Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver is composed of lobules of hepatocytes that are bracketed by vascular structures with the central, or hepatic, veins on one side and the portal tracts (with portal veins and hepatic arteries) on the other side. Oxygenated blood from the hepatic artery mixes with blood from the portal vein in the hepatic sinusoids, which bathe the hepatocytes. The relatively oxygen-rich blood becomes progressively oxygen-depleted as the blood flows through the sinusoids toward the central veins. This oxygen gradient sets up functional “zones” in the lobules of hepatocytes: zone 1—periportal hepatocytes (oxygen-rich); zone 3 hepatocytes (oxygen-poor); and zone 2, which has intermediate oxygenation ( Figs. 66.1 and 66.2 ).
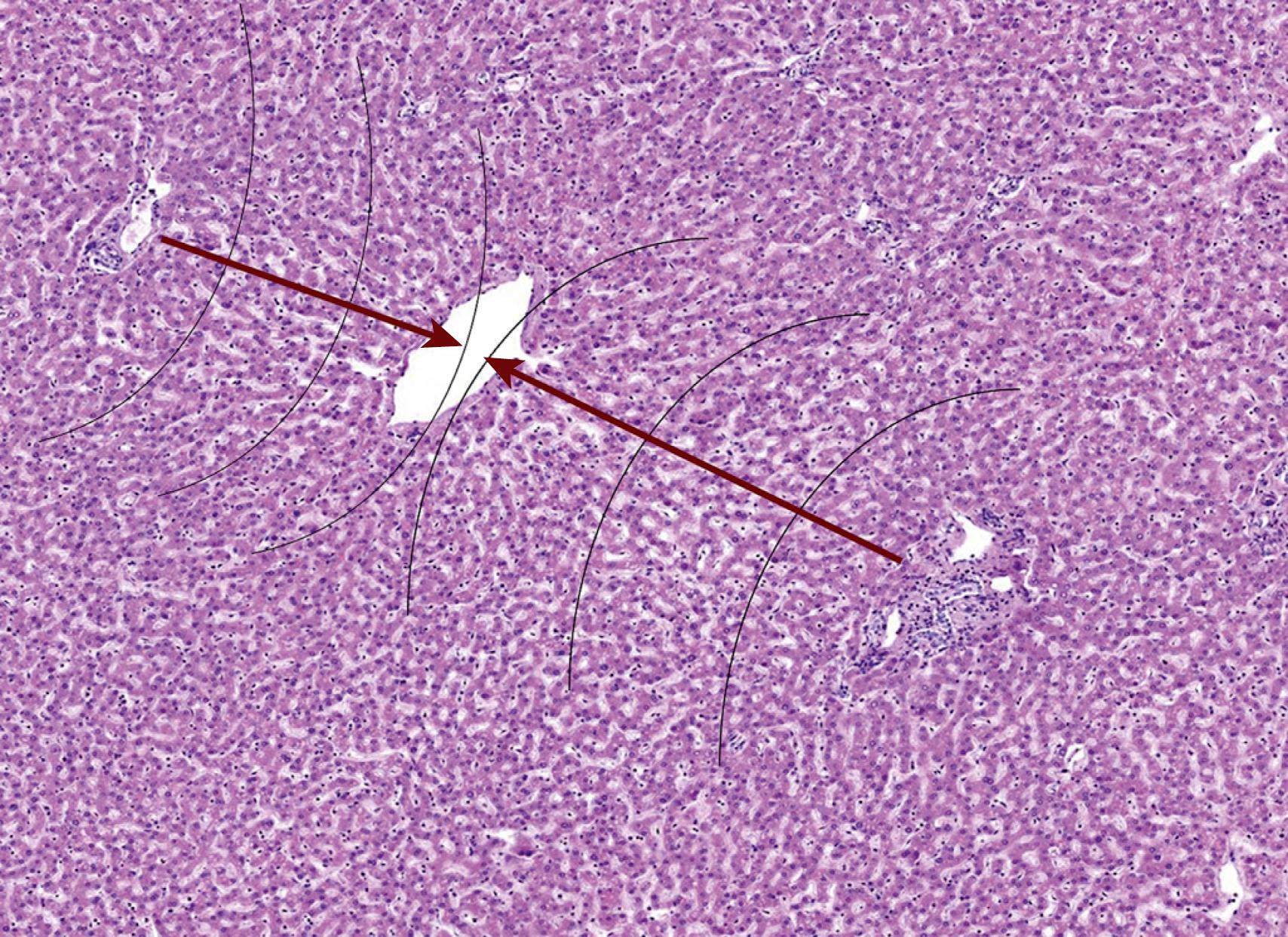
The functional histology of the liver is used every day by pathologists when assessing liver specimens and will be discussed throughout this chapter to highlight key pathologic findings. In essence, liver pathology is a based upon the recognition of patterns, as there are only so many ways the liver can be injured. Once the pattern is recognized, correlation with the clinical impression and laboratory data help to fine-tune the differential diagnosis. This is particularly true when evaluating medical liver biopsies. For example, acute hepatitis primarily affects the hepatic lobules (the zones of hepatocytes) rather than the portal tracts, but the precise cause of the hepatitis usually requires clinical history and laboratory data. The same is true for chronic hepatitis, bile duct loss, biliary obstruction, and most other important patterns in liver pathology.
Rather than attempt at providing an exhaustive review of liver pathology, this chapter is focused on providing a practical summary of the most important issues that face pathologists. The clinical features of the entities are well-covered in other sections of this textbook, so this chapter will highlight key pathologic findings, differential diagnoses, and diagnostic pitfalls of pediatric liver pathology.
In a neonate or young infant with a clinical picture of cholestasis ( Fig. 66.3 ), the first job of the pathologist when evaluating a liver biopsy is to assess the material for signs of biliary obstruction. The histologic features of obstruction are mostly present in the portal tract. Portal edema and ductular reaction, with or without bile plugs in ductules, are the portal hallmarks of biliary obstruction of any cause. These portal-based changes are the key findings in biliary atresia biopsies, but they are not 100% specific. Essentially, any cause of biliary obstruction can generate similar biopsy findings. A pitfall is that the lobular changes in biliary atresia may “outweigh” the portal changes on first look. The pathologist, therefore, must always examine the portal tracts for signs of obstruction no matter what the lobular changes are.
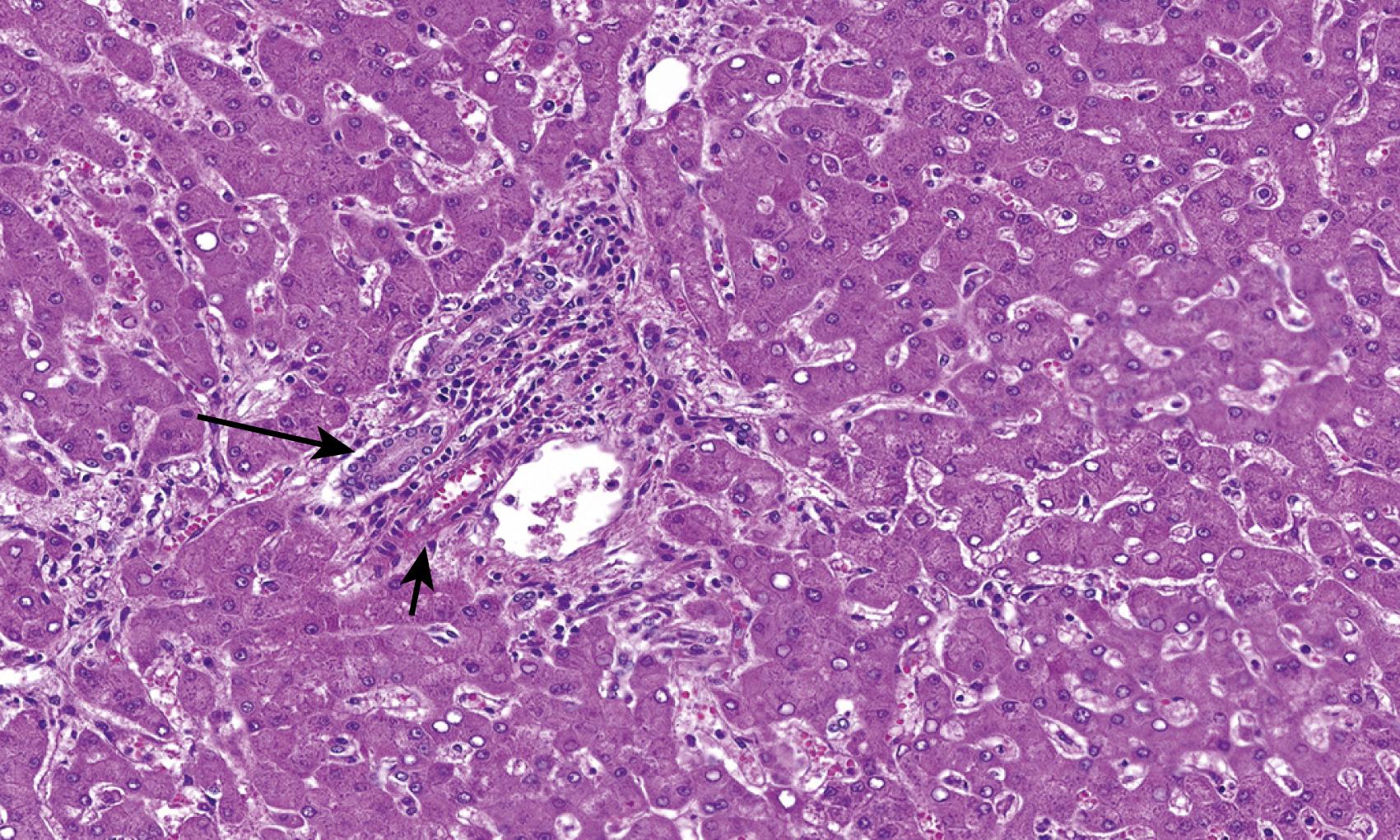
The classic histologic triad of extrahepatic biliary obstruction involves lobular cholestasis in bile canaliculi (canalicular cholestasis), ductular proliferation, and portal edema ( Fig. 66.4 ). Cholestasis is often first identified in a perivenular location (i.e., around central veins). Uniquely, neutrophils are often seen accompanying the proliferating bile ductules; some have termed this phenomenon “pericholangitis.” This should not be confused with true ascending cholangitis, in which the lumina of native interlobular bile ducts (rather than proliferating ductules) are filled with neutrophils.
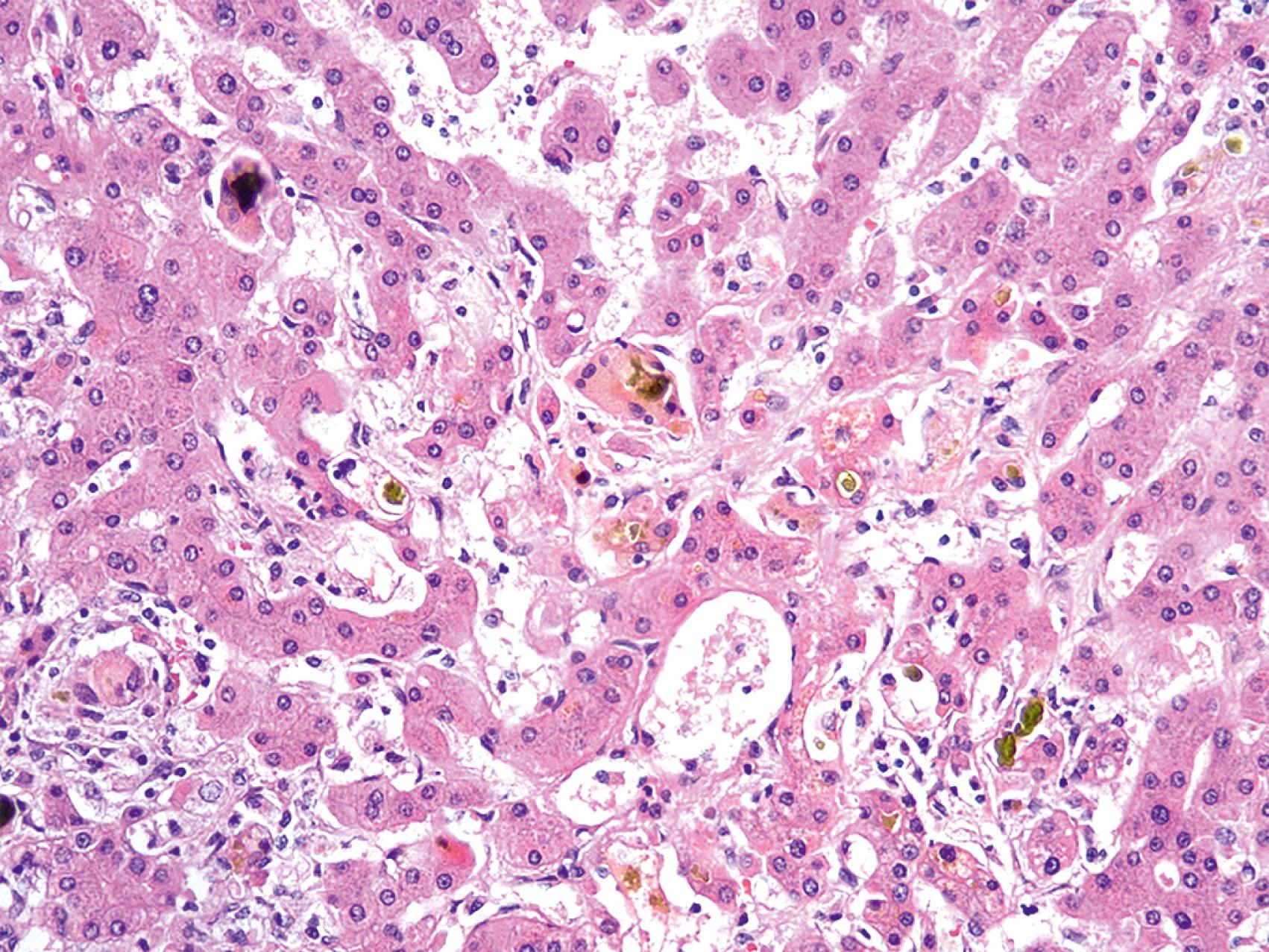
Chronic extrahepatic biliary obstruction causes fibrosis and, eventually, biliary-type cirrhosis. This pattern of cirrhosis is characterized by irregular, or “jigsaw puzzle piece,” cirrhotic nodules with prominent bile ductular proliferation, loss of the native intralobular bile ducts, and abundant cholestasis ( Fig. 66.5 ). The periportal hepatocytes show features of chronic cholestatic injury due to the digestion of cellular elements by bile acids. This chronic cholestatic injury is termed cholate stasis and is characterized by swollen, pale-staining hepatocytes with frequent deposition of Mallory hyaline (i.e., Mallory-Denk bodies). Copper accumulates in the hepatocytes undergoing cholate stasis because copper that is normally exported to the intestines via bile remains in hepatocytes, sometimes to Wilson disease levels.
Portal-based changes of biliary obstruction in biliary atresia are not specific for biliary atresia, and they develop over time. In particular, early biopsies may not demonstrate definitive changes of extrahepatic biliary obstruction. Furthermore, other important causes of biliary obstruction pattern, such as choledochal cyst, alpha-1 antitrypsin deficiency (A1ATD), total parental nutrition (TPN)-induced injury, and others, are often histologically indistinguishable from biliary atresia. Liver biopsy is >90% sensitive at documenting the obstructive features of biliary atresia ; however, since specificity is relatively low, some centers have bypassed preoperative biopsy for the definitive test—intraoperative cholangiogram. This trend of bypassing liver biopsy is likely to continue into the future because treatment outcomes are better with decreasing baby’s age, while, at the same time, histology is less reliable in the youngest patients.
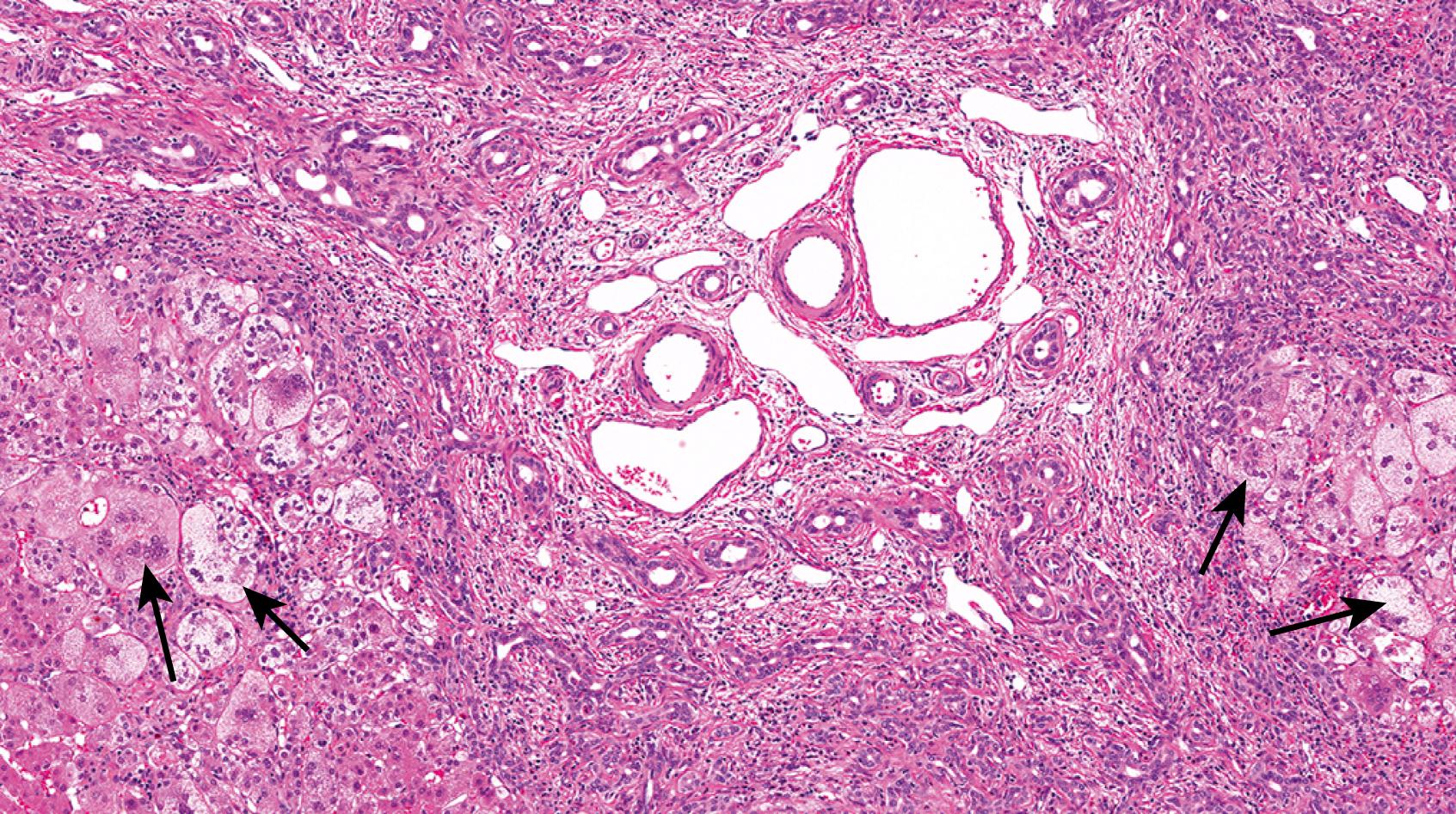
A1ATD is another cholestatic liver disease that can be a diagnostic challenge in young infants. The histology may be variable but most commonly demonstrates a portal-based picture of biliary obstruction. Alternatively, A1AT may also demonstrate a neonatal hepatitis pattern of injury. The pitfall is that the diagnostic A1AT inclusions may not be not well-developed until the patient is 12 weeks of age or older. The A1AT globules are located in the cytoplasm of the periportal hepatocytes. The globules can be seen on hematoxylin and eosin (H&E)-stained sections as slightly darker cytoplasmic inclusions ( Fig. 66.6 ) but are typically nicely accentuated by periodic acid-Schiff stain after diastase digestion (PAS/D; Fig. 66.7 ). A1AT globules are thus PAS positive and diastase resistant. Diastase is important because glycogen that fills all hepatocytes also stains positively with PAS, but glycogen is diastase sensitive. A1AT is an autosomal recessive disorder, so patients that are heterozygous for the A1AT mutant allele also may show accumulation of A1AT globules in the cytoplasm of periportal hepatocytes. On a case-by-case basis, homozygous A1AT deficiency cannot be absolutely distinguished from heterozygous mutation on pathology alone. That said, the heterozygous patients tend to harbor smaller, more granular-appearing globules rather than the larger space–occupying ones in homozygous deficiency.
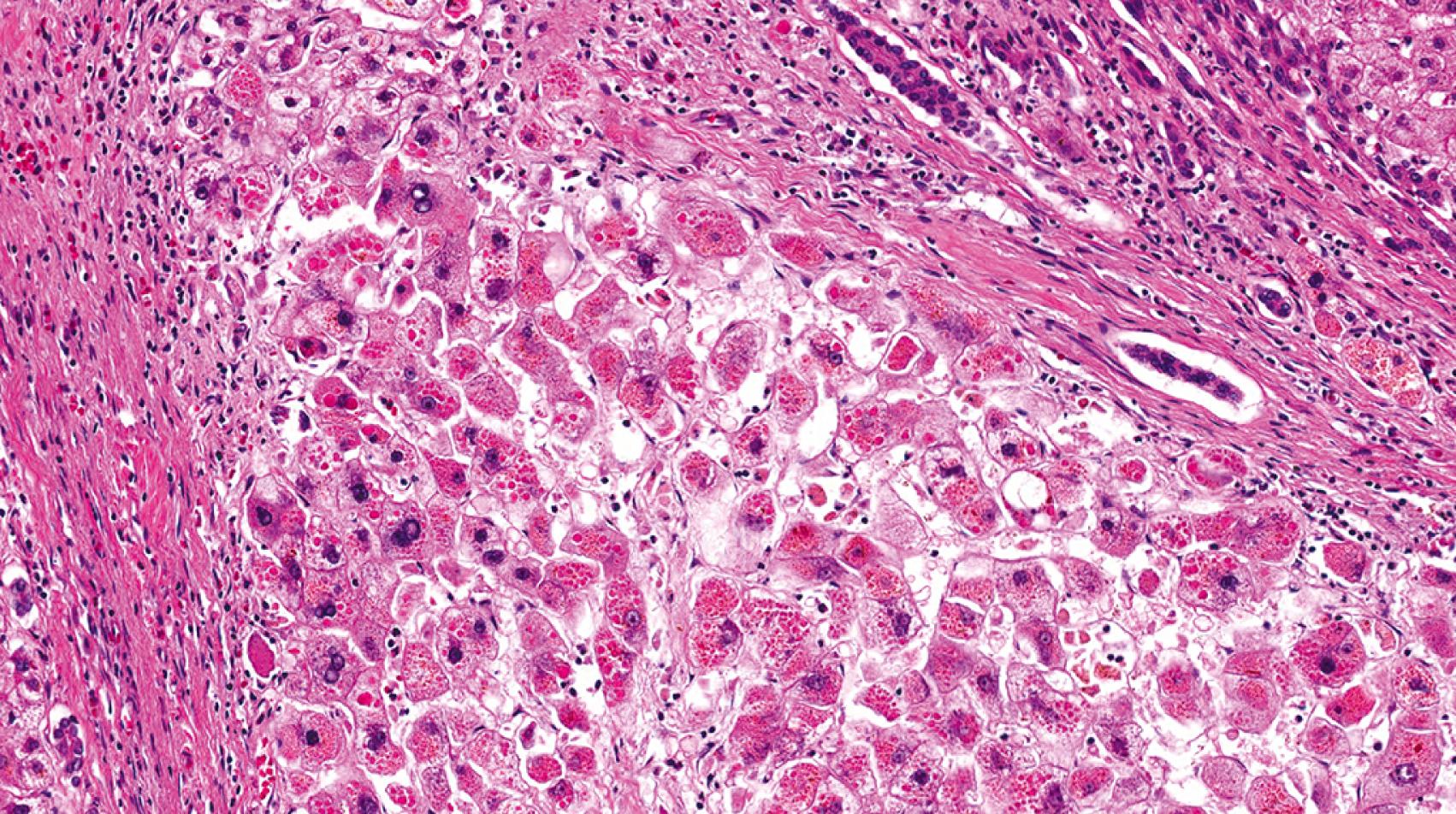
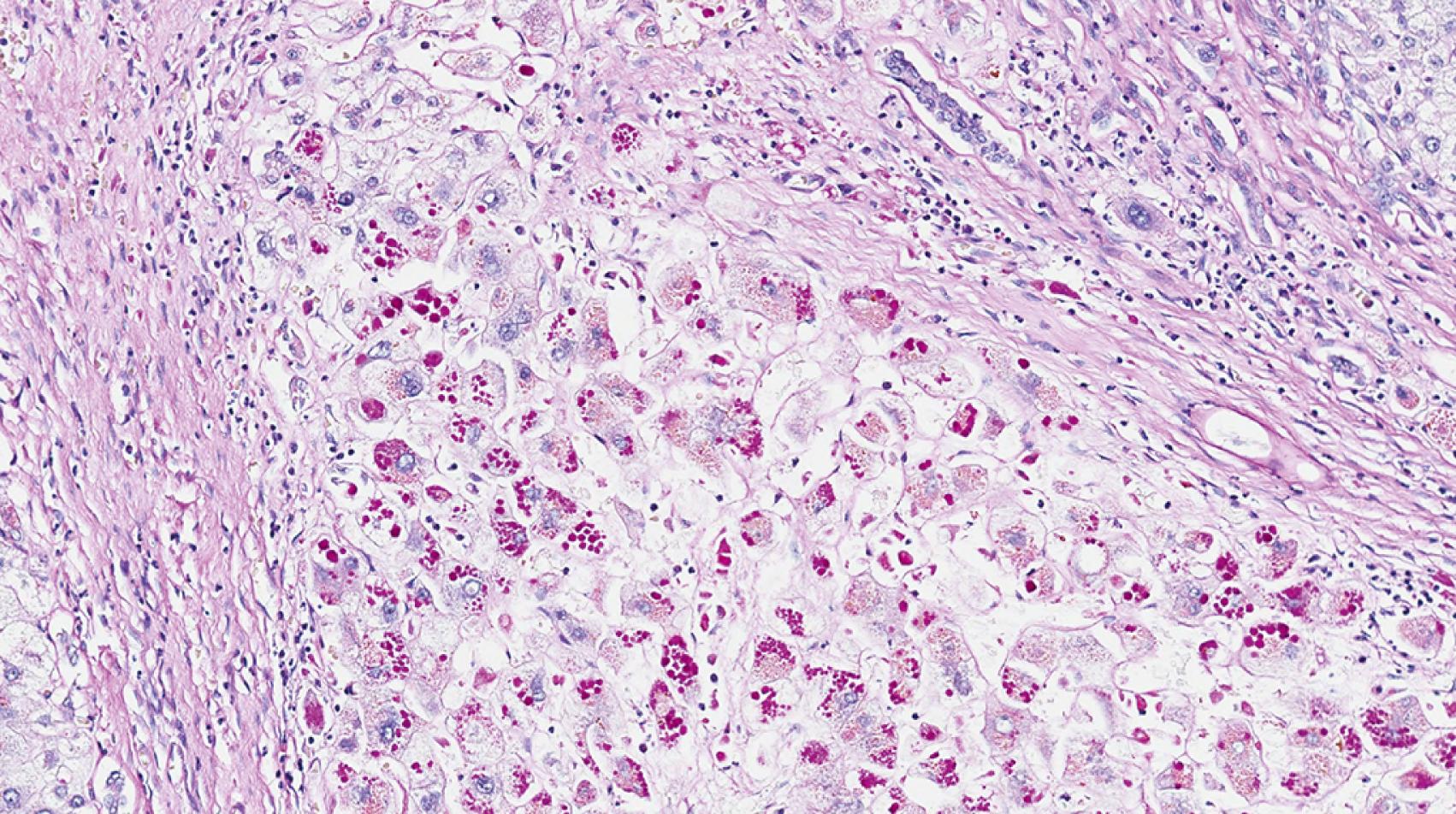
TPN-induced liver disease is another cause of cholestatic/obstructive pattern of injury. Accumulation of fat with or without steatohepatitis may be part of the pathologic spectrum of disease. The incidence of TPN-induced liver disease is higher in children (especially infants) than in adults. Most studies in infants tend to report progression of liver disease (i.e., fibrosis) with duration of TPN ; however others have not found severity of disease to depend on the duration of TPN. , Cholestasis may occur in 2 weeks, and cirrhosis has been reported in at little as 3 months.
When confronted with a liver demonstrating cholestasis and features of biliary obstruction, history is needed to document that the injury is derived from TPN. That said, a recent series of TPN-related liver disease found that ductopenia and perivenular fibrosis in addition to features of cholestasis/obstruction are fairly characteristic of TPN-related liver disease. Ductular cholestasis may also occur.
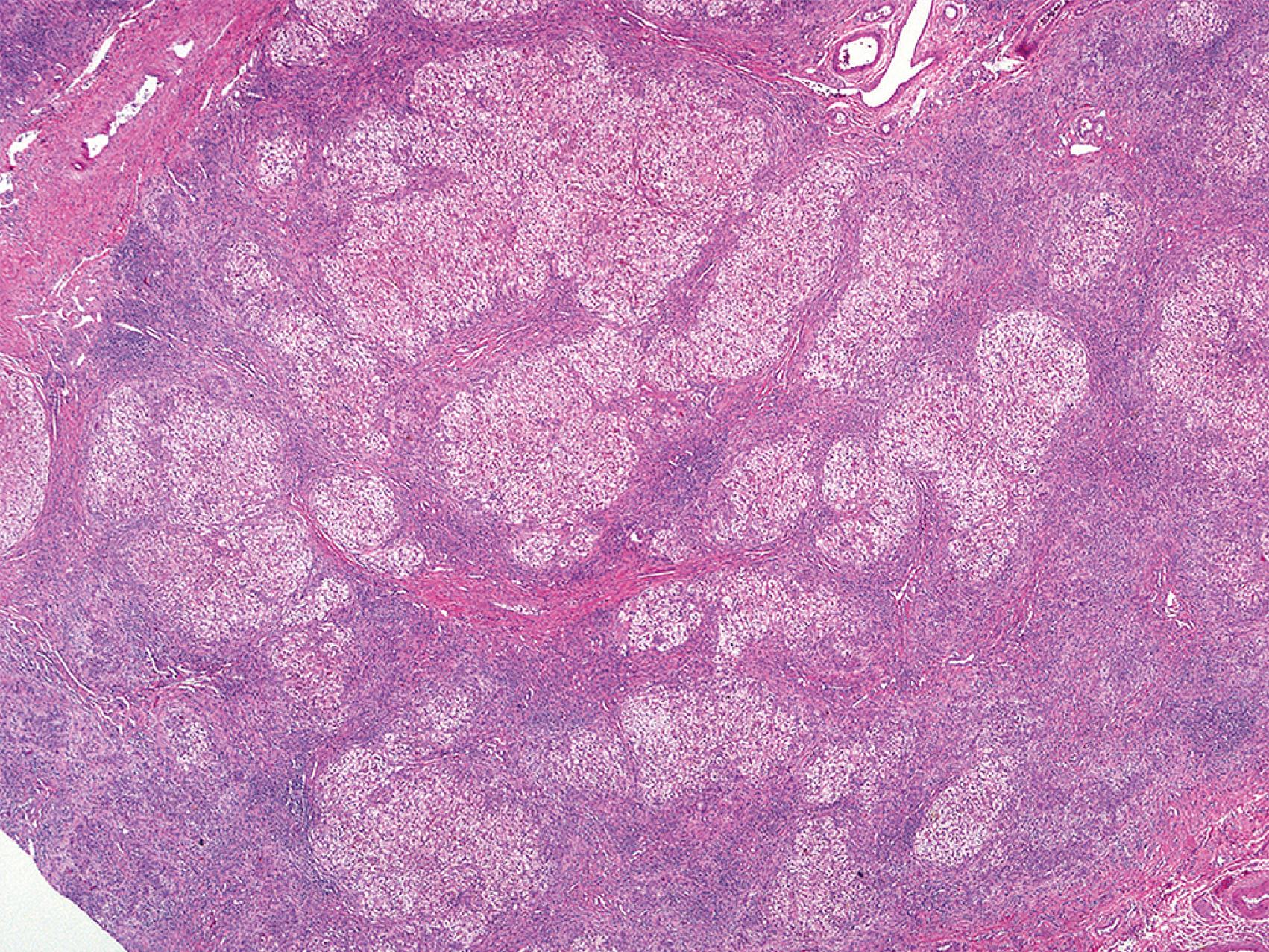
Neonatal giant cell hepatitis is defined by its lobule-predominant pattern of injury. In particular neonatal giant cell hepatitis is characterized by the formation of syncytial giant cells of hepatocytes ( Fig. 66.8 ), variable amounts of inflammation, and cholestasis ( Fig. 66.9 ). As neonatal giant cell hepatitis is a diagnosis of exclusion, other diagnoses must be excluded such as extrahepatic biliary obstruction (biliary atresia), paucity of intrahepatic bile ducts, and infections, among others.
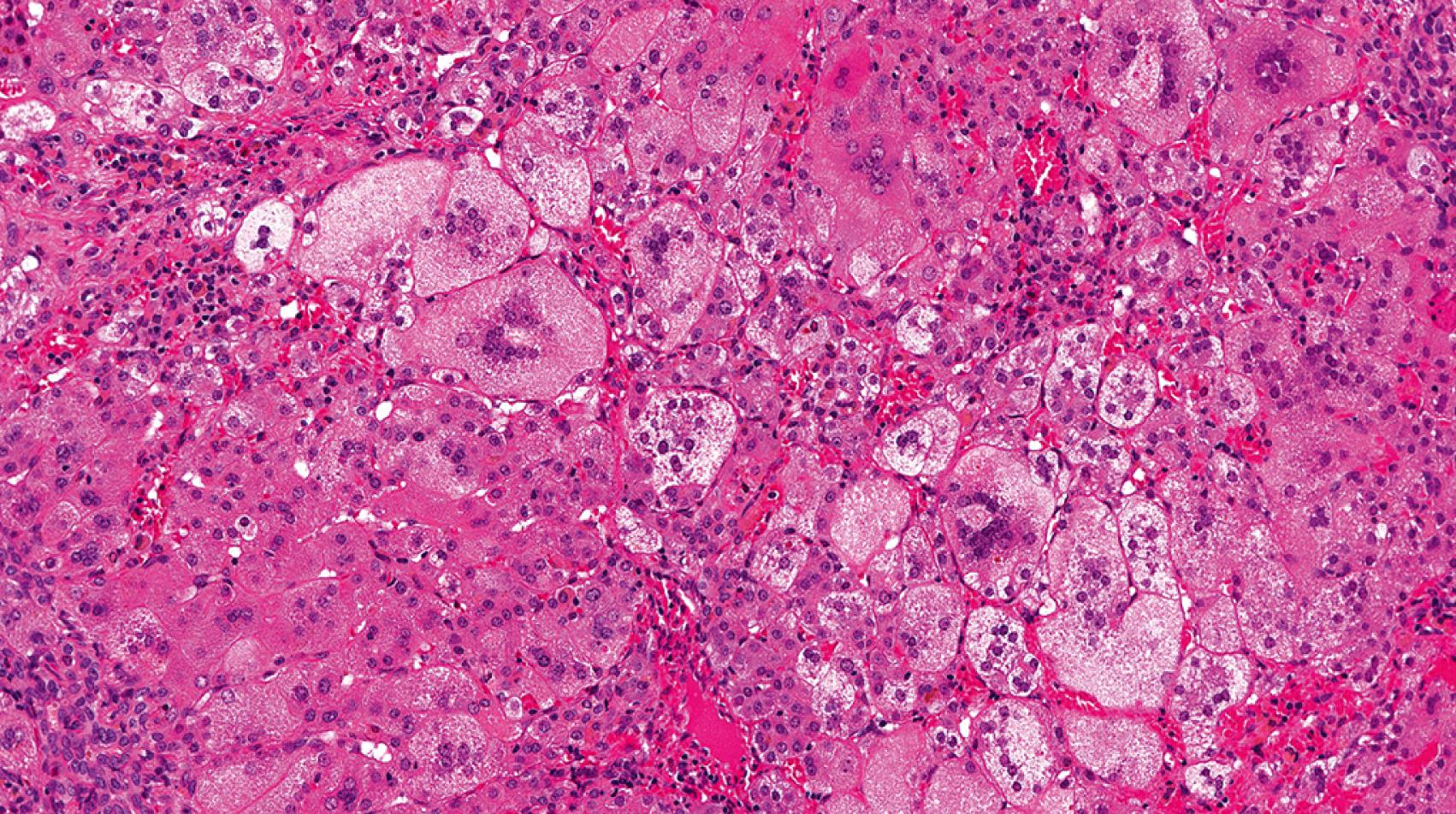
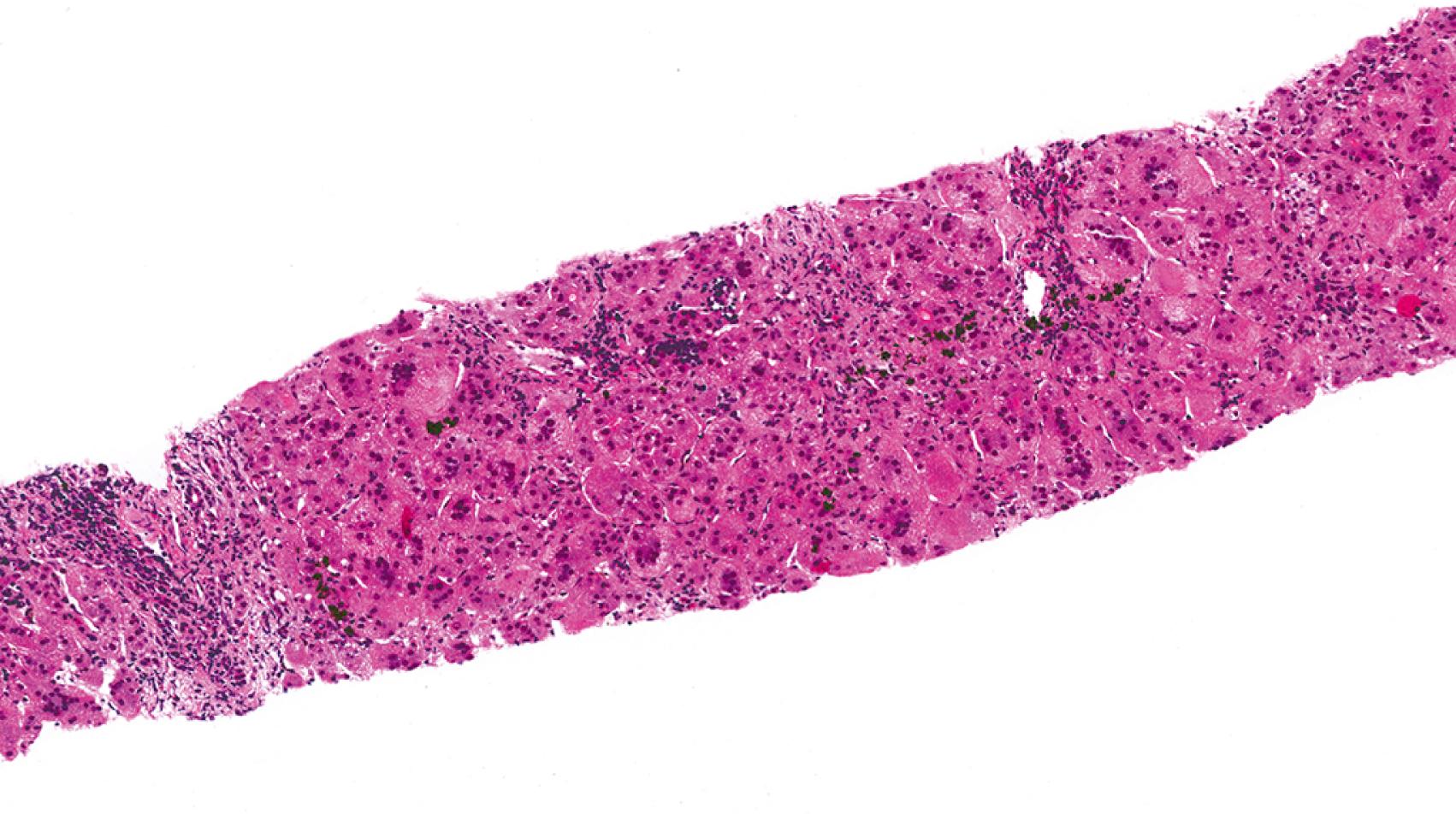
Idiopathic causes used to make up the majority of cases of neonatal cholestasis; however, as the clinical, laboratory, and molecular spectra of other neonatal cholestatic diseases have been increasingly defined, idiopathic neonatal hepatitis has become used less and less. Nevertheless, as suggested by a recent series, the most common cause of neonatal giant cell hepatitis pattern on liver biopsy is idiopathic (49% of cases), with hypopituitarism (16%), biliary atresia (8%), Alagille syndrome (6%), and bile salt defects (6%) being other important associations. It is quite important for the clinician not to assume the patient has the “idiopathic” form until many other possibilities have been excluded. That said, the most common cause of neonatal giant cell hepatitis remains unknown.
Panhypopituitarism may be the most “diagnosable” form of giant cell hepatitis. These patients may have bile duct hypoplasia more than other patients with giant cell hepatitis. Follow-up data is limited in these patients, but one series of nine infants found that cholestasis began at a median age of 13 days, with median age at diagnosis of 1.4 months. GGT levels were only elevated in two of nine patients, so pituitary deficiency is in the differential diagnosis of low gamma-glutamyl transferase (GGT) neonatal cholestasis (as discussed in the following section). Cholestasis resolved with treatment at a median of 88 days, and no cases of cirrhosis were reported.
Progressive familial intrahepatic cholestasis type 2, formerly Byler syndrome, has been renamed bile salt export pump (BSEP) disease because the disease-causing mutation ( ABCB11 ) encodes a BSEP. The BSEP is only expressed in the liver. These patients usually present with cholestasis in the neonatal period with a paradoxically low or normal GGT. Aminotransferase levels are often quite elevated, and progression to cirrhosis is fairly rapid, usually requiring transplant. Giant cell hepatitis with little inflammation is the most characteristic pattern in early biopsies of patients with BSEP disease ( Fig. 66.10 ). , However, a recent study found quite variable histology beyond hepatocellular cholestasis. Another study of biopsy findings found progressive familial intrahepatic cholestasis (PFIC2) as the most common cause of bile duct paucity—even more than biliary atresia or Alagille syndrome. Electron micrography reveals characteristic amorphous bile. Immunohistochemistry can be a useful adjunctive test in the diagnosis, as there are commercially available antibodies that target the BSEP. Not all mutations cause loss of antigen expression, so loss of BSEP by immunohistochemistry is much more meaningful than intact expression. After liver transplant, disease may recur.
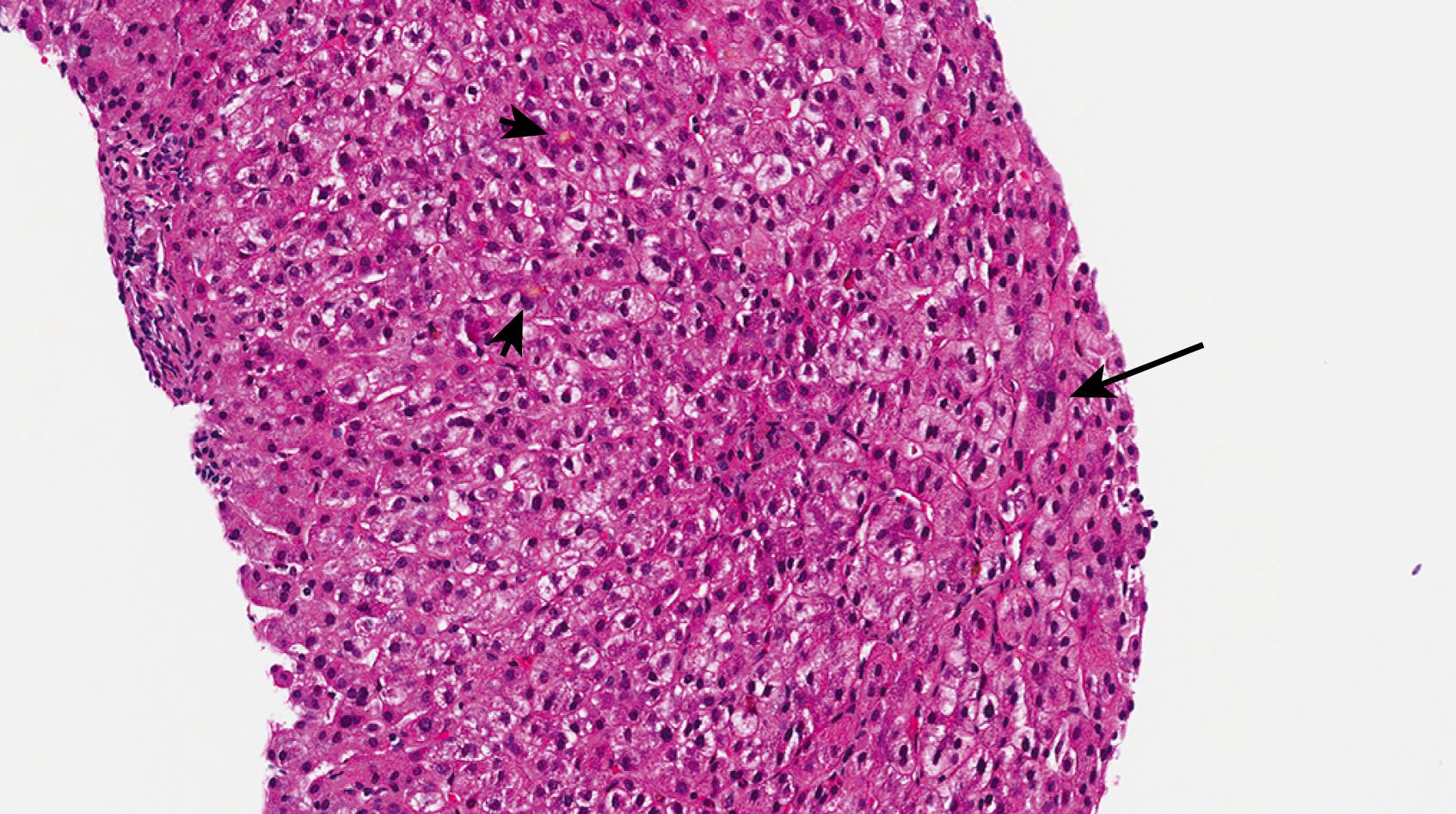
Progressive familial cholestasis type 4, or TJP2 disease, is caused by a homozygous or compound heterozygous mutation in the TJP2 gene, which encodes tight junction protein-2. This autosomal recessive disease presents as a low/normal GGT cholestatic disorder similar to PFIC-2. It tends to be severe with frequent progression to cirrhosis and need for liver transplant in childhood. A few cases of hepatocellular carcinoma (HCC) have been described in these patients. Although the histologic findings are not well described in the literature, the pathologic findings are reportedly similar to PFIC-2 and characterized by cholestatic hepatitis or cholestasis with giant cell transformation.
Bile acids (cholic and chenodeoxycholic acids) are synthesized from cholesterol after undergoing numerous enzymatic steps, with at least seven disease-causing gene mutations. Bile acid synthetic defects (BASD) are characterized by decreased serum bile acids, low/normal GGT levels, and paradoxical absence of pruritus. When these patients present in the neonatal period (most commonly 5β-reductase deficiency and 3β-hydroxy-Δ 5 -C 27 -steroid dehydrogenase deficiency), the patients present with a giant cell hepatitis pattern of injury. These two most common BASD in the neonatal period are both autosomally inherited and are thought to cause injury secondary to impairment of normal bile flow with back-up of toxic bile acid precursors.
Progressive familial intrahepatic cholestasis type 1, formerly Byler disease, has been renamed FIC1 deficiency after the protein (FIC1) that is encoded by the disease-causing mutations in the gene ATP8B1. FIC1 functions as a phospholipid flippase that transfers phosphatidylserine from the outer cell membrane to the inner membrane. There is a disease spectrum under the umbrella of FIC1 deficiency, all of which are inherited in an autosomal recessive manner. Benign recurrent intrahepatic cholestasis (BRIC) is on the indolent side of this spectrum, so it rarely necessitates liver pathology input. Severe forms of FIC1 deficiency tend to present with cholestasis in the first year of life with progressive fibrosis. Cirrhosis occurs at a variable rate. FIC1 protein is also expressed on other organs, such as intestines and pancreas, so these patients may also demonstrate other organ-specific problems such as diarrhea, malabsorption, failure to thrive, and sensorineural hearing loss. GGT levels are typically normal or low, whereas bile acids are markedly elevated.
Precirrhotic histologic changes are characterized by so-called “bland” cholestasis, namely lobular cholestasis without any other significant pathologic findings such as features of biliary obstruction, inflammation, or giant cell hepatitis ( Fig. 66.11 ). Others have described this pattern as pure cholestasis. From the time of the original Byler disease descriptions, the unique qualities of bile have been described, and the term “Byler bile” was introduced shortly thereafter. On light microscopy, the bile in FIC1 deficiency is pale gray rather than green-brown as in other cholestatic disorders. On electron microscopy, the bile has a characteristic coarse granular quality, although this is only evident on tissue that has not undergone formalin fixation with paraffin embedding. If the patient requires liver transplantation, this patient is at risk for hepatic steatosis, steatohepatitis, and even cirrhosis.
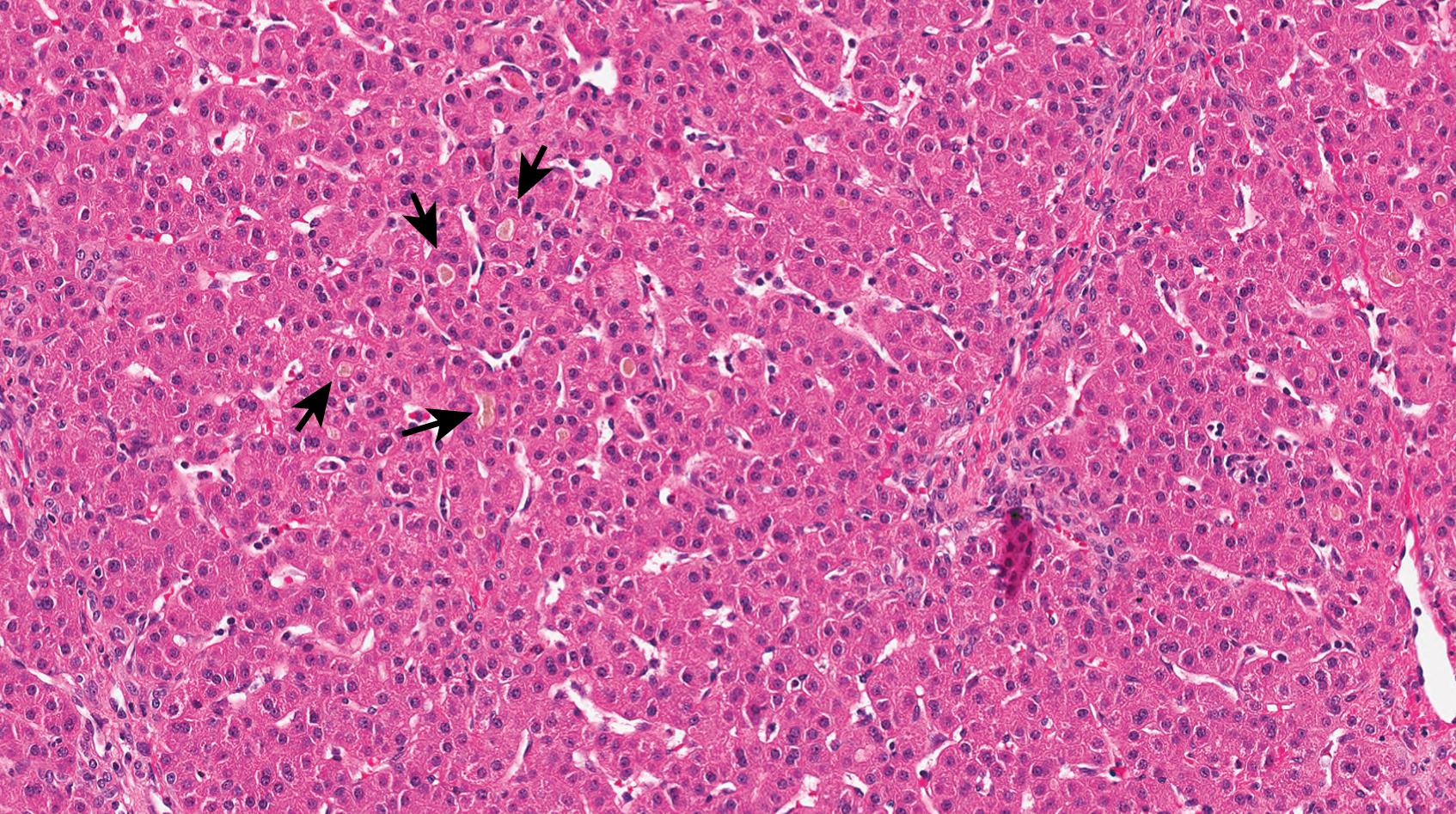
About half of neonates with bacterial septicemia will demonstrate liver enzyme abnormalities, most commonly stemming from cholestatic jaundice, but these numbers may be reflective of gram-negative septicemia alone. For instance, one study found that gram-positive septicemia caused liver enzyme abnormalities in only about 13% of neonates, compared to 46% with gram-negative sepsis. Cholestasis usually manifests a few days after onset of sepsis, and, in most surviving patients, the bilirubin normalizes within 1 to 2 months. Rarely, the direct bilirubin goes beyond 15 mg/dL in septic patients. Cholestasis of sepsis with or without hemolysis may manifest as bland cholestasis (lobular or canalicular cholestasis without other specific features), but the classic finding of cholestasis of sepsis is so-called “cholangitis lenta,” whereby the cholestasis is present within periportal areas and bile ductules (without other features of biliary obstruction). , This finding is probably more common in the setting of autopsy liver pathology, as it is more often seen in severe systemic disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here