Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Glioblastoma (GBM) is one of the deadliest forms of cancer, with a median survival of approximately 15 months. Standard of care treatment of GBM includes maximal safe surgical resection, fractionated radiotherapy, temozolomide chemotherapy, and, most recently, tumor treatment fields. Despite this combination treatment regimen, tumor progression or recurrence occurs after a median of 7 months. Overall survival of patients with GBM is determined by multiple factors, including the patient’s performance status (Karnofsky performance status [KPS]), age, tumor DNA repair status, and extent of surgical resection.
Despite advances in surgical techniques and radiographic imaging, maximal resection has been shown to be achieved in only 35% of cases. Intraoperative brain shift, poorly demarcated tumor margins, and proximity to eloquent brain all affect the surgeon’s ability to achieve the maximal extent of resection (EOR). Therefore, there is renewed focus on improving the intraoperative imaging and visualization of GBM to overcome these inherent limitations during surgery. This chapter investigates the various surgical adjuncts available to aid in the surgical resection of GBM, namely neuronavigation with MRI, diffusion tensor imaging (DTI), functional MRI (fMRI), intraoperative MRI (iMRI), and intraoperative tumor visualization for fluorescence-guided surgery (FGS).
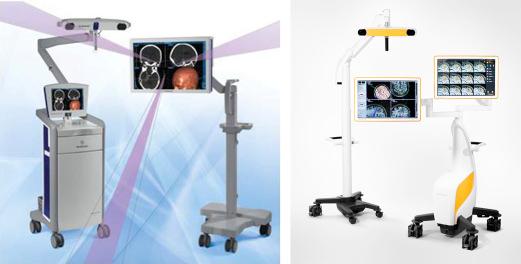
Frameless stereotactic navigation devices have been an indispensable tool for tumor removal by neurosurgeons since they were described by Barnett and colleagues in 1993. At present, 2 commonly used devices are the StealthStation S7 (Medtronic, Minneapolis, MN) and the Brainlab Curve (Brainlab AG, Germany) ( Fig. 14.1 ). Patients undergo high-resolution MRI before surgery. Typical sequences used are postcontrast three-dimensional, T1-weighted gradient-echo (magnetization-prepared rapid gradient-echo) and T1-weighted spin echo. These preoperative MRI scans are subsequently uploaded to the device and registration is performed after the patient’s head is immobilized by 3-pin fixation. Registration is accomplished through the use of skin fiducials (paired-point method) or laser surface registration. The surgeon uses a metal pointer on the scalp to assist with planning for the craniotomy and incision. Intraoperative guidance is performed by using a sterile metal pointer in the surgical field to identify anatomic structures and tumor boundaries ( Fig. 14.2 ). When applied in the surgical field, the average radial accuracy has been reported to be 2.4 ± 1.7 mm. Lesions in the frontal lobe have the highest navigation accuracy, whereas infratentorial lesions have the highest rate of error. Periventricular tumors and those with a volume greater than 30 mL also have a higher rate of registration error.
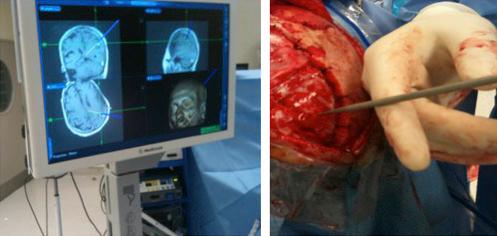
Because frameless stereotactic neuronavigation devices have been present for more than 2 decades at the time of this writing, the main advantages are their time-tested nature and ease of use. Despite these advantages, there are several significant limitations with neuronavigation during GBM resection. First, patient registration can be imprecise, causing delays in operating room workflow. Second, the entry of atmospheric air into the cranial vault, tumor debulking, and the use of hyperosmolar solutions can potentiate brain shift, which exacerbate errors in registration. Navigation errors of a centimeter or more have been reported. These discrepancies can limit the neurosurgeon’s ability to precisely excise the tumor, and increase the frequency of subtotal resections. In addition, the risk of inadvertent violation of eloquent brain is increased with imprecise navigation. Third, there are currently no reliable methods to account for the inaccuracies in navigation caused by brain shift.
With these limitations in mind, tumor neurosurgeons have been progressively examining the use of advanced intraoperative imaging techniques such as DTI, and fMRI (blood oxygen level–dependent fMRI). These advanced magnetic resonance (MR) techniques, which are discussed further in this chapter, have shown promise in improving the EOR and functional outcomes after GBM surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here