Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
MRgLITT is a rapidly evolving technology that is being applied for an increasing number of types of focal epilepsy.
The most common application of MRgLITT in epilepsy continues to be in mesial temporal lobe epilepsy (MTLE).
MRgLITT has also been used in conditions such as focal cortical dysplasia (FCD), insular epilepsy, hypothalamic hamartoma (HH), and, less commonly, tuberous sclerosis, cerebral cavernous malformation, and corpus callosotomy.
MRgLITT has an improved risk profile compared with traditional surgery. Although this is exchanged for a lower likelihood of seizure freedom in certain pathologies such as MTLE, it still provides an acceptable likelihood of seizure freedom and does not preclude further interventions if required.
The term laser ( l ight a mplification by s timulated e mission of r adiation) describes a device that creates, amplifies, and emits a beam of light with coherent photons. Lasers originate from Einstein’s 1916 proposal of “spontaneous emission” wherein an electron that is excited by the addition of energy can resume a more stable lower energy state by emitting photons . Rudolf Ladenburg and Valentin Fabrikant further characterized the phenomenon of light amplification by stimulated emission. In 1951, Charles Towens suggested that stimulated emission at microwave frequencies could produce coherent output following oscillating in a resonant cavity. Three years later, Towens and Gorden revealed the first maser ( m icrowave a mplification by s timulated e mission of r adiation). ,
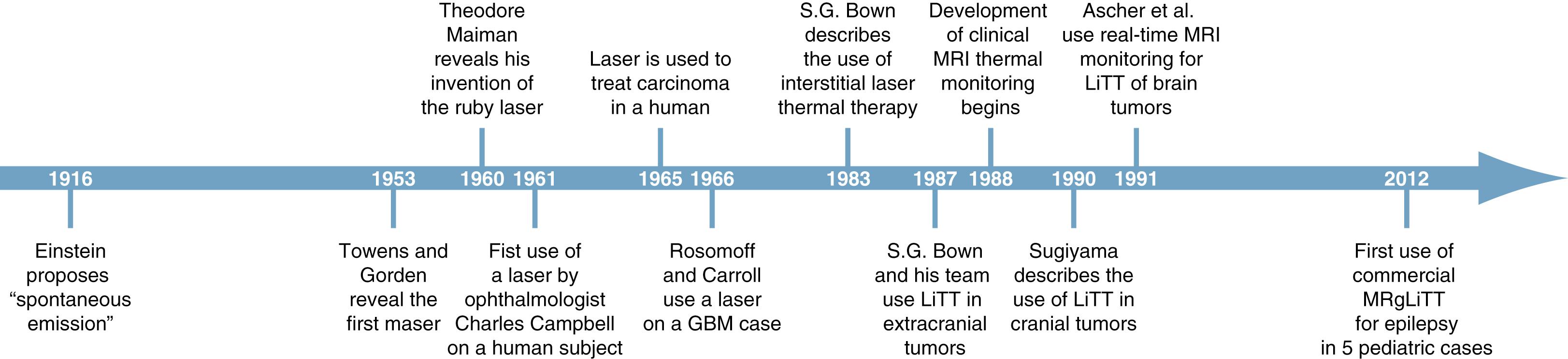
Following the development of the maser, physicists dreamed of extending the principle to higher frequencies and into the optical range. The race was on to build the first laser device. In May 1960, Theodore Maiman revealed his ruby laser at Hughes Research Laboratories and published his initial findings in Nature . Soon afterward, an exponential growth of laser technology attracted multinational corporations as well as defense organizations such as the Pentagon. Maiman described five potential applications of his laser, including concentrating light for industry, chemistry, and medicine. Less than 2 years after his invention, lasers found their first application in human medicine. Ophthalmologist Charles Campbell at Columbia University became the first to photocoagulate the retina of a human subject in November 1961. Only 3 years after the invention, McGuff et al. reported the use of a laser on tumors in rodent models. , Earle et al. and Fine et al. were among the first to study the effects of laser on the brain. In 1965, pioneers at the University of Pittsburgh, including neurosurgeon Hubert Rosomoff, utilized the laser for treating human carcinoma (squamous cell of larynx and lung carcinomatosis). In 1966, Rosomoff and Carroll used a ruby laser in a glioblastoma case and showed that it caused near total cell necrosis in neoplastic tissue; penetration could be achieved through the tumor; and normal brain tissue was relatively resistant to laser injury ( Fig. 98.1 ).
In its earliest incarnations, laser therapy was used externally, that is, the laser was emitted outside the target area. In 1983, S.G. Bown described the concept of interstitial laser therapy in his seminal paper “Phototherapy of Tumors,” along with an extensive review of lasers for tumor therapy. Bown emphasized that with external therapy, care must be taken to keep the tip clean when the fiber tip is stationed above the surface of the target area because a small amount of cellular debris on the tip can absorb energy, heat the tip, and risk destroying it. On the contrary, when the tip is inserted within tissue (a technique he called interstitial therapy ), it is in direct thermal contact with a larger volume. This results in a higher tolerance of the tip of the fiber because a larger quantity of energy is needed to damage it secondary to the conduction of heat by surrounding tissue. This allows nearby tissue to be heated to higher temperatures. The fiber tip acts as a source of energy; thus thermal energy is conducted away from it and the distribution is roughly spherical. The energy delivered is almost entirely absorbed by nearby tissue compared with external delivery in which the absorbed amount is dependent on the nature of the surface under the beam. In 1987, Bown reported the use of intrahepatic laser thermal therapy in rats and promulgated the notion that laser-coagulated tissue is replaced by fibrosis. In 1989, Bown and his team combined real-time imaging with laser therapy for a variety of extracranial tumors in humans. The first report of laser interstitial thermal therapy (LITT) for brain tumors (gliomas or brain metastasis) was from Japan in 1990 and was followed soon after by reports from Europe further describing the efficacy and safety of LITT for brain tumors.
The combination of LITT with real-time MRI guidance was the technological inflection point that transformed the application of this technology. In 1988, Jolesz et al. utilized real-time MRI of laser ablation in a rabbit brain to report reversible tissue changes below 60°C and coagulation from 60°C to 100°C. This was followed by a number of studies characterizing the thermosensitivity of tumor cells, thus MRI thermal monitoring has been optimized for measurements up to 100°C as well as temperatures just below 45°C. In 1991, Ascher et al. utilized real-time MRI monitoring for LITT of brain tumors. Today, LITT is an established stereotactic technique for targeting, heating, and ablating deep structures with real-time MRI guidance and monitoring (MRgLITT). Although the application for epilepsy lagged behind the application for brain tumors, it is now broadly implemented and widely used for medically intractable focal epilepsy.
The earliest descriptions of stereotactic ablation for epileptogenic foci date to the early 1960s in Japan. Variations of this technique with developments in imaging allowed for major improvements in ablation procedures. In 2012, the first report of MRgLITT of epileptogenic foci using a commercially available device approved by the US Food and Drug Administration was published. The utilization of minimally invasive surgery for medically intractable epilepsy has grown exponentially since then, including combinations of LITT with minimally invasive localization of seizures using stereo-electroencephalography (SEEG).
Before the development of MRgLITT, treatment options for minimally invasive approaches for epilepsy included thermocoagulation, radiofrequency lesioning, and stereotactic radiosurgery. Although both radiofrequency ablation and MRgLITT can be used to target deep structures that are challenging to access via open craniotomy, radiofrequency ablation does not provide the real-time feedback on tissue coagulation that MRgLITT offers. Radiofrequency thermal damage relies on tissue conduction and is difficult to control. Furthermore, the radiofrequency energy source may be imprecise. Radiofrequency is thus a “blind” approach. The mismatch between the radiofrequency-ablated volume and the epileptogenic target may be large enough to require multiple probe passes. The inability to accurately target the thermal spread also increases the potential for complications resulting from damage to surrounding structures. Thermal spread is software-controlled by MRgLITT and allows temperature control beyond the tip of the probe, unlike radiofrequency. Concurrent imaging optimizes the ablation zone and minimizes the potential for complications, favoring the use of MRgLITT over radiofrequency thermocoagulation. Here we detail the specific applications of MRgLITT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here