Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Amniocentesis is used from 15 weeks of gestation onwards for prenatal diagnosis of chromosomal abnormalities, single-gene disorders, fetal lung maturity, fetal infections and inflammation.
Chorionic villus sampling (CVS) is used from 10 weeks of gestation onwards for prenatal diagnosis of single-gene defects and chromosomal abnormalities.
Early amniocentesis (<15 weeks) and early CVS (<10 weeks) have been proscribed because of increased risk for fetal loss rate and structural abnormalities
The procedure-related fetal loss rates associated with amniocentesis and first trimester CVS performed by trained specialists at appropriate gestations appear to be low (∼0.1% and 0.2%, respectively).
In equally experienced hands, both the transcervical and the transabdominal routes for CVS have a similar fetal loss rate.
The indications for diagnostic fetal blood sampling and other invasive procedures are now limited because of the availability of less invasive or noninvasive methods.
Chorionic villus sampling (CVS), amniocentesis and to a lesser extent fetal blood sampling are the most common invasive diagnostic procedures performed prenatally. Amniocentesis was first introduced in the early 1880s as a therapeutic procedure to treat polyhydramnios. The technique evolved over the years to be used for the assessment of fetal well-being, including monitoring of Rh-alloimmunised pregnancies. The use of amniocentesis for exclusively genetic indications began in the mid-1950s from early work on fetal sex determination by X-chromatin analysis of amniotic fluid cells (AFCs), to several reports of successful diagnosis of a wide variety of chromosomal and metabolic disorders over the next few years. CVS was introduced and has been used solely for genetic diagnosis and remains the invasive procedure of choice in the first trimester. Fetal blood sampling is used less frequently for genetic diagnosis (generally after 18 weeks), but its main role is in confirming the diagnosis fetal anaemia.
This chapter addresses current technique and the safety of genetic amniocentesis, CVS and fetal blood sampling. Indications and methods of prenatal diagnosis are considered in detail throughout this text. In addition, a brief consideration of the impact of noninvasive prenatal testing on the role of these diagnostic procedures is included.
Whenever possible, preconception counselling to discuss genetic risks and available antenatal testing options before pregnancy should be made available to every couple. When an indication for an invasive diagnostic test has been identified, the couple must be informed about the risks associated with such procedures, the accuracy and limitations of prenatal diagnosis, the time required before results become available, technical problems potentially necessitating a repeat procedure, and the rare possibility of an inability to make a diagnosis.
Amniocentesis should be performed only by a trained obstetrician who has acquired adequate skill and experience in this procedure, has the availability of high-quality ultrasonography and has access to a laboratory with experience in performing prenatal diagnostic tests. The recommendation that the procedure should be performed by an obstetrician is not because of technical difficulty but because the operator must always be prepared to deal with the potential complications of the procedure. According to the American College of Obstetricians and Gynecologists (ACOG), if a serious abnormality is detected and the couple elects to terminate the pregnancy, the obstetrician must either perform the abortion or refer the family to a provider who will act on their request.
Amniocentesis can be performed from about the 15th week of gestation, when the ratio of viable to nonviable cells is greatest. Early amniocentesis (EA) performed before 14 weeks’ gestation, and transvaginal amniocentesis is only of historical interest because of the technical difficulty as well as associated risks of infection and spontaneous abortion.
After a thorough ultrasonographic examination, a needle insertion site is selected. Under continuous real-time ultrasound guidance, the needle is inserted to the optimal pocket of amniotic fluid (AF) while avoiding the fetus. Avoiding needle insertion through the placenta is desirable but not mandatory. Although Tabor and coworkers reported that transplacental needle insertion increased the risk for the procedure, this has not been confirmed by others.
Local anaesthetic is typically not needed. It is uncertain if the site of needle placement affects the level of pain. Counselling before amniocentesis should emphasise that the actual pain and anxiety experienced during the procedure are significantly lower than expected.
The maternal skin is cleansed with an iodine- or alcohol-based solution; sterile drapes are then placed around the needle insertion site to help maintain an aseptic field. A disposable 22-gauge spinal needle with stylet is most frequently used. During the entire procedure, the needle tip should be continuously visualised using two-dimensional real-time ultrasound monitoring. In view of the extensive anterior placenta, this procedure was performed transplacentally. Use of four-dimensional ultrasound guidance has been suggested, but there are no objective data to indicate improved outcomes ( Fig. 23.1 and ![]() , ).
, ).
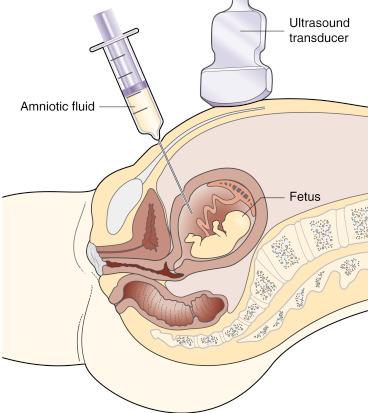
After confirming that the needle is in its proper location, the stylet is removed, and a 10- or 20-cc syringe is attached to the hub of the needle. The initial 1- to 2-mL sample is usually discarded. Ten to 20 mL of AF is usually aspirated into sterile disposable plastic syringes, although as little as 3 to 5 mL of AF has been shown to suffice for reliable prenatal cytogenetic results. Maternal cell contamination appears to occur more frequently in genetic amniocentesis samples that are obtained by physicians who perform fewer than 50 genetic amniocentesis annually. Investigators have described using a vacuum container aspiration technique for amniocentesis, but there appears to be little, if any, advantage over using a syringe technique.
Amniocentesis had been reported to be unsuccessful with rates as high as 5.9% to 10.6%, especially in the era when concurrent ultrasound guidance was not the norm. Because real-time ultrasound guidance has become routine, failure to obtain AF occurs far less. However, this is much more problematic with EA. When performed at 15 to 16 weeks of gestation by experienced practitioners, failure to obtain AF should occur in fewer than 1% of cases.
Training in performing amniocentesis has traditionally been by trainees observing experienced operators followed by the trainees performing the procedure under direct supervision of the mentors. Modern high-fidelity simulator-based models might be useful for teaching amniocentesis because trainees’ performance has been shown to improve with experience on the simulator.
Amniocentesis can be performed successfully in most twin pregnancies perhaps with no increased risk compared with singleton gestations undergoing amniocentesis, although the risk is difficult to quantify accurately because of the lack of randomised studies.
Separate amniocentesis of each sac is used in most centres in the United States to assess individual fetuses irrespective of the chorionicity. Each amniotic sac may be identified if the clinician injects a dye (indigo carmine) immediately after aspiration of the first AF sample but before withdrawal of the needle. After completion of the first amniocentesis, a second amniocentesis is performed in the ultrasonographically located area of the other fetus. Aspiration of clear AF indicates that the second sac was successfully entered; aspiration of blue-tinged AF indicates that the original sac was reentered. However, this is not necessary to perform routinely because visualisation of the membranes separating the sacs is generally possible. Methylene blue dye is proscribed because it has been associated with high risk for small intestine atresia and fetal death.
Single-needle insertion under ultrasound guidance to sample both sacs in twins has been reported. The main concern is that the single-puncture technique could lead to cross-contamination between sacs, resulting in diagnostic inaccuracy. The technique described by Jeanty and colleagues uses a single myometrial needle puncture into the first amniotic sac and then through the membranous septum into the second sac. This technique has been validated by Sebire and coworkers, with no increase in cell contamination between twin fetuses or increased risk for pregnancy loss.
Using the above techniques, experienced investigators have been successful in obtaining information regarding both fetuses in more than 90% to 95%. The reported loss rates after amniocentesis in twins varies from 0.6% to 2.7%.
Amniocentesis has been performed in several triplet pregnancies, with successful aspiration of fluid from all gestational sacs. Still, data are insufficient to make any statement regarding risks of amniocentesis in triplet gestations.
Life-threatening maternal risks are extremely rare. Amnionitis occurs in approximately 1 per 1000 women who undergo amniocentesis. This may lead to fetal loss but is seldom life threatening to the mother.
Minor maternal problems, however, are not rare. Approximately 2% to 3% of women experience transient vaginal spotting or leakage of AF after amniocentesis. Although almost always limited in amount and duration, AF leakage could persist and lead to oligohydramnios and pregnancy loss. Oligohydramnios is a well-known cause of fetal deformation and pulmonary hypoplasia.
Uterine contractions or cramping immediately after amniocentesis are not rare. Again, expectant management and reassurance are generally all that is required.
Potential fetal risks include spontaneous abortion, injuries caused by needle puncture, placental separation, chorioamnionitis, premature labour and injury caused by the withdrawal of AF (e.g., amniotic bands). Rare but reported direct needle injuries include ileocutaneous fistula, peritoneoparietal fistula, gangrene of an arm, ocular trauma, ileal atresia, porencephalic cysts, patellar disruption, brain injuries, peripheral nerve injury and umbilical cord haematoma. Some of these problems are more logically attributed to amniocentesis than others, and all except a few of these case reports are from the era before concurrent use of real-time ultrasound guidance.
Amniocentesis has been associated with an increase in the rate of vertical transmission of human immunodeficiency virus (HIV) type 1. However, with the advent of retroviral chemoprophylaxis, the risk for transmission as a result of amniocentesis has been markedly reduced. Bucceri and coworkers reported nine HIV-infected women who underwent amniocentesis between 16 and 20 weeks of gestation. Six of these women were on chemoprophylaxis, and none of 10 infants born to these women were infected. The International Perinatal HIV Group reported that five of nine HIV-infected women not on chemoprophylaxis undergoing amniocentesis delivered infected infants, but none of five infants born to women taking zidovudine were infected. Recently, a 4-year report from an Italian registry including selected HIV-infected pregnant women receiving combined antiretroviral prophylaxis found no cases of vertical transmission after amniocentesis or CVS. It appears from this limited evidence that HIV-infected women electing to have amniocentesis may benefit from chemoprophylaxis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here