Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The World Health Organization (WHO) defines mesothelioma as a malignant neoplasm arising from the mesothelial cells lining the body cavity surfaces and growing in a diffuse or, less commonly, localized invasive fashion. About 90% of mesotheliomas arise in the pleura, and over 30,000 new cases were reported at this location in 2018. The mean age of affected patients is 73, , however disease in younger patients, including in children, is reported. , Men are more commonly affected at a rate of about 4:1, although the relative proportion of women affected is increasing. Occupational or environmental exposure to asbestos remains the leading risk factor for development of mesothelioma. International efforts to regulate the industrial use of asbestos have led to a decline in the overall proportion of asbestos-related cases in developed countries; however, developing countries are expected to see a rise in mesothelioma incidence over the next decades. ,
Clinical symptoms associated with mesothelioma are nonspecific and include progressive dyspnea, chest pain localized to the involved side, and occasional “B symptoms” including fevers, anorexia, and weight loss. In contrast to primary lung carcinomas, over half of which present with symptoms attributable to metastatic site involvement, mesothelioma is typically localized to the chest cavity at the time of diagnosis. In postmortem studies, just over half of patients who die of mesothelioma have lymph node or distant metastases, with mortality largely attributable to locally invasive disease or pulmonary sequelae including emboli and pneumonia.
At present, there are no specific laboratory test abnormalities that can be used to aid in the diagnosis of mesothelioma. Thrombocytosis is common among patients with mesothelioma but is nonspecific. Serum markers, including megakaryocyte potentiating factor (MPF), mesothelin, osteopontin, fibulin-3, and HMGB1, among others, have been studied but show inadequate diagnostic sensitivity and specificity to justify their use in clinical practice, despite US Food and Drug Administration (FDA) approval of a commercial assay for serum mesothelin detection.
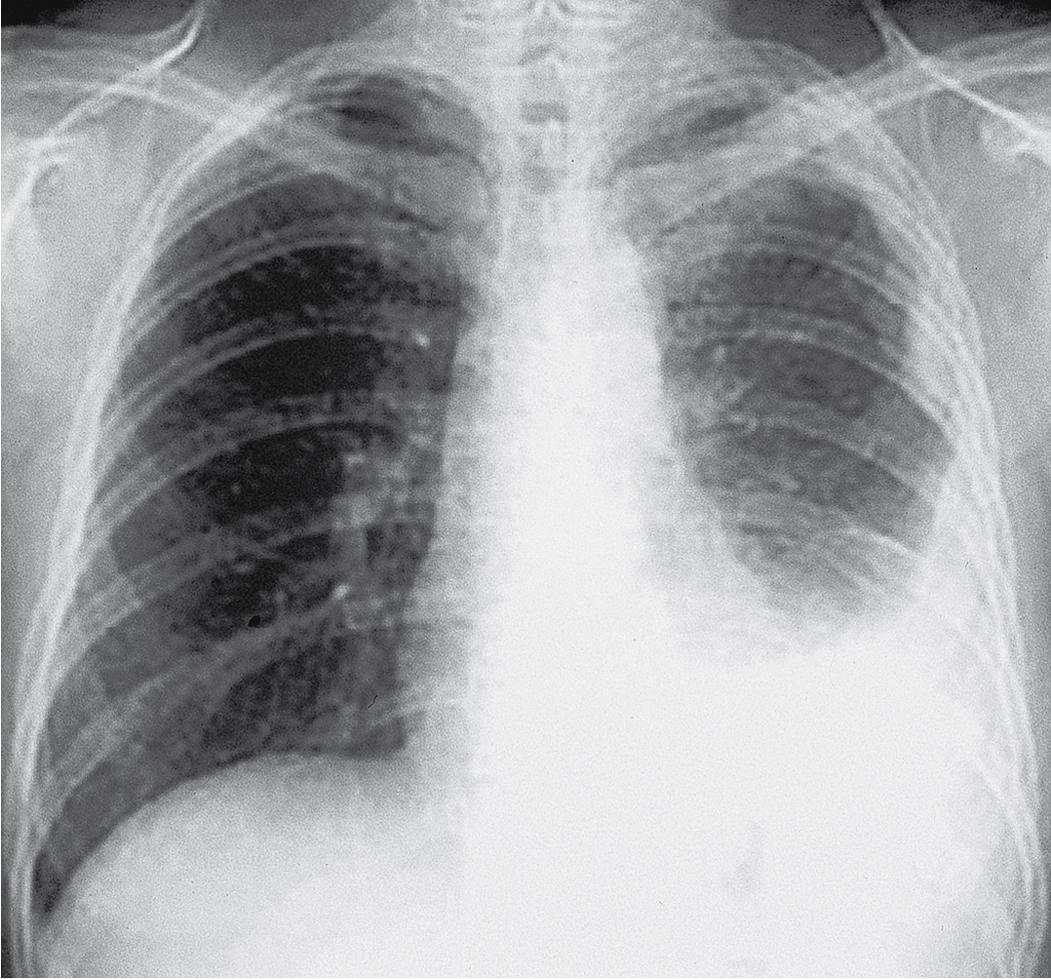
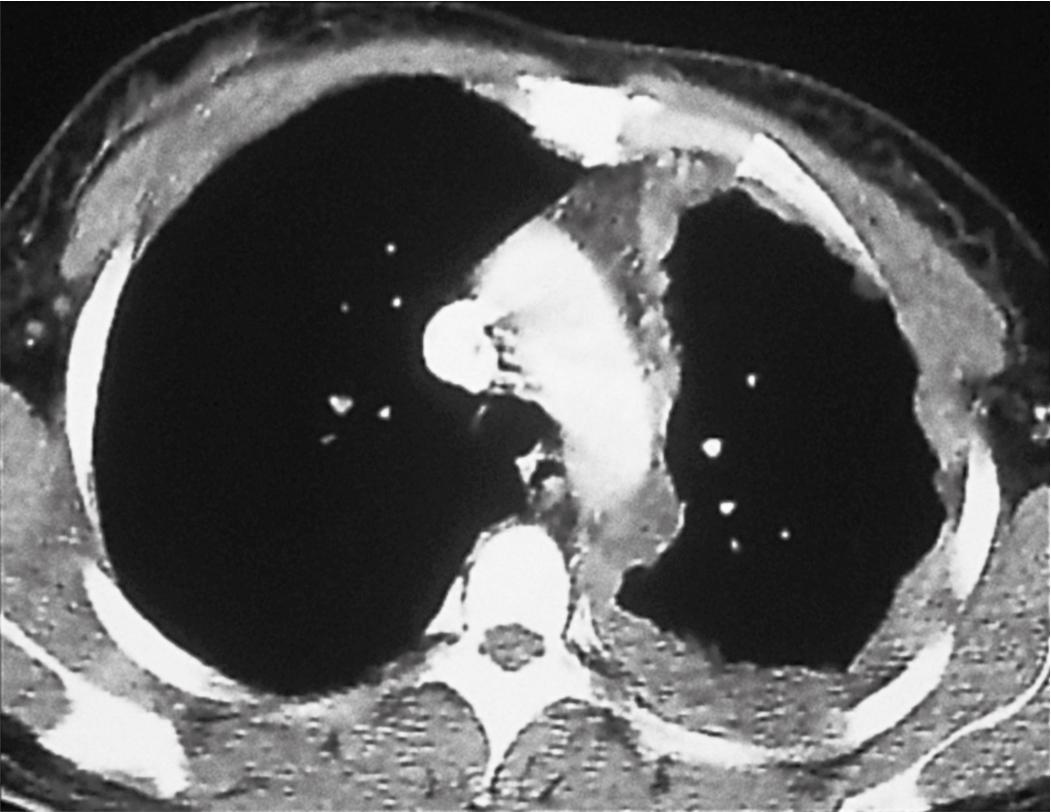
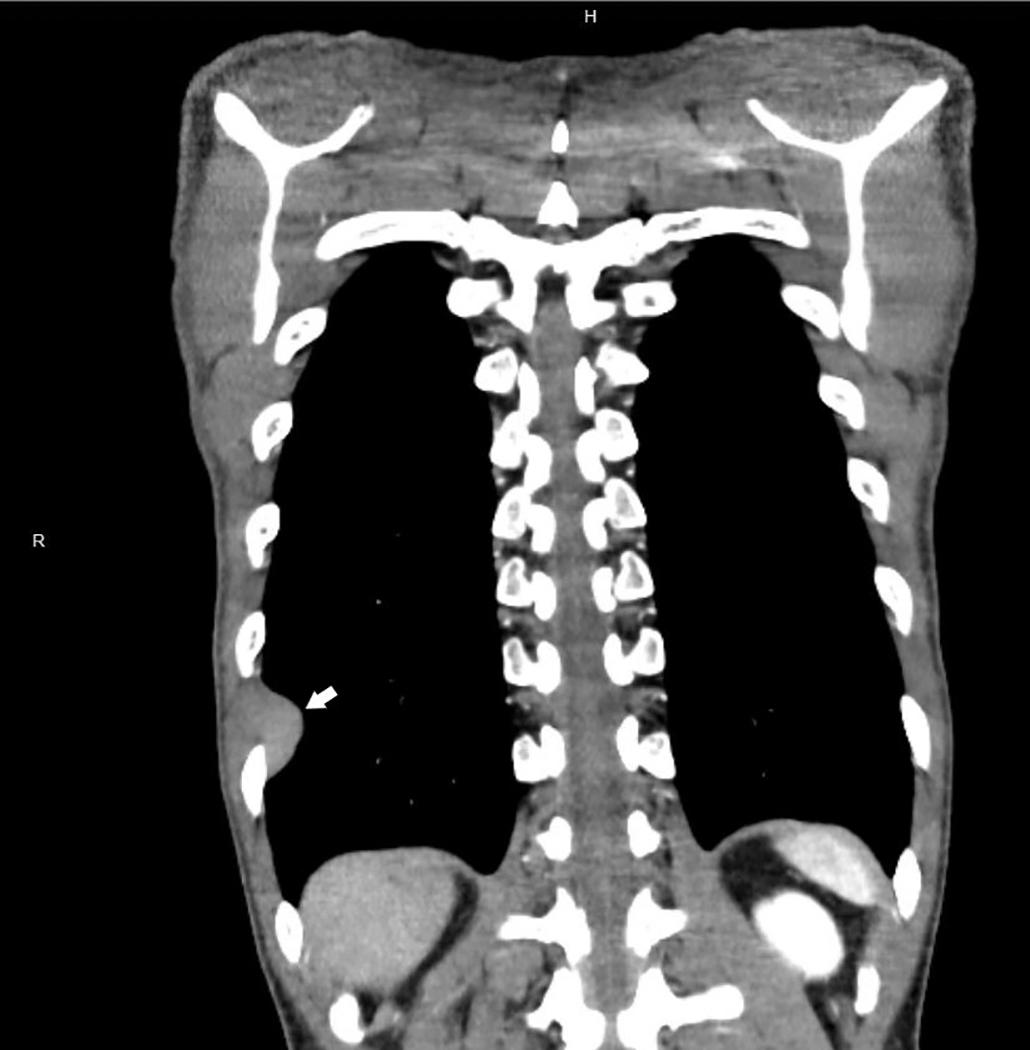
Plain film chest radiographs typically show unilateral pleural effusion ( Fig. 21.1 ) and/or pleural thickening, which may be best visualized following thoracentesis to remove the excess pleural fluid. Computed tomography (CT) scans with contrast and positron emission tomography are recommended to better characterize the location, extent, and functional status of pleural-based disease ( Fig. 21.2 ). Functional magnetic resonance imaging scans may be useful in difficult diagnostic settings, including after talc pleurodesis or for detection of a solitary focus of chest wall or diaphragmatic invasion. , However, none of these modalities is adequate for the diagnosis of mesothelioma, which requires pathologic examination of pleural effusion cytology or tissue biopsy.
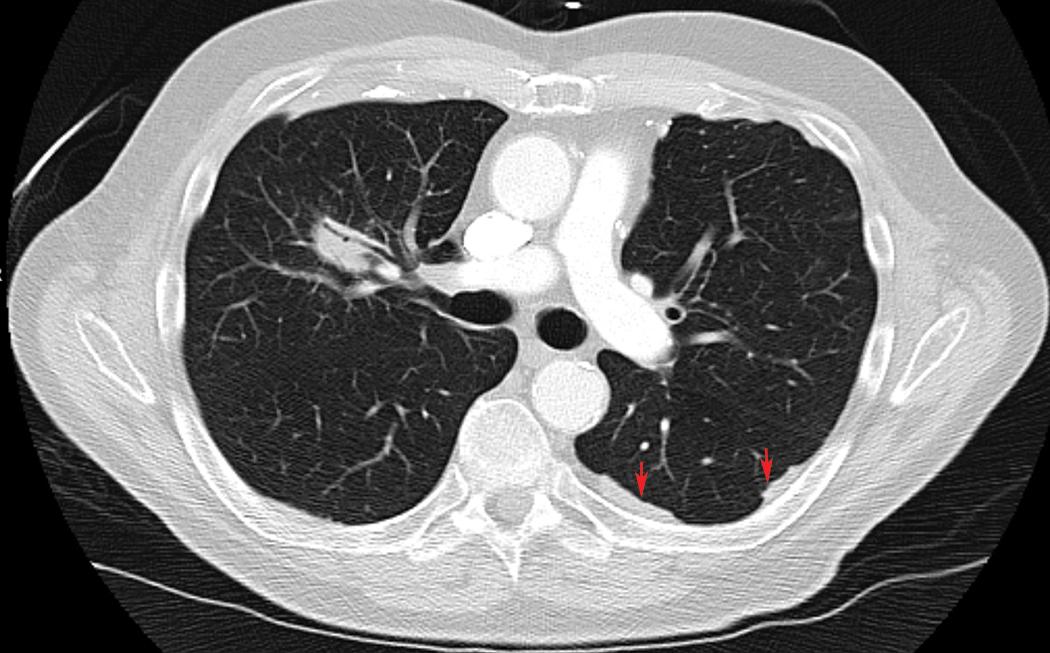
A significant association has been described between detection of pleural plaques on CT scan and the diagnosis of mesothelioma ( Fig. 21.3 ). However, pleural plaques and pleural calcifications are also highly sensitive markers of past asbestos exposure, thus the shared etiology confounds this association, and the utility of radiographic identification of pleural plaques as a marker of increased risk of mesothelioma independent of asbestos exposure history has not been established. As such, international guidelines discourage pursuing further diagnostic procedures to exclude mesothelioma when pleural plaques are detected.
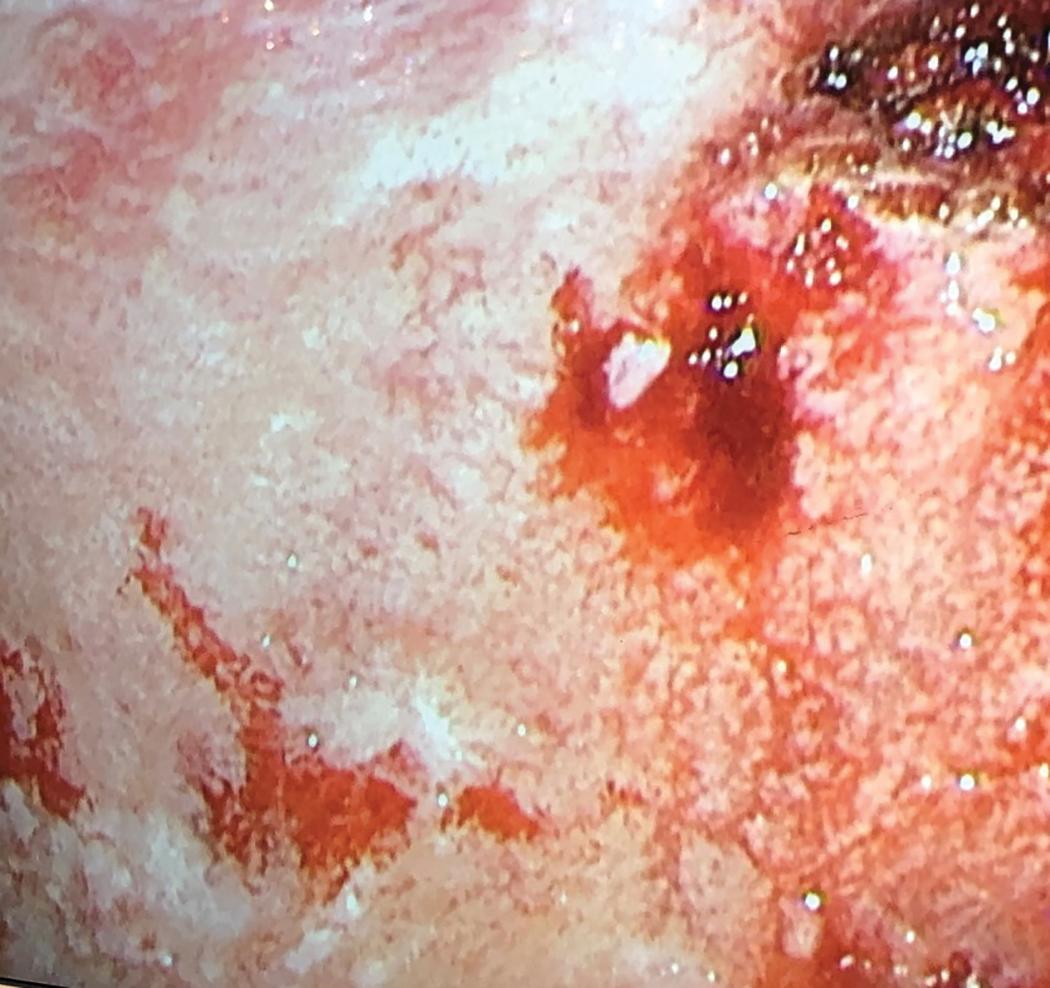
Thoracoscopic biopsies represent the gold standard for mesothelioma diagnosis in the context of pleural effusion of unclear etiology. Biopsies obtained via video-assisted thoracoscopic surgery (VATS), a minimally invasive technique performed under general anesthesia that permits for direct visualization of the pleural cavity, ( Fig. 21.4 ) have been shown to have 95% sensitivity, 100% specificity, and a negative predictive value of 94% in the diagnosis of mesothelioma. Similar performance characteristics have been described for medical thoracoscopy, which is more commonly performed by interventional pulmonologists and other medical specialists in an endoscopy suite using local anesthesia. Thoracoscopic approaches enable clinical staging via evaluation of pleural effusion as well as symptom management with pleurodesis. The diagnostic yield of transthoracic biopsy approaches is lower than that of thoracoscopy, in the order of 57% for blind closed-needle biopsy and 87% for CT image-guided pleural biopsy.
Historically, the diagnosis of mesothelioma depended on identification of pleural or chest wall stromal invasion by tumor cells. However, the advent of robust biomarkers that distinguish reactive from malignant mesothelial proliferations substantially diminishes the need for direct observation of tissue invasion. This opens the door to more routine use of pleural cytology specimens, particularly cell block preparations, for the diagnosis of mesothelioma, although this approach has not been universally embraced in international guidelines and the diagnostic yield is admittedly lower than for tissue biopsies. , The role of cytopathology in the diagnosis of mesothelioma is discussed in detail later.
Mesothelioma grows into needle tracts or VATS port sites following diagnostic transthoracic procedures in 10% and 40% of cases, respectively. , This well-recognized phenomenon historically prompted routine prophylactic radiotherapy of the procedure tracts; however, randomized trials showed that this led to no significant reduction in the rate of procedure tract “metastases.” , Surgeons may, however, excise the skin, subcutis and chest wall muscle around the instrumentation tract at the time of definitive surgery. Nests of mesothelioma may be small and subtle in these specimens and should not be confused with clustered histiocytes, foreign body giant cells, or atrophic skeletal muscle fibers.
Outcomes following the diagnosis of diffuse mesothelioma are grim, and improvements in survival following aggressive surgical intervention, radiation, and systemic therapies (“trimodality therapy”) have been modest. Radical resection via extrapleural pneumonectomy is associated with significant post-operative morbidity and mortality and has been replaced in many centers by pleural decortication, often with intraoperative pleural lavage with hyperthermic chemotherapeutics as well as adjuvant chemotherapy. , This strategy leads to about a 25% five-year overall survival, with improved outcomes predicted by female gender, lower tumor stage, and epithelioid histology. In the community setting, patients with unresectable or metastatic mesothelioma receiving first line standard of care platinum plus pemetrexed therapy have a median overall survival of about 12 months. Immune checkpoint blockade therapies, including anti–PD-1, PD-L1, and CTLA-4 inhibitors, have led to durable responses in a minority of mesothelioma patients; biomarkers predicting increased likelihood of response to these therapies have not yet been identified. , Indeed, tumor PD-L1 expression is not predictive of patient response, but PD-L1 expression itself may have prognostic implications in mesothelioma. In patients with resectable disease, neoadjuvant therapy has shown little benefit and increased risk of postresection mortality.
Asbestos was first identified as a causal agent for mesothelioma in 1960 in a South African population ; this linkage has since been confirmed in countries across the globe. Asbestos has a number of physical properties, including heat and fire resistance, high tensile strength, and chemical resistance that have driven its use in construction and manufacturing over the last century. The WHO now recognizes asbestos as an occupational carcinogen and has committed to global elimination of asbestos-related diseases via education, abatement, and promotion of alternative, non-carcinogenic materials with similar physical properties. Despite this, there is increasing use of asbestos in developing countries. Given the estimated 40-year lag between asbestos exposure and development of mesothelioma, trends in diagnosis are expected to decline in developed countries where asbestos use is banned but increase in rapidly industrializing countries, particularly in Asia. The diseases associated with asbestos exposure include pleural plaques, mesothelioma, and pulmonary asbestosis; the fiber burden is generally lowest among individuals with plaques alone, is relatively higher in those with mesothelioma, and may be as much of an order of magnitude greater in patients with asbestosis. Accordingly, the risk of developing mesothelioma following asbestos exposure is dose dependent, but may be impacted by genetic variations in the affected individual (see “ Molecular Genetics ” section).
The neoplastic risk conferred by asbestos fibers is dictated by physical properties that facilitate passage through the upper and lower airways and deposition and retention within the pleura. These properties include a fiber length-to-diameter ratio of more than 3:1, a diameter below 0.4 μm, and a length of at least 5 to 10 μm; a chemical composition that permits the fiber to persist for decades and generate oxidative damage is also central to its pathogenicity. Asbestos fibers that meet these criteria have been commonplace in industrial applications and include chrysotile (white) asbestos, as well as the amosite (brown) and crocidolite (blue) forms of amphibole asbestos. Crocidolite is the most potent carcinogen of the three. , Chrysotile is the asbestos form most commonly used in industrial applications and of the fibers appears least carcinogenic, possibly due to its flexibility and fragility, permitting fragmentation and phagocytosis by pulmonary macrophages. However, chrysotile is frequently contaminated with amphiboles such as tremolite. Tremolite and erionite (a naturally occurring mineral with physical properties similar to asbestos) are common in the soil in a number of Turkish villages with exceptionally high rates of mesothelioma-associated mortality, , although a genetic component (e.g., through a “founder effect”) has also been proposed.
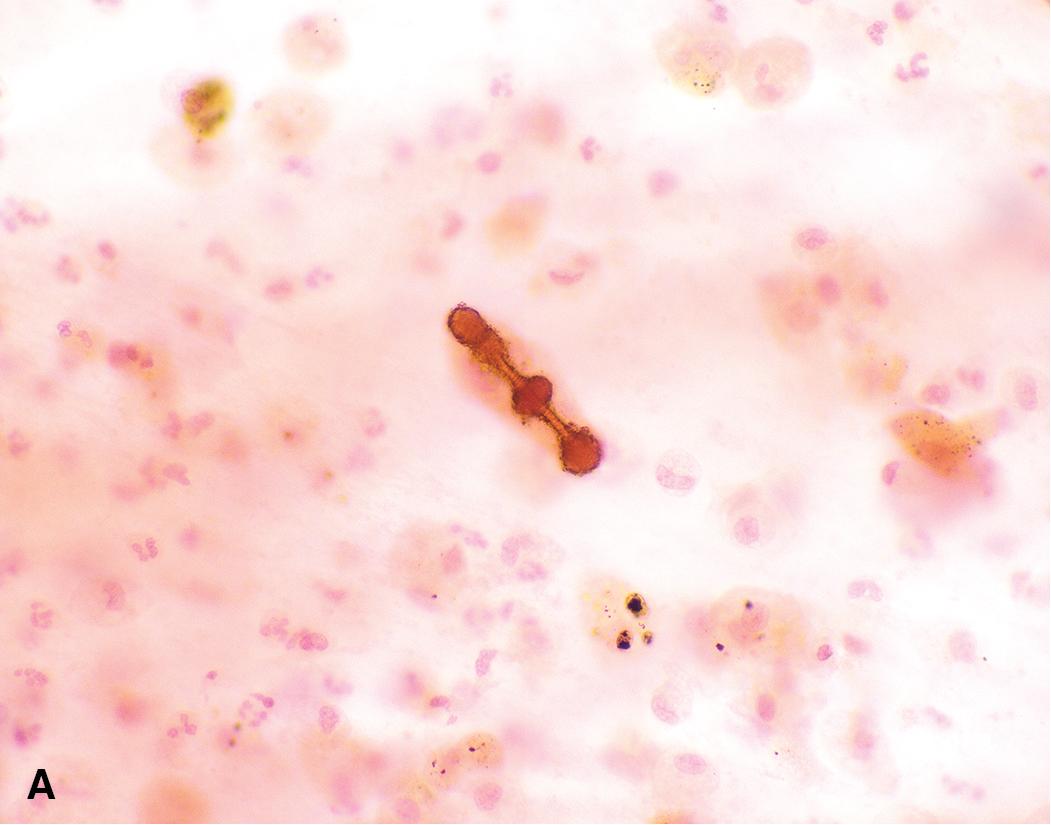
Given the strong causal association between asbestos exposure and mesothelioma, the diagnosis of mesothelioma has significant legal implications for employers and potential for financial compensation for individuals with a probable occupational exposure. In some cases, a legal representative may request evidence of asbestos within the affected tissue. Within the lung tissue, a subset of long amphibole fibers will become coated with ferroproteins to form asbestos bodies ( Fig. 21.5 ). These can be observed on standard hematoxylin and eosin-stained slides, and the fibers can be better highlighted with the use of an iron stain. However, fibers may be difficult to identify by microscopy, which can only detect thicker fibers and cannot discriminate between fiber types. More sensitive approaches include electron microscopy and energy dispersive x-ray analysis following digestion of the surrounding lung tissue. , The concentration of amphibole-type fibers in particular should be compared to a control cohort defined within the laboratory. It is important to note that sampling should be performed from fresh or fixed lung tissue or bronchoalveolar lavage fluid; tumor tissue itself does not contain asbestos bodies.
Asbestos has also been implicated in the development of lung cancer; the combination of asbestos exposure plus a smoking history is estimated to have a multiplicative effect on the risk of lung cancer. However, mesothelioma only rarely presents concurrently with lung cancer, reportedly in just 0.5% to 1% of mesothelioma patients in two large series. ,
Other materials that have been hypothesized to contribute to development of mesothelioma in animal models include synthetic fibers and carbon nanotubes; however, no human cases have been reported with these associations. , Simian virus 40 (SV40), which contaminated polio vaccinations in the 1950s and 1960s, was proposed to contribute to development of mesothelioma based on evidence derived from hamster models and the suggestion that SV40 was detectable in clinical mesothelioma samples. Subsequent studies, however, failed to detect SV40 in tumor tissues, with the discrepancies attributed to false positive results due to contamination by laboratory plasmids.
After asbestos, external beam radiation is the best-established risk factor for development of mesothelioma, with an approximately 4-year latency period. The stated risk of mesothelioma after therapeutic radiation, including for lymphoma, breast cancer, or metastatic testicular cancer, varies between studies, but on average is estimated to be 1.5- to 4-fold greater than in the general population. Younger patients with mesothelioma are more likely to report a history of radiation therapy.
The molecular genetics of mesothelioma are well characterized, thanks to a number of large multi-institutional efforts. In contrast to lung carcinomas, mesotheliomas are characterized by a low tumor mutational burden (<2 nonsynonymous mutations per megabase) and lack of smoking-associated mutational signatures. The most common significantly altered genes in mesothelioma are CDKN2A , BRCA1-associated protein 1 ( BAP1 ), NF2 , SETD2 , TP53 , and LATS2 ( Fig. 21.6 ). , Gene amplification events are rare in mesothelioma, whereas deletions of tumor suppressor genes represent a common feature. Overlapping patterns of inactivating mutations and/or deletions in the significantly altered genes are detected in 80% to 90% of cases. A minor but distinctive molecular subtype is characterized by extensive copy neutral loss of heterozygosity, so-called genomic near-haploidization and frequent TP53 mutations. These cases uniquely possess inactivating alterations in SETDB1 , a histone methyltransferase. Co-occurrence of NF2 and LATS2 inactivation has been proposed to represent another minor mesothelioma subgroup characterized by downregulation of the Hippo pathway, poor prognosis, and a possible enrichment in sarcomatoid histology.
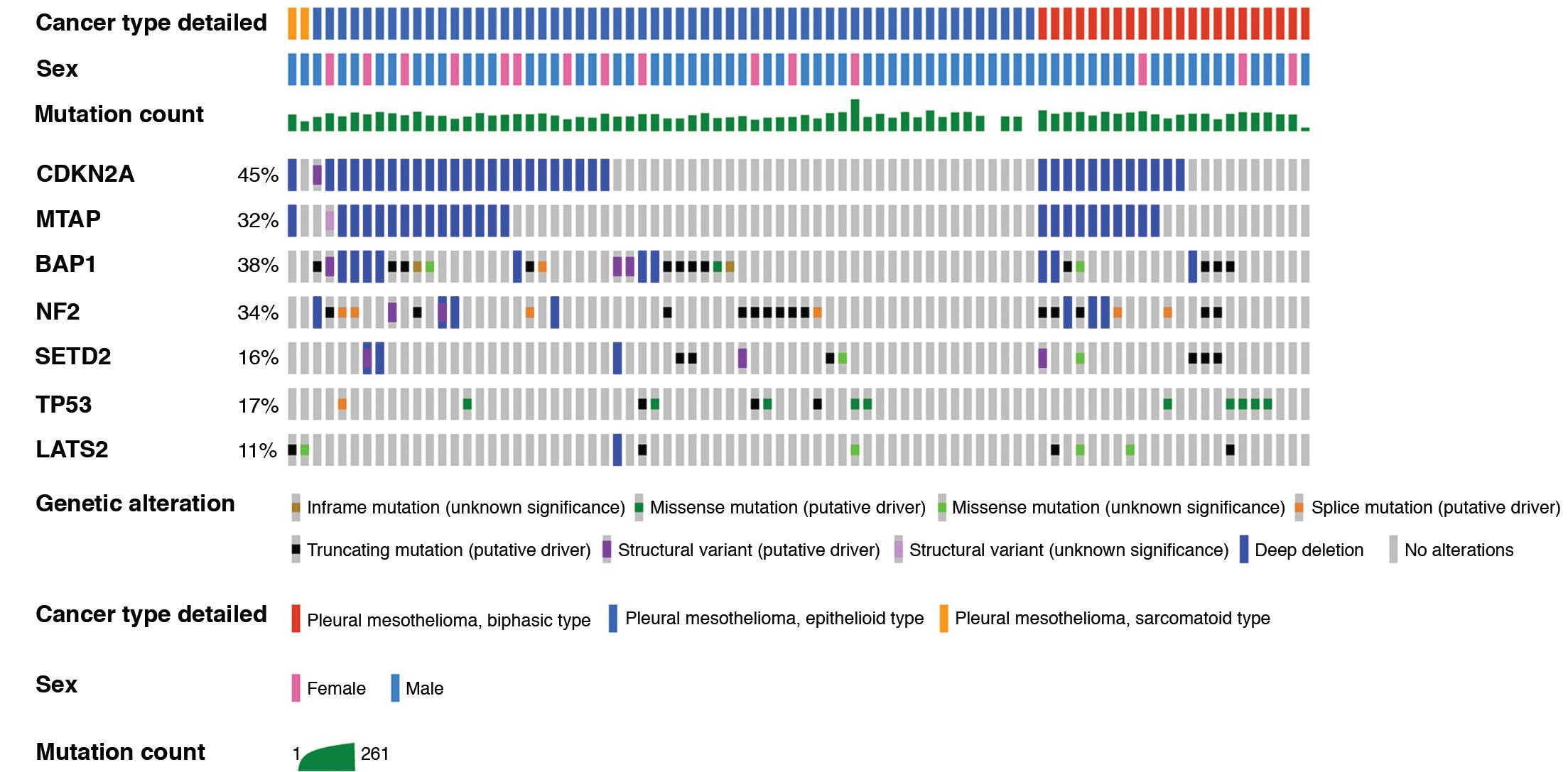
Large scale genomic profiling efforts have been limited by small numbers for sarcomatoid mesothelioma tumors. However, some genotype-phenotype correlations have been noted, including an enrichment of BAP1 inactivation in epithelioid tumors and of CDKN2A deletion and TP53 mutation in biphasic and sarcomatoid tumors. The CDKN2A gene locus (which encodes p16(INK4A) and p14(ARF)) is characteristically altered via deep (two-copy or homozygous) deletions involving chromosome 9p21.3 that often extend to involve nearby genes CDKN2B and methylthioadenosine phosphorylase (MTAP) ( Fig. 21.7 ). P16 immunohistochemistry to detect protein loss of expression does not reliably represent CDKN2A deletion events as detected by fluorescence in situ hybridization (FISH) (a long-standing gold standard testing strategy) , ; however, the high frequency of MTAP codeletion has enabled loss of its protein product to serve as a highly specific if not completely sensitive surrogate for CDKN2A loss. , Specific performance characteristics of immunohistochemistry markers that serve as surrogates for genomic alterations are discussed later.
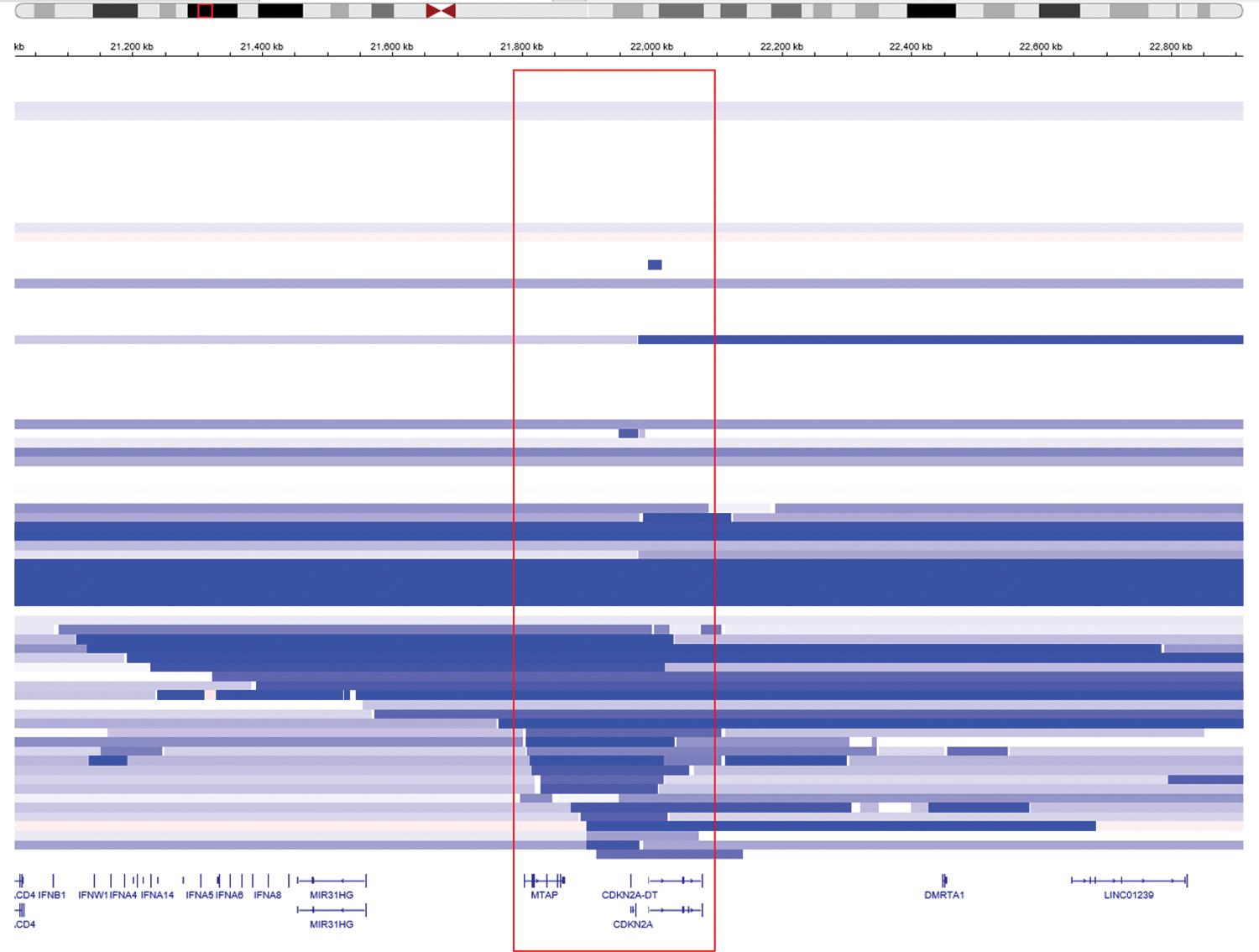
Technical limitations of next generation sequencing may lead to an underestimation of the frequency of specific alterations in mesothelioma cohorts. In particular, short (“minute”) deletions may go undetected by sequencing assays but can be detected using FISH, high density comparative genomic hybridization array, or single nucleotide polymorphism (SNP) array. For the purposes of tumor characterization and discovery, a combination of sequencing and high-resolution array profiling is likely to provide optimal detection of copy number alterations and loss of heterozygosity. Array profiling may also be used in lieu of FISH to detect copy alterations and confirm a malignant diagnosis in challenging cases of atypical mesothelial proliferation; however, this strategy is more expensive and potentially less technically sensitive in samples with abundant stromal contamination.
The observation that mesothelioma sometimes occurs within familial clusters, including in the absence of known exposure to asbestos, prompted linkage analyses that implicated the region at chromosome 3p21-22 and ultimately the BAP1 gene with development of mesothelioma. Studies of families with germline BAP1 mutations led to the description of a BAP1 cancer syndrome, which includes mesothelioma, uveal and cutaneous melanoma, and clear cell renal cell carcinoma. BAP1 is a nuclear protein with an essential role in normal DNA synthesis and replication. Its role varies in different cell types depending on it complexing with tissue-specific transcription factors, including promotion of double stranded DNA repair via homologous recombination, regulation of cell death via apoptosis and ferroptosis, and maintenance of oxidative phosphorylation. Germline BAP1 mutations typically lead to premature truncation through introduction of a nonsense or frameshift event, or through aberrant splicing. ,
The penetrance of any cancer in germline BAP1 mutation carriers appears to be greater than 85%, with a median age of onset of first tumor of 49 years. Mesothelioma is the most common tumor type observed (in about 30% of individuals) and is evenly divided between pleural and peritoneal locations. The presence of a germline BAP1 mutation dramatically increases the risk of carcinogenesis conferred by asbestos exposure. ,
Interestingly, mesothelioma patients with germline BAP1 mutations have seven-fold increased long-term survival compared to patients with sporadic mesothelioma. Outside of BAP1 , germline mutations have been observed in BRCA2 , CDKN2A , MRE11 , BARD1 , RECQL4 , VHL , WT1 , TMEM127 , and MSH3 in patients with mesothelioma, although these alternative germline mutations appear to be enriched in those patients with peritoneal mesothelioma and minimal asbestos exposure. ,
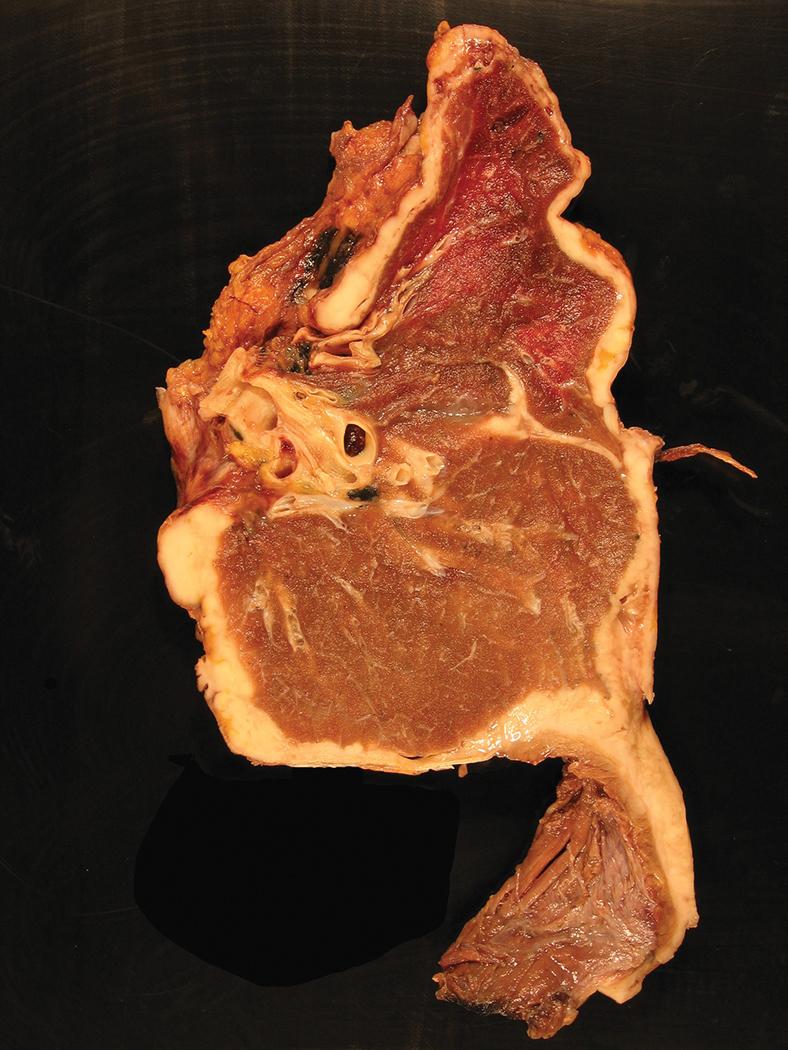
The gross pathologic features of mesothelioma mirror the corresponding radiographic imaging findings. Surgery for mesothelioma typically consists of pleurectomy and decortication, although extrapleural pneumonectomy is performed, albeit uncommonly, in select large medical centers by surgeons with expertise in mesothelioma management. Involvement of the parietal and visceral pleura is variable depending on the diagnostic lead time, from focal studding of the parietal and visceral surfaces by small nodules in early disease, to confluent tumor with complete fusion of the visceral and parietal pleura and encasement of the lung in advanced cases ( Fig. 21.8 ). Comprehensive gross examination of the pleural resection specimen plays a critical role in proper diagnosis and staging of malignant mesothelioma and requires integration with radiographic and surgical findings. Because extrapleural pneumonectomy entails en bloc resection of lung, visceral and parietal pleura, and portions of pericardium, surrounding soft tissues, and/or subjacent diaphragm, these radical resection specimens offer the best opportunity for benchtop orientation and gross examination of tumor extent. In contrast, decortication pleurectomy specimens are often received in multiple containers and are principally oriented by the surgeon’s designations. Nonetheless, in both resection types, careful attention should be paid to the tumor’s color and texture, maximum thickness, gross necrosis, and invasion of associated skeletal muscle, adipose tissue, or other tissues. The parietal pleura is typically more severely affected than the visceral pleura. Tumor may be seen tracking along interlobular septa or invading into lung parenchyma, chest wall, mediastinal fat, or pericardium. Involvement of the diaphragmatic muscle can be seen grossly, including transdiaphragmatic extension into the peritoneal cavity. Although tumor can involve mediastinal structures (e.g., great vessels, myocardium), these are rarely resected. Mesothelioma ranges from creamy white to tan-yellow, with a soft, fleshy, rubbery, or firm texture, and may show hemorrhage or necrosis. If multiple grossly distinct areas are noted, each should be submitted for microscopic examination. In patients who have previously undergone chemical pleurodesis for symptomatic palliation, it may be difficult to grossly distinguish between areas of tumor and iatrogenically induced pleural inflammation and fibrosis, therefore generous sampling may be required to adequately represent the extent of mesothelioma.
Approximately 99% of pleural mesotheliomas diffusely involve the pleural cavity. These are most appropriately termed “diffuse mesothelioma” (although the unqualified diagnosis of “mesothelioma” is generally understood to refer to this diffuse pattern). Rarely, mesothelioma grows as a solitary, circumscribed mass involving the chest wall or visceral pleura, termed “localized mesothelioma”), ranging from 0.5 to approximately 20 cm and often raising clinical concern for primary lung or mesenchymal neoplasms such as solitary fibrous tumor ( Fig. 21.9 ). Localized pleural mesothelioma may be sessile or pedunculated and by definition arises in the absence of tumor involvement in the adjacent pleura. , Because localized and diffuse mesothelioma are histologically indistinguishable, accurate classification is dependent on clinical, radiographic, and gross findings. This distinction is clinically important, as localized mesothelioma has a significantly better prognosis (see also “ Localized Mesothelioma ” later).
Given the significant impact of tumor histotyping on clinical management, the diagnosis of mesothelioma will usually have been rendered on biopsy prior to pleural resection. For patients with inoperable disease, a needle or thoracoscopic biopsy may represent the only tissue available for diagnostic, prognostic, and predictive studies. Accordingly, laboratories should ensure that allocation of biopsies among tissue cassettes is optimized to allow for any necessary immunohistochemical and/or molecular assays.
Although patient history, clinical presentation, and radiographic findings may raise strong suspicion for malignant mesothelioma, the diagnosis is ultimately confirmed microscopically. Microscopic evaluation of a pleural biopsy in the context of suspected malignancy serves to:
Distinguish mesothelioma from histologic mimics, including florid reactive mesothelial hyperplasia and benign or borderline mesothelial tumors (e.g., adenomatoid tumor, well-differentiated papillary mesothelial tumor).
Distinguish mesothelioma from non-mesothelial mimics including primary lung cancers, primary mediastinal tumors (particularly thymoma), metastatic carcinoma from non-thoracic sites, primary mesenchymal neoplasms, hematolymphoid neoplasms, and (rarely) unusual benign heterotopias (see “ Differential Diagnosis ” later).
Provide a comprehensive morphologic analysis and a complete pathology report in the context of confirmed mesothelioma, including all appropriate staging and prognostic information (see “ Staging and Grading ” section later).
While immunohistochemistry plays an increasingly indispensable role in each of these functions, careful microscopic examination of H&E-stained tissue sections remains the cornerstone of accurate diagnosis and proper patient management. Mesothelioma is characterized by infiltrative growth into surrounding tissues, particularly fibroadipose tissue, skeletal muscle, and lung parenchyma ( Fig. 21.10 ). Pleural-based tumors may occasionally invade into pericardium, or directly through the diaphragm into the peritoneum. Optically clear spaces—termed “fake fat”—may occasionally be encountered in reactive pleuritis, mimicking tissue invasion and leading to misdiagnosis as mesothelioma.

Mesothelioma is classified into three morphologic histotypes: epithelioid, biphasic, and sarcomatoid. Histotype is a critical prognostic factor, with progressively poorer outcomes associated with epithelioid versus biphasic versus sarcomatoid tumors. Tumor histotype also determines patient management. Clinical practice guidelines call for complete surgical resection (with or without induction chemotherapy) for epithelioid or biphasic mesothelioma up to stage IIIA (i.e., locally advanced but technically resectable, including with spread to ipsilateral bronchopulmonary, hilar, or mediastinal lymph nodes). , , , In contrast, surgical management is not indicated in sarcomatoid mesotheliomas, although some authors describe a modest survival benefit following surgery for patients with early-stage disease. Epithelioid tumors are also more responsive to chemotherapy than sarcomatoid tumors. Finally, relevant prognostic morphologic features must be reported for each histotype, as detailed later.
In the pleura, approximately 60% to 70% of mesotheliomas are epithelioid, 15% to 30% biphasic, and 5% to 20% sarcomatoid. , , Interobserver agreement for histotyping is generally good, although only fair agreement is achieved in the diagnosis of biphasic mesothelioma. When possible, histotyping is best done on a resection (decortication or pneumonectomy) specimen, as histotyping on a core needle or VATS biopsy is only approximately 80% concordant with that on a subsequent resection. , Diagnostic criteria for biphasic mesothelioma are slightly different in biopsy and resection specimens. In a biopsy, identification of any amount of distinct epithelioid and sarcomatoid components warrants a provisional diagnosis of biphasic mesothelioma, with a diagnostic comment noting the percent contribution of each component. In contrast, biphasic mesothelioma is diagnosed in a resection only when discrete epithelioid or sarcomatoid components each compose 10% or more of the tumor. Although this 10% cutoff is admittedly arbitrary, it allows for standardization of diagnosis and management. If a minor (<10%) but unequivocal sarcomatoid component is present in an otherwise pure epithelioid malignant mesothelioma, current guidelines recommend diagnosing epithelioid subtype, with a comment noting the presence of a small sarcomatoid component and its uncertain clinical significance. The converse applies for a minor epithelioid component in an otherwise purely sarcomatoid tumor.
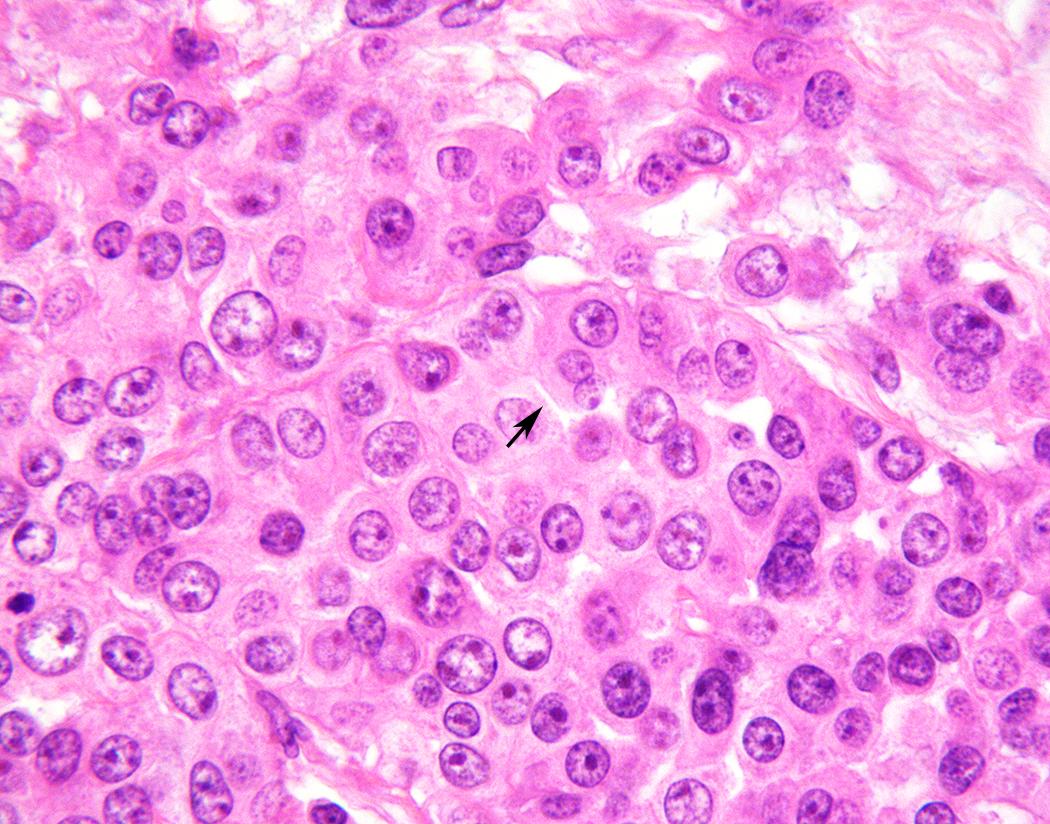
Epithelioid mesothelioma is defined by total or predominant (>90%) tumor composition by cuboidal, ovoid, or round cells. Within this broad definition, epithelioid mesothelioma shows a wide spectrum of cytomorphology and architectural patterns. Tumor cells most often show moderate eosinophilic cytoplasm, and an ectoplasmic “skirt” (more characteristically seen in cytology preparations) may sometimes be perceptible in H&E-stained sections ( Fig. 21.11 ). Most tumors have centrally placed nuclei with mild to moderate nuclear atypia, characterized by relatively smooth, round to ovoid nuclear contours, even to finely vesicular chromatin, and small single nucleoli ( Fig. 21.11 ). , Rare tumors show cytomorphology indistinguishable from benign mesothelium but exhibit infiltrative growth diagnostic of malignancy. At the other end of the spectrum, “pleomorphic mesothelioma” (discussed in detail later) shows marked nuclear atypia. Most tumors have a low mitotic index, less than five mitoses per 10 high-power fields, though occasional tumors show brisk mitotic activity. Atypical mitoses are rarely seen, except in pleomorphic tumors. Tumor necrosis is seen in approximately 30% to 50% of pleural epithelioid mesotheliomas. , Systematic nuclear grading of epithelioid malignant mesothelioma is prognostically relevant, and specific grading criteria are discussed later in “Staging and Grading.”
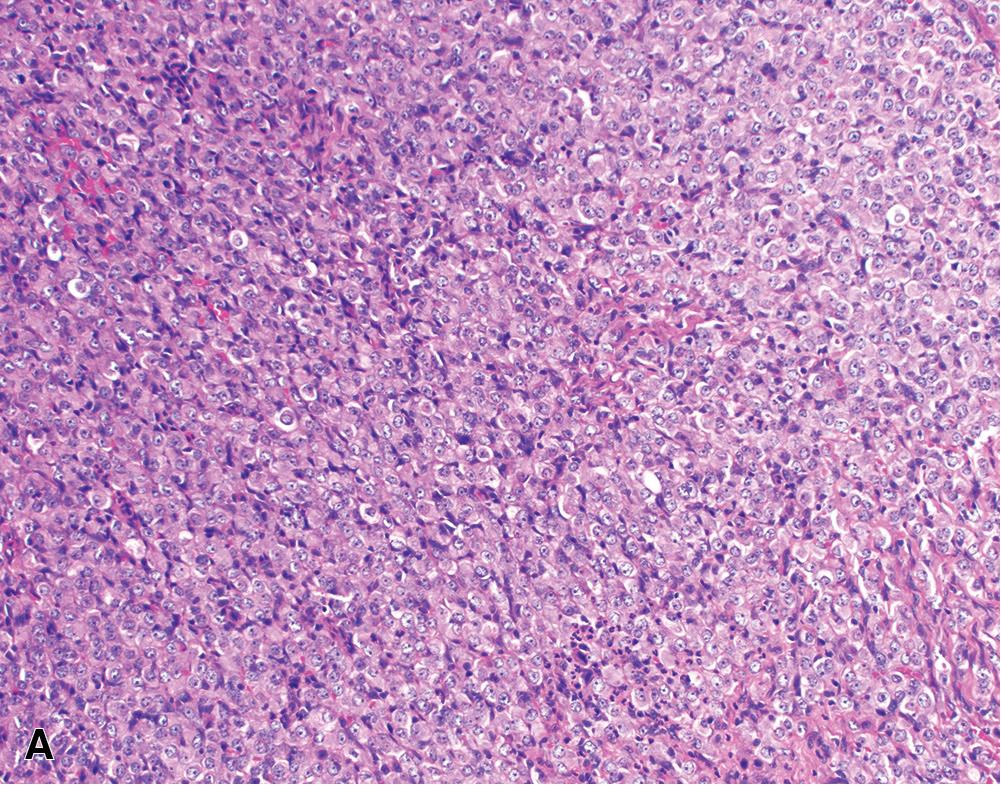
Epithelioid tumors show a broad spectrum of architectural patterns. The principal patterns include solid, trabecular, tubulopapillary, and micropapillary. Solid growth is characterized by sheets or nests of cohesive epithelioid cells ( Fig. 21.12 A), trabecular growth by thin cords or arrays ( Fig. 21.12 B), and micropapillary growth by thin papillae lacking fibrovascular cores ( Fig. 21.12 C). Tubulopapillary architecture is defined by variable combinations of tubules and well-formed papillae with true fibrovascular cores ( Fig. 21.13 A and B). Occasionally, purely papillary or tubular (acinar) patterns may be seen. Psammoma bodies may be present, particularly in tumors with a micropapillary or papillary component ( Fig. 21.13 C). Rarer architectural patterns include single cell (including signet ring-like cells), adenomatoid, and glomeruloid growth ( Fig. 21.14 ). Epithelioid tumors may invade the lung parenchyma in a lymphangitic, lepidic, or intra-alveolar pattern, which may mimic lung adenocarcinoma, organizing pneumonia, or desquamative interstitial pneumonia. Multiple admixed architectural patterns are almost always present. Architectural patterns should be included in the pathology report for epithelioid mesothelioma. The predominant pattern and the percent contribution of each minor pattern (to the nearest 10%) should be indicated in tumors with multiple architectural patterns.
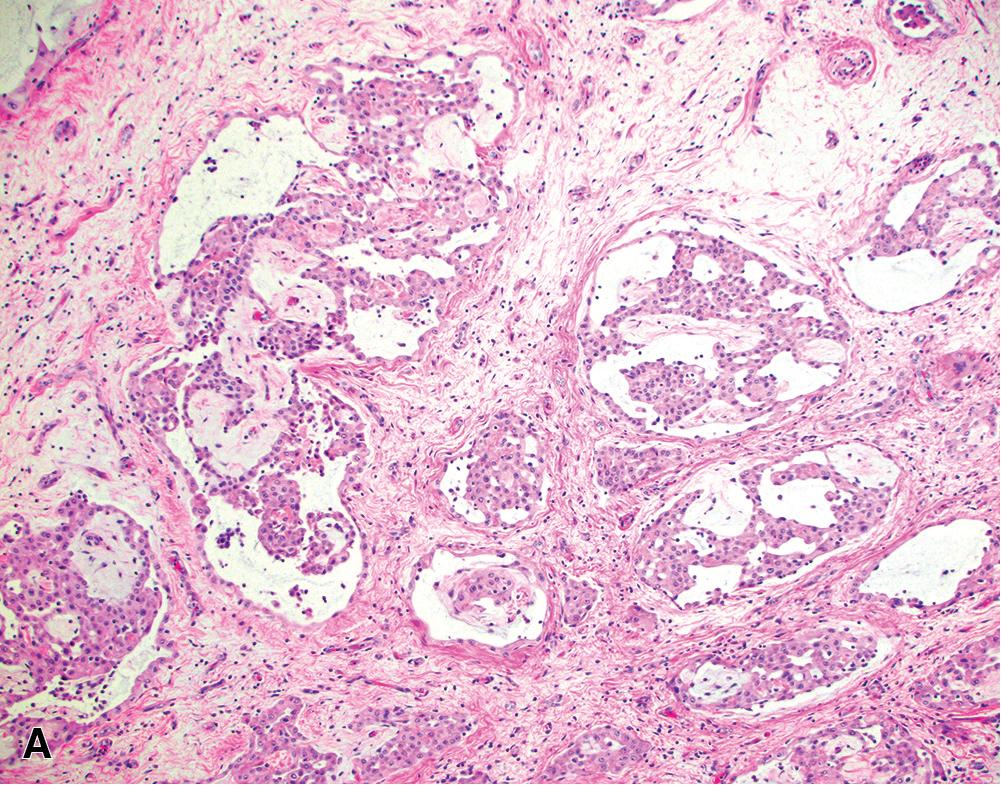
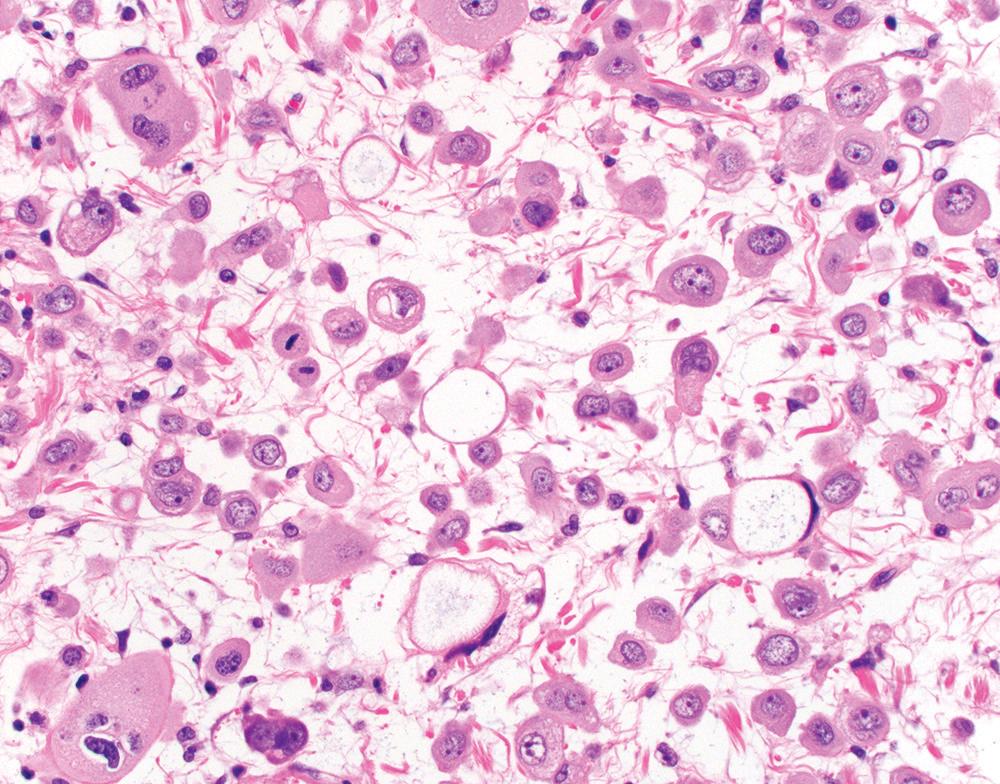
Solid or micropapillary growth are reportedly associated with shorter survival and tubulopapillary and trabecular growth with longer survival, , , , although these associations may not be independently prognostic after accounting for nuclear grade (see also “ Staging and Grading ” later). Micropapillary and single cell pattern are associated with a significantly greater rate of lymphovascular invasion.
Tumor stroma may be hyalinized and paucicellular or may contain a prominent reactive spindle cell population, in which case distinction from biphasic mesothelioma may be challenging. Variable stromal inflammation may be present (see later). Occasionally, prominent myxoid, hyaluronidase-sensitive stroma is present ( Fig. 21.15 ). ,
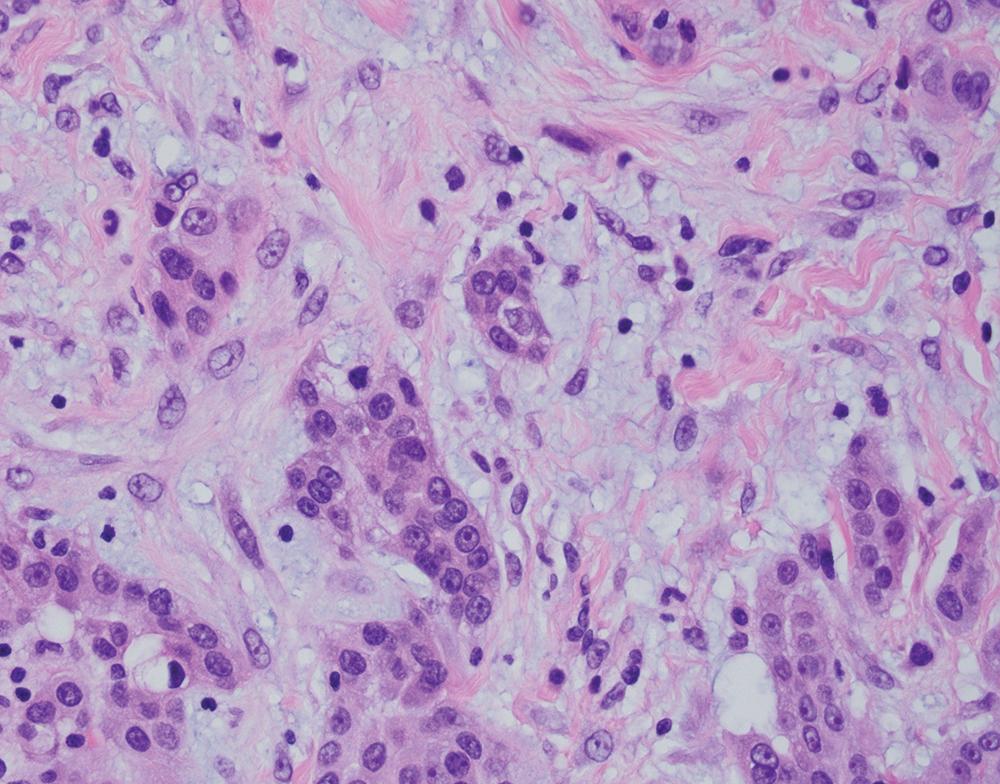
Some unusual morphologic variants of mesothelioma may cause diagnostic confusion; some are more commonly associated with epithelioid disease, whereas others may be seen on a background of epithelioid or sarcomatoid histotypes. These include deciduoid, pleomorphic, rhabdoid, small cell, clear cell, and lymphohistiocytoid subtypes.
A subset of epithelioid mesotheliomas contains abundant eosinophilic cytoplasm, resembling decidua ( Fig. 21.16 ). Although more common in peritoneal tumors, this cytomorphologic pattern also occurs in the pleura. Nuclear cytomorphology may be low- or high-grade, and binucleated cells may be seen. , , Solid growth is typical.
Although tumors with high-grade nuclei have poorer outcomes, deciduoid morphology does not inherently portend a poor prognosis, as originally postulated. The biologic basis for deciduoid morphology is unknown, though one study reported a greater frequency of SMARCB1/INI1 deficiency in deciduoid mesothelioma. Although awareness of this pattern helps avoid diagnostic confusion, deciduoid mesothelioma is best regarded as a morphologic variant of epithelioid mesothelioma, not as a distinct diagnostic entity.
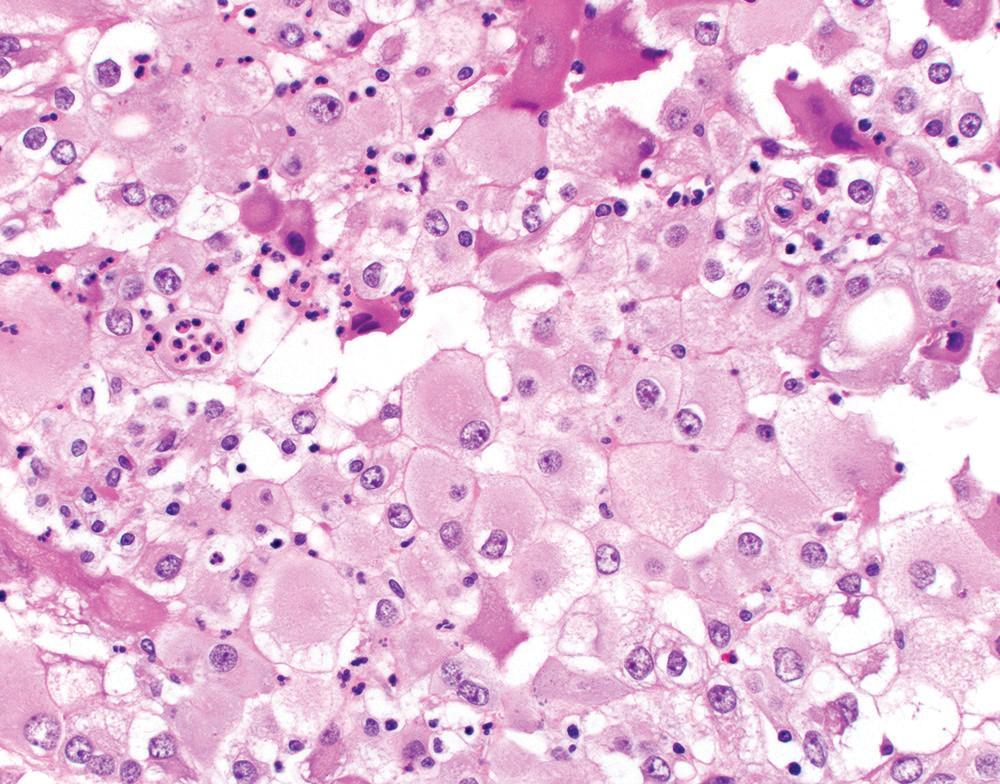
Pleomorphic mesothelioma is increasingly recognized as a prognostically distinct subtype of mesothelioma. , Morphologically, these tumors are characterized by solid sheets of large, often discohesive epithelioid cells with relatively abundant eosinophilic cytoplasm and large nuclei with irregular contours, clumped chromatin, and one or more large nucleoli ( Fig. 21.17 A). Multinucleated tumor giant cells may be present. Mitoses are brisk (>20 per 10 hpf), and atypical mitoses are common. Pleomorphic mesotheliomas are generally positive for mesothelial markers (albeit patchy in some cases) and negative for epithelial tumor markers (including TTF-1), facilitating accurate diagnosis. Ultrastructural examination shows slender microvilli, characteristic of epithelioid mesothelioma.
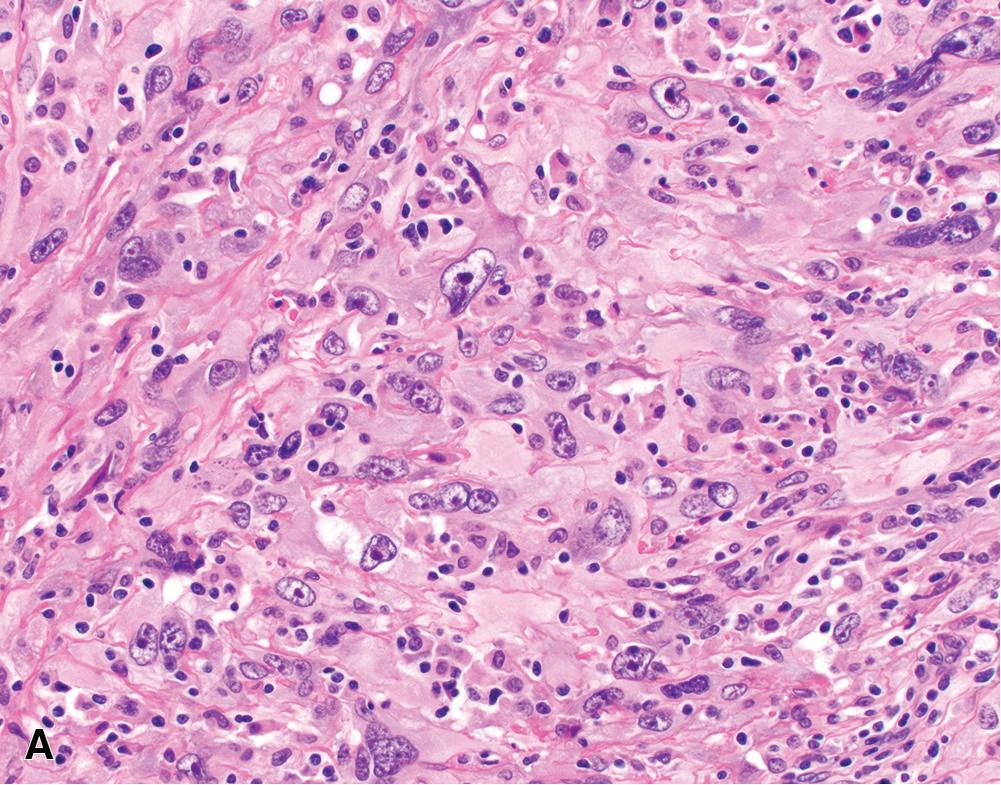
Pleomorphic mesothelioma behaves aggressively, with frequent lymph node metastases. Median survival is approximately 6 to 8 months—significantly worse than other morphologic subtypes of epithelioid mesothelioma (including conventional “high-grade” epithelioid mesothelioma) and more in keeping with biphasic or sarcomatoid mesothelioma. , , , , , , Given their morphologic and ultrastructural features, most authors consider pleomorphic mesotheliomas as a distinctly aggressive subtype of epithelioid mesothelioma , , ; however, pleomorphic features may be seen in biphasic or sarcomatoid tumors ( Fig. 21.17 B). The current WHO classification recommends classifying tumors according to their dominant subtype (epitheloid vs. biphasic vs. sarcomatoid) and reporting the presence of a pleomorphic component, given its adverse prognostic implications.
Rare mesotheliomas contain distinct rhabdoid tumor cells, characterized by abundant eosinophilic cytoplasm and a single rounded eosinophilic cytoplasmic inclusion, which displaces and sometimes indents an eccentrically placed nucleus with a prominent nucleolus ( Fig. 21.18 ). The rhabdoid component in these tumors reportedly ranges from 15% to 75% of tumor cells. The non-rhabdoid component may be epithelioid or sarcomatoid, so these tumors may have epithelioid, biphasic, or sarcomatoid histotype overall. Rhabdoid tumor cells are positive for mesothelial immunomarkers, though expression may be patchy. Loss of SWI/SNF protein expression, which may be associated with rhabdoid morphology in other tumor types, appears to be exceptionally rare in mesothelioma. Muscle markers are negative, and ultrastructural studies confirm the absence of rhabdomyoblastic differentiation.
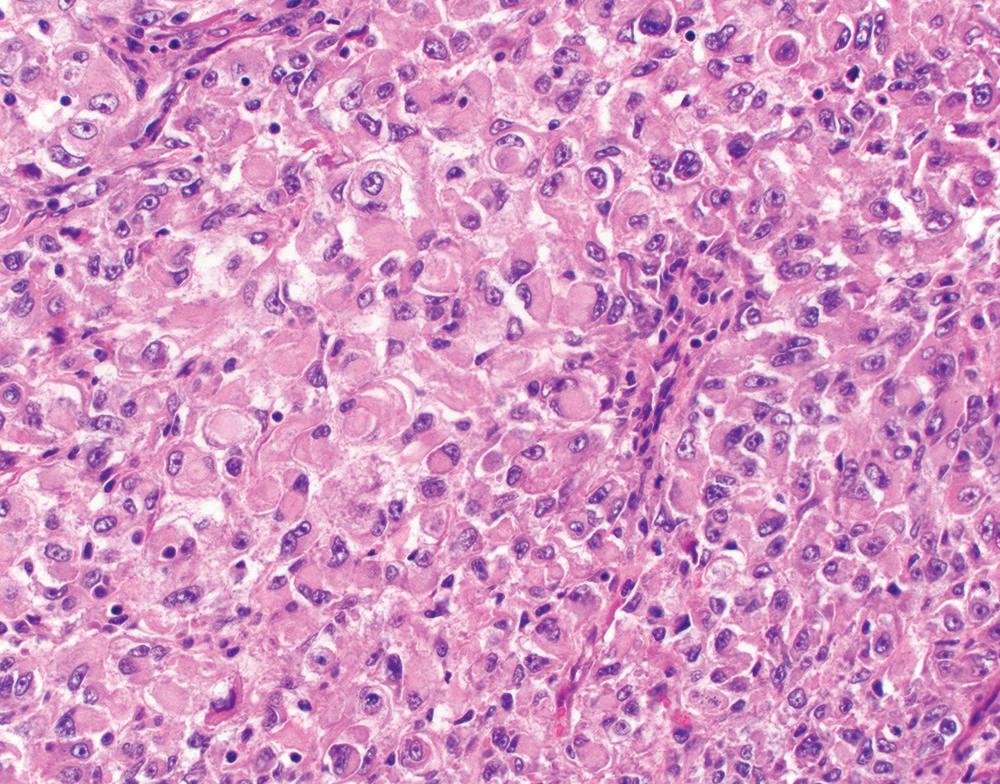
Rhabdoid mesothelioma appears to carry a poor prognosis, with mean survival of approximately 6 months. However, given the rarity of these tumors and the occurrence of rhabdoid cells in epithelioid, biphasic, and sarcomatoid histotypes, the independent prognostic impact of rhabdoid morphology remains unclear. Likewise, reproducibility in distinguishing rhabdoid, high-grade deciduoid, and pleomorphic patterns is uncertain, and these patterns may co-occur. Consequently, one should not be dogmatic in classifying these high-grade variants, but the pathology report should convey the probable association with aggressive clinical behavior.
Rare (<1%) mesotheliomas show “small cell” features, potentially mimicking small cell lung cancer. , These “small cell” foci are characterized by sheets of monotonous cells with scant cytoplasm ( Fig. 21.19 ). However, in contrast to small cell lung cancer, “small cell” mesothelioma is pleural based, shows modest mitotic activity (<5 per 10 hpf), and exhibits even chromatin with conspicuous nucleoli and without nuclear molding. The “small cell” component of such tumors may reportedly comprise between 15% and 95% of the tumor area; however, in all reported cases, more typical (solid, tubular, trabecular, or papillary) mesothelioma morphology is seen at least focally. Rarely, a discrete sarcomatoid component may be present, warranting overall designation of biphasic mesothelioma. “Small cell” foci are positive for mesothelial markers (albeit patchy in some cases) and negative for epithelial markers, helping to confirm the diagnosis. Ultrastructural examination reveals characteristic slender microvilli. No characteristic molecular alterations (e.g., involving TP53 or RB1 ) have been reported for mesothelioma with “small cell” features, although robust studies are lacking.
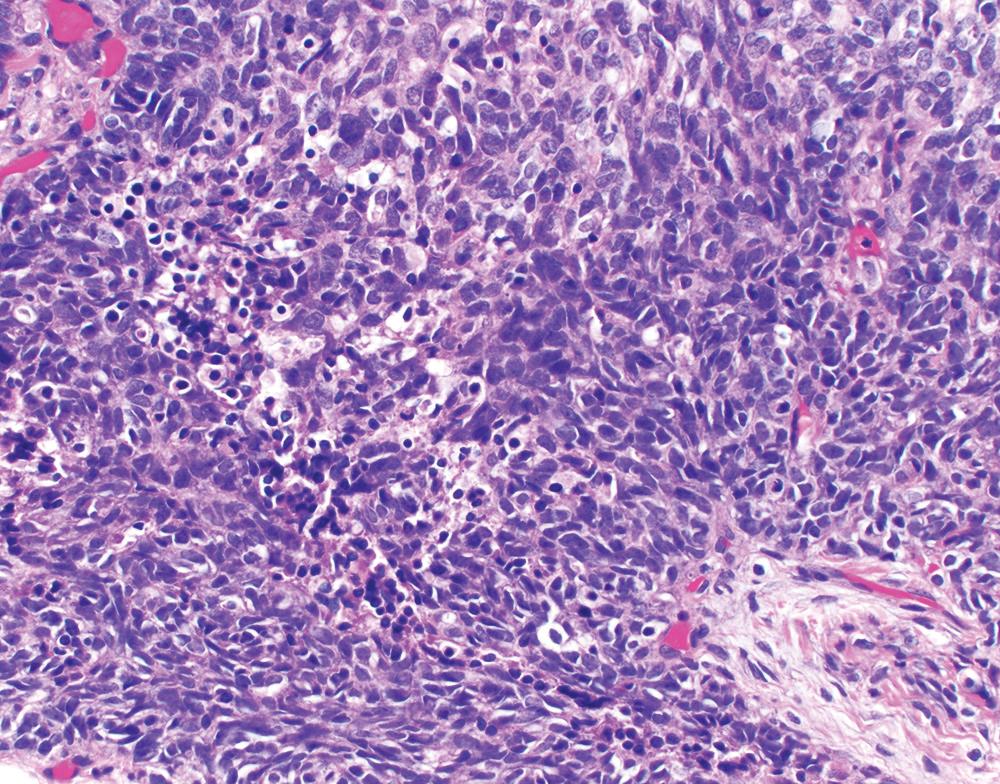
Mesotheliomas with “small cell” features behave aggressively, with mean survival of approximately 8 months. Given the rarity of these tumors, diagnostic criteria and reproducibility are not well established, and these tumors are best diagnosed as a form of epithelioid differentiation with a descriptive diagnostic comment. Furthermore, the term “small cell mesothelioma” is discouraged, so as not to imply that this is a neuroendocrine neoplasm or otherwise related to small cell lung cancer.
Clear cell mesothelioma is an unusual variant of epithelioid mesothelioma characterized by clear to pale vacuolated cytoplasm, which may affect all tumor cells or only a subset ( Fig. 21.20 ). , Cytoplasmic clearing principally reflects glycogen accumulation, although sometimes it results from cytoplasmic lipid, mitochondrial swelling, or cytoplasmic lumen formation. Most reported cases show solid or trabecular architecture and bland nuclear cytology. A characteristic mesothelial immunophenotype aids in correct diagnosis. Median survival (16 months) does not appear to differ from epithelioid mesothelioma overall.

Lymphohistiocytoid is another rare (<1%) morphologic variant of epithelioid mesothelioma, characterized by diffuse sheets of epithelioid to spindled mesothelial tumor cells with a “histiocyte-like” morphology, densely infiltrated by CD8+ cytotoxic T cells and CD68+ histiocytes ( Fig. 21.21 ). By definition, the lymphohistiocytoid areas compose at least 50% of the tumor. The non-lymphohistiocytoid areas may show conventional epithelioid or sarcomatoid morphology. Mesothelial immunomarkers reliably highlight the tumor cells in lymphohistiocytoid foci. However, interpretation of BAP1 and MTAP immunostains requires distinction between tumor cells and non-tumor inflammatory cells, which may be challenging. EBV studies are negative.
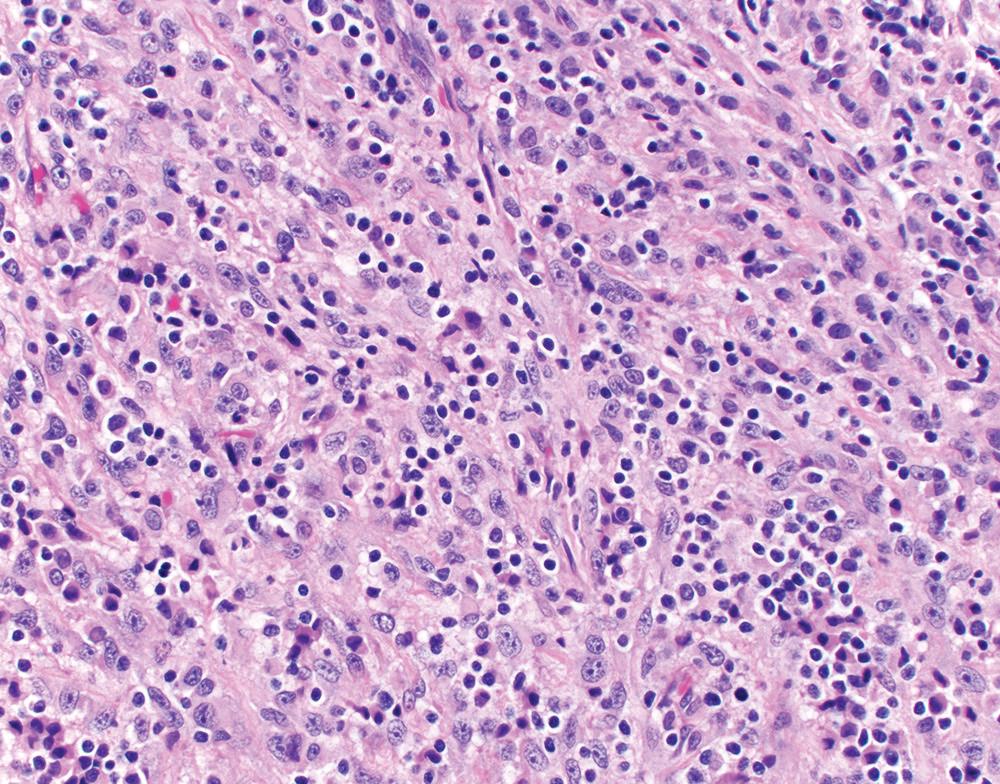
Historically, lymphohistiocytoid mesothelioma was considered a variant of sarcomatoid mesothelioma. However, most cases are now regarded as variants of epithelioid mesothelioma, as they principally comprise epithelioid cells and carry a prognosis comparable to epithelioid mesothelioma overall. Rarely, lymphohistiocytoid mesothelioma may be entirely composed of malignant spindle cells, in which case a diagnosis of sarcomatoid mesothelioma is appropriate. A cytokeratin immunostain may aid in assessment of tumor cell morphology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here