Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We are grateful to Dr. C. Toyoshima (Tokyo, Japan) for providing the image of the SERCA pump structure in Fig. 5.2 and the illustrations in Fig. 5.3 , Dr. L. Raeymaekers (Leuven, Belgium) for providing the structural model of the SPCA pump in Fig. 5.4 , and Dr. S. Pantano (Montevideo, Uruguay) for providing the model of PMCA in Fig. 5.5 .
The authors have been supported by grants from the Italian Ministry of University and Research (FIRB2001 to E.C., PRIN 2003, 2005, 2008 to M.B.), the Telethon Foundation (Project GGP04169), the Italian National Research Council (CNR), and the University of Padova (Progetto di Ateneo 2008, CPDA082825) to M.B. and the FP6 program of the European Union (FP6 Network of Excellence NeuroNe, LSH-2003-2.1.3-3, Integrated Project Eurohear), the Human Frontier Science Program Organization, and the Fondazione Cariparo (Progetti di Eccellenza 2008–2009), ERA-Net Neuron (grant nEUROsyn 2008) to E.C.
Ca 2+ -transporting adenosine triphosphatases (ATPases; Ca 2+ pumps) have been described in animal and plant cells and in the cells of lower eukaryotes. This chapter focuses on those of animal cells and disease processes linked to their dysfunction. The three animal Ca 2+ pumps belong to the large superfamily of P-type ATPases, which have been so defined because their reaction cycle is characterized by the formation of an acid-stable phosphorylated Asp residue (the P intermediate) in a highly conserved sequence (SDKTGT[L/IV/M][T/I/S]). The family now contains hundreds of members and eight subfamilies. The subfamilies have been identified based essentially on the transported substrate specificity, the evolutionary appearance of which has been accompanied by abrupt changes in sequence. The changes, however, do not involve eight structurally and mechanistically conserved important regions that define the core of the superfamily. Five branches have been identified in the phylogenetic tree of the superfamily; two animal Ca 2+ pumps belong to subgroup II A (the sarco/endoplasmic reticulum [SR/ER] Ca 2+ [SERCA] and secretory pathway Ca 2+ [SPCA] pumps) and one to subgroup II B (the plasma membrane Ca 2+ ATPase [PMCA] pump). All P-type ATPases, including the three that transport Ca 2+ in animal cells, are multidomain proteins that share the essential properties of the reaction mechanism, have molecular masses between 70 and 150 kDa, and share the presence of 10 hydrophobic membrane-spanning domains (TMs; some, however, only have six or eight). With the number of TMs being even, the N- and C-termini of all P-type pumps are on the same membrane side (i.e., the cytosol); one exception is a splice variant of the SERCA pump that has 11 TMs. The P-type ATPases also share the sensitivity to the transition state analog orthovanadate and, with some specific differences, to La 3+ . Other inhibitors only affect selected members of the superfamily. The solution of the three-dimensional (3D) structure of the SERCA pump has paved the way for intense molecular modeling work based on the SERCA templates that has indicated that all P-type ATPases share the general principles of 3D structures. In 2017, however, the class PIIB PMCAs were identified as heteromeric complexes assembled from two ATPases and two essential auxiliary subunits (the extracellular immunoglobulin superfamily members neuroplastin [NPTN] and the close paralog basigin [BASI]).
This finding is an important step toward understanding the functional mechanisms of this essential calcium pump family. Instead of being monomers or homodimers as previously envisaged, , , they are now involved in the formation of multiprotein complexes that undergo an additional mechanism of regulation because of the abundance of subunits composition.
This aspect will be discussed in more detail in the discussion of the PIIB PMCA pump.
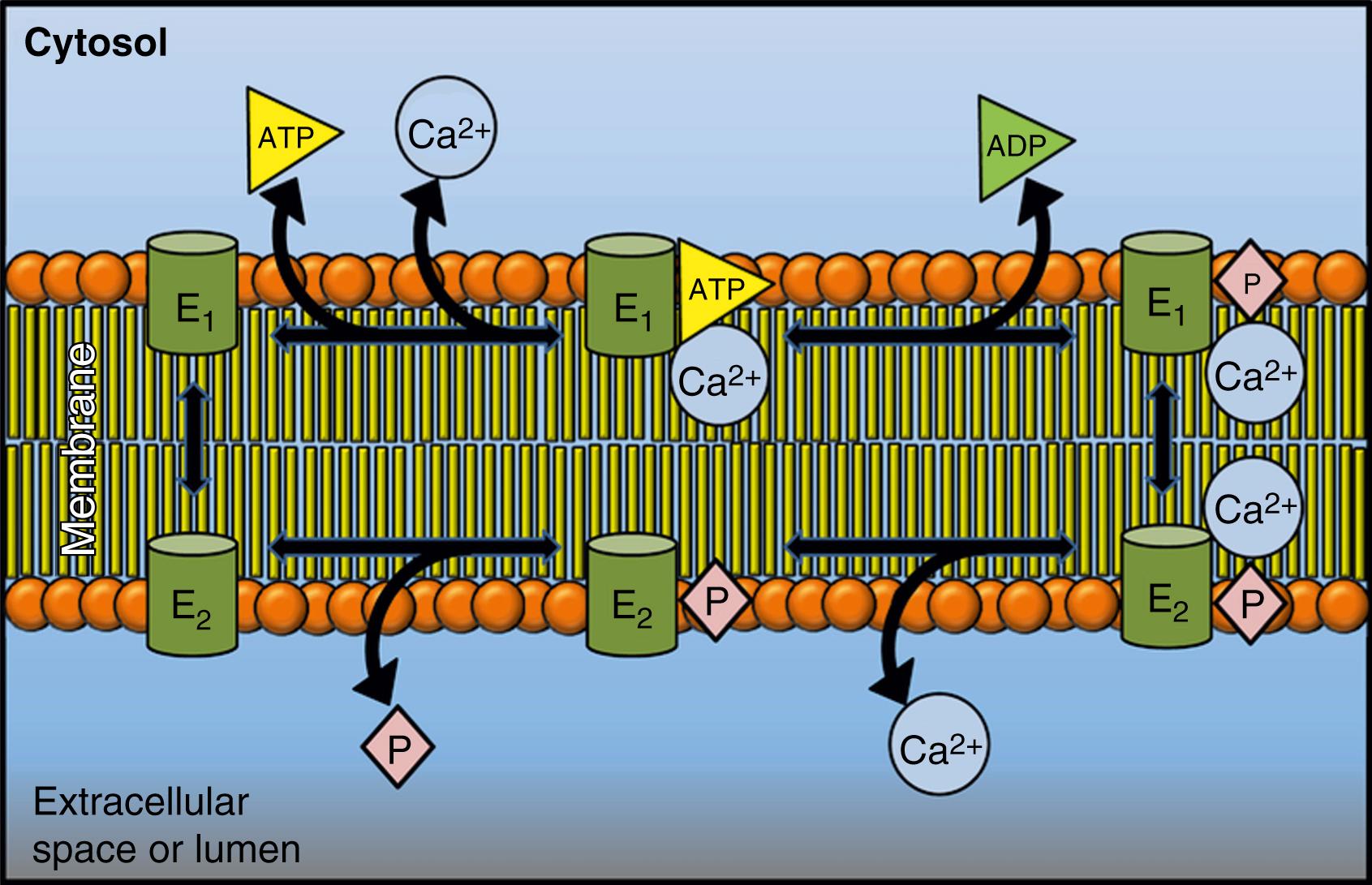
The first description of the reaction cycle of P-type ATPases envisaged two distinct conformations, the E1 and E2 states, which were characterized by different affinities for ATP and the transported ion. As for the Ca 2+ pumps in the E1 state, they engage Ca 2+ with high affinity at one side of the membrane; in the E2 state, its lowered affinity for Ca 2+ releases it to the opposite membrane side. Additional intermediate states were later described to make the reaction cycle much more complex. Importantly, each step of the reaction cycle is reversible, so ATP can be produced by reversing the direction of the ion transport process; reversal of the SERCA pump, with production of ATP, had, in fact, already been demonstrated in one of the first experiments on the transport of Ca 2+ by vesicular preparations of the sarcoplasmic reticulum. A simplified version of the cycle, adapted to the Ca 2+ pumps, is shown in Fig. 5.1 .
Several Ca 2+ pump isoforms have been described in animal cells, differing essentially in tissue distribution, regulatory properties, and some mechanistic peculiarities. The isoform diversity reflects the existence of separate basic gene products but also the occurrence of complex patterns of alternative splicing that very significantly increase the number of variants of each of the three pumps. The analysis of the differential properties of the Ca 2+ pump isoforms is a major focus of investigation because it has important linkages to the general process of cellular Ca 2+ homeostasis, which is regulated in animal cells by a number of nonmembrane Ca 2+ -binding proteins and by membrane-intrinsic Ca 2+ channels and transporters. The transporters interact with Ca 2+ with high or low affinity and thus function either as fine tuners of cytosolic Ca 2+ or come into play whenever the concentration of Ca 2+ increases to levels adequate for their low affinity. The Na/Ca exchanger of the plasma membrane is the low affinity regulator of cytosolic Ca 2+ . The Na/Ca exchanger of the mitochondria and the mitochondrial Ca 2+ uniporter, which are essential for the regulation of mitochondrial Ca 2+ fluxes, also act as low affinity regulators of cytosolic Ca 2+ . The three Ca 2+ pumps on plasma membrane, ER, and Golgi membranes, by contrast, are the high-affinity regulators and control Ca 2+ efficiently even in the low concentrations of the cytosol at rest. Their activity is fundamental to the correct functioning of the machinery of animal cells; dysfunctions, genetic or otherwise, of their operation, may not necessarily induce cell death but invariably generate disease phenotypes. They now define a topic that has undergone tremendous growth.
The SERCA pump is a key protein to adjust the Ca 2+ homeostasis in the ER lumen. Considering that the ER Ca 2+ is involved in a multitude of signaling events and in “housekeeping” functions that control cell growth, differentiation, and apoptosis, the activity of the SERCA pump is crucial to cell wellness.
SERCA pumps are inhibited by La 3+ and orthovanadate, and the discovery of specific inhibitors like thaspigargin, cyclopiazonic acid, and 2.5-di(t-butyl)hydroquinone represented a big advantage in the biochemical and structural characterization of the pump.
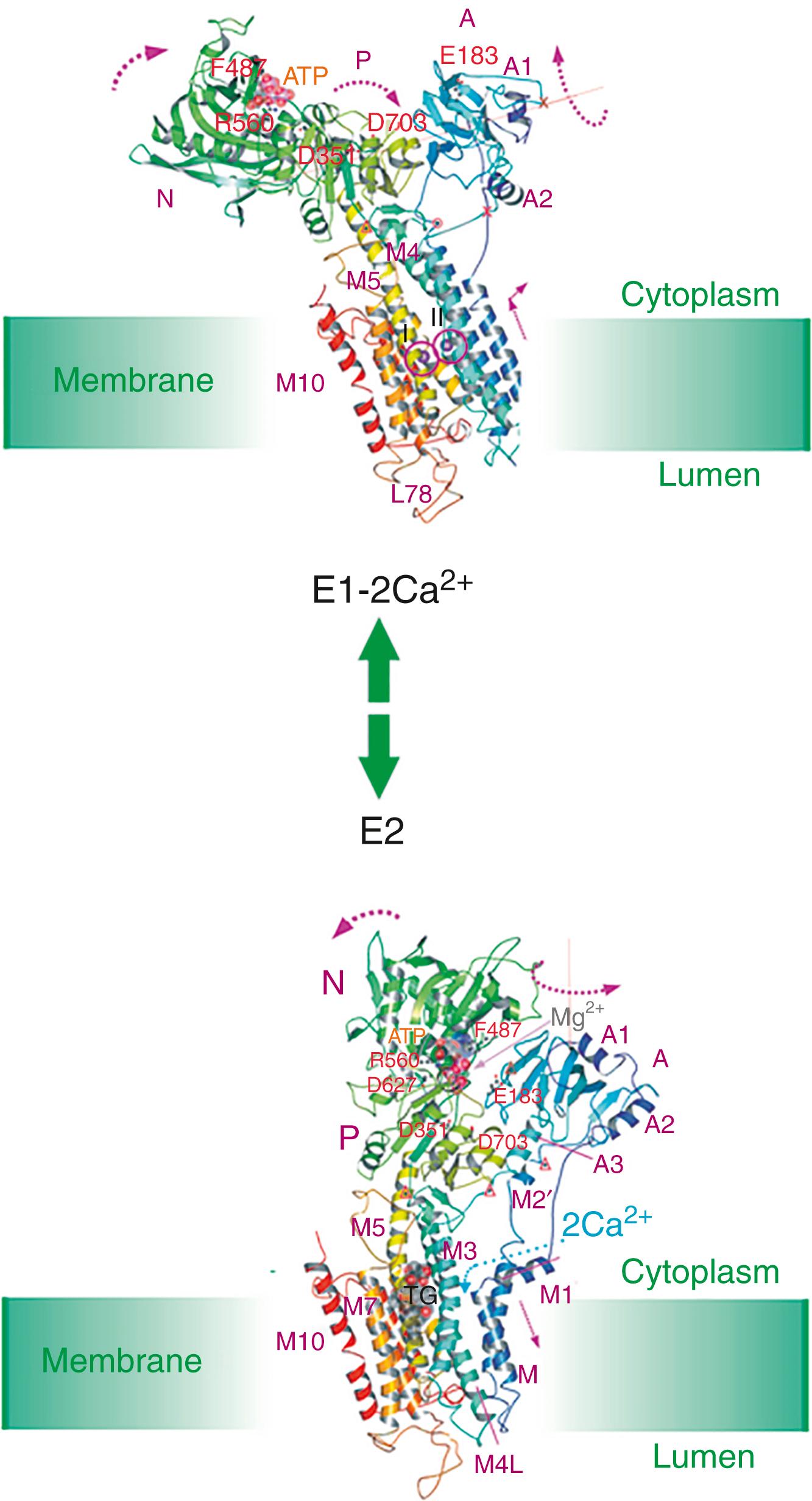
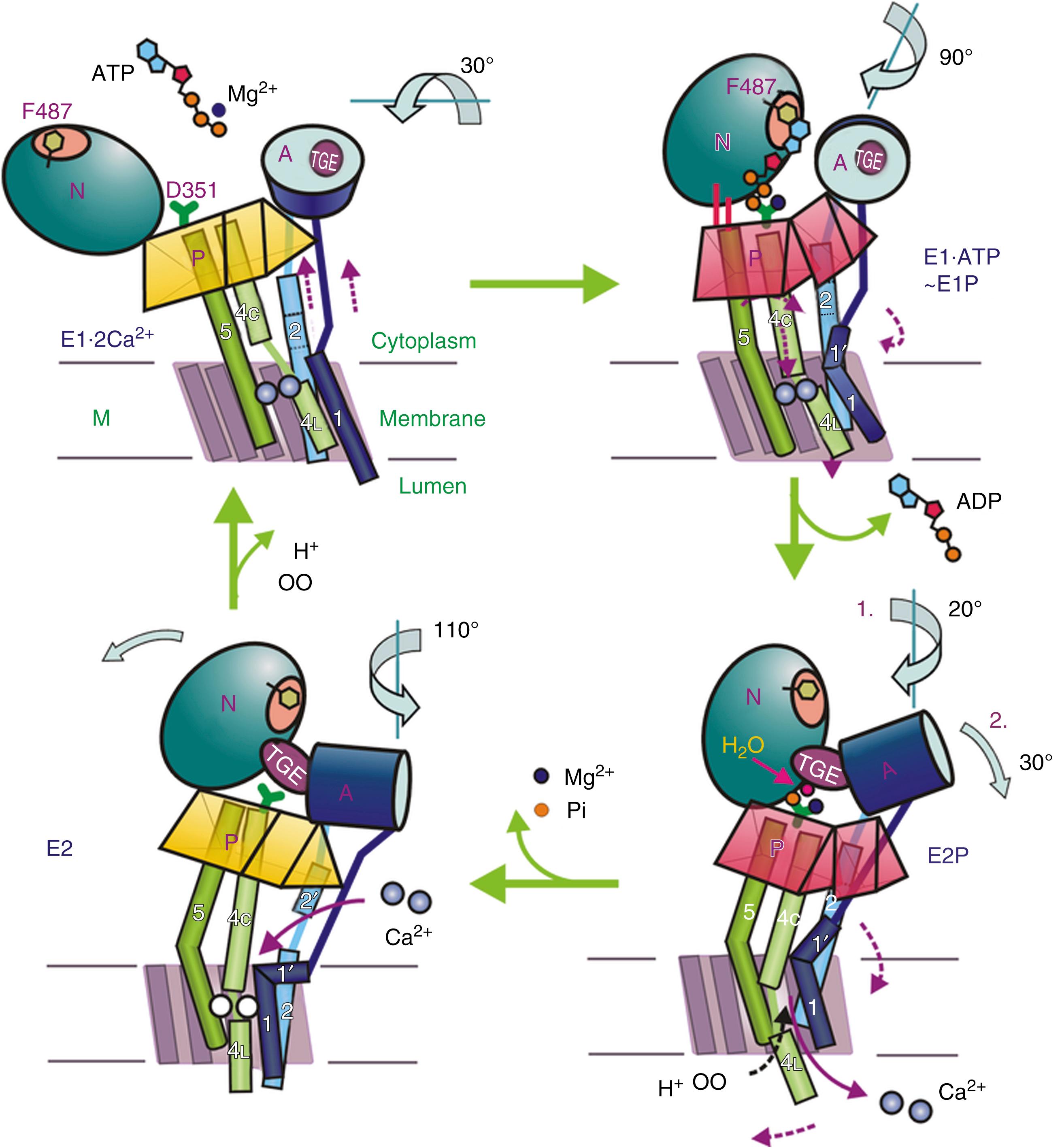
The SERCA protein is organized in the membrane with 10 TMs: numerous mutagenesis studies and the solution of its 3D structure have clarified essential molecular details of its function. They are only briefly summarized here, but the full details are available in a number of more comprehensive reviews. , Analysis of the 3D structure of the SERCA1 pump isoform has revealed that the single polypeptide chain folds in three cytosolic domains and in one transmembrane domain (M) composed of the 10 formerly predicted TMs ( Fig. 5.2 ). The three cytosolic domains have been named according to their role in the reaction cycle: the nucleotide binding domain (N) binds ATP, the phosphorylation domain (P) drives ATP hydrolysis leading the phosphorylation of the catalytic Asp, and the actuator domain (A) catalyzes the dephosphorylation of the P domain. The A and P domains are connected to the transmembrane M domain that contains the two Ca 2+ binding sites, and the SERCA pump transports two Ca 2+ per ATP hydrolyzed. The N domain is instead connected to the P domain. During the cycle, phosphorylation and dephosphorylation events promote conformational changes that control the access of Ca 2+ to the two binding sites (sites 1 and 2), which exist in high- and-low affinity states ( Fig. 5.3 ). The two sites are located near the cytoplasmic surface of the membrane, but site 1 faces the cytoplasmic side and site 2 is closer to the luminal side. Once Ca 2+ becomes bound to site 1, a conformational change increases the affinity of site 2 and permits the phosphorylation of the catalytic Asp by ATP, leading to the transition E2→E1→E1•2Ca 2+ E1P. The binding of ATP crosslinks the P and N domains, permitting the interaction of the P domain with the A domain, which rotates, inducing the opening of the luminal gate that releases Ca 2+ to the lumen, and permits the E1P-E2P transition. The closure of the luminal gate, and thus the E2P→E2 P i transition, then occurs, because a second rotation of the A domain locks it to the P domain. A highly conserved TGES motif (corresponding to the Thr-Gly-Glu-Ser amino acids sequence) in the A domain fills the gap between the N and P domains after the second rotation of the A domain, eventually permitting the release of Ca 2+ into the lumen. The rearrangements of the transmembrane helices M1 to M6 induced by the rotation of the A domain allow protons and water molecules to enter and stabilize the empty Ca 2+ binding sites and induce the retraction of the TGES from the phosphorylation site and the entrance of one water molecule to the phosphorylation site; this induces the release of phosphate (and Mg 2+ ) and the complete closure of the luminal gate.
Three SERCA genes generate three isoforms. Their number is increased by alternative splicing processes. SERCA1 is almost exclusively expressed in muscle tissues, specifically in fast-twitch skeletal muscles. Interestingly, the generation of truncated, less active SERCA1 variants has been described, which contribute to reduce the Ca 2+ concentration in the ER lumen and cause apoptotic cell death. SERCA2b and SERCA2a are the two major SERCA protein isoforms, the former having housekeeping and the latter a more specialized function. SERCA2a is found in slow skeletal and cardiac muscles (it is also expressed in low amounts in smooth muscles and in neurons). The splice variants SERCA2c and SERCA2d have also been found in low amounts in the heart. The expression of the SERCA3 pump, which has a limited cell-type distribution, is variable. In several cell types, this is induced by differentiation and is decreased during tumorigenesis and blastic transformation. In cells of hematopoietic origin and in various epithelial cells, the SERCA2 pump gene is coexpressed with that of the SERCA3 pump. All SERCA3 splice variants (SERCA3a–f) have lower Ca 2+ affinity than the SERCA2 pump, which raises doubts on their role in the presence of higher affinity SERCA pump variants. Differences in their spatial cellular distribution could justify their copresence: the SERCA3 pump is confined to environments with high Ca 2+ concentration, such as those close to the plasma membrane of cardiomyocytes, at basal regions in epithelial cells, and in the membrane of acidic Ca 2+ stores in platelets. Although the SERCA1a pump is the best characterized isoform in terms of structure-function relationship, the regulatory aspects of SERCA pumps have instead been better defined on the SERCA2 isoform.
The SERCA2a pump is the major isoform of developing and adult mammalian heart (SERCA2b is also expressed there, its level being unchanged during the development). SERCA2a is the most abundant protein in the heart SR membrane, and its increased expression during the development paralleled the increasing rate of Ca 2+ uptake by the SR lumen and the shortening of the relaxation time in the adult compared with the neonatal heart. SERCA2a pump levels are higher in atria than in ventricles, partially accounting for the shorter duration of contraction in atrial than in ventricular tissues.
The expression and the activity of the SERCA2a pump have been studied extensively. The primary mechanism of the regulation of the pump is mediated by phospholamban (PLB). PLB is a 52-residue protein composed of a hydrophobic helical C-terminal portion inserted into the SR membrane and a hydrophilic N-terminal region that protrudes into the cytosol and contains phosphorylation sites (Ser16 and Thr17) for protein kinase A (PKA) and, possibly, Ca 2+ /calmodulin-dependent protein kinase II (CAMKII). Dephosphorylated PLB binds to the pump, decreasing its Ca 2+ affinity; phosphorylation by PKA (and, possibly, CaMKII) releases the inhibition and increases the affinity of the pump for Ca 2+ and thus Ca 2+ transport. The hydrophilic N-terminal portion of PLB interacts with a domain close to the active site of the pump and, within the membrane, with transmembrane helices 2, 4, 6, and 9. Steered molecular dynamics and docking experiments indicated that, in addition, polymorphic PLB interactions with novel sites on M3 and with the outside of the SERCA helix M9 can also occur.
It is generally believed that PLB exists both as a pentamer and as a monomer. It is not clear how the conversion between the two forms occurs: the prevailing model involves reversible inhibition of SERCA by monomeric PLN and storage of PLN as an inactive pentamer. Nevertheless, structural observations indicate that pentamers may also interact with the pump, and the interaction could play a structural and functional role in SERCA regulation, which is consistent with the hypothesis that membrane perturbation may facilitate a more rapid turnover rate for SERCA. , Another small (31-residue) transmembrane protein, sarcolipin (SLN), has been recognized to play a role in the regulation of SERCA activity. SLN is predominantly expressed in the atrial compartment of the heart, and its sequence is similar to that of the transmembrane sector of PLB. SLN and PLB share a high degree of homology in their transmembrane domains and their interactions with the SERCA pump may be similar. A projection map of the SERCA-SLN complex at a resolution of 8.5 Å has allowed the direct visualization of an SLN pentamer. The SLN pentamer was found to interact with transmembrane segment M3 of SERCA, although this interaction appeared to be indirect and mediated by an SLN monomer. This SERCA-SLN complex correlated with the ability of SLN to decrease the maximal activity of SERCA. Thus the interactions of the PLN and SLN pentamers with SERCA are different and have opposite functional effects on the maximal activity of SERCA (i.e., PLN increases it, whereas SLN decreases it).
Studies on SLN suggest that it is an uncoupler of the SERCA pump activity and can increase ATP hydrolysis, resulting in heat production.
SLN overexpression in the ventricle cells of animal models (where SLN is essentially absent) caused a decrease in the Ca 2+ affinity of SERCA2a and slowed relaxation, suggesting that SLN may be as effective an inhibitor of SERCA pump activity as PLB. Interestingly, the overexpression of SLN in the heart of PLB null mice caused a decrease in the affinity of the SERCA2a pump for Ca 2+ and impaired contractility. The finding that isoproterenol, a β-adrenergic agonist, relieved the inhibition suggests that SLN could mediate the β-adrenergic response in the heart. Ablation of the SLN gene increases the affinity of the SERCA pump for Ca 2+ , resulting in enhanced rates of SR Ca 2+ uptake.
The SERCA2b pump is the acknowledged housekeeping isoform. It has a dual role. By transporting Ca 2+ from the cytosol to the ER lumen, it contributes to the maintenance of cytosolic Ca 2+ at the low resting levels (about 100 nM), at the same time ensuring the high Ca 2+ levels (in the mM range) in the lumen of ER that make the ER the main intracellular Ca 2+ store that controls cellular activities (e.g., contraction, proliferation, differentiation, and cell death). It also ensures the proper internal Ca 2+ ambient for the ER enzymes (e.g., those involved in protein folding and lipid synthesis).
The SERCA2b pump shares an 85% sequence identity with its SERCA1a counterpart; however, it differs functionally from it and also from the SERCA2a isoform, which is characterized by a unique C-terminal extension (the so-called 2b-tail), which forms a luminal sequence extension and an additional TM segment (TM11). The extension has regulatory properties. The longer pump has a twofold higher affinity for cytosolic Ca 2+ and a lower maximal turnover rate. According to a model based on the SERCA1a structure and on the NMR structure of TM11, the interaction of TM11 with TM7 and TM 10 has been proposed to stabilize the pump in the Ca 2+ -bound E1 conformation, with the high-affinity Ca 2+ -binding sites facing the cytosol. The TM11 has also been proposed as a novel regulator of the SERCA pump. The core constitution of the 18-residue-long TM11 with the SERCA1a protein in vitro reduced the V max and increased the Ca 2+ affinity of the latter. The crystal structures of SERCA2b in an E1 state revealed that the TM11 is located adjacent to TM10 and weakly interacts with a part of the L8/9 loop and the N-terminal end of TM10, thereby inhibiting the SERCA2b catalytic cycle. Accordingly, mutational disruption of the interactions between TM11 and its neighboring residues caused SERCA2b to display SERCA2a-like ATPase activity. Thus TM11 serves as a key modulator of SERCA2b activity by fine-tuning the intramolecular interactions with other transmembrane regions, possibly acting as an intramolecular uncompetitive inhibitor. In this respect, SERCA2b regulation resembles the calmodulin dependent regulation of the plasma membrane Ca 2+ pump.
The regulation of the SERCA pump by PLB and SLN, and by the 2b-tail in the 2b variant, resembles the interaction of the β- and γ-subunits of the Na + /K + ATPase with the catalytic subunit α. It also resembles the regulatory interaction of the PMCA pump with calmodulin (see later), suggesting operationally similar molecular mechanisms of P-type pump regulation. It could also be mentioned that the C-terminal portion of the PMCA pump can, to some extent, replace PLB as an inhibitor of the SERCA pump. Apart from PLB and SLN, which are the best-studied regulators of the SERCA pump, other proteins interact with it. Those interacting at the luminal side have an important role. The two chaperons calreticulin and calnexin contain a globular N-domain that binds carbohydrates, an extended P-domain that mediates the binding of ERp57 (see later), and an acidic C-terminal domain, that in the case of calreticulin binds 25 mol of Ca 2+ per molecule of protein with low affinity (K D , 2 mM). Luminal Ca 2+ buffering by calnexin is less significant, and the acidic C-terminus of the protein protrudes into the cytosol. Calreticulin and calnexin have been suggested to interact through their N-domains with a putative glycosylated residue present in the C-terminal tail of the SERCA2b pump but absent in SERCA2a.
ERp57, a member of the protein disulfide isomerase (PDI) family of proteins with thio-oxidoreductase activity, is recruited by the SERCA2b pump/chaperone complex to catalyze the formation of an inhibitory disulfide bridge between Cys875 and Cys887 in the luminal loop L7-8 of the SERCA2b pump. It could be a regulatory mechanism, but the proposal is controversial because mutations of either or both Cys residues resulted in loss of Ca 2+ transport but not of the activity in SERCA1.
Two other luminal Ca 2+ -binding proteins have been shown to interact with the SERCA2 pump: the ubiquitously expressed calumenin (CALU) and the histidine-rich Ca 2+ -binding protein (HRC). Both decrease the apparent Ca 2+ affinity of the pump. HRC binds Ca 2+ with high capacity and low affinity and could mediate both SR Ca 2+ uptake and release through its interaction with SERCA, when the SR Ca 2+ is low, and with triadin, which is part of the ryanodine receptor (RyR) Ca 2+ release complex when it is saturated by Ca 2+ .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here