Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We are thankful to Mr. Michael R. Czachowski for his help with coregistration of various imaging modalities.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Magnetoencephalography/magnetic source imaging (MEG/MSI) has the ability to do the following:
Noninvasively localize abnormal epileptic activity with a spatial resolution of less than 5 mm
Provide nonredundant localizing information that guides and improves phase 2 evaluations
Localize eloquent cortices of interest noninvasively in patients with or without structural abnormalities
Identify displaced anatomic landmarks (e.g., central sulcus, sylvian fissure)
Assist in optimizing the surgical trajectory for an intraoperative navigation and operation
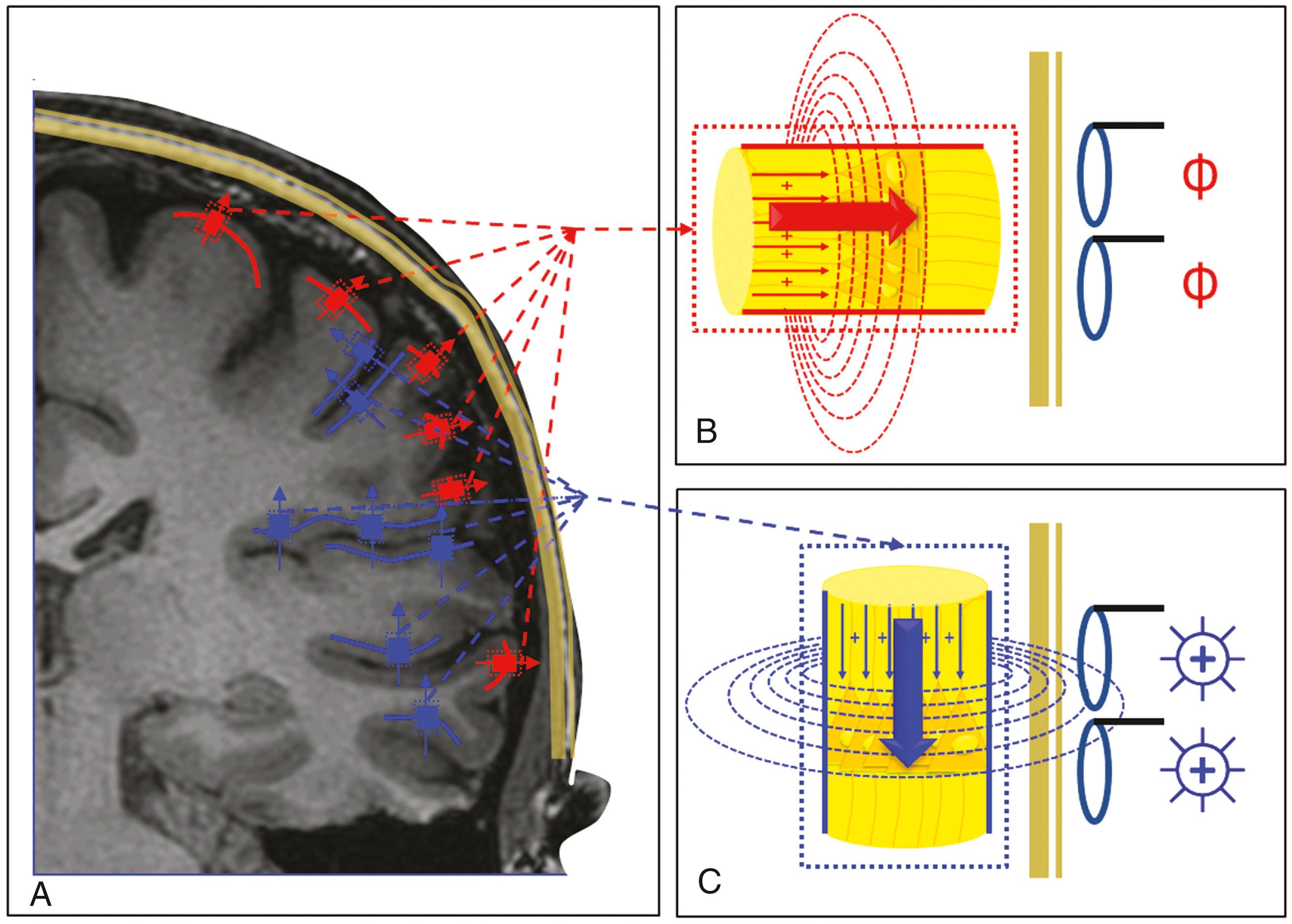
Magnetoencephalography (MEG) is a noninvasive and painless procedure that involves no external magnetic field, electricity, x-rays, or radioactivity. It is also known as magnetic source imaging (MSI) based on its most established form of clinical application in which source estimates (dipoles) are coregistered with the patient’s magnetic resonance image ( Fig. 86.1 ). Although, strictly speaking, MSI is a specific type of MEG application and not necessarily its synonym, interchangeable use of these two terms currently prevails. , MEG is a direct, noninvasive neurophysiologic technique for studying the brain based on measuring magnetic fields primarily associated with the summated postsynaptic intradendritic electric currents subtending normal and pathologic cerebral processes that are reflected outside of the skull. Directed flow of the electrically charged particles involved in normal or pathologic interactions of neurons in the form of electric currents is inseparably associated with magnetic fields that are recorded using supersensitive magnetic sensors. Parallel orientation of the dendrites (cortical pyramidal cells) is the critical prerequisite for a spatial summation of these otherwise undetectable electric potentials (and corresponding fields), and synchronous firing is critical for temporal summation of the infinitesimal electric field generated by each individual cell to produce the still miniscule but summated magnetic fields that are detectable by the supersensitive sensors used in MEG systems (see Fig. 86.1 ). Because the strength of the magnetic fields produced by the brain is extremely low, very specialized instrumentation is required to detect these signals as well as to be shielded from the overpowering magnetically hostile (e.g., hospital) environment. , , These sensing devices contain small coils (or chips) that function as flux transformers and are coupled to superconducting quantum interference devices (SQUIDs) that operate on two phenomena of quantum physics: superconductivity and tunneling. , , , Most modern systems include more than 300 of these specialized sensors arranged in a helmet-shaped configuration. , By analyzing the complex patterns of the signals recorded by the sensors, the location, strength, and orientation of the sources can be estimated , (see Fig. 86.1 ). However, the true neuronal source extent cannot be assessed even with currently available advanced source imaging techniques. , In today’s clinical practice related to epilepsy, it is preferable to use a combination of the localization results from both the magnetoencephalogram and the simultaneously recorded electroencephalogram , , because they are complementary and most informative when combined appropriately.
MEG uses extensive spatial sampling that is enabled by hundreds of sampling locations surrounding the brain, in contrast with the 20 to 30 electrodes used in traditional routine electroencephalography (EEG). This sampling volume along with the fact that magnetic fields by their nature are not filtered (smeared), weakened (reduced), or distorted by the intervening tissues (cerebrospinal fluid, meninges, skull, and scalp)—that is, the tissues surrounding the brain are “transparent” for magnetic fields—make MEG a superior technique for localizing the brain’s activity very accurately using simple models. , This is particularly helpful when dealing with a complex clinical reality involving altered anatomy, as in postoperative and posttraumatic scenarios in which EEG signals are distorted and may provide misleading localization.
Unlike EEG, which measures an amplified potential difference between two electrodes placed on the scalp, MEG measures summated, very weak magnetic fields. SQUIDs, used to measure these fields, require cooling to attain the state of superconductivity. This is currently achieved by their immersion in liquid helium, which attains a temperature near absolute zero (−269°C, −452°F, or 4.2°K). An important practical consideration is the necessity for a weekly consumption of 80 to 100 L of helium to keep the system functional, and this is a considerable cost. Different helium recycling systems are available but as of yet are not widely used in the MEG field.
Dealing with extremely weak signals is just one problem that MEG technology has to solve; the other is the overpowering and fluctuating magnetic “noise” of the external environment, which is 10 7 to 10 9 times greater than the magnetic field of the brain. (Of note, the magnetic field of Earth is about 50 million times stronger than that of the brain, but because it is a steady field, it is not recorded by the MEG sensors.) This cardinal problem of environmental noise is solved by placing the MEG system in a magnetically shielded room that deflects the magnetically hostile influence sufficiently to enable an adequate recording. In some locations, MEG systems are installed near extremely unfriendly magnetic environments such as underground railways; such placement may require additional means of “active shielding” in which current is used to cancel the detected magnetic noise from the environment.
As the fundamental laws of physics (i.e., Maxwell’s equations) teach us, the relationship between the electric current and its inseparable corresponding (induced) magnetic field is governed by the right-hand rule: If one curls the fingers around the current-conducting wire and extends the thumb pointing in the direction of the current flow, the curled fingers point in the direction of the magnetic field. This depicts the orthogonal mutual perspective from which EEG (concerned with electric current) and MEG (concerned with magnetic field) are “viewing” the same cerebral sources. Thus the role of geometry is very important in MEG, and the sources are divided into tangential or radial with respect to the overlying skull (see Fig. 86.1 ). According to the right-hand rule, a tangential source (one that has the mean geometric orientation parallel to the overlying skull) will be associated with a magnetic field that spreads orthogonally through the skull and will most likely be detected by the MEG sensors. A radial (“outward”) source (one that has the mean geometric orientation orthogonal to the overlying skull) will induce a magnetic field that will spread parallel to the overlying skull and will most likely remain inside the skull and thus not reach the MEG sensors. Consequently, MEG is sensitive to the sources in cerebral sulci (e.g., sylvian fissure, superior temporal sulcus) and large cortical planes (e.g., inferior temporal or frontal) because they have tangential orientation, as opposed to EEG, which prefers the gyral and fissural sources that have exclusively or predominantly radial ordination (see Fig. 86.1 ).
Even more than 40 years after the first SQUID MEG recording by Cohen, the most common clinical use of MEG is in MSI. , MSI provides a best estimate of the location of cortical activity that is occurring at any instant in time. This application uses a sphere as the head model, and a pointlike dot called an equivalent current dipole (ECD) as a source model in the iterative statistical computation process that generates possible source locations based on the measured magnetic field data (see Fig. 86.1 ). It has been demonstrated that MEG can attain localization accuracy of 2 to 3 mm. Of course, cerebral sources are not dots, and many sources can coexist; “dots” on magnetic resonance imaging (MRI), sometimes called “a probability cloud,” may be misleading because they indicate not the extent but rather the complexity of the source ( Fig. 86.2 ).
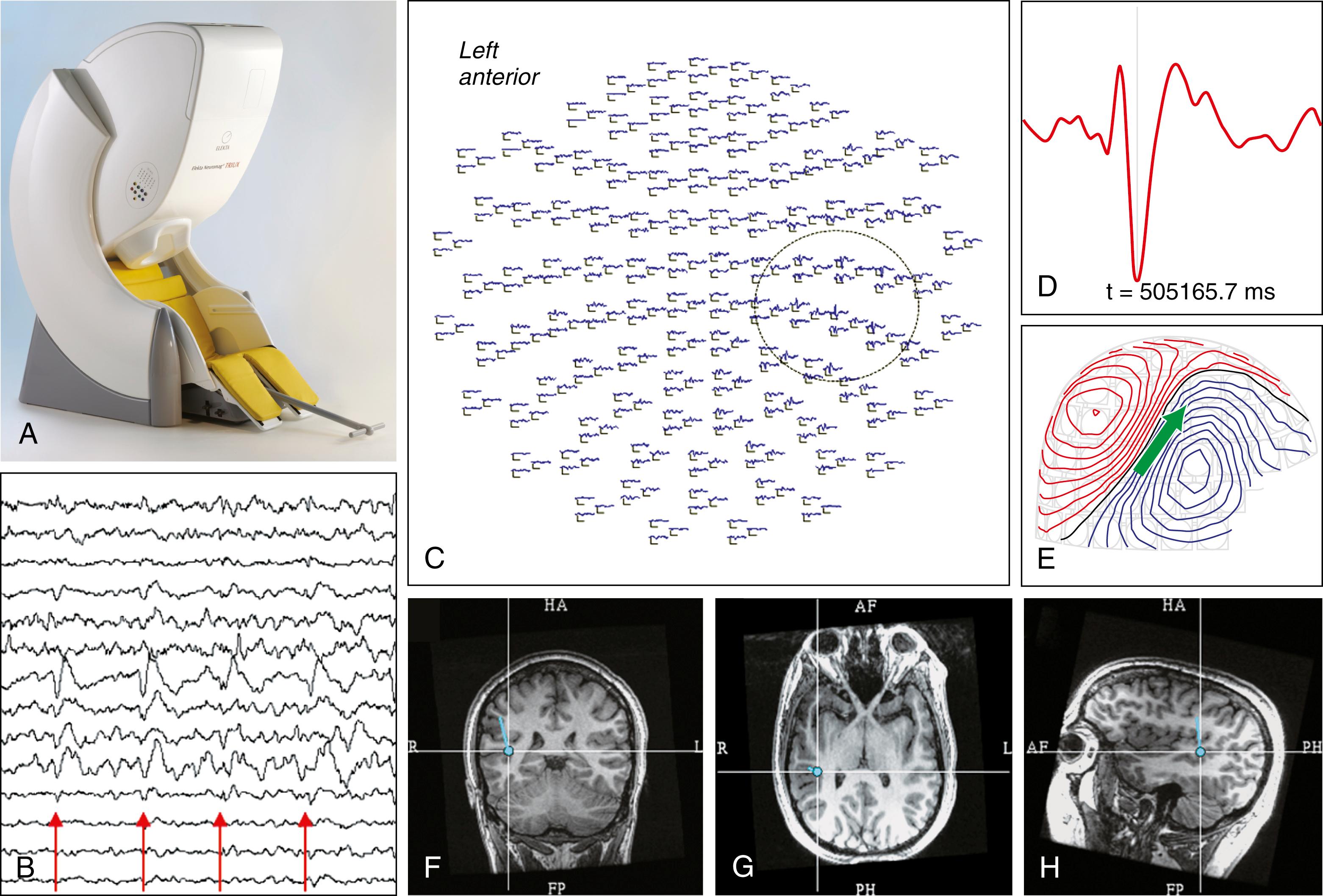
In addition to single ECDs, many other methods of MEG analysis are utilized in research, and some are advancing into clinical practice. Synthetic aperture magnetometry is a beam-forming technique that applies a spatial filter to the recorded magnetoencephalogram to determine the cortical location; minimum norm estimates are a current distribution technique that determines where the current is flowing in the cortex ; and MUltiple SIgnal Classification (MUSIC) is an algorithm used for frequency estimation. , Emerging evidence , suggests that other methods besides single ECD may yield similar results. More recent advances in signal processing are inspiring software packages and approaches that can use the MEG data to detect the underlying functional and effective connectivity in the brain.
Compared with EEG, the advantages of MEG are that it is free of biologic references, requires no electrodes, and is able to detect a smaller activated area (about 6 cm 2 versus >10 cm 2 ). Also, its signals are not distorted by the skull or other intervening tissues, it is amenable to simpler clinically useful source modeling, and it provides superior spatial sampling because it covers the whole head with a few hundred sensors. , , On the negative side, it is an immobile, expensive instrument that must be housed inside a magnetically shielded room and kept “cold,” even when not in use. In addition, it is susceptible to metallic and movement artifacts, it is sensitive to tangential sources only, it has a lower sensitivity to deep sources, and it involves labor-intensive data analysis procedures to provide imaged results. ,
Because MEG is considered and used clinically primarily as a localizing tool, a frequently asked question is “What is the localization accuracy of MEG?” Overall, the source extent, complexity, and depth have a considerable effect on its localization accuracy. Probably some of the best answers came in the early days of the clinical use of MEG, in the 1980s. The position of a virtual current dipole placed at a known location in a model was determined with an accuracy of approximately 2 mm, and an accuracy of approximately 3 mm in locating a current dipole source was demonstrated in different scenarios by several groups. , , Romani and colleagues were able to “separate sources” a few millimeters apart while studying a tonotopic map of the human auditory cortex, and Hari and associates demonstrated “millimeter accuracy” while studying the organization of the somatosensory cortex. However, it is worth repeating that—at this time—irrespective of its claimed and/or true sensitivity and localizing accuracy, MEG does not provide even a rough estimate of the source size.
Generally speaking, for patients with epilepsy, this tool enables identification of epileptogenic tissue noninvasively , (see Table 86.1 ). Alone or combined with presurgical mapping of eloquent cortices of interest ( Box 86.1 ), MEG creates neuronavigational maps that can be used by surgeons in real time during a surgical procedure, and that assist in delineation of parts of the brain to preserve or remove.
| Evidence-Based MEG Indication | Evidence-Based Justification | Remarks |
|---|---|---|
|
Additional nonredundant localizing information is provided by MEG in about one-third of cases undergoing presurgical evaluation. , MEG-exclusive spikes are seen in 47% of patients without EEG spikes. Retrospective MSI-guided review of MRI may disclose previously concealed lesions in up to 50% of patients. , , Areas of interictal MEG spiking are associated with PET abnormalities in MRI-negative TLE. ECD properties correlate with cortical thinning in left mesiotemporal epilepsy. |
Without MEG, these patients are frequently excluded from a complete presurgical evaluation or may be exposed to extensive invasive investigations that may not be entirely appropriate. |
|
MSI-guided re-review of MRI may lead to a positive finding in up to 50% of seemingly negative MRIs. MEG can detect mesial temporal spikes in about 85% of patients with mesial TLE. MEG spike orientation may help in distinguishing mesial TLE from lateral TLE. , Spike orientation may predict epileptogenic side across cerebral sulci. Vertical or horizontal MEG spikes in the anterior temporal pole indicate a higher chance of mesial TLE. |
MEG findings may help in identifying more surgical candidates, obviate a need for ICM in some cases, or at times lead to a direct resection. |
|
An example of this is a patient with TS in whom MEG was shown to be more accurate than an ictal scalp EEG in identifying the epileptogenic zone, as well as recognizing the most active lesion (rarely identifiable with a scalp EEG), , , and overall very useful in identifying suitable candidates for surgery , , with a favorable long-term outcome. | In the not-too-distant past, these patients would not have been considered for resective surgery. MEG contributed considerably to the change in clinical practice leading to identifying more surgical candidates in TS and similar populations, at times completely avoiding or better planning ICM, and performing more complete resections leading to more favorable surgical outcomes. |
|
Large lesions introduce diagnostic complexity by changing the anatomy and making a scalp EEG unreliable. MEG can identify an (the most) active part of a lesion or perilesional tissue. MEG is more accurate than ictal scalp EEG in patients with large lesions and altered anatomy. MSI can guide a choice of an optimal access trajectory and helps delineate the extent of resection of the epileptogenic zone. |
Because large lesions may not be amenable to complete resections, it is critical to know what part of perilesional areas are (most) active and thus tailor the degree of resection according to the highest likelihood of seizure improvement or full control in spite of incomplete resection. |
|
Operative or traumatic skull defects distort electric fields, making spike identification difficult and EEG susceptible to erroneous localization. MEG is more accurate than ictal scalp EEG in patients with altered anatomy because magnetic fields are relatively unaffected by the structure of the skull, and MEG sensitivity and localization accuracy remain unaltered. |
MEG is not only overall superior to EEG in this setting, but may identify and localize incompletely resected parts of the original target area or a separate previously unsuspected epileptogenic focus (see Figs. 86.3E and 86.5 ). |
|
Propagation of interictal epileptiform EEG activity can lead to erroneous source localizations. MEG helps distinguish between the primary focus and propagated activity, , , , and MEG spikes propagation may have prognostic implications for epilepsy surgery. |
These may be among the most complex patients considered for epilepsy surgery. In these settings, a working hypothesis may not be supported sufficiently to accept the risks associated with ICM, and having a clarification of localizing ambiguity may be a decisive factor in making an optimal decision. |
|
In spite of their overall complex anatomy, the major horizontal intrasylvian cortices (e.g., Heschl’s gyrus and planum temporale) facilitate MEG sensitivity to the respective sources. MEG can identify intrasylvian spikes in EEG-negative cases , or a single intrasylvian epileptogenic focus in patients with ambiguous EEG, such as in LKS, where MEG sensitivity is high (68%–100%) , and MSI-aided surgery may lead to significant clinical improvement. , |
Without MEG, these patients are frequently excluded from a complete presurgical evaluation or may be exposed to extensive invasive investigations that may not be entirely necessary. |
|
MEG can detect spikes from the interhemispheric area, , , is more sensitive to frontal and occipital sources than EEG, and can lateralize medial frontal and occipital spikes, even when the EEG is nonlateralizing. Almost 90% of ECoG spikes were associated with MEG spikes. |
Because frontal lobe epilepsy has the most unfavorable surgical outcome, and various EEG ambiguities may occur, the acquisition of additional noninvasive localizing information is essential. |
|
Because it is suspected that insular epilepsy may be accountable for failures of a significant number of TLE surgeries, , and MEG spikes can be captured in over 64% of patients with insular epilepsy, , MEG findings can improve diagnostic accuracy, substantiate ICM, and justify its risks. , | As a result of the low sensitivity of scalp EEGs to insular spikes, and the suspected frequent misdiagnosis of insular epilepsy as temporal, leading to a failure of anterior temporal lobectomy, the value of MEG is very high and may be decisive in justifying the risks associated with ICM and avoiding surgical failure. |
|
MEG identifies interictal spikes in 47% of patients without EEG spikes. | It is very important to avoid the misconception that patients with no spikes on EEG are unlikely to benefit from MEG and that MEG should be ordered only for “frequent spikers.” |
Localizes eloquent cortices of interest noninvasively in patients without structural abnormalities. , , , ,
Establishes spatial relations of the lesion(s) with eloquent cortices noninvasively. a
a References 62, 125, 135, 141, 180, 284.
Detects the functional tissue within the tumor (e.g., diffusely growing gliomas).
Identifies displaced anatomic landmarks (e.g., central sulcus, sylvian fissure). , , ,
Assists in choosing an optimal surgical trajectory. , ,
Overall, defines the key elements of a comprehensive neuronavigational map as a prerequisite for planning an optimal surgical trajectory and safe intraoperative navigation and operation. b
b References 61, 62, 125, 132, 133,265.
Although MEG has been in clinical use for a few decades, the road to greater advances from a research to a clinical tool is still evolving. So far, over 175 MEG systems have been installed worldwide; the current cost of the system, with a magnetically shielded room, is close to $3 million. In the United States, an annual service contract ranges from $80,000 for the basic preventive maintenance and minimal support, to $125,000 for complete maintenance (planned and unplanned), including replacement parts, full support, and guaranteed uptime. Helium prices fluctuate periodically and remain vastly disparate across MEG sites and suppliers. The need for 80 L/wk plus extra for the annual maintenance procedure amounts to more than $80,000 annually in some regions.
In the United States, only two indications are approved for reimbursement within the Current Procedural Terminology (CPT) codes (American Medical Association, Chicago, IL). One is the use of MEG for recording of spontaneous epilepsy localization, billable as CPT 95965: Magnetoencephalography (MEG), recording and analysis; for spontaneous brain magnetic activity (epileptic cerebral cortex localization). The other is the use of MEG for presurgical mapping of eloquent cortices (language, sensory, motor, auditory, and visual), billable as CPT 95967: Magnetoencephalography (MEG), recording and analysis; for evoked magnetic fields, single modality (e.g., sensory, motor, language, or visual cortex localization). Epilepsy surgery, particularly nonlesional neocortical, has been the key driver of the dissemination and wider acceptance of MEG in clinical practice in the preceding two decades. Recently, MEG has been increasingly used in presurgical mapping ( Table 86.1 and Fig. 86.3 ).
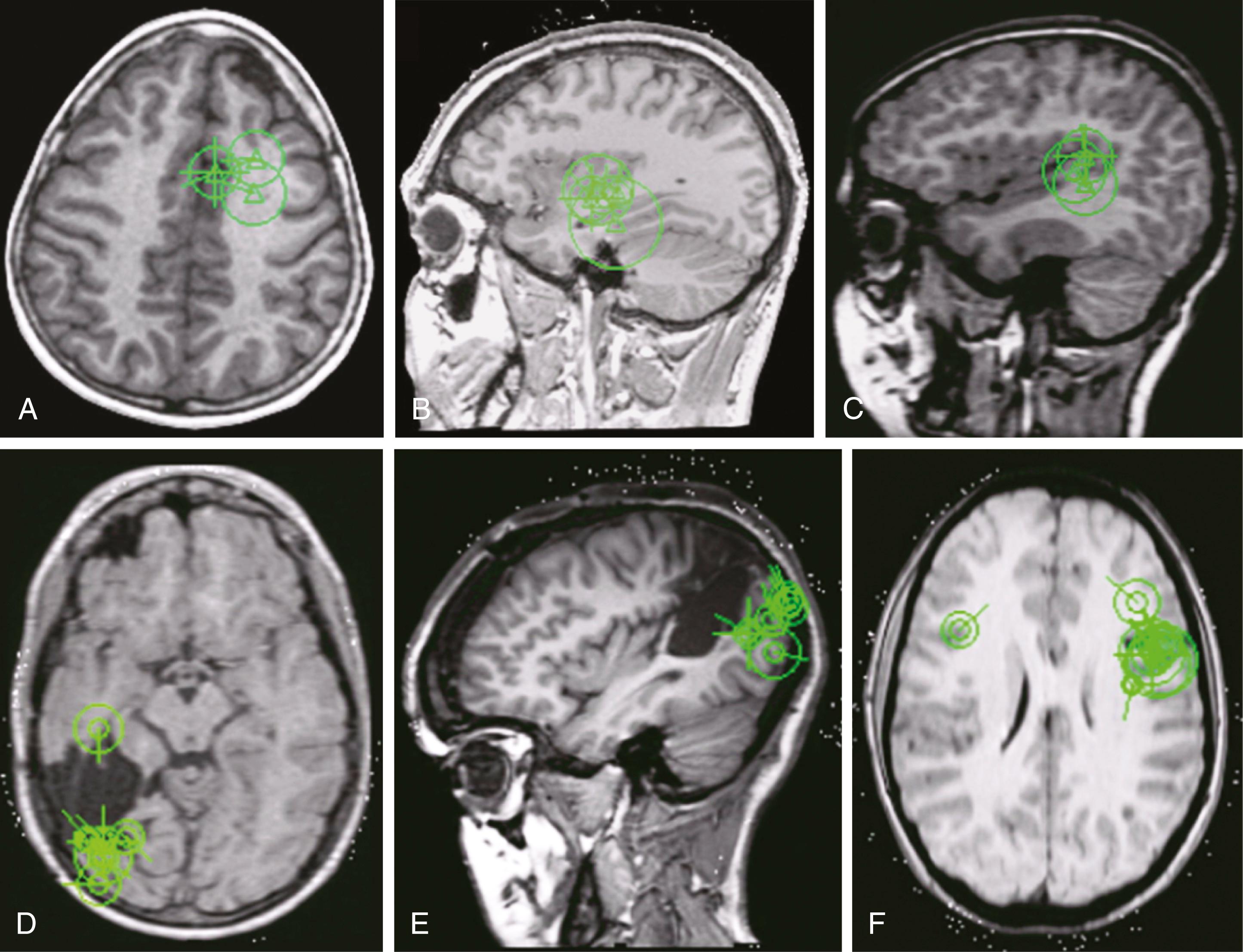
Subject preparation is a critical step before a MEG scan and involves a comprehensive survey for any ferromagnetic sources, ranging from any implants in patients (e.g., a vagus nerve stimulator, rods, screws, plates, shunts, pumps, tooth fillings, dentures, nonremovable jewelry) to clothes, shoes, hair accessories or jewelry, and hair sprays, mousses, and gels. Most magnetic artifacts can be prevented by the removal of the potential source(s) and/or by a demagnetization procedure (also known as degaussing ). If unavoidable artifacts are recorded, particularly those from extracranial sources (e.g., heartbeats, vagus nerve stimulator–related sources), these can be removed after the recording using specialized software such as independent component analysis, principal component analysis, or Maxfilter. Most scans require the patient to keep very still; to this end, sleep is a powerful ally for the personnel working on the data acquisition, and appropriately instructed parents may help calm and control children while sitting or resting next to them in a magnetically shielded room without causing considerable artifacts. Usually patients are sleep deprived before coming in for the MEG scan. Cognitively impaired subjects who are unable to cooperate sufficiently and very young children may need to be sedated to ensure a technically adequate recording. If the best approach—a still patient—fails, continuous head position tracking tools are now a standard part of advanced whole-head MEG systems, , and their practical usefulness has been demonstrated. ,
Clinicians should be aware of some potential drawbacks of exploiting MEG results. If multifocal epilepsy is thought to be present, MEG may fail to detect epileptogenic zone(s) in the areas of its suboptimal sensitivity (e.g., deep sources such as those in the cingulate gyrus) or may disclose epileptogenic tissue that is not responsible for the patient’s habitual seizures (e.g., propagated activity or secondary foci) and thus should not be used to exclude the epileptogenic potential of those areas. This requires placing competently interpreted MEG results in the specific clinical context expertly. Conversely, if a single epileptogenic focus is presumed based on consistently stereotypical semiology, MEG may identify propagated or secondary activity without disclosing the primary source and thus result in a different type of false localization. Because benign variants are not yet systematically studied and understood in MEG, this may be an explanation for at least some “bilateral” or “contralateral” MEGs. Ultimately, a spikeless (i.e., negative) MEG study should be considered with the full appreciation of its methodologic limitations and not as definite proof of the absence of epileptogenic potential.
Considering its nature, MEG provides interictal recordings and identifies an irritative zone. However, seizures are captured in approximately 10% of MEG recordings, and this may assist in localizing an ictal onset zone, although this is not the primary purpose of MEG. , If no interictal activity is captured, pharmacologic activation of interictal epileptiform discharges may be considered to increase the yield of MEG-EEG recordings, but this is not accepted practice. , , Repeating a study after sleep deprivation with or without antiepileptic drug manipulation may be considered a more plausible option. , , Importantly, patients with a negative scalp EEG should be referred for a MEG study and not deprived of it because 47% of those without EEG spikes may have MEG spikes. , A very brief general overview of the role of MEG in epilepsy surgery is provided here, with detailed specific clinical examples illustrated by the figures and described in their captions. For clarity, 10 common evidence-based MEG indications in presurgical evaluation of patients with drug-resistant epilepsy (DRE) are detailed in Table 86.1 .
Irrespective of still-evident variability in presurgical evaluation of patients with DRE, , and of discouraging dilemmas in the interpretation of diagnostic accuracy studies during a presurgical workup for epilepsy surgery, , four general roles for MSI in (pre)surgical decision making have been proposed :
Supplementing sufficient new and/or supporting clinical information to propel the patient further along presurgical evaluation (“Eligibility to go forward in surgery evaluation”)
Guiding selection of appropriate patients for proceeding directly to intracranial monitoring (ICM) or surgery (“Patient selection beyond eligibility”)
Improving the accuracy and overall yield of ICM and surgical outcome (“Affect probability of cure”)
Complementing other noninvasive tests with sufficient nonredundant information to obviate the need for ICM (“Skip intracranial EEG”)
Multiple supporting examples for each of these four roles are detailed in Table 86.1 , Figs. 86.2 to 86.5 , and Fig. 86.10 (see later).
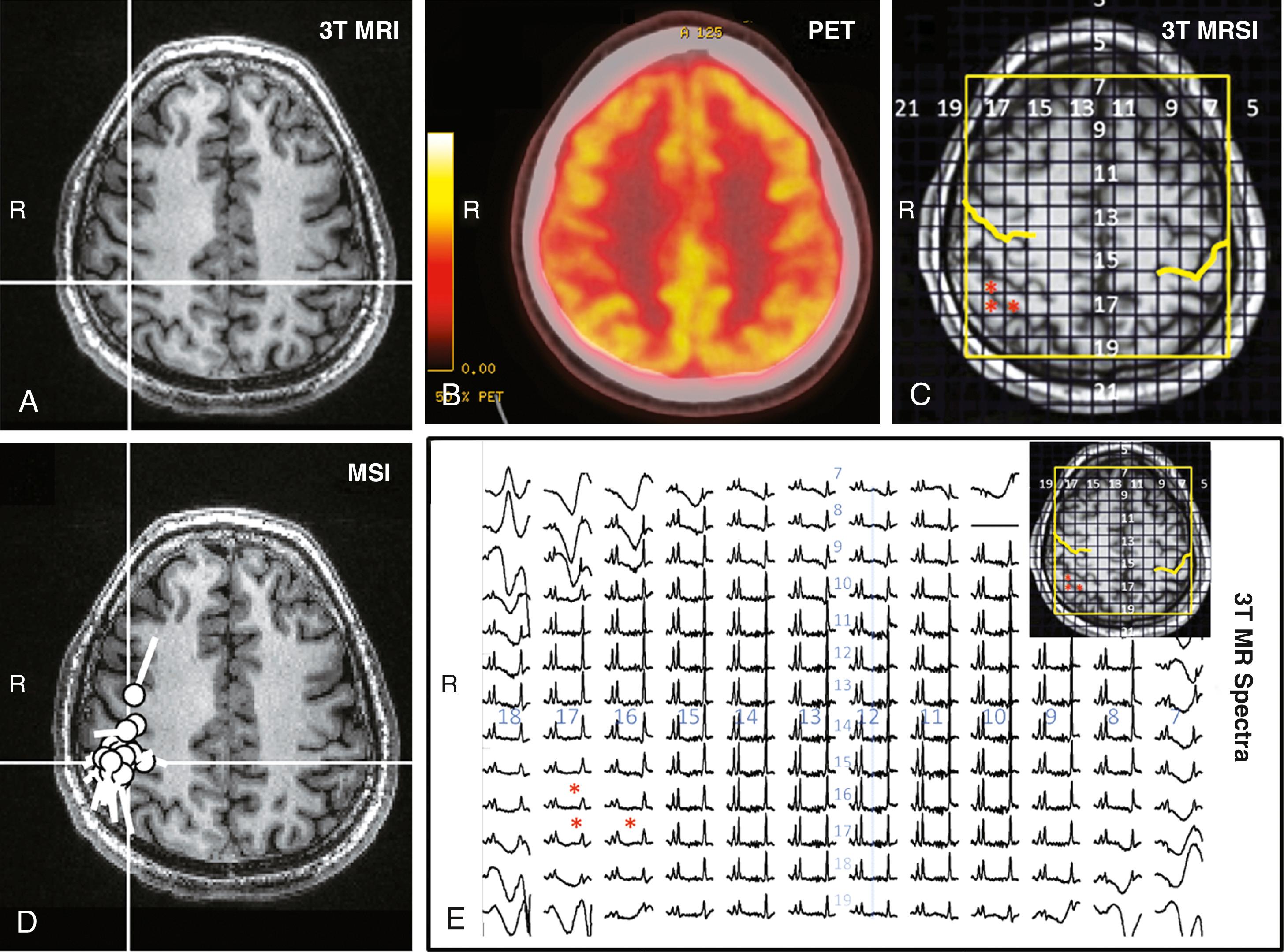
In evaluation of DRE, MEG is typically utilized during phase 1 of the presurgical evaluation, which includes long-term scalp video-EEG monitoring, MRI, neuropsychological testing, and eventually positron emission tomography (PET) and/or single-photon emission computed tomography (SPECT). , , , In this phase, MEG is used to determine early if surgery is a feasible option. , , Currently, MEG/MSI is primarily used in patients who are not obviously safe surgical candidates, such as those with normal or nonlesional MRI findings, conflicting electroclinical and radiologic data, or dual or multifocal pathologies, or those with lesion(s) adjacent to eloquent cortices. , MEG can be used to screen patients with MRI-detected focal or hemispheric lesions and concordant electroclinical data with an acceptable risk-benefit ratio for surgical candidacy as well as to formulate a working hypothesis about the physiology of the underlying seizures , , (see Table 86.1 , Figs. 86.3 to 86.5 , and Fig. 86.10 [see later]). Even in patients with clear lesions on MRI, the MRI finding itself may not be sufficient to proceed to surgery because association of the lesions with the epilepsy must be proven. , , MEG provides evidence for and also distinguishes between epileptogenic and nonepileptogenic lesions in cases of several or diffuse lesions. , , In patients with seemingly normal MRI scans, MEG can disclose subtle structural changes that are reflected in abnormal electromagnetic activity a
a References 5, 8, 20, 86, 89, 90.
and suggest further diagnostic workup, including an expert visual reevaluation of existing or differently protocolled MRI ( Fig. 86.4 ) or the use of postprocessing methods such as voxel-based morphometry.
Patients with ambiguity regarding a hypothesized epileptogenic zone may require ICM (also known as phase 2 presurgical evaluation ). , , The very high sensitivity of ICM is limited by its “tunnel vision”: an electrode has a very limited field of view (i.e., several millimeters), and a seizure onset zone may be easily missed if placement of electrodes is not optimal, , which seems to be a relatively frequent occurrence. The results of preoperative MEG analysis can be used not only to direct the placement of intracranial EEG electrodes but also to optimize the extent of resection and improve surgical outcomes. , Multiple studies of different populations of epilepsy patients confirmed, in various clinical contexts, that the complete removal of MEG spike-generating regions correlates with a more favorable surgical outcome , , , ( Fig. 86.5 ) and that single and tighter clusters of MEG dipoles herald a more favorable surgical outcome. , , Possible practical implications of these findings are that tightly clustered spikes designate epileptogenic tissue involved in seizure inception (i.e., seizure onset zone), and scattered MEG spikes reflect irritated cortex. , , Consequently, in spite of some passionate proponents of the poorly understood and somewhat dispraised term (complete) clusterectomy, , this may suggest that removal of the irritated cortex may not always be required for seizure freedom.
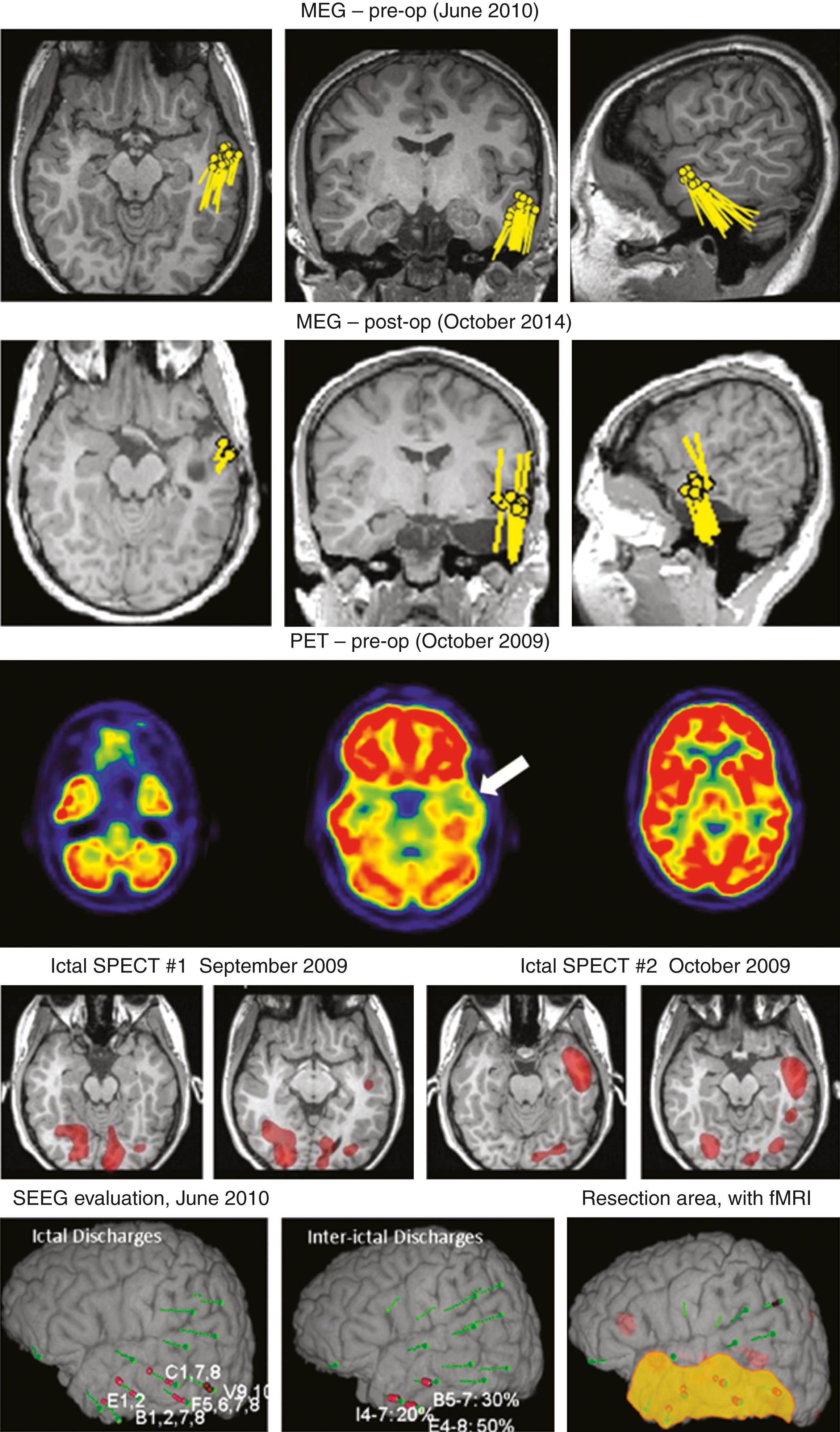
Overall, referral of patients for epilepsy surgery is suboptimal , in spite of class I evidence, but this may explain only one aspect of what may be multifactorial underutilization of MEG. , , Physicians’ insufficient familiarity with the evidence for the value of MEG in various clinical scenarios, the lack of understanding of its pertinent end user practicalities, reluctance to depart from “what we have always done,” and occasional logistic limitations may also be involved. Considerable progress would be made if at least all epilepsy patients undergoing ICM and those who have failed surgery and may benefit from reoperation would be referred for MEG , ; in these patients, MEG may also contribute to prognostication and counseling (see Table 86.1 ). Importantly, experts suggest that no patient should be denied surgery solely based on the results of a MEG study within the context of current clinical MEG practice.
Although MEG is not currently used routinely for diagnosing epilepsy but rather as a localizing tool that advances neurophysiologic and topologic understanding of a particular clinical reality, a study that assessed the routine use of MEG as a component of the primary diagnostic process for epilepsy gleaned relevant findings supporting the earlier use of MEG in presurgical evaluation. Specifically, in a tertiary referral center outpatient cohort of 51 patients with presumed neocortical epilepsy and inconclusive EEG, MEG yielded a higher diagnostic gain (63% versus 57%) toward the clinical diagnosis as the “gold standard” and a lower false-negative rate (27% versus 38%) compared with the diagnostic standard of care—a sleep-deprived EEG. However, even in its most established form as a localizing tool, MEG remains underutilized in the presurgical evaluation for epilepsy surgery.
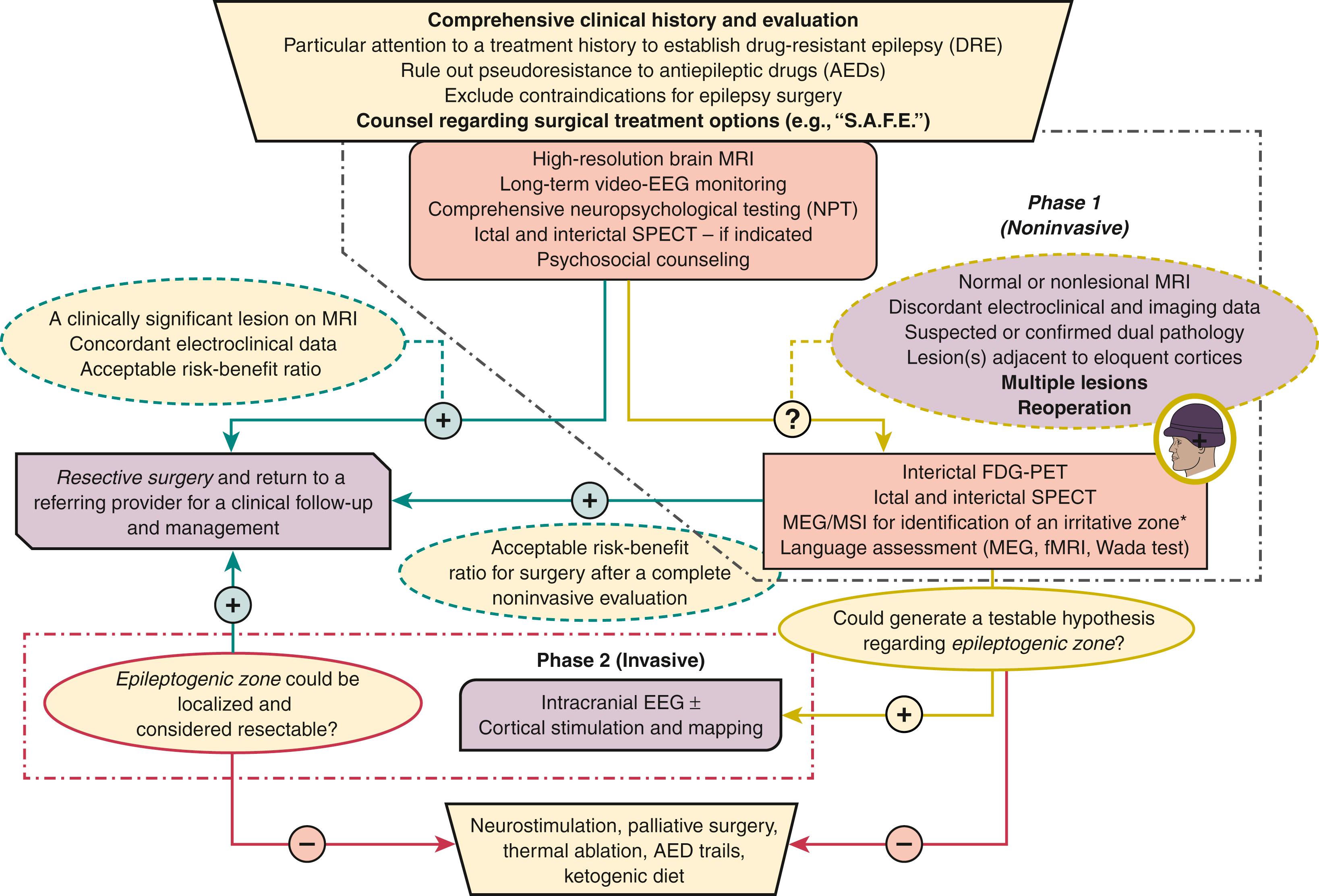
Here we include a comprehensive algorithm of presurgical evaluation of patients with DRE for the purpose of illustration of the place and role of MEG in this process ( Fig 86.6 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here