Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primary lung neoplasms in children are rare, occurring much less frequently than congenital malformations, inflammatory masses, and metastatic tumors. In one published review of pulmonary masses at a large pediatric hospital, the ratio of primary neoplasms to metastatic lesions to nonneoplastic lesions was 1:5:60. In most pediatric series, primary malignant pulmonary neoplasms outnumber benign neoplasms. However, numbers vary depending on patient population and definition of benign neoplasm, with some series including vascular/lymphatic malformations and inflammatory myofibroblastic tumors (IMTs) in this category. Primary pulmonary malignancies represent approximately 0.2% of all pediatric malignancies.
Although almost all adult-type pulmonary epithelial neoplasms have been reported in childhood, less than 0.2% of lung cancers occur in the first two decades of life. Endobronchial tumors, including carcinoid and mucoepidermoid tumors, are the most common pulmonary epithelial malignant tumors of childhood, seen most frequently in older children and adolescents. These endobronchial tumors are typically low grade, with rare case reports of neuroendocrine carcinoma and high-grade mucoepidermoid carcinoma. Acinic cell carcinoma is exceedingly rare. Endobronchial tumors are more likely to represent IMT in a young child or infantile hemangioma in an infant. Common types of adult lung cancers are extremely rare in children. In most reviews of pediatric lung tumors, adenocarcinoma, squamous cell carcinoma, and basaloid carcinoma are in young adults older than 17 years of age. There are rare reports of adenocarcinoma in situ in children with congenital pulmonary airway malformation (CPAM) type I, including one at 6 years of age. Squamous cell carcinoma has been reported in the bronchus of a 6-year-old child. Pulmonary blastoma (distinct from pleuropulmonary blastoma [PPB]) is rarely found in childhood. Laryngotracheal papillomatosis, associated with human papillomaviruses, occurs in childhood and may rarely result in spread to pulmonary airways.
IMT occurs throughout the body and at all ages, with 25% of pediatric tumors located in the lung. In the pediatric population, IMT is most frequent in the second decade of life but can occur in the newborn period. This mesenchymal tumor, recently reclassified as of intermediate biologic potential, is the most common endobronchial tumor of childhood.
Pulmonary tumors that are essentially limited to childhood include PPB, congenital peribronchial myofibroblastic tumor (CPMT), and fetal lung interstitial tumor (FLIT). This group of tumors shares features of apparent origin from primitive pulmonary mesenchyme and early age at presentation (all occurring in the perinatal period/early infancy, with PPB also presenting at a later age). Clinical behavior, however, is vastly different. CPMT and FLIT represent benign tumors, whereas PPB follows the course of an aggressive malignant neoplasm. These three tumors are described in the following sections.
Although rare, PPB is the most common malignant pulmonary tumor of childhood, with approximately 25 new cases reported to the International Pleuropulmonary Blastoma Registry (IPPBR) per year and 350 cases listed to date. This mesenchymal tumor, first described as an entity in 1988, is a dysembryonic/dysontogenetic tumor analogous to Wilms tumor, retinoblastoma, and hepatoblastoma. Previous case reports have used nomenclature such as rhabdomyosarcoma and malignant mesenchymoma arising in a congenital pulmonary cyst. The tumor has also been reported as “pulmonary blastoma”; however, PPB lacks a malignant epithelial component and is clinically distinct from the biphasic pulmonary blastoma.
Malignant pulmonary mesenchymal tumor of infancy and childhood, associated with DICER1 familial tumor predisposition syndrome
Rare, although the most common malignant pulmonary tumor in children
Type I is more common in males; otherwise, no gender predominance
Median age: type I—8 months, type II—35 months, type III—41 months
Extremely rare in adults, with exception of type Ir PPB
Chest pain, fever, cough, respiratory distress, pneumothorax (cystic tumors)
Unilocular or multilocular cysts in type I; solid component present in type II (partially cystic) and type III
Tumors can be multifocal and bilateral
Pneumothorax and effusions may be present
Prognostic factors include tumor type and presence or absence of metastases
Type I PPBs are treated with complete surgical excision; the role of adjuvant chemotherapy is uncertain
Type II and type III PPBs are treated with complete surgical excision when possible, immediately followed by chemotherapy; neoadjuvant chemotherapy is sometimes used
Local recurrence is more common with types II and III PPBs than with type I PPBs; type I PPBs can recur with a solid component
Distant metastasis, especially to the brain, spinal cord, or bone, is not unusual in patients with type II or type III PPB
Survival is dependent on type: 5-year survival rates are 91% for type I, 59% for type II, and 37% for type III (including solid tumors with uncertain cystic component)
PPB is the sentinel disease of the DICER1 familial tumor predisposition syndrome, and 25% of PPBs are associated with this syndrome. Two-thirds of PPBs have heterozygous germline mutations in DICER1, a gene involved in mesenchymal-epithelial interactions of various organs during early development. Malignant transformation is thought to involve a “second hit” with somatic DICER1 mutation. In addition to PPB, some of the numerous tumors associated with the syndrome include cystic nephroma, ovarian sex cord stromal tumors, botryoid embryonal rhabdomyosarcoma (ERMS), thyroid tumors, and nasal chondromesenchymal hamartoma. In addition to DICER1 mutation, trisomies 8 and 2 have been found in some PPBs.
Type I: thin-walled unilocular or multilocular cysts, without gross thickening of cyst wall
Type II: solid and cystic components
Type III: solid tumor, gelatinous white with areas of hemorrhage and necrosis
Cyst walls are lined by benign nonciliated epithelium and contain subepithelial condensations of small primitive cells with or without rhabdomyoblastic differentiation
Nodules of immature cartilage may be present
Fibrous, vascular cyst walls without condensed immature mesenchyme or sarcoma; fibrous nodules are sometimes present
Thickened areas of sarcomatous or blastematous overgrowth within septa
Solid component similar to that of type III PPB
Mixture of sarcomatous and blastematous elements
Sarcomatous areas include rhabdomyosarcoma, fibrosarcoma, chondrosarcoma, and undifferentiated sarcoma
Anaplasia and marked pleomorphism are usually present
Immunohistochemical staining depends on represented sarcomatous components
Blastema stains weakly for vimentin and smooth muscle actin
Type I PPB
Type IV congenital pulmonary airway malformation
Cystic synovial sarcoma
Synovial sarcoma
Various sarcomas arising from the chest wall (rhabdomyosarcoma, Ewing sarcoma/peripheral neuroectodermal tumor)
Congenital infantile fibrosarcoma
PPB is subclassified into type I (entirely cystic), type II (cystic and solid), and type III (entirely solid) tumors, which demonstrate a biologic progression from nonmetastasizing purely cystic lesions to highly aggressive malignant tumors. More recently, a type Ir lesion has been culled out from classic type I tumors. Type Ir lesions lack immature mesenchyme and malignant histologic features and are considered to be a regressed or nonprogressed cystic PPB.
Type I tumors represent a third of PPBs and are more common in males than females. Classic type I tumors occur at a median age of 8 months and almost all present before 3 years of age. Cystic PPBs have been diagnosed in the third trimester by fetal ultrasound, and one tumor was reported at 21 weeks’ gestation by screening ultrasound. In contrast to classic type I PPB, type Ir tumors present from infancy to adulthood, with a median age at diagnosis of 46.5 months. Pneumothorax is a common presentation for both classic type I and Ir tumors. Purely cystic tumors have not been associated with metastases; however, both type I and Ir PPBs can recur as more aggressive tumors with a solid component.
Type II and III PPBs present at a median age of 35 and 41 months, respectively, with an equal male-to-female ratio. Most tumors are diagnosed before 7 years of age, with a few in older children. Only one case has been reported in an adult to date (type II, at 36 years of age). Type II and III tumors are extremely rare in infancy, with only one reported before a year of age. Presenting symptoms include chest pain, fever, cough, and respiratory distress. Pneumothorax is less common than with type I tumors. Approximately 10% of patients present with distant metastases, most commonly in the central nervous system, followed by bone.
Images of type I tumors show unilocular or multilocular air-filled cysts within the lung or, less frequently, arising from pleura ( Fig. 6.1 ). Cysts are usually unifocal but can be multifocal or bilateral. When a solid component is present (type II and III), there may be infiltration of diaphragm, chest wall, mediastinum, or great vessels. Pneumothorax and pleural effusions may be present.
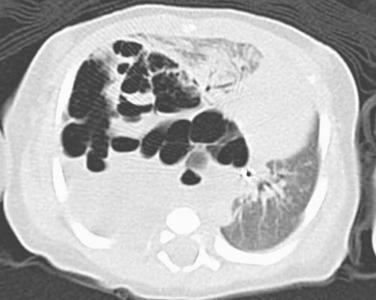
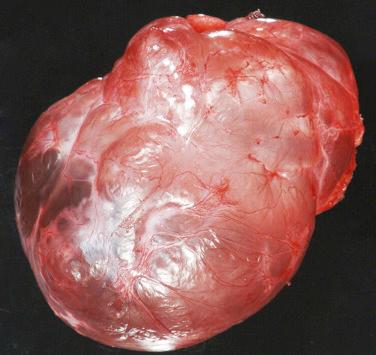
Type I PPB consists of a thin-walled unilocular or multilocular cyst, without thickened or nodular walls ( Figs. 6.2 and 6.3 ). Type II tumors have both cystic and solid components. The solid components of type II and III tumors are gelatinous and white, with areas of hemorrhage and necrosis. Tumors may be greater than 10 cm in size.

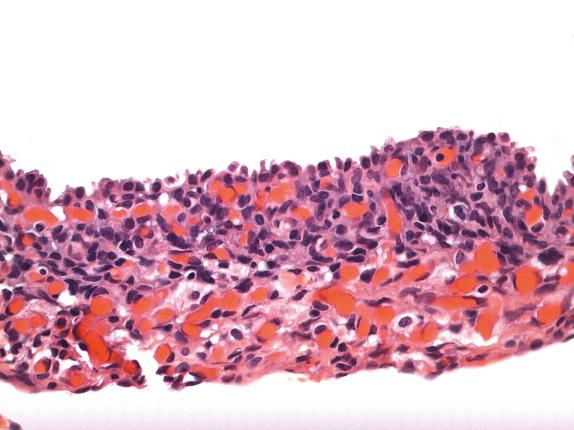
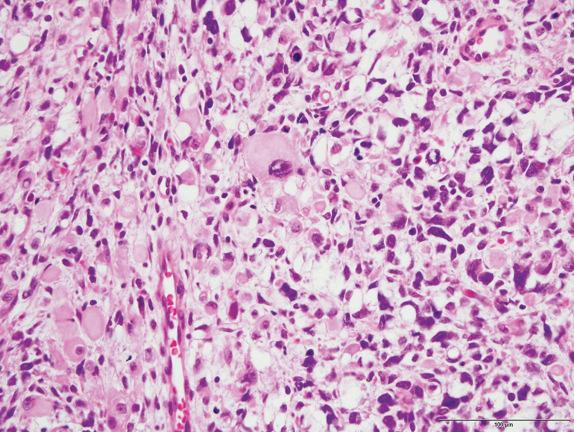
Type I PPB is composed of thin, delicate septa lined by bland, flattened to cuboidal nonneoplastic epithelium. Underlying this epithelium is a condensation of primitive mesenchymal cells (blastemal-like) with enlarged hyperchromatic nuclei or frankly malignant sarcoma, without gross thickening of the septal wall. This condensation of atypical cells may form an obvious subepithelial cambium but is sometimes extremely subtle, with only rare small nodules of immature cells ( Figs. 6.4 and 6.5 ). In the latter case, microscopic examination of the entire lesion may be required for correct diagnosis. Sarcomatous foci may include ERMS or fibrosarcoma. Rhabdomyoblast-like strap cells and nodules of immature cartilage may be present. Anaplasia is rare. The tumor transitions to normal lung parenchyma without intervening capsule.
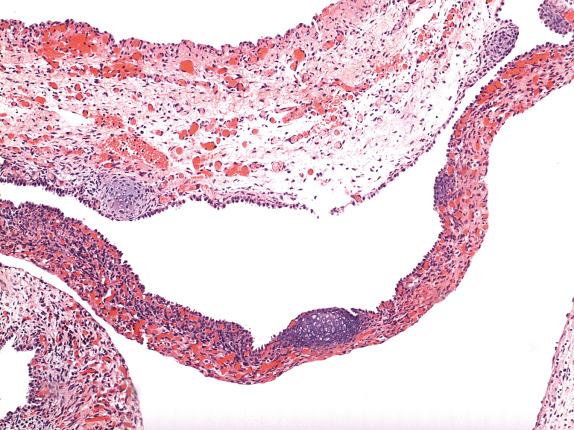
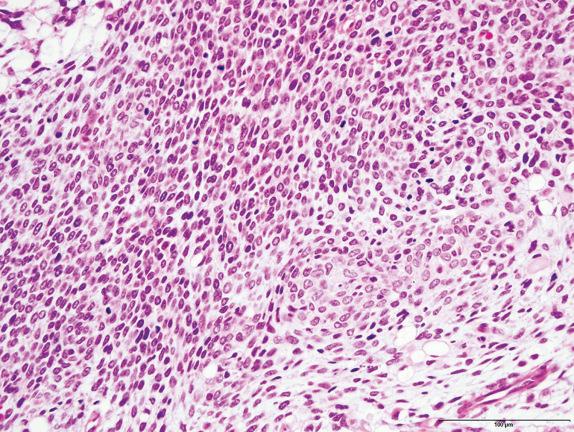
The cystic areas of type II PPB are similar to type I tumors, but solid, expansile areas are also present. The solid areas of types II and III PPB contain blastema with immature cartilage and sarcoma, frequently of diverse types, including RMS, FS, and chondrosarcoma ( Figs. 6.6, 6.7, and 6.8 ). Anaplasia is usually present.
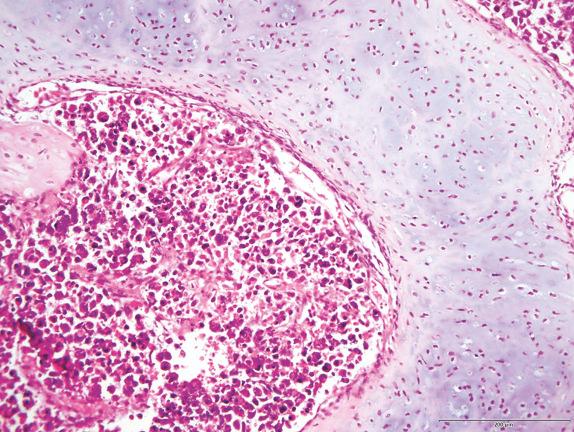
Type Ir PPB consists of thin, fibrous vascular septa, without foci of primitive mesenchyme or sarcoma ( Fig. 6.9 ). Necrosis and fibrous nodules may be present. Histology resembles type IV CPAM, and it is likely that most type IV CPAMs are actually regressed/nonprogressed cystic PPB.
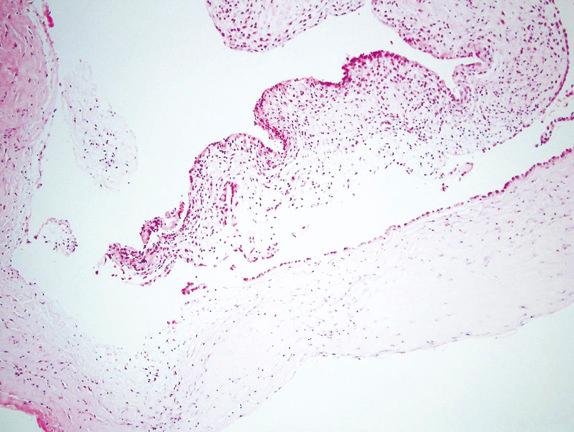
The main differential diagnosis of type I cystic PPB includes type II and IV CPAM. Type II CPAM is distinguished from type I PPB by the presence of cystic bronchiolar-like structures lined by columnar respiratory epithelium with intervening alveoli. As mentioned, type IV CPAMs most likely represent type I PPB. Reports of PPB arising in the setting of incompletely resected presumed CPAM support this viewpoint. The diagnosis of type I/Ir PPB should not be excluded without careful microscopic examination of presumed type IV CPAM.
PPBs with solid components are extremely rare in infancy. In the perinatal period and infancy, the differential diagnosis for pulmonary solid tumors on imaging includes type III CPAM (microcystic), IMT, FLIT, and CPMT. These tumors are histologically distinct from solid PPBs (see later sections). Congenital infantile fibrosarcoma is distinguished from solid PPB by its presentation at a younger age and more likely origin from chest wall. The differential diagnosis of solid PPB in older children includes synovial sarcoma, RMS, and Ewing sarcoma/peripheral neuroectodermal tumor (ES/PNET). Location of tumor is important in distinguishing PPB from these tumors, because RMS and ES/PNET are much more likely to originate from the chest wall than the lung. Types II and III PPBs usually have a mixture of sarcoma types; however, PPB with a predominantly spindle cell component, can be challenging to diagnose, especially in a limited biopsy specimen. Immunohistochemical and molecular studies may be needed to distinguish synovial sarcoma from PPB. Biphasic pulmonary blastomas typically present in adults but can occur in children. Unlike PPB, pulmonary blastomas have both malignant epithelial and mesenchymal elements.
Type I cystic PPB is treated by surgical resection. Adjuvant chemotherapy has been used in some patients; however, the role of chemotherapy in type I lesions is uncertain. The most recent IPPBR studies show no difference in disease-free survival (DFS) or overall survival (OS) with the addition of chemotherapy. Metastatic disease has not been reported with type I PPB, but 9% of cases recur as more aggressive type II or III tumors. Five-year DFS and OS are 82% and 91%, respectively. Metastatic workup is not needed with type I PPB; however, it is important to determine whether there are other tumors in the patient or family members that may suggest DICER1 familial predisposition syndrome. Early screening of DICER1 mutation carriers for cystic PPB may help prevent progression of cystic tumors to more aggressive solid tumors.
Type II and III PPBs are treated by surgical resection when possible, followed by multiagent chemotherapy. Neoadjuvant chemotherapy has been used in large nonresectable tumors. High-dose chemotherapy and stem cell rescue have been used in some cases. Radiation treatment does not appear to have an impact on patient survival. The 5-year DFS and OS for type II PPB are 71% and 59%, respectively. The 5-year DFS and OS rates for type III PPB (and solid tumors with uncertain cystic component) are 53% and 37%, respectively.
Prognostic factors include tumor type and presence of metastatic disease. Studies have not shown prognostic significance for the presence of anaplasia or DICER1 mutation. The significance of complete gross resection is not certain due to incomplete data on surgical margins.
The name CPMT was first used in 1993 to describe a group of rare, perinatal mesenchymal tumors with nonmalignant clinical behavior. CPMT is thought to arise from peribronchial mesenchyme found at 12 weeks’ gestation. Histologically, CPMT is composed primarily of a fascicular, spindle cell proliferation with myofibroblastic phenotype; however, tumors show marked variation in cellularity and mitotic activity. Nomenclature for the tumor in case reports before 1993 reflects this histologic variability, with names such as bronchopulmonary fibrosarcoma, bronchopulmonary leiomyosarcoma, hamartoma, mesenchymal tumor, and congenital mesenchymal malformation. CPMT has been suggested to be analogous to mesoblastic nephroma of the kidney and congenital spindle cell tumor of the intestine. The tumor also shows some resemblance to metanephric stromal tumor of the kidney, which can present in the perinatal period.
Perinatal benign pulmonary tumor arising from primitive peribronchial mesenchyme
Extremely rare
More frequent in males
Reported from 20 weeks’ gestation to 8 weeks of age
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here