Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary lymphoproliferative disorders encompass a spectrum of benign and malignant lymphoid proliferations. Benign proliferations include intraparenchymal lymph nodes, follicular bronchiolitis, lymphoid interstitial pneumonia (LIP) (sometimes encompassed under the term diffuse lymphoid hyperplasia ), localized pulmonary “nodular” lymphoid hyperplasia, and pulmonary involvement by IgG4-related disease. Malignant lymphoproliferative disorders include pulmonary marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT), pulmonary lymphomatoid granulomatosis (LYG), posttransplant lymphoproliferative disorder, diffuse large B-cell lymphoma, primary pulmonary Hodgkin lymphoma, intravascular lymphoma, and secondary involvement of the lung by leukemia and lymphoma. Other rare types of lymphoproliferative disorders may also present primarily within the lung and include extramedullary plasmacytoma and primary pulmonary Ki-1+ anaplastic large cell lymphoma. Two distinctive types of lymphoma may develop within the pleural cavity and include primary effusion lymphoma and diffuse large B-cell lymphoma associated with chronic inflammation (pyothorax-associated lymphoma).
Lymph nodes occurring within the pulmonary parenchyma
Up to 18% of lungs at autopsy
Found in males and females of any race
Tend to be found in older individuals
Identified as single or multiple subpleural or paraseptal nodules on chest x-ray and computed tomography (CT) scan
Resected to exclude the possibility of carcinoma
Benign incidental finding with no morbidity or mortality
No treatment is required
Solid, round, or oval-shaped nodule(s) in a subpleural or paraseptal distribution
Appear similar to other lymph nodes with primary and secondary follicles and sinus histiocytosis
Deposits of anthracotic material are often prominent
Metastatic carcinoma should be sought in patients with known lung cancer
Intrapulmonary thymoma
Nodular lymphoid hyperplasia
Pulmonary lymph nodes may be biopsied to exclude the possibility of carcinoma. This possibility has been enhanced by their increased detection on radiographic imaging procedures.
Intraparenchymal lymph nodes are often discovered as an incidental finding on radiographic studies. Patients are usually asymptomatic or have symptoms related to other types of disease. Most patients are smokers, a habit that is postulated to predispose them to development of these lymph nodes. In one study, intraparenchymal lymph nodes were found in 18% of patients undergoing autopsy.
Intrapulmonary lymph nodes usually appear as single or multiple well-circumscribed nodules in a subpleural or paraseptal location. They may occasionally have radiographic features that are worrisome for either primary or metastatic disease (eg, spiculation, pleural indentation, fuzzy margins, and vascular involvement) ( Fig. 22.1 ).
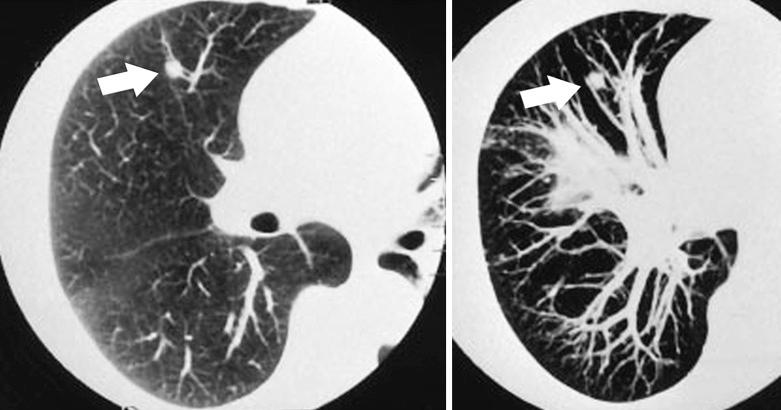
Intraparenchymal lymph nodes appear as solid, subpleural, round or ovoid nodules beneath the pleural surface or adjacent to interlobular septa. In one series, they ranged in size from 7 to 12 mm in maximum diameter.
Histologically, intrapulmonary lymph nodes appear similar to lymph nodes in other areas of the body. They contain both primary and secondary germinal centers ( Fig. 22.2 ). Histiocytes containing anthracotic pigment are often prominent in nodal sinuses.
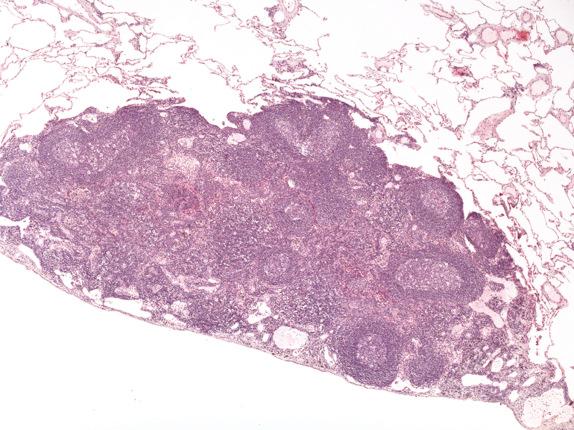
Intrapulmonary lymph nodes are usually histologically distinct and not readily confused with other entities. Metastatic carcinoma should be sought in patients with known lung cancer. Intrapulmonary thymoma and nodular lymphoid hyperplasia may occasionally enter the differential diagnosis. Intrapulmonary thymoma is histologically similar to mediastinal thymoma. Unlike intrapulmonary lymph nodes, thymomas are usually larger (0.5–12 cm) and contain variable mixtures of lymphocytes and epithelial cells, usually subdivided into lobules by fibrous bands. If needed, immunohistochemical staining for cytokeratin will highlight admixed epithelial cells and readily discriminate between these two entities. Although nodular lymphoid hyperplasia shares some histologic features with intrapulmonary lymph nodes, lesions of nodular lymphoid hyperplasia are usually larger (2–4 cm), lack the architecture of a lymph node, contain variable amounts of fibrosis, and may efface the underlying pulmonary parenchyma.
The term lymphoid hyperplasia encompasses follicular bronchiolitis and LIP as part of a spectrum of reactive hyperplasia of bronchus-associated lymphoid tissue (BALT). BALT is part of the spectrum of MALT, which also occurs in the thyroid, salivary glands, and gastrointestinal tract. In the lung, BALT occurs as aggregates of lymphoid tissue along bronchioles and lymphatics. Although follicular bronchiolitis and LIP have distinct histologic patterns, they frequently coexist, and their clinical associations are similar. Interaction of inhaled antigens with lymphocytes of BALT may initiate both T-cell and B-cell effector mechanisms important to normal pulmonary homeostasis. The histologic pattern of lymphoid hyperplasia, therefore, presumably reflects the different types of cellular responses. LIP is characterized by an interstitial expansion predominantly of T-lymphocytes, whereas follicular bronchiolitis consists predominantly of B-cell follicles adjacent to bronchi and bronchioles. Cases of LIP with prominent peribronchial follicles and germinal centers reflect both a B- and T-cell response. The association of LIP with chronic infections (Epstein-Barr virus [EBV] and human immunodeficiency virus [HIV]), autoimmune diseases, drug reactions, and bone marrow transplantation presumably reflects the effects of long-standing antigenic stimulation occurring in these settings.
Distinct histopathologic pattern of hyperplasia of BALT, characterized by development of primary and secondary germinal centers in a peribronchiolar distribution
Rare
Most cases are associated with an underlying connective tissue disease (eg, rheumatoid arthritis and Sjögren syndrome), immunodeficiency state, or hypersensitivity disorder
Females and males affected equally
Affects adults most often (median age is 44), but may affect children
Symptoms include progressive dyspnea and cough, sometimes accompanied by fever, weight loss, or recurrent pneumonia
Spirometry may show either obstructive or restrictive defects
Chest x-ray: bilateral reticular or reticulonodular infiltrates
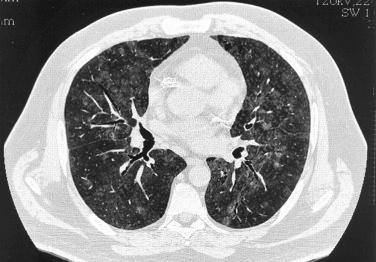
High-resolution CT scan: bilateral small (less than 3 mm) centrilobular and sometimes peribronchial nodules; occasionally larger (3–12 mm) nodules and focal areas of ground-glass opacity may be present
Prognosis is variable but generally good; rarely a cause of death
Treatment consists of corticosteroids, with variable responsiveness
In patients with associated collagen vascular disease, immunodeficiency (eg, HIV), or concurrent drug reaction (eg, amiodarone), treatment is directed at the underlying cause
Primary and secondary lymphoid follicles in peribronchiolar locations
Lymphoid follicles also often present along interlobular septa and pleura in a lymphatic distribution
Lymphoid follicles stain for pan–B-cell markers (CD20) and lack staining for BCL-2
Lymphoid interstitial pneumonia
Low-grade lymphoma of BALT
Pulmonary involvement by chronic lymphocytic leukemia
Nodular lymphoid hyperplasia
Follicular bronchiolitis is one pattern of hyperplasia of BALT. Follicular bronchiolitis is thought to represent hyperplasia of BALT in response to variable antigenic stimuli. In support of this view, lesions similar to follicular bronchiolitis have been induced in experiments with animals exposed to Mycoplasma pneumoniae, respiratory syncytial virus, and intratracheal installation of bacille Calmette-Guérin.
Follicular bronchiolitis occurs in children and adults, although adults are affected most often (median age of 44). Symptoms include progressive dyspnea and cough, sometimes accompanied by fever, weight loss, or recurrent pneumonia. Obstructive or restrictive defects may be present on spirometry. Most cases of follicular bronchiolitis are associated with an underlying connective tissue disease (eg, rheumatoid arthritis or Sjögren syndrome), an underlying immunodeficiency state (eg, HIV infection), or hypersensitivity disorder (eg, an unusual manifestation of amiodarone toxicity). Occasional cases are idiopathic. Peripheral blood eosinophilia has been noted in some idiopathic cases.
Follicular bronchiolitis may also occur as a secondary finding in patients with bronchiectasis, chronic infections, chronic bronchitis, or cystic fibrosis. In these cases, the pathologic and clinical features of the disease are dominated by the primary disease process.
Bilateral reticular or reticulonodular infiltrates are usually present on chest x-ray. High-resolution CT scan shows bilateral small (less than 3 mm) centrilobular and sometimes peribronchial nodules. Occasional larger (3–12 mm) nodules and focal areas of ground-glass opacity are present in some patients ( Fig. 22.3 ).
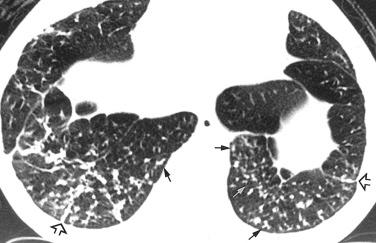
Gross features of follicular bronchiolitis are nondescript. Cases in which follicular bronchiolitis is a secondary finding are dominated by the gross features of the primary disease (eg, bronchiectasis, associated abscess, etc.).
Follicular bronchitis/bronchiolitis consists of lymphoid follicles, often with prominent germinal centers, in a peribronchiolar or peribronchial distribution ( Fig. 22.4A ). The hyperplastic follicles often reside between bronchioles and pulmonary arteries and can compress the bronchiolar lumens ( Fig. 22.4B ). Lymphocytes may be present in the adjacent bronchiolar epithelium. Lymphoid follicles may also be present in a lymphangitic distribution along the interlobular septa and beneath the pleura ( Fig. 22.4A ). These findings can also be present in patients with LIP (see later), and there are cases where both of these patterns occur concurrently.
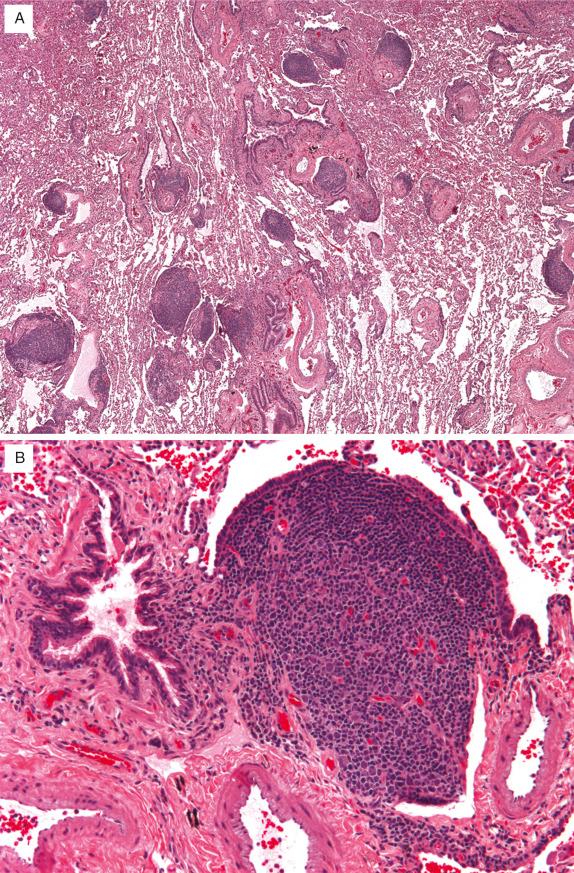
Like germinal centers in reactive lymph nodes, cells in the reactive germinal centers in follicular bronchiolitis express CD20 and lack BCL-2.
The differential diagnosis of follicular bronchiolitis includes LIP, low-grade lymphoma of BALT, pulmonary involvement by chronic lymphocytic leukemia, and nodular lymphoid hyperplasia. Although LIP may show peribronchial and peribronchiolar lymphoid follicles (eg, focal areas of follicular bronchiolitis), this feature is usually overshadowed by the conspicuous diffuse chronic interstitial inflammatory infiltrate that defines this pattern. Although there may be focal involvement of alveolar septa by a chronic interstitial inflammatory infiltrate in follicular bronchiolitis, it is not a prominent feature. Nonetheless, there is substantial overlap between the histologic patterns and clinical conditions associated with follicular bronchiolitis and LIP, and in some cases, the distinction is somewhat arbitrary. Low-grade lymphoma of BALT may have follicles with germinal center formation. However, these lymphomas usually contain confluent areas of monomorphous lymphocytes without germinal centers. Lymphoid cells may permeate cartilage and cause effacement of other normal structures. Similarly, pulmonary involvement by chronic lymphocytic leukemia is distinguished by a monomorphous infiltrate of small lymphocytes with a lymphatic distribution. In contrast to follicular bronchiolitis, nodular lymphoid hyperplasia usually presents as a well-demarcated mass consisting of a confluent proliferation of germinal centers admixed with sheets of interfollicular plasma cells.
The prognosis of idiopathic cases of follicular bronchiolitis is generally good, but it may be worse in patients under age 30. Patients are usually treated with corticosteroids, with variable results. In patients with follicular bronchiolitis associated with a collagen vascular disease or immunodeficiency, treatment is directed at the underlying cause.
Histopathologic pattern in the spectrum of pulmonary lymphoid hyperplasia, characterized by a diffuse, interstitial, polymorphous, predominantly mononuclear inflammatory cell infiltrate
Rare disorder that is often associated with other conditions, including connective tissue diseases (eg, Sjögren syndrome, rheumatoid arthritis), other autoimmune disorders (eg, autoimmune hemolytic anemia), immunodeficiency (eg, AIDS, common variable immune deficiency), infections, drug usage, and allogeneic bone marrow transplantation
Idiopathic LIP is extremely rare
Women are affected more often than men
Usually develops in older patients, but may occur at all ages from infants to very old; median age at diagnosis is in the fifth to sixth decade
Considered an AIDS-defining illness in children less than 13 years old
Symptoms include cough and dyspnea
Spirometry usually shows a restrictive pattern of abnormalities
Laboratory studies may reveal a polyclonal gammopathy or hypogammaglobulinemia
Chest x-ray: patchy bilateral reticulonodular infiltrates with a predilection for the lower lobes
High-resolution CT scan: areas of ground-glass opacity, often accompanied by poorly defined centrilobular and subpleural nodules; cystic spaces or lymph node enlargement may be present
Prognosis is variable and usually determined by the associated disease or condition
Between 30% and 50% of patients die within 5 years; causes of death include infectious complications from treatment, end-stage pulmonary fibrosis, or, rarely, evolution into lymphoma
Treatment consists of corticosteroids with variable results, and antiretroviral agents are used in cases associated with AIDS
Dense, predominantly mononuclear inflammatory cell infiltrate that diffusely expands alveolar septa
Infiltrate consists of lymphocytes, histiocytes, and plasma cells
There may be associated germinal centers along bronchovascular bundles and interlobular septa
Occasional poorly formed granulomas or multinucleated giant cells may be present
Later lesions show variable amounts of fibrosis or even honeycombing
Interstitial lymphocytes are predominantly T-cells that express CD3
Germinal centers along bronchovascular bundles are predominantly B-cells and express CD20
Background plasma cells show a polyclonal pattern of expression of kappa and lambda light chains
Immunoglobulin heavy chain gene rearrangement studies typically show a polyclonal pattern; occasional cases may show oligoclonal bands
Follicular bronchiolitis
Low-grade lymphoma of BALT
Pulmonary involvement by chronic lymphocytic leukemia
Nodular lymphoid hyperplasia
Hypersensitivity pneumonitis
NSIP
Usual interstitial pneumonitis
LIP is a histologic pattern of lung disease characterized by a diffuse, interstitial, polymorphous, predominantly mononuclear inflammatory cell infiltrate. It was first described by Liebow and others in the early 1970s, although many of the original cases would now be considered examples of low-grade lymphoma of BALT. As in the preceding discussion on follicular bronchiolitis, LIP is considered part of the spectrum of pulmonary lymphoid hyperplasia.
Females are affected more often than males. The disease usually affects older individuals with a median age of diagnosis in the fifth to sixth decade. Clinical symptoms include chronic cough and dyspnea, weight loss, and fatigue. Fever, chest pain, and hemoptysis are less common. Laboratory studies may reveal a polyclonal gammopathy or, less frequently, hypogammaglobulinemia or monoclonal gammopathy. Pulmonary function studies most often show a restrictive pattern.
LIP may be associated with or represent the pulmonary manifestation of a number of diseases, including connective tissue diseases (eg, Sjögren syndrome, rheumatoid arthritis), other autoimmune disorders (eg, autoimmune hemolytic anemia), immunodeficiency disorders (eg, acquired immunodeficiency syndrome [AIDS], common variable immune deficiency), and infections. It can develop as a drug reaction and in a setting of allogeneic bone marrow transplantation ( Table 22.1 ). LIP is prevalent in children (but not adults) with AIDS and since 1987 has been considered an AIDS-defining illness in those younger than 13 years old. Cases of idiopathic LIP are rare.
| Connective tissue diseases | Sjögren syndrome Rheumatoid arthritis Systemic lupus erythematosus |
| Other immunologic disorders | Autoimmune hemolytic anemia Pernicious anemia Myasthenia gravis Hashimoto thyroiditis Primary biliary cirrhosis Celiac sprue Dysproteinemia |
| Immunodeficiency | AIDS, particularly in children Common variable immunodeficiency |
| Infections | Pneumocystis jirovecii Legionella pneumonia |
| Chronic active hepatitis | |
| Drug induced/toxic exposure | Dilantin (phenytoin) |
| Allogeneic bone marrow transplantation | |
| Familial | |
| Idiopathic |
Chest radiographs show patchy bilateral reticulonodular infiltrates with a predilection for the lower lobes. On high-resolution CT scan, areas of ground-glass opacity are often accompanied by poorly defined centrilobular and subpleural nodules ( Fig. 22.5 ). Occasionally cystic spaces and lymph node enlargement may also be identified.
LIP is characterized by a dense, interstitial, predominantly mononuclear cell infiltrate that diffusely expands alveolar septa ( Fig. 22.6A ). The infiltrate consists primarily of lymphocytes, histiocytes, and plasma cells ( Fig. 22.6B ). In some cases, there are germinal centers along bronchovascular bundles and septa. The histologic features in these cases overlap with the pattern of follicular bronchiolitis. Other findings occasionally encountered include poorly formed granulomas and multinucleated giant cells, and secondary alveolar changes can include collections of proteinaceous fluid, scattered mononuclear inflammatory cells, and foamy macrophages or giant cells. In later lesions, there may be variable amounts of fibrosis and even honeycombing, ultimately resulting in end-stage lung disease.
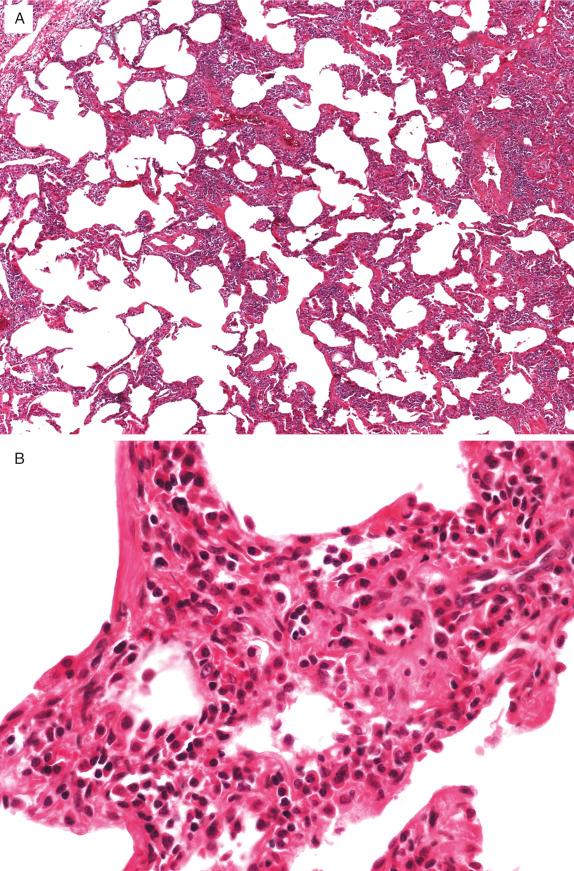
Interstitial lymphocytes consist predominantly of CD3-expressing T-cells, whereas germinal centers in bronchovascular bundles are composed predominantly of CD20-expressing B-cells. Accompanying plasma cells are polyclonal and express kappa or lambda in similar proportions.
A polyclonal pattern is typically present on polymerase chain reaction for immunoglobulin heavy chain gene rearrangement. Occasional cases may show oligoclonal bands. Identification of a clonal B-cell pattern should prompt consideration of a low-grade lymphoma.
The differential diagnosis of LIP is broad. It includes all disorders in which there is a prominent lymphoid infiltrate: low-grade lymphoma of BALT, pulmonary involvement by chronic lymphocytic leukemia, angioimmunoblastic lymphadenopathy, nodular lymphoid hyperplasia, follicular bronchiolitis, hypersensitivity pneumonitis, nonspecific interstitial pneumonitis (NSIP), and in chronic forms, usual interstitial pneumonitis. Pulmonary involvement by low-grade lymphoma or chronic lymphocytic leukemia is distinguished from LIP by the monomorphism of the lymphoid infiltrate. Infiltration of bronchial cartilage and pleura may be present in low-grade lymphoma of BALT, but these are absent in LIP. In cases where morphologic distinction is difficult, immunohistochemical stains and polymerase chain reaction (PCR) analysis for IgH heavy chain rearrangement can be helpful. Interstitial lymphocytes in low-grade lymphomas of BALT and chronic lymphocytic leukemia consist predominantly of B-cells that express CD20. Kappa or lambda light chain restriction can sometimes be demonstrated by immunohistochemical staining of associated plasma cells. PCR analysis for IgH chain gene rearrangements will frequently show a clonal rearrangement.
Angioimmunoblastic lymphadenopathy can occasionally involve the lung in a diffuse pattern. However, this disorder is usually suspected based on the clinical and radiographic features, which include generalized lymphadenopathy combined with hepatosplenomegaly, autoimmune hemolytic anemia, and skin rash. Nodular lymphoid hyperplasia is readily distinguished from LIP by its presentation as a well-demarcated mass, as opposed to the diffuse interstitial involvement of LIP. Although follicular bronchiolitis may show focal extension of the lymphoid cells into the peribronchiolar interstitium, the diffuse interstitial involvement seen in LIP is lacking. Nonetheless, many cases of LIP have associated lymphoid follicles with germinal centers along bronchioles and, in occasional cases, the histologic features overlap, making the distinction between the two somewhat subjective. Hypersensitivity pneumonitis can be distinguished from LIP by its patchy peribronchiolar accentuation; patchy, poorly formed granulomas; and occasional foci of organizing pneumonia. Although interstitial lymphocytic infiltrates are characteristic of cellular NSIP, the density of the lymphoid infiltrate is less than LIP. Nonetheless, NSIP and LIP may be difficult to distinguish morphologically, and many cases of LIP in the older literature would now be reclassified as NSIP. Given their similar etiologies and associations, LIP, in fact, may be considered just a more exuberantly cellular expression of NSIP. Finally, particularly in immunocompromised patients (eg, those with AIDS), infections should be considered as potential etiologies for LIP. In these patients, evidence of EBV, cytomegalovirus, Pneumocystis jirovecii, and mycobacteria should be sought by special stains and/or molecular testing.
The prognosis of LIP is variable. There is an inconsistent response to corticosteroids, and between 30% and 50% of patients die within 5 years. Antiretroviral agents may be effective in cases associated with HIV infection. Causes of death include infectious complications of treatment or advanced pulmonary fibrosis.
A localized form of lymphoid hyperplasia that forms a discrete pulmonary mass
Extremely rare
Males and females are affected equally
Usually diagnosed in older individuals with a median age of 65 (range 19–80)
Usually asymptomatic, although some patients may experience cough, dyspnea, or pleuritic chest pain
Chest x-ray and CT scan show an isolated nodule or mass (64%) or multiple nodules or masses (36%)
Benign disorder without risk of recurrence
Excised to establish the diagnosis
Gray, white, or tan; solid; well-demarcated nodule(s), most often in a subpleural location
Mean dimension is 2 cm, but can measure up to 6 cm
Well-demarcated mass consisting of an aggregate of lymphoid follicles with reactive germinal centers
Sheets of interfollicular plasma cells and foci of interfollicular fibrosis
Lesions lack amyloid, bronchial, or cartilaginous permeation by lymphocytes, Dutcher bodies, and lymphoepithelial lesions
Germinal center cells stain for B-cell markers (CD20)
Interfollicular lymphocytes consist primarily of T-cells, which express CD3 and CD5
Interfollicular plasma cells show a polyclonal pattern of kappa and lambda light chain expression
A significant increase in IgG4-positive plasma cells is present in a subset of cases
Analysis for immunoglobulin heavy chain gene rearrangements is negative
Low-grade lymphoma of BALT
Lymphoid interstitial pneumonia
Follicular bronchiolitis
Inflammatory pseudotumor
Extramedullary plasmacytoma
Nodular lymphoid hyperplasia is considered to represent a localized form of lymphoid hyperplasia, which presents as a discrete pulmonary mass. Perspectives on nodular lymphoid proliferations in the lung have evolved considerably. Although most nodular lymphoid proliferations in the lung were originally considered benign (“pseudolymphomas”), with the recognition of lymphomas of MALT/BALT, it became apparent that most of these lesions represented low-grade lymphomas. Subsequently, however, other reports have presented cases that lacked histologic features of lymphoma and did not show either light chain restriction or immunoglobulin heavy chain gene rearrangement by rigorous immunohistochemical and genetic evaluation. These studies showed that the entity of pulmonary nodular lymphoid hyperplasia, although rare, does exist and is distinct from low-grade lymphoma. The factors triggering this reactive polyclonal expansion of BALT are unknown, although they appear to be different from factors associated with other types of hyperplasia of BALT (LIP and follicular bronchiolitis). There is no association with connective tissue diseases such as Sjögren syndrome or underlying viral infections such as HIV. Based on the presence of acute inflammatory foci in occasional cases of nodular lymphoid hyperplasia, it has been postulated that inflammatory stimuli underlie the development of these reactive lymphoid masses. Other studies have revealed increased numbers of IgG4-positive plasma cells and an increase in the average IgG4/IgG ratio in some cases, compared with low-grade BALT lymphoma, intraparenchymal lymph nodes, and lymphoid hyperplasia. These findings support the concept of pulmonary nodular lymphoid hyperplasia as a benign lymphoplasmacytic proliferation distinct from low-grade BALT lymphoma and raise the possibility, in some cases, of an association with IgG4-related disease.
Men and women are affected in approximately equal numbers. Median age at diagnosis is 65 (range 19–80). Pulmonary symptoms such as cough, dyspnea, and/or pleuritic chest pain are present in a minority of patients.
Chest x-ray and CT scan show an isolated mass (64%) or occasionally multiple nodules (36%). Hilar or mediastinal adenopathy may be present in some patients.
Most lesions consist of gray, white to tan, solid, well-demarcated nodules. The mean diameter is 2 cm, but they can measure up to 6 cm.
Nodular lymphoid hyperplasia is a well-demarcated nodule consisting of aggregates of lymphoid follicles with reactive germinal centers and sheets of interfollicular plasma cells, most often in a subpleural location ( Fig. 22.7A ). Plasma cells may contain Russell bodies. Foci of interfollicular fibrosis may be present and may focally efface the underlying pulmonary parenchyma. Significantly, Dutcher bodies, bronchial or pleural permeation, lymphoepithelial lesions, and amyloid are absent. Resected hilar or mediastinal nodes, when present, show reactive lymphoid hyperplasia.
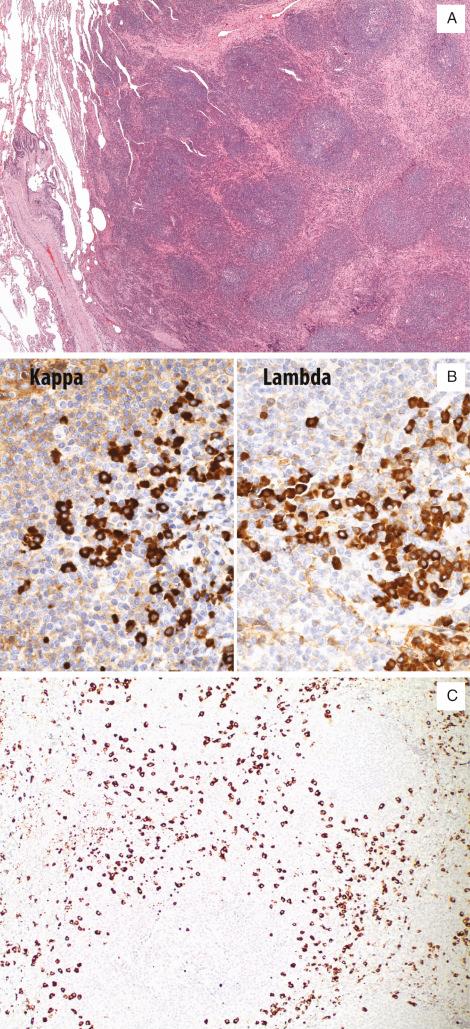
Immunohistochemical stains for lymphoid markers are consistent with a reactive process. Germinal centers of follicles stain for B-cell markers (CD20), whereas interfollicular lymphocytes consist primarily of T-cells that stain for CD3 and CD5. BCL-2 expression is absent in germinal centers, but present in the mantle zone and interfollicular T-cells. A polyclonal pattern of expression of kappa and lambda light chains is present in interfollicular plasma cells ( Fig. 22.7B ). A subset of cases may show an increase in IgG4-positive plasma cells (average 78/hpf, range 15/hpf–167/hpf in one study) ( Fig. 22.7C ).
PCR analysis for immunoglobulin heavy chain gene rearrangements shows a polyclonal pattern.
The main entity in the differential diagnosis of nodular lymphoid hyperplasia is MALT (BALT) lymphoma. Other entities to be considered include LIP, follicular bronchiolitis, inflammatory pseudotumor, and plasmacytoma. Nodular lymphoid hyperplasia can usually be distinguished from MALT lymphoma by a number of findings ( Table 22.2 ). MALT lymphomas tend to show infiltration of pleura and bronchial cartilage, features that should not be seen with nodular lymphoid hyperplasia. Lymphangitic spread is usually prominent in MALT lymphomas but mild and focal in nodular lymphoid hyperplasia. Lymphoepithelial lesions are absent in nodular lymphoid hyperplasia but variably present in BALT lymphoma. Likewise, intranuclear inclusions (Dutcher bodies) are absent in nodular lymphoid hyperplasia but variably present in MALT lymphoma. Immunohistochemical staining for kappa and lambda light chain restriction shows a polyclonal pattern in nodular lymphoid hyperplasia but a monoclonal pattern in most cases of MALT lymphoma. Staining of lymphocytes in lymphoepithelial lesions for CD43 and CD20 can be found in low-grade lymphomas of MALT but not nodular lymphoid hyperplasia. IgG4-positive plasma cells may be increased in pulmonary nodular lymphoid hyperplasia but not in cases of MALT lymphoma. Additionally, immunoglobulin heavy chain gene rearrangements can be identified in most cases of MALT lymphoma but are not present in nodular lymphoid hyperplasia. Recent studies have suggested that plasmacytoma is a form of MALT lymphoma. Unlike nodular lymphoid hyperplasia, it consists of a monomorphous population of plasma cells that show light chain restriction that can be detected by immunohistochemistry.
| Histology and Immunohistochemistry | Nodular Lymphoid Hyperplasia | MALT Lymphoma |
|---|---|---|
| Architecture | Well-circumscribed lesion, usually localized | May be localized, but is infiltrative, often invading the pleura and bronchial cartilage |
| Lymphangitic spread | Focal, mild | Prominent |
| Cellularity | Reactive germinal centers with interfollicular small lymphocytes and plasma cells | Polymorphic: monocytoid B-cells, centrocyte-like (cleaved) atypical lymphocytes and plasma cells |
| Germinal centers | Reactive, with no follicular colonization by neoplastic cells | Reactive, with colonization by neoplastic cells |
| Lymphoid population | Polymorphous | Monomorphous or polymorphous |
| Monocytoid B-cells | Inconspicuous or absent | May be conspicuous |
| Plasma cells | Usually not extensive | May be extensive |
| Intranuclear inclusions (Dutcher bodies) | Absent | Variably present |
| Amyloid | Absent | May be abundant |
| Lymphoepithelial lesions | Absent | Variably present |
| Plaquelike pleural infiltration | Absent or inconspicuous | Variably present |
| Kappa/lambda reactivity | Polyclonal | Monoclonal in approximately 40% of cases |
| Bcl-2 reactivity | Negative germinal centers | Negative in the follicular center cells but positive in the colonizing neoplastic lymphocytes |
| Immunoglobulin heavy chain gene rearrangement | Negative | Positive in 60% |
Nodular lymphoid hyperplasia is usually easily distinguished from LIP and follicular bronchiolitis. The latter two entities typically involve the lung in a diffuse manner, as opposed to the discrete mass seen in nodular lymphoid hyperplasia. Nodular lymphoid hyperplasia can be distinguished from inflammatory pseudotumor (inflammatory myofibroblastic tumor) of the lung by the absence of an associated myofibroblastic or fibrohistiocytic spindle cell proliferation.
Nodular lymphoid hyperplasia is a benign lesion that does not recur if it is excised, although surgical excision is usually needed to enable correct diagnosis.
Spectrum of fibroinflammatory lesions affecting various organ systems, including the pancreas, bile duct, gallbladder, retroperitoneum, salivary gland, aorta, lacrimal gland, kidney, mediastinum, and lung
Pulmonary involvement may occur either alone or in association with involvement of other organ systems
Rare
Males are affected more often than females
Affected patients are typically middle age or older (range 40–86)
Many patients lack pulmonary symptoms. When present they are generally mild and include cough, dyspnea, hemoptysis, chest discomfort, and pleural effusion
Levels of IgG4 are increased in 80% to 90% of patients
Spirometry is normal in most patients (64%) but may also show obstructive or restrictive patterns (24% and 12%, respectively)
Findings on CT scan are variable and include:
Nodules, solitary or multiple
Thickening of bronchovascular bundles, interlobular septa and pleura
Single or multiple round, ground-glass opacities
Areas of consolidation
Honeycombing
Increased uptake is present on FDG-PET
Prognosis is generally excellent
Treatment consists of steroids
Rare patients may undergo spontaneous remission
Discrete gray nodular lesions
Firm lung parenchyma and pleural thickening
Ill-defined nodular lesions up to 1.5 cm in diameter
Fibroinflammatory masses in a lymphangitic distribution along the pleura, interlobular septa, and bronchovascular bundles
Variable associated lymphoplasmacytic infiltrate
Intimal endothelial inflammation commonly present in both arteries and veins
Increase in IgG4-positive plasma cells (range 24–229 in three high-powered fields)
Increase in IgG4-to-IgG ratio (range 16%–71%)
Subset of cases of other disease patterns: inflammatory pseudotumor, grade I LYG, NSIP, pulmonary hyalinizing granuloma, and pulmonary nodular lymphoid hyperplasia also show an increase in IgG4-positive plasma cells and thereby may represent different expressions of the disease
Not usually necessary
NSIP
Grade I LYG
Follicular bronchiolitis
Inflammatory pseudotumor
Pulmonary nodular lymphoid hyperplasia
Pulmonary involvement by Rosai-Dorfman disease
Pulmonary involvement by Erdheim-Chester disease
IgG4-related disease consists of a spectrum of fibroinflammatory lesions that affect various organ systems, including the pancreas, bile duct, gallbladder, retroperitoneum, salivary gland, aorta, lacrimal gland, kidney, mediastinum, and lung. Affected organs show tumorlike masses and/or fibrosis with infiltration by numerous IgG4-positive plasma cells and lymphocytes. High levels of serum IgG4 are often found in affected patients, who often respond readily to corticosteroid therapy.
Pulmonary manifestations of IgG4-related disease have been reported both in patients with and without concurrent autoimmune pancreatitis. Many of these patients also show elevated serum levels of IgG4. As in other organ systems, these cases are generally characterized by fibroinflammatory masses with an associated increase in IgG4-positive plasma cells. Other lesions suggested as potentially within the spectrum of IgG4-related disease include the plasma cell–rich variant of inflammatory pseudotumor, an interstitial pneumonitis resembling idiopathic NSIP, some lesions formerly thought to represent grade I LYG, pulmonary hyalinizing granuloma, and a subset of cases of pulmonary nodular lymphoid hyperplasia.
Males are affected more often than females. Affected patients are typically middle aged or older, ranging from 40 to 86 years of age. Reported symptoms of pulmonary involvement by IgG4-related disease include cough, hemoptysis, dyspnea, chest discomfort, and pleural effusion. In approximately half of cases, patients are asymptomatic with abnormalities identified on chest imaging studies.
Patterns on CT are variable and include thickening of bronchovascular bundles and interlobular septa, single or multiple round ground-glass opacities, solitary or multiple areas of consolidation, nodules (solitary or multiple), and honeycombing. These patterns generally correspond with histologic features. For example, the areas of ground-glass opacity resemble NSIP both radiographically and pathologically. In addition to these findings, hilar and mediastinal lymphadenopathy are typically present.
Positron emission tomography (PET) in five patients showed high-grade accumulation of 18-fluorodeoxyglucose (FDG) (three of three patients) and accumulation of gallium citrate (two of two patients). FDG-PET has been proposed as a means of assessing the extent of IgG4-related disease and its response to therapy in other organ systems.
Macroscopic features are only described in rare reports based on the appearance of surgical lung biopsies. Gross findings are variable but reflect the findings on imaging studies. Biopsies may show discrete gray nodular lesions, firm lung parenchyma, and pleural thickening or ill-defined nodular lesions up to 1.5 cm in diameter.
Histologic features of IgG4-related disease involving the lung vary. Generally, affected patients have fibroinflammatory masses ( Fig. 22.8A ). These have a characteristic morphology consisting of a lymphohistiocytic inflammatory infiltrate with numerous admixed plasma cells accompanied by fibrosis in a lymphangitic distribution along the pleura, interlobular septa, and bronchovascular bundles ( Fig. 22.8B ). Intimal endothelial inflammation often affects both arteries and veins ( Fig. 22.8C ). A subset of cases of other disease patterns, such as the plasma cell–rich variant of inflammatory pseudotumor, grade I LYG, pulmonary hyalinizing granuloma, and pulmonary nodular lymphoid hyperplasia, show an increase in IgG4-positive plasma cells and are postulated to potentially represent, in some cases, different morphologic expressions of IgG4-related disease.
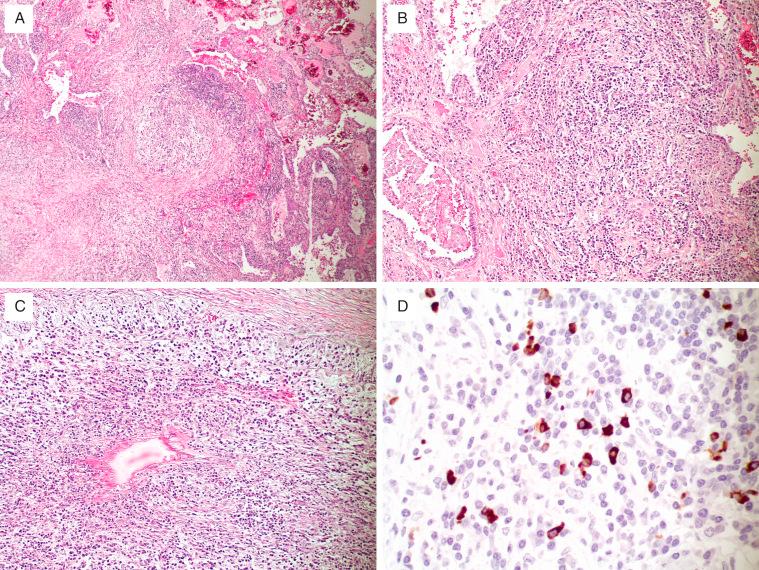
An elevated serum level of IgG4 is identified in 80% to 90% of patients with pulmonary involvement by IgG4-related disease.
Immunohistochemical stains show an increased number of IgG4-positive plasma cells (range 24–229 in three high-power fields), as well as an increased IgG4-to-IgG plasma cell ratio (range 16%–71%) ( Fig. 22.8D ). A recent consensus statement on the pathology of IgG4-related disease in various organs has suggested a minimum of 20 IgG4-positive plasma cells/hpf in small biopsies and 50 IgG4-positive plasma cells/hpf in surgical lung biopsies are required to support the interpretation of IgG4-related disease. These counts are only helpful in the appropriate clinical and pathologic contexts. Other characteristic histopathologic features—namely, fibrosis or a dense lymphoplasmacytic infiltrate—should be present. Other diseases (eg, malignancies) may also harbor increased numbers of IgG4-positive plasma cells.
Cases of grade I LYG, plasma cell–rich inflammatory pseudotumor, pulmonary hyalinizing granuloma, and pulmonary nodular lymphoid hyperplasia may have an increased number of IgG4-positive plasma cells on immunohistochemistry. The significance of these findings is not completely clear. They may represent alternative morphologic expressions of IgG4-related disease or, alternatively, a nonspecific increase in IgG4-positive plasma cells occurring through some other mechanism. Higher-grade lesions of LYG (grade II and III) are easily distinguished by the presence of atypical cells, which stain positively for EBV by in situ hybridization techniques. Inflammatory myofibroblastic tumor is in the differential of plasma cell–rich variants of inflammatory pseudotumors. These are true neoplasms with a fascicular proliferation of spindled cells. The number of associated IgG4-positive plasma cells is usually lower as well.
A pattern resembling NSIP may be present in some patients with IgG4-related sclerosing disease. Biopsies show a diffuse interstitial lymphoplasmacytic infiltrate, which expands alveolar septa, is present along bronchovascular bundles, and may involve the pleura. The infiltrate also involves small arterioles and venules, which may show obliterative arteritis and phlebitis. Abundant IgG4-positive plasma cells are present on immunohistochemical staining. This histologic pattern generally corresponds to those cases with the radiographic findings of ground-glass infiltrates. It is distinguished from idiopathic cases of NSIP by the increased numbers of IgG4-positive plasma cells present.
Erdheim-Chester disease and pulmonary involvement by Rosai-Dorfman disease are similar to pulmonary involvement by IgG4-related disease in that they have a prominent lymphangitic distribution. Erdheim-Chester disease, however, generally lacks the marked lymphoplasmacytic inflammatory background and vascular inflammation found in IgG4-related disease. An increase in IgG4-positive plasma cells is not identified. Moreover, in contrast to cases of Erdheim-Chester disease, characteristic radiographic findings in long bones are absent. Some cases of Rosai-Dorfman disease do show an increase in IgG4-positive plasma cells and can pose difficulty in the differential diagnosis. Rosai-Dorfman disease typically presents clinically with massive bilateral cervical lymphadenopathy. It is characterized by an infiltrate of large histiocytes with eosinophilic cytoplasm containing entrapped lymphocytes. This latter feature has been termed emperipolesis. Emperipolesis may be present focally in pulmonary involvement by IgG4-related disease but is usually not as prominent as in Rosai-Dorfman disease. Nonetheless, the similarity in some of the histologic and immunohistochemical features between Rosai-Dorfman disease and IgG4-related disease has prompted some authors to suggest these disorders overlap.
Prognosis in pulmonary involvement by IgG4-related disease is excellent and similar when it involves other organs. Patients generally respond well to steroids, although radiographic abnormalities may remain. Rare patients have undergone spontaneous remission.
Postfollicular low-grade B-cell lymphoma arising from BALT; considered part of the spectrum of extranodal marginal zone lymphomas arising in association MALT of diverse organs (lung, gastrointestinal tract, salivary gland, thyroid)
Rare, but it is the most common type of primary pulmonary lymphoma (95%)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here