Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intracranial suppurations may be primary or occur as a secondary complication of surgery. Postsurgical intracranial suppuration may be considered a mode of direct inoculation (iatrogenic) of infective agent into the craniotomy surgical field. It may be subdural or intracerebral or involve only the meninges. Intracranial infections and suppurations complicate the postoperative course, possibly leading to serious consequences affecting patient recovery and well-being. Such complications may lead to adverse outcomes of surgery, with significant added cost and less favorable neurological results. This paradigm has global implications for neurosurgical care, with relevant peri-operative preventative measures and postoperative approaches for management, elucidated in this chapter.
Pyogenic brain abscess is a focal collection of pus within the brain. This potentially fatal condition continues to be the subject of neurosurgical concern and global discussion. The incidence of pyogenic brain abscesses is 8% of intracranial masses in the developing countries, whereas in the West the incidence is 1% to 2%, with male predominance. , This condition is rare, largely because of the brain’s natural resistance to infection, a property mediated by its abundant vascular supply, the relative impermeability of the blood-brain barrier, and improvement in the treatment of ear, sinus, and orofacial infections during the latter half of the 20th century. An active neurosurgical service in a hospital in a developed country can expect to see 4 to 10 cases annually. , Peak ages vary depending on predisposing factors.
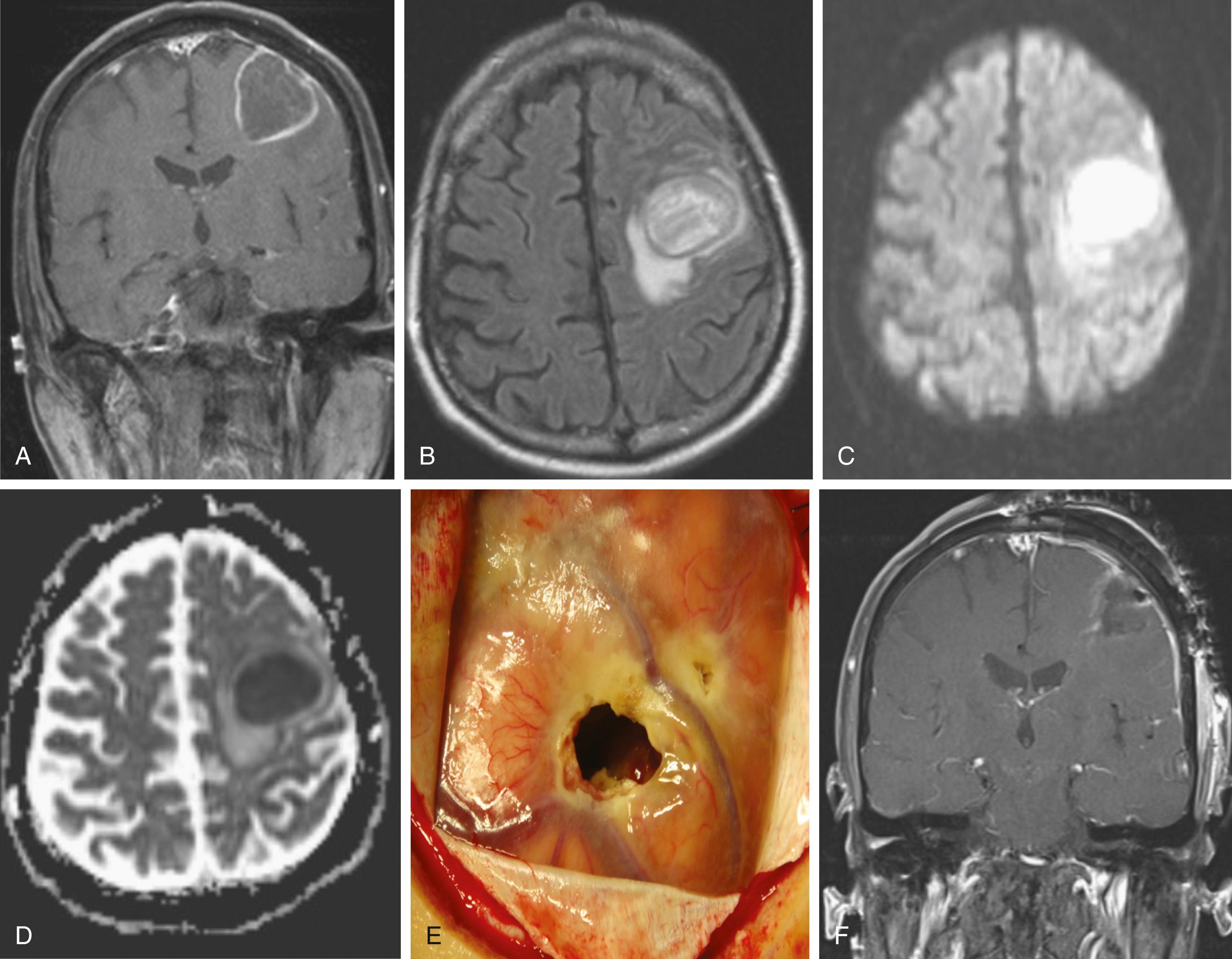
Brain abscess development is often characterized by four stages with a generally predictable window for each phase (elucidated later in the text): early cerebritis (1 to 4 days); late cerebritis (4 to 10 days); early capsule formation (11 to 14 days); and late capsule formation (>14 days). , Brain abscess formation is initiated when bacteria gain entry into cerebral tissues with trauma, contiguous spread from a suppurative focus, or hematogenous dissemination from a distant infection. Imaging modalities are the mainstay for staging abscess formation (discussed below). Many cases (40% to 60%) are the result of contiguous spread of infection from the middle ear, oropharynx, and paranasal sinuses ( Fig. 128.1 ). These lesions are usually solitary, and their distribution in the brain reflects the predisposing lesion ( Table 128.1 ). Seeding the brain probably occurs via the valveless emissary veins draining contiguous areas, allowing microorganisms to flow into the venous system of the brain from adjacent sites. Middle ear infections, chronic otitis media, or chronic mastoiditis can lead to temporal lobe or cerebellar abscesses by direct spread via the tegmen tympani or by translabyrinthine spread. Paranasal sinusitis can lead to frontal or temporal lobe abscesses by retrograde thrombophlebitis of the diploic veins. Frontal sinus infection can also lead to frontal lobe brain abscess when complicated by osteomyelitis of the frontal bone of the skull with dehiscence of the posterior table. Suppurative chronic obstructive pulmonary disease and dental infections are important sources of disseminated hematological spread into the intracranial cavity. ,
| Predisposing Lesion | Intracranial Location | Predicted Bacteriology |
|---|---|---|
| Paranasal sinusitis | Frontal lobe | Microaerophilic ( Streptococcus intermedius group) and anaerobic strep, Haemophilus, Bacteroides, Fusobacterium, and Prevotella species |
| Otitis media, mastoiditis | Temporal lobe or cerebellum | Aerobic and anaerobic streptococci, Enterobacteriaceae, P. aeruginosa, Prevotella species, Bacteroides fragilis |
| Dental sepsis | Frontal lobe | S. viridans and anaerobic streptococci, Bacteroides, Fusobacterium, Prevotella, and Actinomyces species |
| Penetrating trauma | Related to site of wound | S. aureus, aerobic streptococci, Clostridium species, Enterobacteriaceae |
| Postoperative trauma | Related to site of surgery | Staphylococcus epidermidis, S. aureus, Enterobacteriaceae, P. aeruginosa |
| Congenital heart disease | Multiple abscesses, most commonly in distribution of middle cerebral artery | Microaerophilic and aerobic strep |
| Infective endocarditis | Same as in congenital heart disease | S. aureus, S. viridans, Enterococcus species |
| Pulmonary infection (lung abscess, empyema) | Same as in congenital heart disease | Microaerophilic and anaerobic streptococci, Actinomyces, Fusobacterium, Nocardia, and Prevotella species |
| Intra-abdominal infection Compromised host (AIDS, cancer chemotherapy, chronic steroids, lymphoma) |
Same as in congenital heart disease | Streptococcus species, B. fragilis , Enterobacteriaceae toxoplasmosis, Nocardia species, EBV lymphoma, TB, fungi |
Many risk factors can predispose patients to bacterial brain abscess across a range of circumstances. Various considerations for classification criteria include: age (i.e., neonatal, pediatric, adult, and elderly), source of infection (i.e., direct penetrating inoculation, local extension of nearby infection, and hematological spread from distant location), comorbidities (i.e., immune status), and causative organism (i.e., bacterial, fungal, parasitic, and mixed).
Penetrating cranial trauma (such as open cranial fracture with dural tear) is a well-described, though relatively infrequent, cause of pyogenic brain abscess, accounting for less than 10% of these infections. , , A distinctive form of posttraumatic brain abscess that occurs largely in young children results from penetrating injuries secondary to a foreign body injury, such as to the orbital region, transnasal-tansethmoid and, less commonly, other areas of the skull, from pencil tips, wooden sticks, and sharp toys. The interval from injury to clinical presentation varies, and may extend from days to years. Treatment, as in other penetrating cranial injuries, often involves early surgical debridement.
An increasingly important problem occurs in intravenous drug users with infected valvular vegetations delivering septic emboli, resulting in cerebral infarction, cerebral hemorrhage, brain abscess formation, and spinal epidural abscesses. This population is also prone to toxin-mediated diseases (tetanus and botulism) with inoculation of these agents at injection sites. In general, however, transient bacteremia is unlikely to result in brain abscess in the absence of breaches in the blood-brain barrier that predispose cerebral lesions, such as previous stroke or primary or metastatic neoplasms. ,
Pediatric brain abscesses are most common between the ages of 4 to 7 years, and the minority of these children have congenital heart disease. Cerebral abscess complicates cyanotic congenital heart disease in 2% to 6% of cases. Cyanotic congenital heart disease is a leading underlying cause of pediatric brain abscess, accounting for 6% to 50% of cases. Tetralogy of Fallot and transposition of the great vessels underlie most cases, although any cardiac defect that results in significant right-to-left shunting appears to increase the risk. The pathogenesis probably involves increased blood viscosity as a result of chronic hypoxemia (due to right-to-left shunting), leading to areas of microinfarction within the brain that act as nidi for infection. The mortality from pyogenic brain abscess in this setting is high. Intrasellar, brain stem, basal ganglia, and thalamic abscesses are rare.
Intrasellar abscesses generally occur in the setting of preexisting pituitary or sellar lesions, such as adenomas, craniopharyngiomas, or Rathke cleft cysts, or as a complication of trans-sphenoidal surgery. , These lesions may also occur as a result of intrasellar extension from sphenoid sinusitis. Abscesses of the brain stem are generally hematogenous in origin and may extend longitudinally over several levels of the brain stem. The incidence of these lesions appears to have decreased over the past decades, probably as a result of improvements in the management of pediatric ear and sinus infections. Twenty percent of such brain abscesses are cryptogenic; many of these cases are the result of non-treated dental infections.
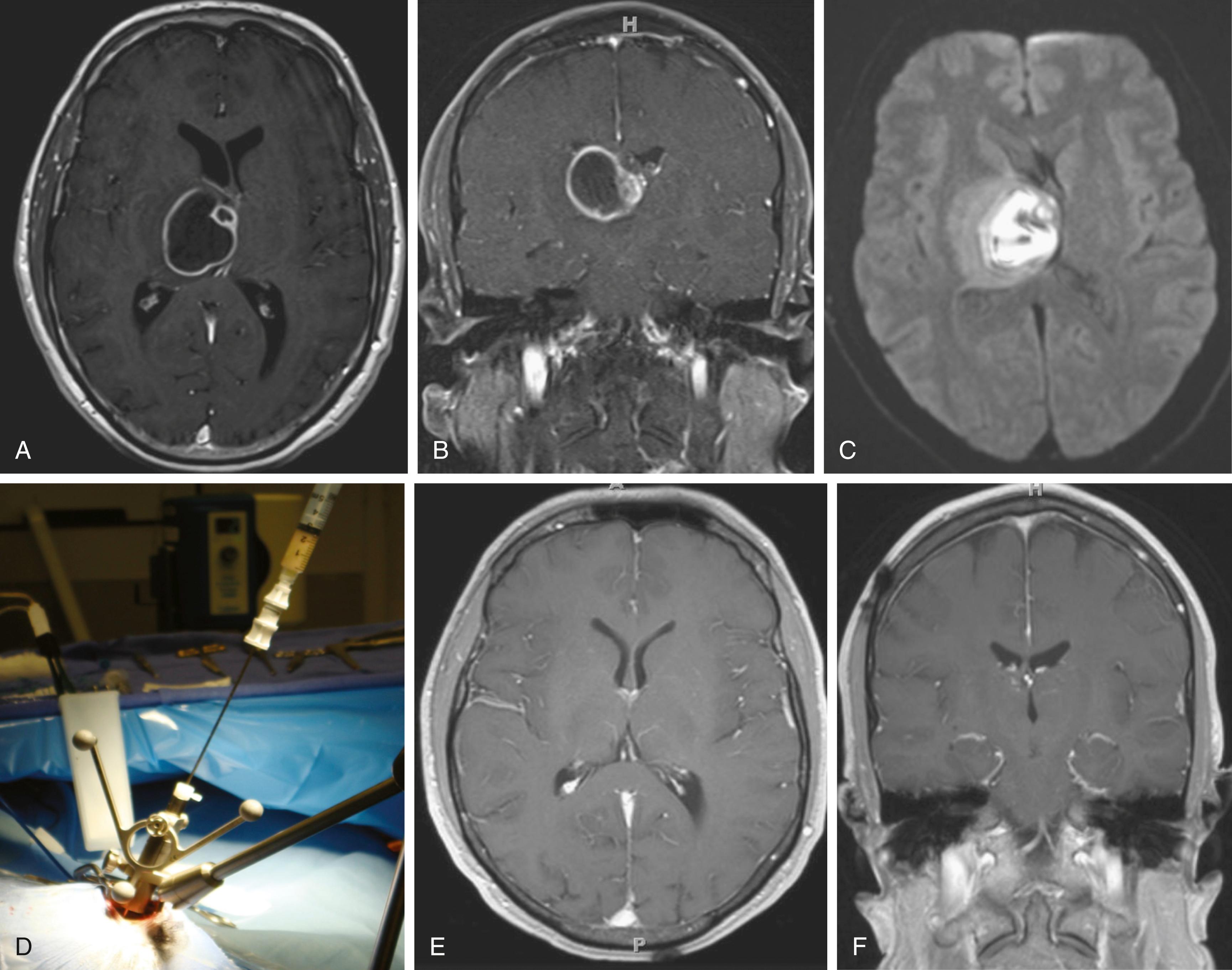
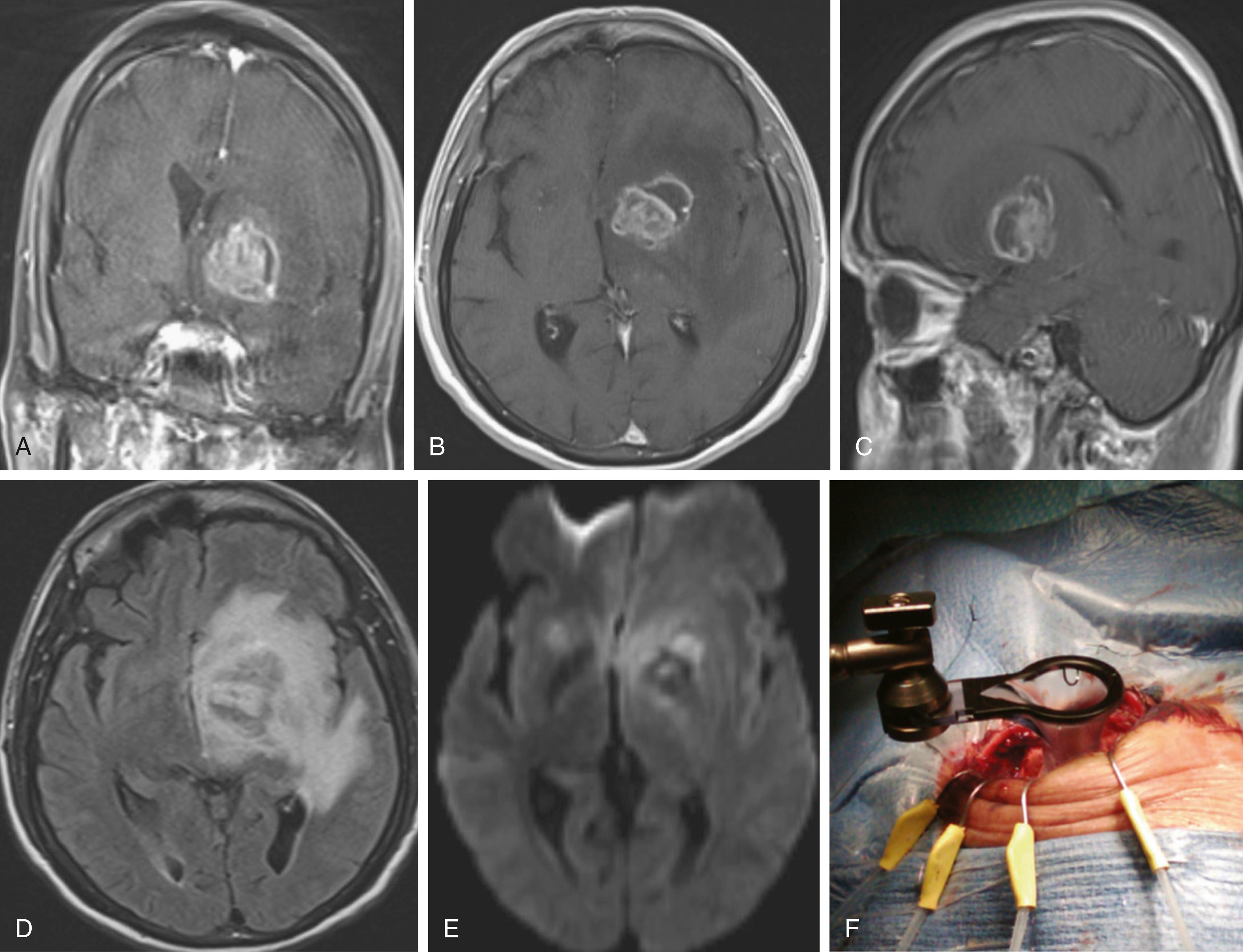
The bacteriology of intracranial infection is determined by the initial site of infection (see Table 128.1 ). Streptococci ( Streptococcus milleri and Streptococcus viridans ) are the most common cause of pyogenic brain abscesses, involved in nearly two thirds of cases, because of their extension from the nasopharynx and oropharynx, as well as from endocarditis (S. viridans). , Staphylococcus aureus accounts for 10% to 21% of cases, generally in the setting of trauma and postoperatively, but it is also seen among patients with brain abscesses resulting from endocarditis. , Methicillin-resistant S. aureus (MRSA) should be considered in hospitalized patients or among those who were known as MRSA colonized. Anaerobic bacteria ( Bacteroides, Prevotella, Peptostreptococcus, Fusobacterium, and Actinomyces species) are also major causes of brain abscesses, usually as a part of polymicrobial infection. Brain abscesses due to Actinomyces species are commonly associated with pulmonary and odontogenic infections. Gram-negative bacilli such as Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Serratia species, and Proteus species are associated with genitourinary or intra-abdominal infections, as well as commonly isolated from brain abscesses following head trauma and postoperative infection. , , Pseudomonas species can cause brain abscess that results from otitis media or otitis externa. , Other bacterial species may be cultivated from brain abscesses in various clinical settings: Clostridium species, in association with an underlying malignancy or hemolytic–uremic syndrome ; Propionibacterium acnes in the postneurosurgical patient ; Nocardia species such as N. asteroides and N. farcinica comprise 1% to 2% of all cerebral abscesses, with a mortality rate of 31%. They are frequently seen in immunocompromised patients but can occur in immunocompetent patients. Nocardia brain abscess ( Fig. 128.2 ) may be an isolated cranial pathology or may result from dissemination of cutaneous or pulmonary infection. The number of case reports describing Nocardia brain abscess have been increasing; this may be related to better diagnostic techniques, but it is most likely due to increased incidence. Fungal brain abscesses can be caused by yeast such as Candida or Cryptococcus species; dimorphic fungi such as Histoplasma, Coccidioides, or Blastomyces species; and molds such as Aspergillus or Rhizopus species, which are associated with immunocompromised states. Protozoa and helminths can cause parasitic brain abscesses and may be relevant in certain cases, such as cat exposure or endemic areas. Toxoplasma gondii can cause central nervous system (CNS) toxoplasmosis and brain abscess ( Fig. 128.3 ), and Taenia solium can cause neurocystcercosis.
Brain abscess in the neonatal period has a distinctive bacteriologic profile, with most of these lesions caused by Proteus species and Citrobacter diversus. In contrast to meningitis caused by other pathogens, neonatal meningitis caused by these organisms is complicated by brain abscess in 40% to 75% of cases, so early computed tomography (CT) evaluation of neonates with meningitis or bacteremia caused by Proteus or Citrobacter species is recommended. Interestingly, the most common bacterial causes of acute pyogenic meningitis ( Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis ) are rarely associated with brain abscess. ,
Most patients with pyogenic brain abscess have symptoms for less than 2 weeks, although the disease can present indolently. The presenting features of brain abscess depend on the size and location of the lesion(s), the virulence of the infecting agents, the immunologic status of the host, and the cerebral edema elicited by the expanding intracranial mass lesion. The classic triad of fever, headache, and focal neurologic deficit is present in less than 50% of cases. Headache, usually dull and poorly localized, is present in 50% to 70% of cases yet is nonspecific and a potential cause of diagnostic delays. , , , , , Sudden worsening of a preexisting headache in a patient with a brain abscess, especially if accompanied by the acute onset of meningeal signs, suggests either herniation or intraventricular rupture of the abscess.
Fever occurs in 25% to 50% of adults , , , , , and is more common in children. Symptoms and signs related to any underlying disease (e.g., paranasal sinusitis or otitis media), if present, may aid in the diagnosis. Altered levels of consciousness are often present. Focal neurologic signs depend on the location of the lesions within the brain and the extent of cerebral edema : frontal and parietal lobe abscesses are commonly associated with hemiparesis and aphasia; temporal lobe presentations may include aphasia or visual field disturbances; intrasellar lesions tend to mimic pituitary tumors, and cerebellar abscesses often present with ataxia and nystagmus. Seizures occur in roughly 30% of cases, without a clear distribution pattern. Patients present with multiple brain abscesses in approximately 10% of cases.
A moderate peripheral leukocytosis is found in most patients with brain abscesses and intracranial infections; however, blood cultures are rarely (∼10%) positive. Regardless, blood cultures should be performed prior to antimicrobial initiation. Erythrocyte sedimentation rate and C-reactive protein levels are elevated in the majority of the patients and although nonspecific indicators of inflammation, levels are used to monitor patient response. Lumbar puncture in the setting of brain abscess with mass effect is strongly contraindicated and rarely provides useful clinical information. The best opportunity to obtain a specific microbiologic diagnosis is via direct biopsy. Consultation and coordination of efforts between neurosurgeons and infectious disease specialists are crucial to ensure the appropriate specimens are obtained, and cultures of abscess material are optimally handled and processed to maximize the chances of identifying the pathogen(s). The broad-range bacterial ribosomal DNA polymerase chain reaction (PCR) method, combined with DNA sequencing, has been used to examine pus and tissue from neurosurgical patients with suspected meningitis, brain abscess, spondylitis, or spinal epidural abscess.
CT scan is excellent for the diagnosis of brain abscess, anatomic localization of the lesion, and evaluation of cerebral edema. Its value in identifying cerebritis is improved by a delayed contrast-enhanced scanning technique. The contrast-enhanced CT appearance of brain abscess is a hypodense lesion surrounded by ring enhancement with a variable peripheral zone of cerebral edema; dense ring enhancement is not a constant feature of abscesses but depends on the maturity of the lesion. Although the sensitivity of CT scanning for brain abscess is 95% to 99%, the specificity is compromised by the inability of this modality to reliably distinguish brain abscess from metastatic tumor or some vascular lesions. , Indium-111 ( 111 In)–labeled leukocyte scanning may be used to complement CT scanning. Radiolabeled leukocytes accumulate in foci of active inflammation, enhancing the chances of distinguishing abscess from metastasis in inconclusive cases.
A series of histopathologic stages has been described that appear to parallel the evolution of CT findings in human brain abscesses. , Early cerebritis (days 1 to 4) is a poorly circumscribed lesion characterized by acute inflammation and cerebral edema associated with bacterial invasion. Later (days 4 to 10), the zone of cerebritis expands, and necrosis develops, with pus forming at the center of the lesion. CT scanning reveals some ring enhancement with diffusion of contrast material into the necrotic center. The early capsule stage (days 10 to 14) demonstrates the establishment and maturation of a well-formed collagenous capsule associated with a reduction in the degree of cerebritis and some regression in the local edema. At the late capsule stage (days 14 and beyond), there is continued maturation of a thick capsule with extracapsular gliosis and dense ring enhancement with little contrast diffusion on the CT scan. ,
Capsule formation and ring enhancement on imaging studies are generally thinner and less complete on the ventricular side of the abscess. This pattern is likely related to the relatively poor vascularity of the deep white matter and reduced migration of fibroblasts into the area. The thinner area of capsule predisposes to ventricular rupture of the abscess.
The nature of the infecting organism influences encapsulation. Models of Bacteroides species show delayed capsule formation with multiple daughter abscesses, suggesting incomplete containment of the infection, whereas S. aureus experimental abscesses were larger, demonstrated delayed healing, and were associated with marked extracapsular abnormalities. The route of infection also appears to affect capsule formation: abscesses resulting from hematogenous spread tend to have less extensive encapsulation than those arising from a contiguous focus of infection. This pattern is probably the result of microinfarcted areas of the brain arising from metastatic emboli, leading to tissue hypoxia, impaired angiogenesis, and impeded fibroblast migration.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here