Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Melanocytic neoplasms of the central nervous system (CNS) arise from the population of leptomeningeal melanocytes that are scattered throughout the arachnoid membranes. Lesions can manifest as diffuse disseminations within the subarachnoid space or as solid masses, and they range in histologic grade from benign to malignant. Meningeal melanocytoma (benign mass forming proliferation), meningeal melanoma (malignant mass forming proliferation), meningeal melanocytosis (benign diffuse meningeal melanocytic proliferation), and meningeal melanomatosis (malignant diffuse meningeal melanocytic proliferation) belong in this category of diseases. The term neurocutaneous melanosis refers to a group of rare genetic disorders that combine giant single and/or numerous smaller congenital nevi with diffuse leptomeningeal melanosis and increased risks of malignant transformation to melanoma.
Diseases derived from leptomeningeal melanocytes have intrigued pathologists for generations. Their original descriptions were contributed by the founding fathers of pathology, including Virchow, who was the first to describe meningeal melanoma in 1859, and Rokitansky, who described neurocutaneous melanosis not much later in 1861. Low-grade dura-based melanocytomas were once thought to represent “melanotic meningiomas” until ultrastructural investigations demonstrated that tumor cells were melanocytic in origin, rather than meningothelial.
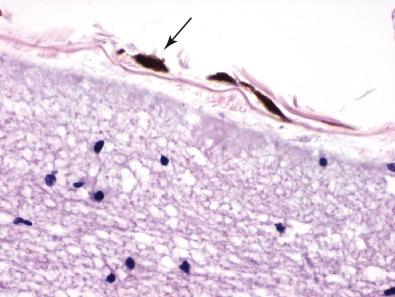
Melanocytes are normal, neural crest–derived constituents of the human leptomeninges that are intimately associated with pia and arachnoid membranes ( Fig. 19.1 ). Individuals with darkly pigmented skin have greater numbers of leptomeningeal melanocytes. Their highest density is found overlying the ventral surface of the superior spinal cord, brainstem, and base of the brain, where they can be noted grossly as a faint, gray-brown discoloration and microscopically as thin, elongated, heavily pigmented individual cells. The neoplasms that arise from leptomeningeal melanocytes are likewise localized to the coverings of the brain, where they produce mass lesions or diffuse disseminations.
Circumscribed tumors that are well differentiated are classified as meningeal melanocytoma to distinguish them from the highly malignant melanoma. They occur most often in adults (mean, 45–50 years old), but age of presentation ranges from 9 to 73 years. There is a slight female predominance (female to male ratio of 1.5:1). These tumors are rare and represent only 0.06% to 0.1% of all primary brain tumors, with an estimated annual incidence of 1 case per 10 million. The majority arise in the extramedullary, intradural compartment at the cervical and thoracic spinal levels, where they are compressed against the dura. In these locations they can be associated with nerve roots and spinal foramina. Less frequently, they arise from the leptomeninges in the posterior fossa and supratentorial compartments. Meckel's cave, in particular, is a site with a peculiar predilection for primary melanocytic neoplasms, and there is an association of this location with ipsilateral nevus of Ota (unilateral oculodermal melanocytosis involving the distribution of the ophthalmic and maxillary branches of the trigeminal nerve and, frequently, the sclera). Melanocytomas are generally associated with focal neurologic symptoms due to compression of adjacent CNS parenchyma. In case reports, a prolonged evolution of symptoms prior to surgical resection (from 5–10 years) has been documented.
The nodular form of primary CNS melanoma presents as a solitary, discrete neoplasm with a slight predilection for the spinal cord and posterior fossa—the most abundant source of precursor melanocytes—yet also arises in the supratentorial compartment. Patients range from 15 to 71 years, with an estimated annual incidence of 0.5 cases per 10 million. Primary melanomas cause focal neurologic symptoms related to their location, with symptoms typically evolving more rapidly than melanocytomas.
By neuroimaging, melanocytomas are extra-axial and well circumscribed, sharing many features with the more common meningioma. One distinguishing feature of melanocytoma is its melanin content. The unique paramagnetic properties of melanin result in a relatively specific magnetic resonance imaging (MRI) pattern of hyperintensity on T1-weighted images and isointensity or hypointensity on T2-weighted images ( Fig. 19.2 ). The degree of tissue destruction and T2 hyperintensity in adjacent CNS parenchyma associated with melanocytomas is generally low.
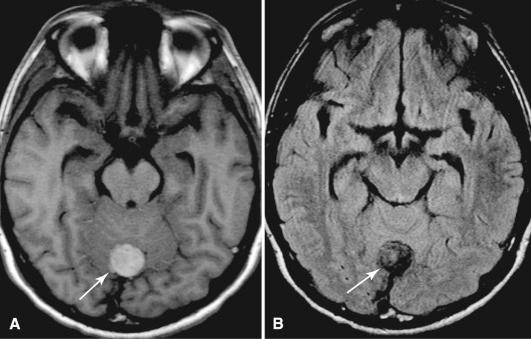
MRI of nodular forms of melanoma also demonstrates a solid, usually dural-based mass. T1- and T2-weighted signal abnormalities may be seen and depend on the amount of melanin present, which varies considerably among primary CNS melanomas. Those with heavy melanin show the greatest T1 hyperintensity and T2 hypointensity. The degree of contrast enhancement also varies. In both diffuse and solid forms of melanoma, the rapid growth rate is usually reflected in the adjacent brain, where vasogenic edema is seen as T2 hyperintensity.
The majority of melanocytomas and melanomas arise within the leptomeninges and grow as extra-axial masses. Variable pigment production results in lesions that are grossly gray, brown, or black. Malignancy is typically associated with frank invasion of the underlying CNS parenchyma that can be noted grossly. Less commonly, primary melanoma presents as an intra-axial mass and secondarily disseminates throughout the leptomeninges. These localized diseases involve the spinal cord and posterior fossa most frequently, but also arise in the supratentorial compartments.
Meningeal melanocytomas are solitary, discrete, and low grade, with no invasion of surrounding structures. The major architectural pattern takes the form of tight nests of slightly spindled, well-differentiated melanocytes containing variable amounts of melanin ( Fig. 19.3 ). In this arrangement, which shows a superficial resemblance to the whorls of meningioma, heavily pigmented cells are seen at the periphery of nests. In other variants, tumor cells are arranged in vasocentric fascicles or in sheets and some contain a dominant population of epithelioid tumor cells. In all cases, nuclei are oval or bean shaped, typically with small nucleoli and often showing grooves, but lacking any appreciable cytologic atypia. Mitoses are generally absent (on average, <1 per 10 high-power field [HPF]), and the MIB-1 proliferation index is less than 2%. Most melanocytomas are heavily pigmented, containing populations of both melanin-producing melanocytes and large, pigment-filled melanophages. Rare melanocytomas do not produce histologically detectable melanin (i.e., amelanotic ), and their recognition relies on a familiarity with cytologic, histologic, immunohistochemical, and/or ultrastructural properties.
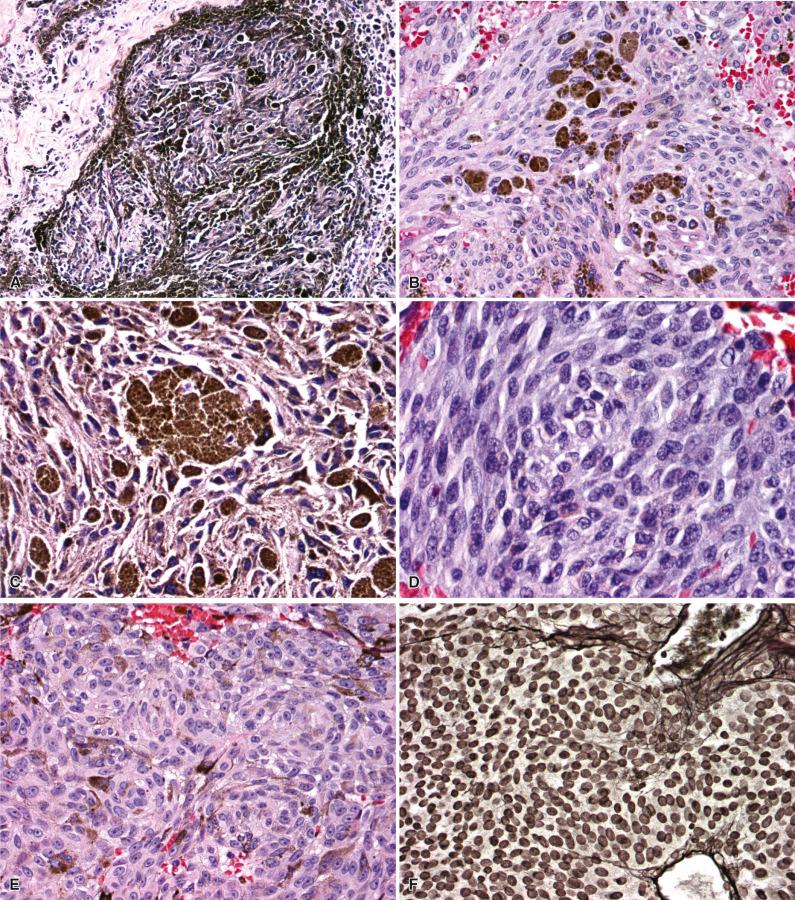
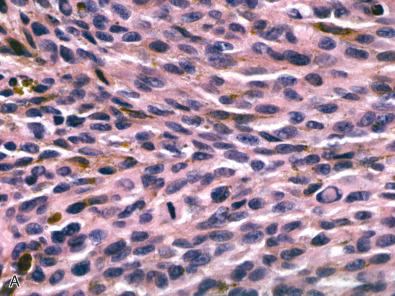
Meningeal melanoma is a faithful histologic mimic of melanomas arising in other sites. Lesions consist of anaplastic spindled or epithelioid cells arranged in loose nests, fascicles, or sheets, with variable cytoplasmic melanin pigment ( Fig. 19.4 ). Tight nesting such as that seen in melanocytomas is not a general feature of melanoma. Melanomas have a greater cellular density than melanocytomas and also show significantly more cellular pleomorphism and nuclear atypia. In distinction to melanocytoma, melanoma will often demonstrate unequivocal tissue invasion or coagulative necrosis. The cytologic features of melanoma are known to vary substantially. One subset of melanoma consists of large cells with bizarre nuclei, numerous typical and atypical mitotic figures, significant pleomorphism, and large cherry red nucleoli ( Fig. 19.4B and C ). Another set are densely cellular and less pleomorphic, usually consisting of tightly packed spindle cells with high nuclear to cytoplasmic ratios. Meningeal melanomatosis may arise from the diffuse spreading of a primary CNS melanoma throughout the subarachnoid space.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here