical dermatofibrosarcoma.
Benign myxoid mesenchymal tumor of the skin and subcutis
Rare
Sporadic cutaneous myxoma: trunk, legs, head/neck
Carney complex-associated myxoma: eyelids and ear, may be multiple
None
Most often occur in middle-aged men
Polypoid or papular skin lesion
May recur locally
Complete excision
Poorly circumscribed, gelatinous, small
Vaguely lobular
Much more vascular than other myxomas; may exhibit well-developed arborizing vasculature
Bland spindled cells
Abundant myxoid matrix
Stromal neutrophils and entrapped adnexal units in some
Typically express only vimentin
May exhibit PRKAR1A loss
Cutaneous myxomas are benign but may recur locally, and should be excised completely.
Cutaneous mucinosis, digital mucinous cyst, aggressive angiomyxoma, nerve sheath myxoma (neurothekeoma), myxoid neurofibroma, myxoid dermatofibrosarcoma protuberans.
Angiomyofibroblastoma is an uncommon, benign, non-recurring myofibroblastic lesion of the vulva and pelviperineal region. The vast majority of cases are observed in the vulva of middle-aged women, less frequently in the vagina (10–15% of cases), perineum, or inguinal region. Angiomyofibroblastomas present as slow-growing, painless masses, which are usually considered clinically to be Bartholin’s gland cysts.
Angiomyofibroblastomas are well-circumscribed but usually non-encapsulated lesions, 1 to 10 cm in size (4 cm on average). The consistency is soft, and the cut section of the lesion is gray-pink with a glistening appearance.
Angiomyofibroblastoma, which develops in the pelviperineal subcutaneous tissue, is composed of small-to-medium-sized well-formed vessels, round to spindled myofibroblasts arranged around the vessels and loose edematous stroma in varying proportions ( Fig. 15.4A ). The tumor cells have eosinophilic cytoplasm and bland nuclei, occasionally with smudged chromatin. Epithelioid to plasmacytoid neoplastic cells may be present as well as bi- or multinucleate cells ( Fig. 15.4B ). Mitotic figures are rare and there is no tumor necrosis. Interstitial mast cells are commonly observed. Approximately 10% of cases also contain mature adipocytes. Fibrosis and perivascular hyalinization are common in long-standing lesions. Some cases show some morphologic overlap with cellular angiofibroma, but it is now understood that the pathogenesis of cellular angiofibroma is related to RB1 loss, whereas angiomyofibroblastomas are not.
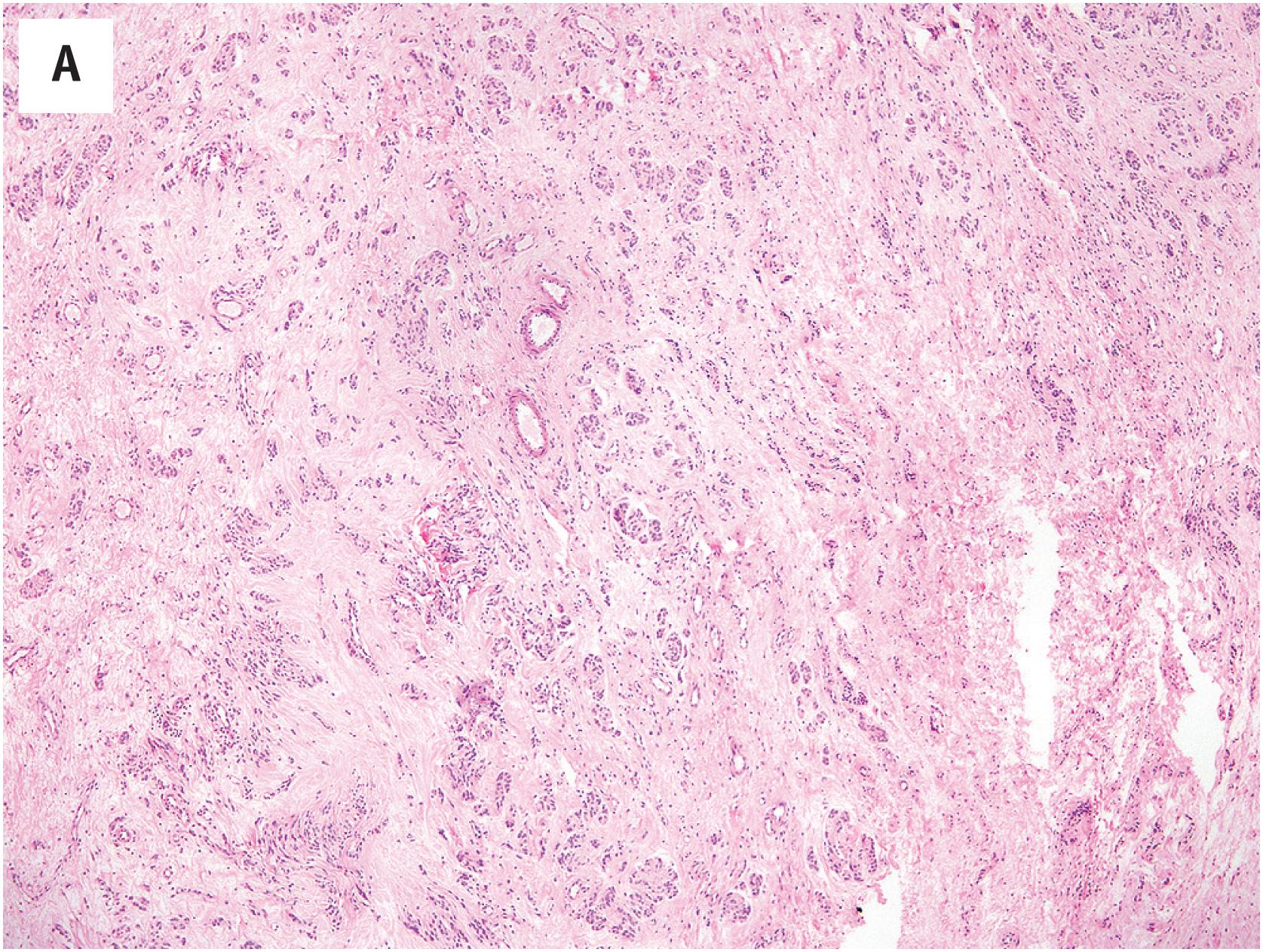
Most angiomyofibroblastomas are strongly positive for desmin and focally positive for smooth muscle actin and CD34. Desmin staining may be reduced or absent in postmenopausal cases. Positivity for estrogen and progesterone receptors is the rule. S-100 protein, keratins, and EMA are not expressed. RB1 protein expression is retained (normal).
Angiomyofibroblastoma is a small, cellular, superficially located lesion with numerous small vessels, unlike deep (aggressive) angiomyxoma, a large, deeply situated, hypocellular myxoid lesion with gaping vessels. Epithelioid smooth muscle tumors are more solid, eosinophilic tumors which typically exhibit at least small areas of more typical smooth muscle, and are diffusely smooth muscle actin-, caldesmon-, and desmin-positive. Cellular angiofibromas tend to show more uniform spindled growth around the hyalinized blood vessels and exhibit the loss of RB1 expression. Solitary fibrous tumors have more abundant collagen, a pronounced branching, staghorn, vascular pattern, a “patternless” growth pattern, and express STAT6 in a nuclear pattern.
Benign myofibroblastic lesion of the vulva and pelviperineal regions
Angiomyofibroblastoma:
Rare
Mostly observed in the vulva, less frequent in the vagina
Benign, non-recurring neoplasms
Middle-aged women
Slow-growing painless nodule
Benign, non-recurring neoplasms
Well-circumscribed
Small (2–3 cm on average)
Numerous small to medium-sized vessels
Round to spindle-shaped myofibroblasts concentrated around vessels
Epithelioid-appearing, plasmacytoid, bi- and multinucleate cells frequent
Loose edematous stroma
Mitotic figures rare
Intralesional mature adipocytes in approximately 10% of cases
Strongly positive for desmin, ER, and PR; less frequently smooth muscle actin and CD34-positive
Retained (normal) expression of RB1 protein
Aggressive angiomyxoma, epithelioid smooth muscle tumors, cellular angiofibroma, solitary fibrous tumor
Angiomyofibroblastomas are benign non-recurring lesions. Simple excision is curative. Malignant angiomyofibroblastomas have been reported but are exceptional.
Cellular angiofibroma and mammary-type myofibroblastoma are morphologically similar tumors with a similar immunophenotype which are characterized genetically by deletions of 13q, with the loss of RB1 expression. These very likely represent slightly different manifestations of a single entity and will be considered together.
Cellular angiofibromas present as small, slowly-growing masses of the vulva of middle-aged women, less frequently in the vagina (10–15% of cases), perineum, or inguinal region. Cases in males have been reported as “angiomyofibroblastoma-like tumors of the male genital tract”; such lesions typically involve the scrotum, spermatic cord, or inguinal region. This terminology is now discouraged.
Approximately 50% of mammary-type myofibroblastomas occur in the inguinal or groin region, including the vulva/vagina, perineum, and scrotum. Identical cases may be observed in the breast and in a wide variety of somatic soft tissue locations. The great majority involve the skin and subcutis.
Both present as variably sized (minute to >20cm), well-circumscribed, solid masses.
Cellular angiofibroma is generally well-circumscribed and is composed of proliferating bland spindle cells, arrayed about numerous small-to-medium-sized vessels with hyalinized walls, perivascular fibrinoid deposits and/or intraluminal thrombi ( Fig. 15.5A and B ). In typical examples, the mitotic activity is low and necrosis is absent. Very rare cases of cellular angiofibroma with apparent morphologic progression to sarcoma (or pseudosarcomatous nuclear changes) have been reported; such cases tend to resemble pleomorphic liposarcoma or undifferentiated pleomorphic sarcoma ( Fig. 15.5C ). Some cellular angiofibromas display striking stromal myxoid change ( Fig. 15.5D ). The morphological features of mammary-type myofibroblastoma are quite similar, although in their classical form these lesions tend to have a more distinctly nested architecture, with bands of refractile collagen circumscribing nests of bland spindled cells with ovoid to pointed nuclei ( Fig. 15.5G ). A variable component of mature fat is present in both tumors.
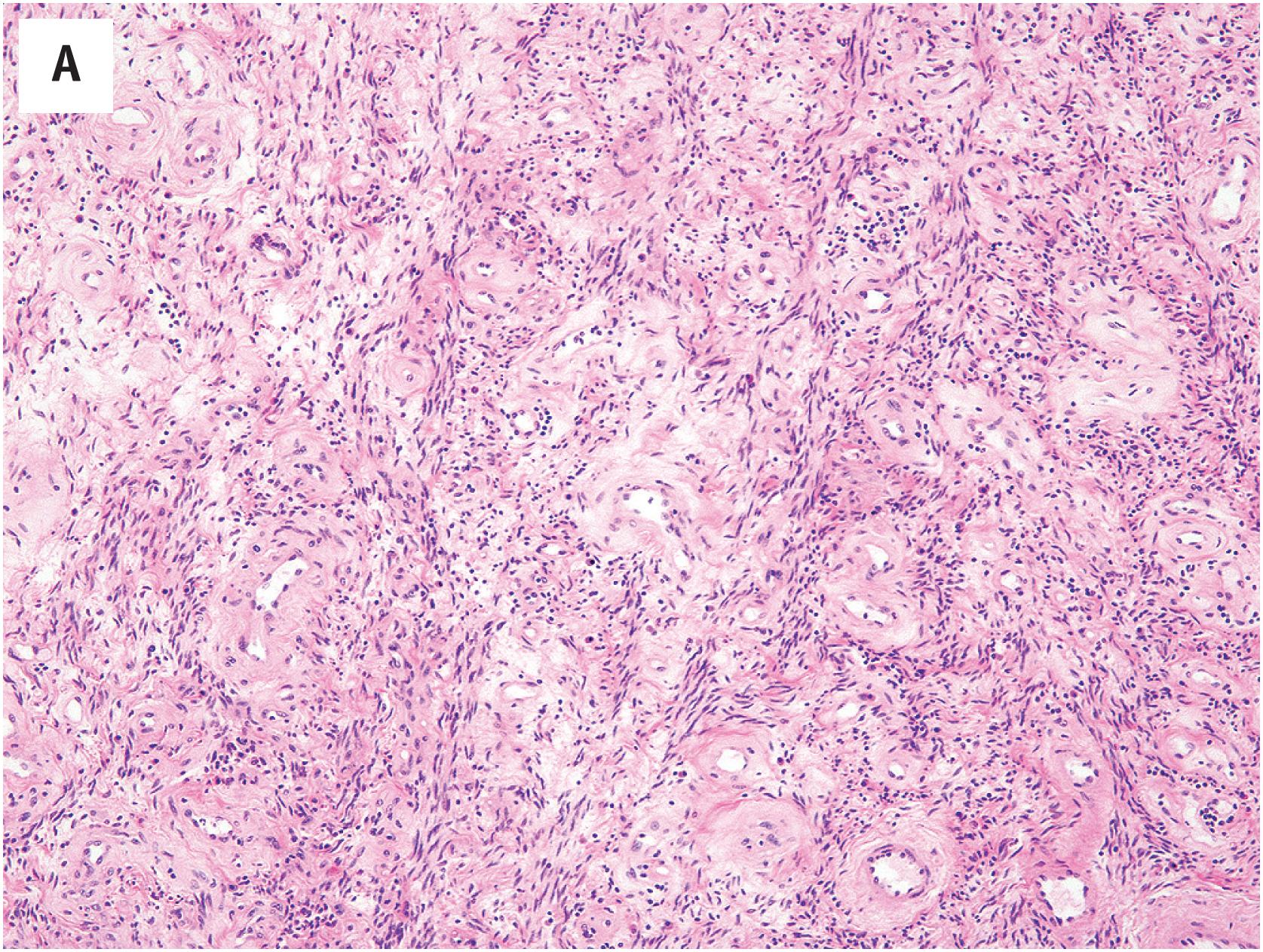
Cellular angiofibromas tend to be negative for smooth muscle actin and desmin, are variably positive for CD34, and are usually strongly positive for estrogen and progesterone receptors. The diffuse co-expression of CD34 and desmin is typically seen in mammary-type myofibroblastoma. Both display the loss of RB1 expression ( Fig. 15.5E and F ).
Angiomyofibroblastomas are more myxoid, have small gaping blood vessels, are composed of epithelioid cells, and have retained expression of RB1. Deep angiomyxomas are deeply situated, large, extensively myxoid lesions lacking the compact spindle cell growth seen in both cellular angiofibroma and myofibroblastoma. The distinction of both from spindle cell lipoma is difficult and possibly arbitrary. The presence of areas of typical cellular angiofibroma distinguishes very rare cellular angiofibromas with morphologic progression to sarcoma from primary soft tissue sarcomas of the inguinopelvic region. Solitary fibrous tumors lack desmin expression, have retained RB1 expression, and express STAT6 in a nuclear pattern.
Cellular angiofibromas and mammary-type myofibroblastomas are benign and do not typically recur. Recurrences and/or metastases have not as yet been reported in sarcomatous cellular angiofibromas.
Cellular angiofibromas are usually smooth muscle actin- and desmin-negative, with variable expression of CD34. Mammary-type myofibroblastomas typically have strong CD34 and desmin co-expression. Both express ER and PR and display the loss of RB1 expression.
Benign, RB1-deficient myofibroblastic lesions of the vulva, inguinoscrotal, and pelviperineal regions, as well as a wide variety of somatic soft tissue sites and the breast
Rare; cellular angiofibromas are most often located in the vulva in women, and in the inguinoscrotal region in males
Approximately 50% of mammary myofibroblastomas occur in the inguinopelvic region, with the remained involving the breast and various somatic soft tissue locations
Benign, non-recurring neoplasms
Middle-aged women and men
Slow-growing painless mass of widely variable size
Benign, non-recurring neoplasms
Sarcomatous cellular angiofibromas have behaved in a clinically benign fashion to date
Well-circumscribed masses, ranging in size from minute to very large
Bland spindle cells with ovoid to pointed nuclei, resembling those seen in spindle cell lipoma
Numerous small- to medium-sized, hyalinized vessels with fibrinoid deposits and sometimes organizing intraluminal thrombi
Variable component of mature fat
Packeted growth pattern with bands of refractile collagen in classic mammary-type myofibroblastoma
Extremely rare cellular angiofibromas contain foci resembling pleomorphic liposarcoma or undifferentiated pleomorphic sarcoma
Angiomyofibroblastoma, angiomyxoma, spindle cell lipoma, solitary fibrous tumor.
Deep angiomyxomas were previously known as “aggressive angiomyxomas,” but it is now understood that their natural history is much less aggressive than was previously thought.
Most deep angiomyxomas occur in the genital, perineal, and pelvic region of reproductive-aged women, although rare cases also occur in the pelvis, spermatic cord, or scrotum of men (female/male ratio 6:1). These tumors typically present as slowly-growing, deeply situated masses which may infiltrate the adjacent structures, including the soft tissues surrounding the rectum and vagina. Owing to their infiltrative growth and myxoid nature, deep angiomyxomas may be difficult to excise completely, and have been reported to recur in up to 30% of cases. More recent series have, however, emphasized that correctly diagnosed and appropriately treated angiomyxomas have a much lower rate of local recurrence (<10%) and that almost all patients are cured with a single re-excision. Metastases are not observed.
Deep angiomyxomas typically appear as soft, myxoid, variably circumscribed masses of up to 60 cm in size. Some tumors may be polypoid.
Deep angiomyxomas are composed of very bland, spindled to stellate cells widely dispersed in a myxoid matrix ( Fig. 15.6A ). A characteristic feature is the presence of numerous blood vessels, ranging from capillaries up to large, gaping vessels with hyalinized walls ( Fig. 15.6B ). In general, these vessels tend to be nonarborizing. Small aggregates of conventional appearing smooth muscle cells often are observed in close proximity to the blood vessels ( Fig. 15.6C ). Cellularity is typically low, mitotic figures are very rare, and necrosis is absent. Mast cells and lymphoid aggregates may be present.
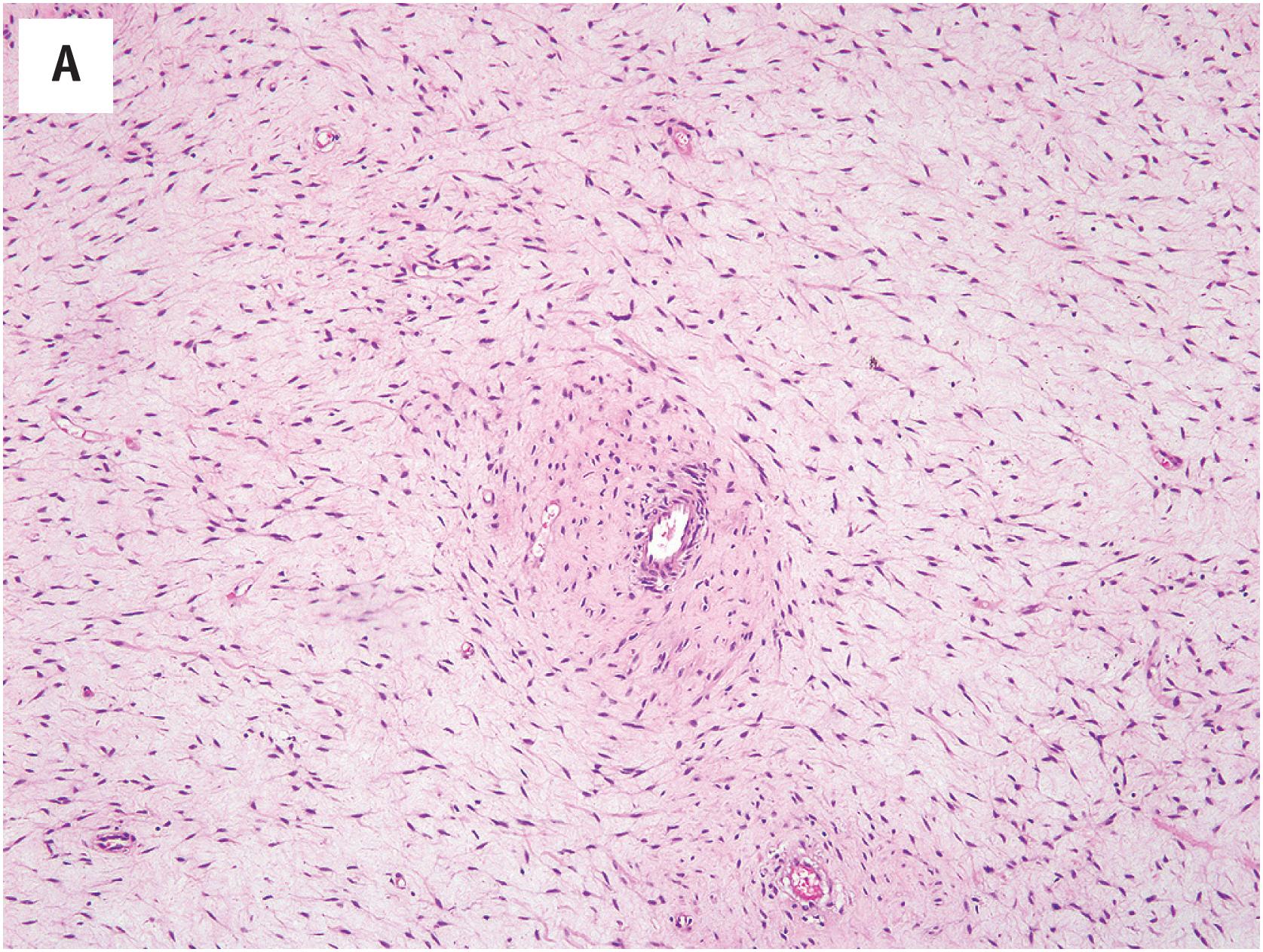
Most angiomyxomas express both desmin and smooth muscle actins, with some also expressing CD34. S-100 protein and cytokeratins are absent. Estrogen and progesterone receptors are positive. The strong expression of HMGA2 is observed, but is not entirely specific for angiomyxoma ( Fig. 15.6D ).
Deep angiomyxomas are typified by clonal aberrations involving the HMGA2 gene, located at 12q13-15.
Extensively myxoid leiomyomas may closely mimic deep angiomyxoma, but are typically better circumscribed, contain larger areas of more typical smooth muscle, and have larger, thick-walled blood vessels. Angiomyofibroblastomas are superficially located and smaller, and consist of epithelioid cells arranged in a perivascular distribution. Myxoid change is very unusual in angiomyofibroblastoma. Fibroepithelial polyps of the genital region blend into the overlying epidermis or mucosa, without a Grenz zone, and are usually much more cellular and cytologically variably than are angiomyxomas. Myxofibrosarcomas are almost always subcutaneous tumors of the extremities, which display much greater cytological atypia and have a well-developed, arborizing, thick-walled vasculature. Myxofibrosarcoma-like histology in a deeply situated lesion should suggest dedifferentiated liposarcoma. Myxomas, including cutaneous myxoma, are very unusual in the genital region and have distinctive histological features, as described earlier in this chapter.
Despite its original name, deep angiomyxoma is not an especially aggressive lesion. It does, however, have significant potential for local recurrence, owing to its infiltrative growth pattern and the difficulty of completely resecting extensively myxoid tumors. As noted above, incompletely excised deep angiomyxomas have risk of recurrence of approximately 30%; this risk decreases with more complete initial resection. Deep angiomyxoma has no potential for distant metastasis or malignant transformation.
Positive for desmin, smooth muscle actins, and HMGA2; often negative for CD34, S-100 protein, and cytokeratins.
A distinctive myxoid tumor of the genital region, most often seen in reproductive-aged women
Rare
Perineum, pelvis, and genital region in women
Scrotum, spermatic cord, and pelvis in men
Benign neoplasm with significant potential for local recurrence
Much more common in women than in men (6:1)
Reproductive-aged women and adult males
No race predilection
Slowly-growing, deeply situated mass
Significant potential for local recurrence if incompletely excised
Requires wide excision
No known role for adjuvant therapies
Variably circumscribed but infiltrative
Large (up to 60 cm)
Myxoid
Infiltrative
Grenz zone present if near skin/ mucosa
Abundant myxoid matrix
Bland spindled to stellate cells
Prominent vasculature: capillaries and dilated, hyalinized larger vessels
Small smooth muscle aggregates near blood vessels
Lymphoid aggregates and mast cells
Angiomyofibroblastoma, myxoid leiomyoma, other myxomas, myxofibrosarcoma, fibroepithelial polyp.
Angiofibroma of soft tissue (AFST) is a rare, recently described soft tissue tumor that may contain foci closely resembling those of myxoid liposarcoma and solitary fibrous tumor.
These tumors typically present as painless masses of the extremities, frequently involving or adjacent to the large joints, and may be either subcutaneous or intramuscular. Most occur in middle-aged adults, with a slight female predominance.
The tumors appear as nondescript, sometimes myxoid soft tissue masses.
Microscopically, these tumors show a variably myxoid to collagenized stroma, with a moderately cellular proliferation of bland, fibroblastic spindled cells ( Fig. 15.7A ). They are most notable for their elaborate vasculature, with some areas containing innumerable small, arborizing vessels, reminiscent of myxoid liposarcoma, and others having larger, branched vessels, similar to those seen in solitary fibrous tumor ( Fig. 15.7B ). Perivascular collagen deposition and marked hyalinization or fibrinoid necrosis of the medium-sized vessel walls is often present. Foamy macrophages, hemosiderin deposition, and chronic inflammation are also often present.
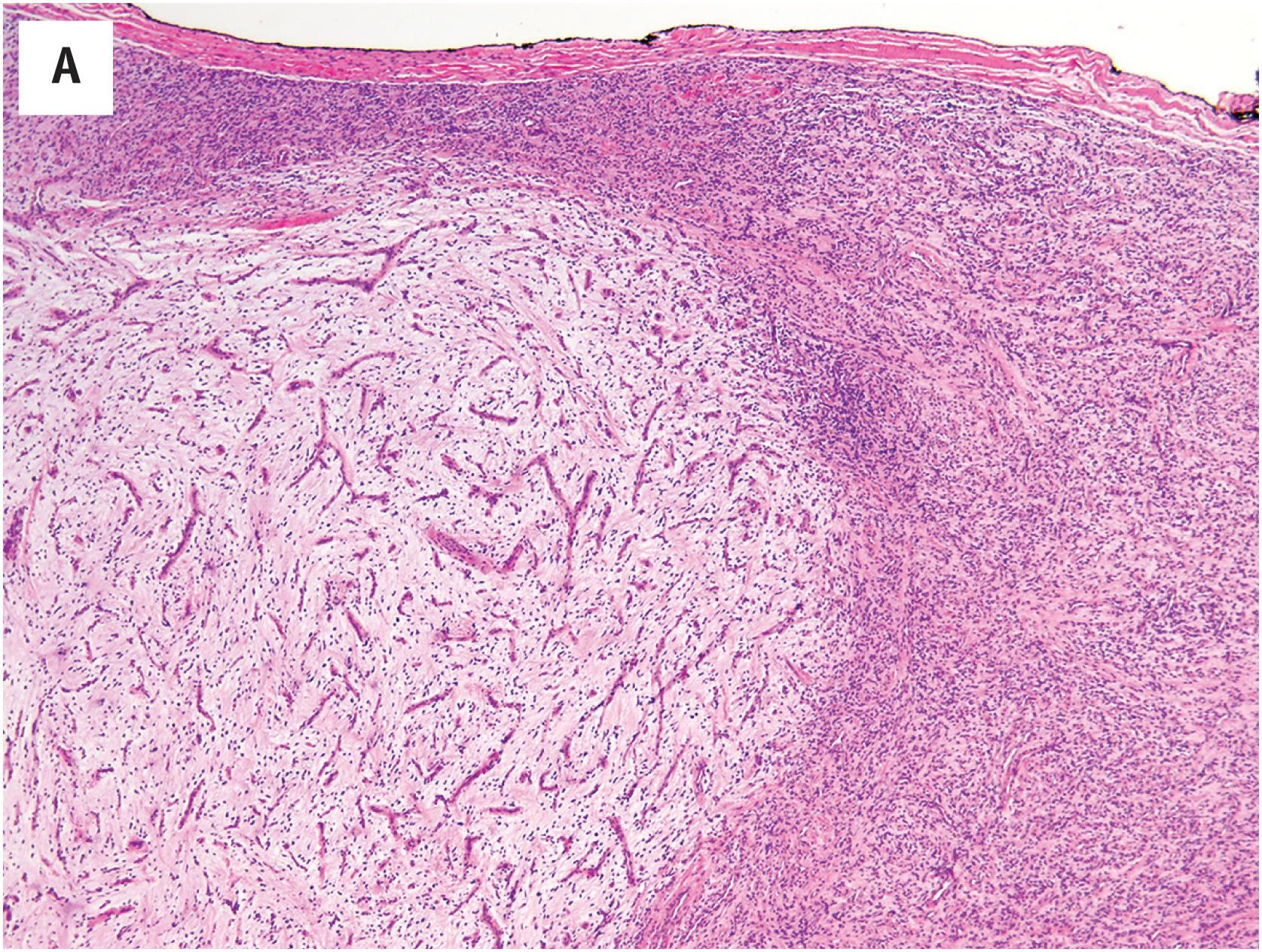
Benign mesenchymal tumor of the subcutis and muscle showing mixed features of solitary fibrous tumor and myxoid liposarcoma
Rare
Most common around knees but may occur in any somatic soft tissue location
None
Most common in middle-aged women
Painless soft tissue mass, often around the knee
Benign, rarely recurs locally
Variably myxoid, non-descript soft tissue mass
Myxoid to collagenized stroma
Moderately cellular; bland, fibroblastic spindled cells
Highly vascular, with innumerable small, arborizing vessels (myxoid liposarcoma-like) and thicker, branching vessels (solitary fibrous tumor-like)
Perivascular collagen deposition, hyalinization, and/or fibrinoid necrosis of vessel walls
Limited CD34 expression
STAT6-negative
May contain desmin-positive dendritic cells
By immunohistochemistry, the neoplastic cells display limited expression of CD34 and are STAT6-negative.
Most cases of AFST are characterized by the AHRR- NCOA2 fusion gene. Less commonly, GTF2I-NCOA2 or GAB1-ABL1 fusions are found instead.
Historically, AFST were likely confused with solitary fibrous tumors. However, AFST lacks the “patternless” proliferation of bland fibroblastic spindled cells and the abundant hyalinized collagen that characterizes solitary fibrous tumors, contains areas with diffuse proliferation of small-caliber vessels, and lacks STAT6 expression. The elaborate vasculature present in AFST may also suggest myxoid liposarcoma, but lipoblasts and mature fat are absent.
AFST are benign tumors with limited capacity for local recurrence.
AHRR-NCOA2 fusion; less commonly GTF2I-NCOA2 or GAB1-ABL1 fusions.
Solitary fibrous tumor, myxoid liposarcoma.
EWSR1-SMAD3 -rearranged fibroblastic tumor is a recently described tumor, apparently displaying fibroblastic differentiation.
Tumors arise over a wide age range, are more common in women, and arise in the dermis or subcutaneous tissue of the distal extremities. They have some potential for local recurrence but have not been reported to metastasize.
EWSR1-SMAD3 -rearranged fibroblastic tumors are small, generally not exceeding 1.5 cm.
EWSR1-SMAD3-rearranged fibroblastic tumors typically involve the dermis and subcutis ( Fig. 15.8A and B ), and display an infiltrative growth pattern, with the entrapment of adnexal structures or subcutaneous fat. Histologically the tumors are comprised of interlocking, cellular fascicles of fibroblasts admixed with distinctive, hypocellular hyalinized zones, which may be calcified ( Fig. 15.8C ). Cellular and hypocellular areas may be intermingled or display a zonal pattern, with hyalinized areas at the center. Lesional cells display bland nuclear features, with elongated, sometimes wavy nuclei, without atypia or mitotic activity ( Fig. 15.8D ).
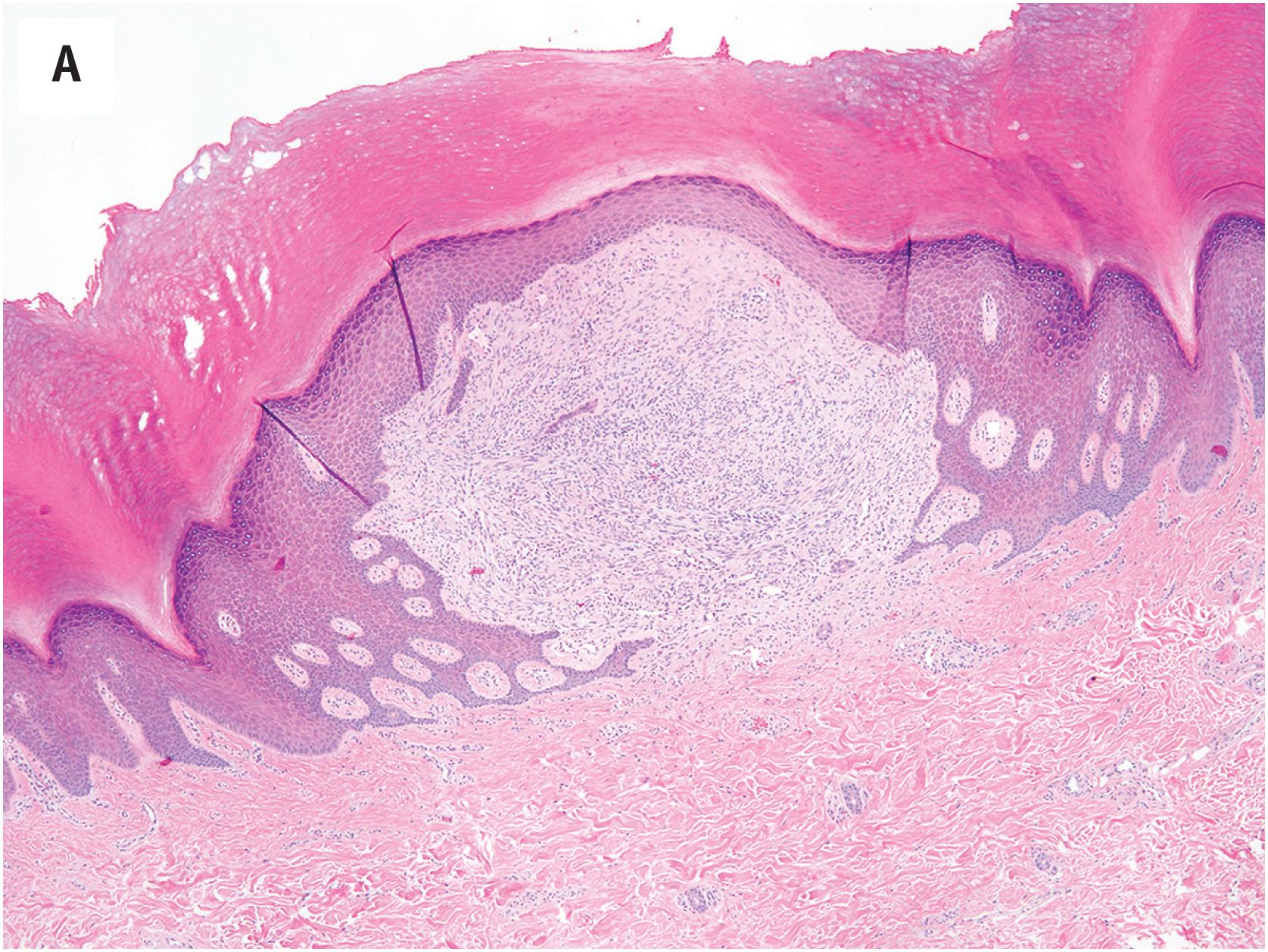
EWSR1-SMAD3 -rearranged fibroblastic tumors are strongly positive for ERG in a nuclear pattern ( Fig. 15.8E ), and negative for CD34, EMA, and smooth muscle actin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here